Abstract
Digoxin (10−7 – 10−5 M) or digitoxin (10−7 – 10−5 M) decreased the basal and human chorionic gonadotropin (hCG)-stimulated release of progesterone from rat granulosa cells.
Digoxin (10−5 M) or digitoxin (10−5 M) attenuated the stimulatory effects of forskolin and 8-bromo-cyclic 3′ : 5′-adenosine monophosphate (8-Br-cAMP) on progesterone release from rat granulosa cells.
Digoxin (10−5 M) or digitoxin (10−5 M) inhibited cytochrome P450 side chain cleavage enzyme (cytochrome P450scc) activity (conversion of 25-hydroxyl cholesterol to pregnenolone) in rat granulosa cells but did not influence the activity of 3β-hydroxysteroid dehydrogenase (3β-HSD).
Neither progesterone production nor P450scc activity in rat granulosa cells was altered by the administration of ouabain.
Digoxin (10−5 M) or digitoxin (10−5 M), but not ouabain, decreased the expression of P450scc and steroidogenic acute regulatory (StAR) protein in rat granulosa cells.
The present results suggest that digoxin and digitoxin decrease the progesterone release by granulosa cells via a Na+,K+-ATPase-independent mechanism involving the inhibition of post-cyclic AMP pathway, cytochrome P450scc and StAR protein functions.
Keywords: Digitalis, rat granulosa cells, progesterone, P450scc, StAR protein
Introduction
It has been reported that some steroids and their glycosides have specific actions on contractility and electrophysiology of the heart (Antman & Smith, 1985). Some glycosides are obtained from leaves of the foxglove, Digitalis purpurea or Digitalis lanata. Digoxin and digitoxin are two major components of digitalis. It has long been known that these substances produce a profound beneficial effect on failing heart muscle. Indeed, digoxin, digitoxin and related drugs have widespread clinical use in the treatment of heart failure and atrial dysrhythmias (Antman & Smith, 1985). The direct positive effects of both digitalis and ouabain have been attributed to the inhibition of Na+-K+-ATPase, an enzyme system that provides the energy for active transport of Na+ and K+ across the cell membrane (Blanco & Mercer, 1998). The primary sexual problems reported in male patients taking cardiac glycosides for cardiovascular disease are decreased sexual desire and excitement (Neri et al., 1987).
Patients receiving long-term digoxin therapy show decreased plasma testosterone and luteinizing hormone levels (LH) (Neri et al., 1980; Stoffer et al., 1973) which may account for the inhibition of sexual desire and excitement observed in these subjects. Recently, we found that digoxin inhibits the production of testosterone via a decrease of adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP) in rat testicular interstitial cells (Lin et al., 1998a). Therapy with digitalis glycosides has also been associated with gynaecomastia in women (LcWinn, 1953). In addition, decreased urinary excretion of gonadotropin in postmenopausal women (Burckhardt et al., 1968), breast enlargement (Capeller et al., 1959), and cornification of vaginal epithelium (Britsch et al., 1963; Navab et al., 1965) have been reported in several studies. Since progesterone is an important ovarian hormone which causes thermogenesis by raising the basal metabolic rate, and also plays a major role in preparing the reproductive tract for zygote implantation and the subsequent maintenance of the pregnant stage (Hadley, 1995), we have investigated the effects of digitalis on the production of progesterone by ovarian cells.
It has been shown that the luteinizing hormone (LH)-increased production of progesterone (Denning-Kendall & Wathes, 1994; Lahav et al., 1996; Liu & Hsueh, 1986) correlates with increased generation of cyclic AMP (Denning-Kendall & Wathes, 1994; Lahav et al., 1996). An increased expression of the cytochrome P450 side chain cleavage (P450scc) enzyme (Lahav et al., 1996; Lauber et al., 1993), and 3β-hydroxysteroid dehydrogenase (3β-HSD) (González Reyes et al., 1997) by cyclic AMP in granulosa cells has also been demonstrated. The conversion of cholesterol to pregnenolone is the rate-limiting step in the final formation of progesterone and this step is regulated by mitochondrial enzyme P450scc (Dirami & Cooke, 1998; Too et al., 1984; Waterman & Simpson, 1985). The steroidogenic acute regulatory (StAR) protein is thought to facilitate cholesterol transfer through the mitochondria membrane, making it available to P450scc (Reinhart et al., 1999), and it is believed to be the key regulator of the biosynthesis of steroid hormones. The conversion of pregnenolone to progesterone is catalysed by microsomal enzyme 3β-HSD. Progesterone is the main product of the ovarian granulosa cells, which diffuses into theca cells to serve as a substrate for biosynthesis of androgens (Hadley, 1995). The theca cells provide androgens, whereas granulosa cells convert androgens to oestrogens by 17β-HSD and cytochrome P450 aromatase (P450arom) (Hadley, 1995).
In the present study, the effects of digoxin, digitoxin, and ouabain on the basal and human chorionic gonadotropin (hCG)-stimulated release of progesterone from ovarian granulosa cells were examined. We found that both digoxin and digitoxin, but not ouabain, inhibit the production of progesterone and the activities of P450scc in granulosa cells and decrease the expression of P450scc and StAR protein. These data suggest that the inhibitory roles of digoxin and digitoxin on progesterone release are exerted distal to the formation of cAMP via a Na+-K+-ATPase-independent mechanism.
Methods
Reagents
Chemicals and reagents including pregnant mares serum gonadotropin (PMSG), Dulbecco's modified Eagle medium (DMEM)/F12, fatty acid-free bovine serum albumin (BSA), N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), penicillin-G, streptomycin sulphate, insulin, medium-199 (M199), L-glutamine, 8-bromo-cAMP (8-Br-cAMP), 25-OH-cholesterol, pregnenolone, and phenylmethylsulphonyl fluoride (PMSF) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Lauryl sulphate (SDS), bromophenol blue, and dithiothreitol were purchased from Research Organics Inc. (Cleveland, OH, U.S.A.). Proteinase inhibitor cocktail tablets were purchased from Boehringer Mannheim (Mannheim, Germany). Cell culture plasticware was obtained from Falcon Labware (Lincoln Park, NJ, U.S.A.). The anti-pregnenolone antiserum was purchased from Biogenesis (Poole, England, U.K.). The peroxidase-conjugated IgG fraction to mouse IgG and peroxidase-conjugated IgG fraction to rabbit IgