Abstract
We have examined the role of nitric oxide (NO) in a model of functional angiogenesis in which survival of a skin flap depends entirely on angiogenesis to provide an arterial blood supply to maintain tissue viability.
The different effects of nitric oxide synthase (NOS) inhibitors on rat skin flap survival appeared to be explained on the basis of their NOS isoform selectivity. Skin flap survival was decreased by iNOS-selective (inducible NOS) inhibitors, S-methyl-isothiourea, aminoguanidine and aminoethylthiorea; unaffected by the non-selective inhibitor nitro-imino-L-ornithine; and enhanced by the cNOS (constitutive NOS, that is endothelial NOS (eNOS) and neuronal NOS (nNOS)) inhibitor, nitro-L-arginine methyl ester.
Skin flap survival was reduced in mice with targeted disruption of the iNOS gene (iNOS knockout mice), and the administration of nitro-L-arginine methyl ester significantly increased flap survival in iNOS knockout mice (P<0.05).
iNOS immunoreactivity was identified in mast cells in the angiogenic region. Immunoreactive vascular endothelial growth factor (VEGF) and basic fibroblast growth factor were also localized to mast cells.
The combination of interferon-γ and tumour necrosis factor-α induced NO production and increased VEGF levels in mast cells cultured from bone marrow of wild-type, but not iNOS KO mice.
The increased tissue survival associated with the capacity for iNOS expression may be related to iNOS-dependent enhancement of VEGF levels and an ensuing angiogenic response. Our results provide both pharmacological and genetic evidence that iNOS activity promotes survival of ischaemic tissue.
Keywords: Skin flap surgery, angiogenesis, nitric oxide, nitric oxide synthase inhibitors, vascular endothelial growth factor
Introduction
Angiogenesis in the adult occurs physiologically in wound healing and in the menstrual cycle, but may also occur pathologically in conditions such as cancer and chronic inflammation (e.g. rheumatoid arthritis) (Folkman, 1995). There is intense interest in the possibility of therapeutic modulation of angiogenesis to facilitate wound healing and inhibit tumour growth or chronic inflammation (Fan et al., 1995). By contrast, the importance of angiogenesis in reconstructive surgery (transfer of skin grafts, skin flaps and prefabricated tissues) and replantation of limbs or digits has received little attention. Enhancement of the angiogenic process is potentially a major contribution to the safety and speed of these tissue transfers and broadens the scope of re-vascularizations.
Non-developmental angiogenesis involves increases in production and release of angiogenic factors such as vascular endothelial growth factor (VEGF) which directly stimulate endothelial cells to degrade the extracellular matrix, migrate, proliferate and organize endothelial cells to form capillaries (Neufeld et al., 1999). A vast number of pro-inflammatory endogenous mediators including tumour necrosis factor-α (TNF-α) influence angiogenesis by mobilizing inflammatory cells such as macrophages and mast cells (Fan et al., 1995). In contrast, angiogenic responses to basic fibroblast growth factor (bFGF) and VEGF are not dependent on recruitment of inflammatory cells.
Nitric oxide (NO), a mediator with a complex profile of biological activity was first implicated in angiogenesis by Pipili-Synetos et al. (1993) who reported that the nitric oxide synthase (NOS) inhibitor, nitro-L-arginine methyl ester (L-NAME) enhanced vessel growth in the chick chorioallantoic membrane (CAM) model (Pipili-Synetos et al., 1993). Additional studies indicated that NO donors reduced tissue angiogenesis and tumour neovascularization (Pipili-Synetos et al., 1995; Sakkoula et al., 1997). However, opinion on the role of NO in angiogenesis has been polarized by disparate observations in the literature. Ziche et al. suggest that NO directly stimulates endothelial cell proliferation (Ziche et al., 1993a), that NOS inhibitors inhibit angiogenesis in the rabbit corneal model and that NO donors promote angiogenesis in this model (Ziche et al., 1993b). Furthermore, tumour specimens exhibiting high levels of NOS activity induced angiogenesis in the rabbit corneal assay and when NO production was blocked, tumour angiogenesis and growth were suppressed (Gallo et al., 1998). Endothelial NOS activity (eNOS) has been implicated in the proliferative effects of VEGF (Ziche et al., 1997a) through activation of extracellular signal-related kinase (Erk) (Parenti et al., 1998). In addition to acute stimulation of eNOS activity (Ziche et al., 1997a) VEGF also increases eNOS expression (Hood et al., 1998). Even though bFGF-induced proliferation of endothelial cells occurs independently of NO (Ziche et al., 1997a), NO appears to exert its effect through upregulation of bFGF production by endothelial cells which in turn stimulates proliferation and urokinase-type plasminogen activator expression (Ziche et al., 1997b). The foregoing observations clearly implicate eNOS activity as an intermediate in the angiogenic cascade between VEGF and bFGF, but the role of iNOS in angiogenesis has not been delineated.
A new model of angiogenesis has been developed to avoid confounding influences, such as developmental angiogenesis in foetal tissue or vessel growth into a normally avascular area, that are associated with other models of angiogenesis (Theile et al., 1998). In this new model, the developing vessels are required to provide blood flow to a skin/muscle flap, mimicking the clinical process of angiogenesis in reconstructive surgery. A further advantage of this new model of functional angiogenesis is the use of skin flap survival as a functional measure of the extent of angiogenesis, thereby avoiding the contentious aspects of measurement of the angiogenic response histologically (Jain et al., 1997).
We have evaluated the role of iNOS in the angiogenic process subserving skin/muscle flap survival by using a series of NOS inhibitors having different selectivity for inhibition of iNOS or constitutive NOS (eNOS and nNOS) isoforms. In addition, mice with targeted disruption of the iNOS gene (iNOS knockout (MacMicking et al., 1995) and their corresponding wild-type control (C57BL/6 strain) have been used to establish the relative importance of iNOS in a more definitive manner than can be achieved using moderately selective pharmacological inhibitors. Our observations provide evidence for an as yet undescribed mechanism by which NO derived from iNOS promotes survival of ischaemic tissue through angiogenesis, possibly by increasing the availability of mast cell VEGF within the angiogenic zone.
Methods
Surgical procedure and drug treatment in rats
These experiments received the prior approval of the St Vincent's Hospital Animal Ethics Committee and conformed to the NHMRC guidelines for care and maintenance of animals. The surgical technique for the creation of the model of angiogenesis has been characterized and described in detail (Theile et al., 1998). Briefly, in the first operation adult male Sprague-Dawley rats (200 – 250 g) were anaesthetized by intra-peritoneal injection of sodium pentobarbitone (30 – 45 mg kg−1), the right and left inferior epigastric vascular pedicles were isolated and the artery and nerve cauterized to create a gap of 7 mm between the ends of the artery. The vein was left intact to prevent further retraction of the divided arteries. Commencing on the day of operation (day 1), rats received daily i.p. injections of saline (1 ml kg−1), nitro-L-arginine methylester hydrochloride (L-NAME, 30 mg kg−1), which has a limited degree of selectivity for cNOS compared to iNOS (Gross et al., 1991; Hickey et al., 1997). S-methylisothiourea sulphate (SMT, 3 mg kg−1), aminoethylthiorea bromide (AET, 3 mg kg−1), and aminoguanidine bicarbonate (AG, 30 mg kg−1), all of which are relatively selective as iNOS inhibitors (Hickey et al., 1997; Reutten & Thiemermann, 1996; Southan et al., 1995) or nitro-iminoethyl-L-ornithine hydrochloride (L-NIO, 30 mg kg−1), which inhibits both iNOS and cNOS to similar degrees (Southan et al., 1995). After 7 days, a second operation was performed: on the left side, a standard sized (4×3 cm) island skin flap, sustained by the regenerated epigastric artery, and intact vein, was raised (i.e. dissected away from the underlying tissue) on the abdominal wall. This flap, now solely supplied with blood via the epigastric vessels, was sewn back into position. The right epigastric pedicle was harvested for histological analyses. After a further 6 days, per cent flap survival was established by tracing necrotic areas and total flap area and measured by computer-based planimetry.
Surgical procedure in mice
Adult C57BL/6 wild-type (WT) or iNOS KO mice of either sex weighing 20 – 30 g were anaesthetized using chloral hydrate (40 mg kg−1, i.p.) and underwent two operations as outlined for the rat. However, the gap in the mouse epigastric artery after cauterization (first operation) was 4 mm. After periods of 0, 5, 7, 10, 14 or 21 days, a flap (3×1.5 cm) was raised (second operation). Flap survival was evaluated after a further 6 days.
Measurement of skin flap survival
In mice, the necrotic skin flap area was revealed after intra-muscular injection (into the tongue) of fluorescein (400 mg kg−1), since the black skin colour precluded direct visual assessment of necrosis. Fluorescein, identified under UV illumination, was detected in blood-perfused skin. Necrotic (absence of fluorescein) and surviving flap areas were traced and the percentage survival was determined using the Videopro 32 image analysis system (Faulding Imaging, Clayton, Victoria, Australia).
Assessment of morphological changes
Epigastric pedicles removed from the right side of rats in the second operation were immersion-fixed in buffered formol saline (BFS) for a minimum of 24 h and processed for final embedding in paraffin. Prior to final embedding, the angiogenic zone of the pedicle was transfected and the cross-sectioned surface placed face down in the block to allow 5-μm-thick pedicle cross sections to be cut. These sections were placed on glass slides and stained with haematoxylin and eosin or toluidine blue (1% w v−1 in 50% isopropanol) for identification of mast cells. In addition, four epigastric pedicles were removed from two unoperated rats, fixed and processed as described above for comparison with operated (angiogenic) pedicles.
Immunohistochemistry
Sections (5 μm) of the paraffin-embedded pedicles were mounted on gelatin-coated glass slides and stained for bFGF, VEGF, iNOS with an indirect immunohistochemical method. The antibodies used to detect iNOS and VEGF were monoclonal isotypes IgG2a and IgG1 respectively, whilst bFGF was a polyclonal. Antibodies of irrelevant specificity 1gG2a anti-smooth muscle α-actin, 1gG1 anti-EC NOS (endothelial) and collagen II rabbit polyclonal antibody were used as controls. In brief, the sections were dewaxed, rehydrated and washed in distilled water followed by a phosphate buffered saline (PBS, pH 7.4) wash (10 min). Endogenous peroxidase activity was blocked by incubation with hydrogen peroxide (3% in methanol) for 15 min at room temperature. The sections were incubated with diluted sheep serum (1 : 20). The primary antibodies were incubated on the sections overnight at room temperature (rabbit anti-human bFGF, diluted 1 : 200; mouse anti-VEGF, diluted 1 : 640; mouse anti-iNOS, diluted 1 : 25 or antibodies of irrelevant specificity at a dilution similar to their specific antibody match). Negative control slides were prepared by substituting sheep serum for the primary antibody. After 24 h, the slides were washed with PBS and incubated with the secondary antibody (1 : 100 dilution of: sheep anti-rabbit horseradish peroxidase-conjugated antibody (for polyclonal primary antibodies) and with sheep and mouse horseradish peroxidase-conjugated antibody (for monoclonal primary antibodies) for 30 min at room temperature). The peroxidase reaction was developed in PBS (containing 3% hydrogen peroxide), diaminobenzidine (DAB) tetrahydrochloride (0.5 mg ml−1) for 3 – 5 min. The sections were washed and selected sections were counterstained with Mayer's haematoxylin.
In vitro culture of mouse-derived mast cells
Bone marrow cells from the femoral bone of either WT or iNOS KO mice were harvested by lavage and aspiration. The harvested cells were cultured for 4 – 6 weeks in RPMI 1640 media containing 100 u ml−1 penicillin, 100 μg ml−1 streptomycin, 2 mM L-glutamine, 10% foetal calf serum and 20% Walter and Eliza Hall Institute-3 D cell conditioned media as described previously (Hartmann et al., 1997). After 4 weeks more than 99% of the cells in culture were identified as mast cells (MCs) by metachromatic staining with toluidine blue. Mast cells were stimulated at a concentration of 105 cells ml−1 for analysis of nitrite in the cell culture media and at 2×105 cells ml−1 for VEGF analysis in the presence or absence of TNF-α (3 nM) and IFNγ (100 u ml−1) for 24 h. Nitrite concentration was analysed by measuring the absorbance (550 nm) of cell culture supernatants after addition of an equal volume of the Griess reagent as described (Green et al., 1982). For VEGF measurements, Triton X-100 (0.01%) was used to lyse the cells and total VEGF was measured by a commercially available ELISA according to the manufacturer's protocol (R&D Systems, Minneapolis, U.S.A.).
Materials
Unless otherwise stated all chemicals and drugs were purchased from Sigma chemical company (St Louis, MO, U.S.A.). Rats were purchased from Monash University Animal Services (Melbourne, Australia). Mice WT and iNOS KO (129/SVEVx C57BL/6JF2) were internally bred from original breeding pairs provided by Dr G. Karupiah (John Curtin School of Medical Research, ANU, Canberra, Australia) and originally produced by Dr J. MacMicking (Merck, Rahway, NJ, U.S.A.) and Professor C. Nathan (Cornell University, New York, U.S.A.). Walter and Eliza Hall Institute 3D- cells were obtained from The Walter and Eliza Hall Institute (Parkville, Australia). The following antibodies were used: rabbit anti-human bFGF (Sigma Chemical Company, St Louis, MO, U.S.A.), VEGF monoclonal, (generous gift, Genentech Inc, San Francisco, CA, U.S.A.), mouse anti-iNOS (macrophage inducible, Transduction Laboratories Inc, Lexington, Kentucky, U.S.A.), anti-smooth muscle α-actin (Dako Corporation, Carpinteria, CA, U.S.A.), anti-ECNOS (endothelial) (Transduction Laboratories Inc., Lexington, KY, U.S.A.) and collagen II rabbit polyclonal antibody (Novocastra Laboratories Ltd, U.K.). Sheep anti-rabbit, horseradish peroxidase-conjugated antibodies raised against rabbit primary antibodies; and sheep, anti-mouse, horseradish peroxidase-conjugated immunoglobulin raised against murine monoclonal primary antibodies were from Silenus Laboratories (Hawthorn, Australia). RPMI 1640 media and foetal calf serum were from Commonwealth Serum Laboratories - Biosciences (Parkville, Australia), VEGF ELISA kit was from R & D Systems (Minneapolis, U.S.A.).
Statistical evaluation
Time-course and NOS inhibitor experiments on skin flap survival of rats and mice were analysed by ANOVA followed by Dunnett's test for multiple comparisons after initial arcsine transformation of the data to normalize the ratios of skin flap survival. For in vitro experiments using bone marrow-derived mast cells, Student's paired t-test was used. Differences were considered to be statistically significant when P<0.05.
Results
Effect of NOS inhibitors on rat angiogenic pedicles
In saline-treated rats in which 7 days was allowed for bridging angiogenesis, there was a significant flap survival of 39±7% (n=17, P<0.05 compared with 0% survival when no time was allowed for angiogenesis) (Theile et al., 1998). Treatment with the NOS inhibitors had a distinct effect depending on the inhibitor selectivity (Figure 1). L-NAME significantly increased flap survival (P<0.05, n=12) whereas the non-selective NOS inhibitor L-NIO had no effect (P>0.05, n=6). The iNOS-selective inhibitor, SMT significantly reduced flap survival (P<0.05, n=10). Flap survival in rats treated with the iNOS-selective inhibitors, AET (n=6) or AG (n=5) was not significantly different to that in the SMT- or saline-treated rats. Furthermore, flap survival in rats treated with iNOS-selective inhibitors did not differ significantly from 0% (P>0.05) (Figure 1).
Figure 1.
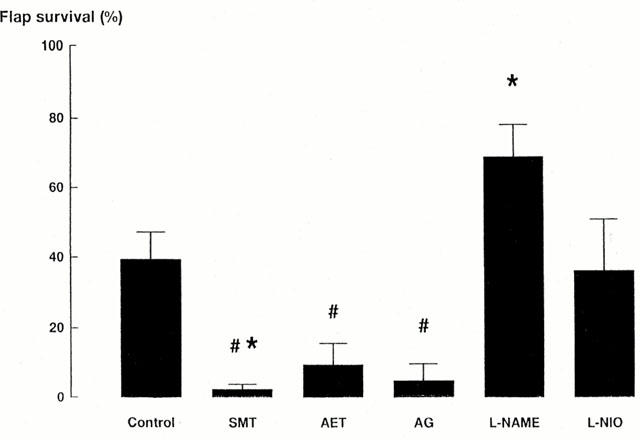
Rat skin flap survival represented as the percentage of the skin flap area that survives 6 days after flap elevation. Rats were treated by daily intraperitoneal injection of saline (1 ml kg−1, control, n=12) or one of the following NOS inhibitors: nitro-L-arginine methylester (L-NAME, 30 mg kg−1, n=12); S-methylisothiourea (SMT, 3 mg kg−1, n=10), aminoethylthiorea (AET, 3 mg kg−1, n=6); aminoguanidine (AG, 30 mg kg−1, n=5); nitro-iminoethyl-L-ornithine (L-NIO, 30 mg kg−1, n=6). *Indicates significant differences (P<0.05) between saline-treated controls and drug treatments. # Indicates non-significant differences (P>0.05) between drug treatments and 0% survival.
Effect of targeted iNOS gene disruption on angiogenesis-dependent skin flap survival in mice
The inhibition of flap survival caused by the three iNOS-selective inhibitors in the rat strongly supported the possibility of a pro-angiogenic role for iNOS, but the selectivity of these compounds for iNOS is less than would be required to draw a definite conclusion. Therefore, we used the iNOS KO mouse (MacMicking et al., 1995) to provide a more definite examination of the role of iNOS in functional angiogenesis. The time course of flap survival in wild-type mice (Figure 2) was similar to that observed in rats (Theile et al., 1998). In iNOS KO mice, flap survival remained below 30% for 21 days and was significantly less than that in wild-type controls, which showed a progressive increase in survival to approximately 85% by 21 days. An additional experiment was conducted examining daily administration of L-NAME (30 mg kg−1 per day, i.p.) in iNOS KO mice on flap survival in which 5 days was allowed for angiogenesis prior to flap elevation. L-NAME treatment increased skin flap survival to 57±15%, compared with saline treated iNOS KO mice 8±4% (Figure 3).
Figure 2.
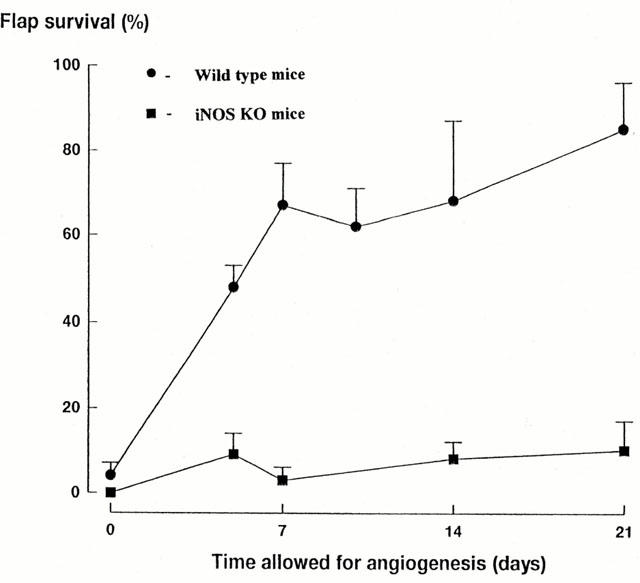
A comparison of the time-course of flap survival in wild-type (n=6 – 9) and iNOS KO mice (n=6 – 9) allowed up to 21 days for angiogenesis to support skin flap survival. The time-dependent increase in skin flap survival in wild-type mice is not observed in iNOS KO mice.
Figure 3.
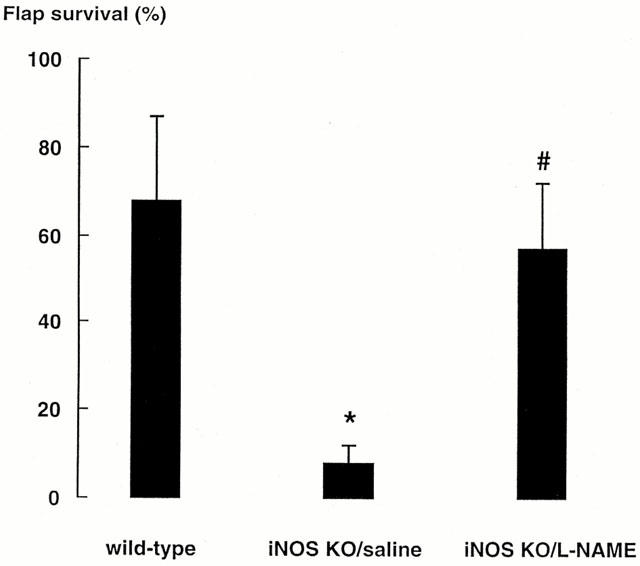
Effects on skin flap survival of L-NAME treatment in iNOS KO mice allowed a 5 day period for angiogenesis. *Indicates significant difference (P<0.05) between L-NAME-treated iNOS KO mice and saline-treated iNOS KO mice. # Indicates a lack of difference (P>0.05) between L-NAME-treated iNOS KO mice and saline-treated wild-type mice.
Morphology of the angiogenic zone
In saline-treated (control) rats cross-sections of the reconstituting vascular pedicle revealed that the epigastric vein was surrounded by areas of vascular connective tissue containing microvessels of varying calibre (Figure 4a). In toluidine blue stained sections, large granular metachromatically staining cells, i.e. mast cells, were frequently present adjacent to microvessels (Figure 4b).
Figure 4.
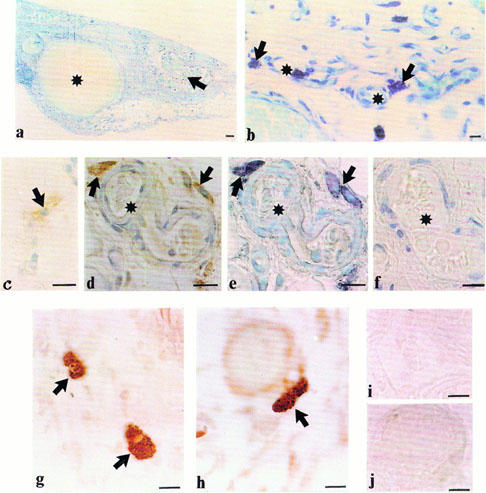
(a,b) Toluidine blue-stained cross-section of the angiogenic zone of an epigastric vascular pedicle obtained 7 days post-cauterization from a saline-treated rat. In (a) note the epigastric vein (asterisk) and surrounding areas of vascularized connective tissue (arrow). (b) A higher power micrograph of the area indicated by the arrow in (a). Large granular purple cells (mast cells, arrows) lie adjacent to microvessels (asterisks). Five-micron-thick paraffin sections. Scale bar in (a)=100 μm, scale bar in (b)=10 μm (c) Slight iNOS immunoreactivity in a large granular cell (arrow) in an epigastric pedicle from an unoperated rat. (d – f) The angiogenic zone of an epigastric vascular pedicle obtained 7 days post-cauterization from a saline-treated rat, microvessel indicated by asterisk. (d) Stronger iNOS immunoreactivity in large granular cells (arrows) surrounding an arteriole. (e) The same tissue section as (d) now additionally stained with toluidine blue. Purple (metachromatic) staining indicating mast cells (arrows) is confined to the iNOS immunoreactive cells confirming iNOS occurs in mast cells of the angiogenic zone. (The DAB deposits of iNOS positive granules now appear black). (f) Negative control section (without primary antibody). All sections counterstained with haematoxylin. Scale bars for (c – f)=10 μm. (g – j) Micrographs of the angiogenic zone of an epigastric vascular pedicle obtained 7 days post-cauterization from a saline-treated rat. (g) Immunoreactive VEGF within granules in mast cells (arrows). (h) Dense bFGF immunoreactive granules in a mast cell (arrow). (i) Negative control (without primary antibody) for (g) and (j) negative control for (h). Scale bars for (g – j)=10 μm.
Immunoreactive iNOS and growth factor localization in mast cells within the angiogenic pedicle of rat and mouse
Inducible NOS immunoreactivity was more apparent in the operated (Figure 4d) compared with the unoperated pedicle (Figure 4c) and was frequently associated with large, mononuclear granular cells (Figure 4d). These iNOS-positive cells were further identified as mast cells by staining of adjacent sections with toluidine blue (Figure 4e). Macrophages in the rat reconstituting vascular pedicle (identified by their haemosiderin granules) were not immunoreactive for iNOS. The angiogenic pedicles were also subjected to immunohistochemistry for VEGF and bFGF. Both angiogenic factors were localized to mast cells granules (Figure 4g,h). Antibodies of irrelevant specificity, but of identical isotypes to iNOS, VEGF and bFGF did not stain mast cells.
Inducible NOS-regulated VEGF expression in mouse bone marrow-derived mast cells
The observed co-expression of VEGF and iNOS in mast cells of the reconstituting vascular pedicle together with the anti-angiogenic effects of iNOS inhibitors raise the possibility that iNOS activity may be linked to expression in VEGF. Thus we examined VEGF levels in mast cells cultured from the bone marrow of wild-type and iNOS KO mice. There were no differences in basal supernatant levels of nitrite between wild-type (4.7±0.7 nmol 106−1 cells) and iNOS KO (4.1±1.2 nmol 106−1 cells) bone marrow-derived mast cells. Moreover, this basal nitrite level was derived largely from the foetal calf serum content of the medium. Incubation of wild-type mast cells with interferon γ (IFNγ, 100 u ml−1) and TNF-α (3 nM) for 24 h induced a significant increase (P<0.05, n=4) of 1.9±0.3 nmol nitrite 106−1 cells in the cell culture supernatant. The cytokine-induced increase in nitrite concentration was not observed when iNOS KO-derived mast cells were incubated in the same cytokine mixture (non-significant change from the basal level of 0.3±0.2 nmol 106−1 cells, n=3). The cytokine treatment significantly increased total cell VEGF (P<0.05) in wild-type mast cells, but had no effect on VEGF content of mast cells cultured from iNOS KO mice (Figure 5).
Figure 5.
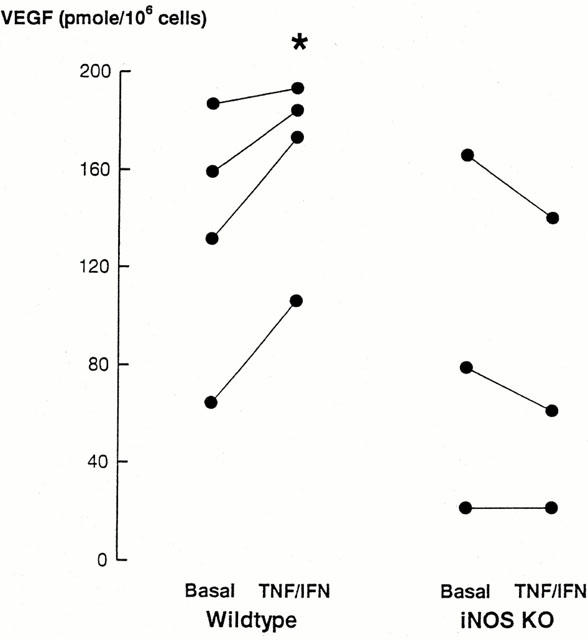
Bone marrow-derived mast cell VEGF content. VEGF was measured in the media of cells incubated in the absence or presence of tumour necrosis factor-α and interferon γ (TNF-α/IFNγ). Cells were obtained from either wild-type or iNOS KO bone marrow. * Indicates a significant (P<0.05) increase in levels of VEGF compared with basal levels.
Discussion
In an in vivo model which incorporates a pathophysiological type of angiogenesis in the adult (Theile et al., 1998), we have provided pharmacological and genetic evidence that iNOS activity enhances skin flap survival, and by direct inference, angiogenesis. Three lines of evidence implicated iNOS as pro-angiogenic. Firstly, whilst the non-selective NOS inhibitor (L-NIO) had no effect on skin flap survival, the iNOS-selective inhibitors were anti-angiogenic. Secondly, iNOS KO mice, which provide a more definitive experimental paradigm than the relatively selective pharmacological iNOS inhibitors, showed a prolonged delay in angiogenesis compared with wild-type mice. Finally, increased iNOS expression was observed in the operated vascular pedicle of the rats and wild-type mice compared with unoperated control pedicles. In contrast to the anti-angiogenic effects of iNOS inhibition, treatment with L-NAME (selective inhibitor of constitutive NOS isoforms, i.e. eNOS and nNOS) enhanced skin flap survival in rats and iNOS KO mice.
In the INOS KO mice the interpretation of the pro-angiogenic action of L-NAME is restricted to an effect on constitutive NOS, consistent with NO derived from constitutive NOS having anti-angiogenic activity. However, the possibility that L-NAME has unreported actions unrelated to NOS activity cannot be discounted. Further studies using eNOS knockout mice are required to resolve the importance of this NOS isoform.
NO has been implicated in angiogenesis on the basis of observations in different models of angiogenesis, including the chick chorioallantoic membrane (Pipili-Synetos et al., 1993), the rabbit cornea (Ziche et al., 1993b) and different tumour types (Gallo et al., 1998). However, these studies offer conflicting conclusions as to whether NO promotes or inhibits angiogenesis. The proposition that NO has both pro- and anti-angiogenic roles is not difficult to accept when consideration is given to the well documented differences in amount, location and duration of NO production by cNOS compared with iNOS isoforms (Knowles, 1996). The iNOS KO mouse exhibits impaired wound healing compared with wild-type mice (Nathan, 1997), consistent with a role for iNOS in wound healing-associated angiogenesis. This impaired wound healing appears to be one of the few phenotypic changes induced by iNOS KO in these extensively studied mice (Nathan, 1997). The developmental and physiological normality of the iNOS KO mice indicate that the role of iNOS in angiogenesis is likely to be largely restricted to inflammatory or pathophysiological conditions. Our results may help to explain, but do not directly reconcile, the polarized conclusions of previous studies. The restricted role of iNOS in angiogenesis associated with pathophysiological conditions may partly explain the model-dependent roles of NOS isoforms, as these different models are likely to depend to variable degrees on developmental angiogenic mechanisms. The chorioallantoic membrane model, in which inhibition of NOS activity is associated with enhanced angiogenesis, could be considered as having the closest relationship to developmental angiogenesis of any of the models in common use.
Previous studies by Ziche et al. (1997a,1997b) have addressed the importance of NO in angiogenesis, and potential sites of action have been identified. Thus, NO appears to act downstream of VEGF and proximal to bFGF release to induce endothelial cell proliferation (Parenti et al., 1998; Ziche et al., 1997a,1997b). There are potentially a number of additional roles for NO in the angiogenic process. When rat aortic endothelial cells were cultured in a monolayer, NOS inhibitors had no influence on EC proliferation, but culture in a collagen matrix induced iNOS and inhibitors of iNOS impaired capillary tube formation suggesting a role for NO in the morphogenesis of neovessels (Papapetropoulos et al., 1997). Loss of inhibition of platelet activation by NO with a consequent increase in growth factor release may explain the pro-angiogenic effects of NOS inhibition by L-NMMA in the chorioallantoic membrane model (Pipili-Synetos et al., 1993) and the pro-angiogenic action of L-NAME in the current study.
The mast cell is a significant source of bFGF (Qu et al., 1995) and all four isoforms of VEGF have recently been identified in the human mast cell tumour line HMC-1, and in isolated human dermal mast cells (Grutzkau et al., 1999). Mast cells and macrophages have well-established roles in angiogenesis as sources of angiogenic factors. Immunoreactive iNOS, VEGF and bFGF were co-localized to mast cells in the angiogenic zone of the reconstituting vascular pedicle. In assessing the likelihood that mast cell-derived NO directly influences angiogenesis, it is relevant to consider that the levels of iNOS-derived NO produced by bone marrow-derived mast cells (∼2 nmol 106 cells−1) in vitro are less than one tenth of those produced by macrophages.
Furthermore, in view of the low tissue density of mast cells, it seems unlikely that mast cell-derived NO is a direct mediator of angiogenesis. We considered the possibility that the influence of mast cell iNOS activity was indirect due to an influence on the release of potent angiogenic factors.
In view of the evidence linking NOS activity and VEGF action (Parenti et al., 1998; Ziche et al., 1997a,1997b), and that linking VEGF and NO release (Hood et al., 1998; Tuder et al., 1995) we examined the influence of iNOS-derived NO on mast cell VEGF levels using mouse bone marrow-derived mast cells. Induction of iNOS was associated with upregulation of levels of VEGF in wild-type, but not KO mice. Furthermore, our previous experiments using the RBL-2H3 mast cell line indicated that lower concentrations of peroxynitrite (ONOO−) enhanced mast cell degranulation (Barker & Stewart, 1998). This latter observation is in contrast to the well established inhibitory effects of NO on mast cell degranulation. Thus NO either directly or by conversion to peroxynitrite, increases both the amount and the release of VEGF from mast cells, thereby facilitating angiogenesis. While further work is required to elucidate the mechanism underlying the iNOS-mediated upregulation of VEGF in mast cells, a similar effect of iNOS activity on VEGF was recently observed in human carcinoma cell lines (Ambs et al., 1998). In murine peritoneal macrophages, LPS/IFNγ-induced NOS activity appeared to be responsible for upregulation of VEGF release (Xiong et al., 1998). In addition, exogenous NO increased VEGF expression in non-exercised rat skeletal muscle (Benoit et al., 1999). NO also appears to have a role in hypoxic induction of VEGF in vascular smooth muscle (Tsurumi et al., 1997). Although our data support a role for iNOS in facilitating angiogenesis, impairment of angiogenic responses in eNOS KO mice (Lui et al., 1998) suggests that proangiogenic effects of NO are dominant, irrespective of the NOS isoform which produces the NO.
Our observations suggest that VEGF expression and release are upregulated in part by iNOS induction. This mechanism is distinct from, but does not conflict with the model advanced by Ziche and colleagues which suggests that increased endothelial NOS activity in response to VEGF receptor activation leads to endothelial cell proliferation (Parenti et al., 1998; Ziche et al., 1997b). Given the complexity of the in vivo model used in the present study, a combination of these mechanisms could contribute to the effects of NOS inhibitors or iNOS gene knockout on flap survival. Moreover, given the multitude of components of angiogenic processes that are affected by NO, it is not surprising that the overriding influence of NO on angiogenesis is both context and NOS-isoform dependent. Thus, the relationship between NO, VEGF and angiogenic responses is influenced by the context (physiological, injury, hypoxia), amount and location of NO production.
Currently, there is considerable interest in the systemic treatment of chronic inflammatory disease with iNOS inhibitors. Our findings suggest that inhibition of angiogenesis may contribute significantly to the therapeutic effect of iNOS inhibitors in inflammation, but also indicate the possibility that such use of iNOS inhibitors will be accompanied by significantly delayed wound healing (Nathan, 1997). The therapeutic potential of NOS isoform-selective inhibitors for control of angiogenesis in inflammatory conditions and tumour growth warrants further investigation.
Acknowledgments
This work has been supported by the National Health and Medical Research Council of Australia, Grant Number: 990490, and St Vincent's Hospital Melbourne.
Abbreviations
- AET
aminoethylthiourea
- AG
aminoguanidine
- bFGF
basic fibroblast growth factor
- DAB
diaminobenzidine
- ELISA
enzyme linked immuno-sorbent assay
- Erk
extracellular signal-related kinase
- IFNγ
interferon γ
- L-NAME
nitro-L-arginine methyl ester
- L-NIO
nitro-imino-L-ornithine
- LPS
lipopolysaccharide
- MC
mast cell
- NO
nitric oxide
- NOS
nitric oxide synthase
- cNOS
constitutive nitric oxide synthase
- eNOS
endothelial nitric oxide synthase/nNOS: neuronal nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- iNOS KO mice
inducible nitric oxide synthase knockout mice
- ONOO−
peroxynitrite
- PBS
phosphate buffered saline
- RPMI 1640
Roswell Park Memorial Institute Culture Media 1640
- SMT
S-methyl-isothiourea
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor
- WT
wild-type
References
- AMBS S., BENNETT W.P., MERRIAM W.G., OGUNFUSIKA M.O., OSER S.M., KHAN M.A., JONES R.T., HARRIS C.C. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br. J. Cancer. 1998;78:233–239. doi: 10.1038/bjc.1998.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER J.E., STEWART A.G. Differing effects of nitric oxide compared with peroxynitrite on mast cell degranulation in vitro. Br. J. Pharmacol. 1998;123:305P. [Google Scholar]
- BENOIT H., WAGNER J.H., WAGNER P.D. Effect of NO, vasodilator prostaglandins, and adenosine on skeletal muscle angiogenic growth factor gene expression. J. Appl. Physiol. 1999;86:1513–1518. doi: 10.1152/jappl.1999.86.5.1513. [DOI] [PubMed] [Google Scholar]
- FAN T.D., JAGGAR R., BICKNELL R. Controlling the vasculature-angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol. Sci. 1995;16:57–66. doi: 10.1016/s0165-6147(00)88979-8. [DOI] [PubMed] [Google Scholar]
- FOLKMAN J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- GALLO O., MASINI E., MORBIDELLI L., FRANCHI A., FINISTORCHI I., VERGARI W.A., ZICHE M. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl. Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate, nitrite and [15N]-nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- GROSS S.S., JAFFE E.A., LEVI R., KILBOURN R.G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem. Biophys. Res. Commun. 1991;178:823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- GRUTZKAU A., KRUGER-KRASAGAKES S., BAUMEISTER H., SCHWARZ C., KOGEL H., WELKER P., LIPPERT U., HENZ B.M., MOLLER A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol. Biol. Cell. 1999;4:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMANN K., WAGELIE-STEFFEN A.L., VON STEBUT E., METCALF D. Fas (CD95, APO-1) antigen expression and function in murine mast cells. J. Immunol. 1997;159:4006–4014. [PubMed] [Google Scholar]
- HICKEY M.J., SHARKEY K.A., SIHOTA E.G., REINHARDT P.H., MACMICKING J.D., NATHAN C., KUBES P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- HOOD J.D., MEININGER C.J., ZICHE M., GRANGER H.J. VEGF upregulates ecnos message, protein, and no production in human endothelial cells. Am. J. Physiol. 1998;43:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- JAIN R.K., SCHLENGER K., HOCKEL M., YUAN F. Quantitative angiogenesis: Progress and problems. Nat. Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- KNOWLES R.G. Nitric oxide synthases. Biochem. Soc. Trans. 1996;24:875–878. doi: 10.1042/bst0240875. [DOI] [PubMed] [Google Scholar]
- LIU Y.X., CHRISTOU H., MORITA T., LAUGHNER E., SEMENZA G.L., KOUREMBANAS S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J. Biol. Chem. 1998;273:15257–15262. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- MACMICKING J.D., NATHAN C., HOM G. , CHARTRAIN N., FLETCHER D.S., TRUMBAUER M., STEVENS K., XIE Q.W., SOKOL K., HUTCHINSON N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- NATHAN C. Inducible nitric oxide synthase: What difference does it make. J. Clin. Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUFELD G., COHEN T., GENGRINOVITCH S., POLTORAK Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- PAPAPETROPOULOS A., GARCIA-CARDENA G., MADRI A., SESSA W.C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARENTI A., MORBIDELLI L., CUI X.L., DOUGLAS J.G., HOOD J.D., GRANGER H.J., LEDDA F., ZICHE M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal related kinase (1/2) activation in postcapillary endothelium. J. Biol. Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- PIPILI-SYNETOS E., SAKKOULA E., MARAGOUDAKIS M.E. Nitric oxide is involved in the regulation of angiogenesis. Br. J. Pharmacol. 1993;108:855–857. doi: 10.1111/j.1476-5381.1993.tb13476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPILI-SYNETOS E., PAPAGEORGIOU A., SAKKOULA E., SOTIROPOULOU G., FOTSIS T.X.K.G., MARAGOUDAKIS M.E. Inhibition of angiogenesis, tumour growth and metastasis by the NO-releasing vasodilators, isosorbide mononitrate and dinitrate. Br. J. Pharmacol. 1995;116:1829–1834. doi: 10.1111/j.1476-5381.1995.tb16670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QU Z., LIEBLER J.M., POWERS M.R., GALEY T., AHMADI P., HUANG X.N., ANSEL J.C.X.B.J., PLANCK S.R., ROSENBAUM J.T. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am. J. Pathol. 1995;147:564–573. [PMC free article] [PubMed] [Google Scholar]
- RUETTEN H., THIEMERMANN C. Prevention of the expression of inducible nitric oxide synthase by aminoguanidine or aminoethyl-isothiourea in macrophages and in the rat. Biochem. Biophys. Res. Commun. 1996;225:525–530. doi: 10.1006/bbrc.1996.1206. [DOI] [PubMed] [Google Scholar]
- SAKKOULA E., PIPILI-SYNETOS E., MARAGOUDAKIS M.E. Involvement of nitric oxide in the inhibition of angiogenesis by interleukin-2. Br. J. Pharmacol. 1997;122:793–795. doi: 10.1038/sj.bjp.0701436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABO C., THIEMERMANN C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEILE D.R.B., KANE A.J., ROMEO R., MITCHELL G.M., CROWE D., STEWART A.G., MORRISON W.A. A model of bridging angiogenesis in the rat. Br. J. Plast. Surg. 1998;51:243–249. doi: 10.1016/s0007-1226(98)80016-7. [DOI] [PubMed] [Google Scholar]
- TSURUMI Y., MUROHARA T., KRASINSKI K., CHEN D.F., WITZENBICHLER B., KEARNEY M., COUFFINHAL T., ISNER J.M. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat. Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- TUDER R.M., FLOOK B.E., VOELKEL N.F. Increased gene expression for VEGF and the VEGF receptors kdr/flk and flt in lungs exposed to acute or to chronic hypoxia – modulation of gene expression by nitric oxide. J. Clin. Invest. 1995;95:1798–1807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG M., ELSON G., LEGARDA D., LEIBOVICH S.J. Production of vascular endothelial growth factor by murine macrophages – regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am. J. Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZICHE M., MASINI M.E., GRANGER H., GEPPETTI P., LEDDA F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem. Biophys. Res. Commun. 1993a;192:1198–1203. doi: 10.1006/bbrc.1993.1543. [DOI] [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI L., CHOUDHURI R., ZHANG H.T., DONNINI S., GRANGER H.J., BICKNELL R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Invest. 1997a;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI L., PARENTI A., AMERINI S., GRANGER H.J., MAGGI C.A. Substance-P increases cyclic GMP levels on coronary postcapillary venular endothelial cells. Life Sci. 1993b;53:L229–L234. doi: 10.1016/0024-3205(93)90556-i. [DOI] [PubMed] [Google Scholar]
- ZICHE M., PARENTI A., LEDDA F., DELLERA P., GRANGER H.J., MAGGI C.A., PRESTA M. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ. Res. 1997b;80:845–852. doi: 10.1161/01.res.80.6.845. [DOI] [PubMed] [Google Scholar]


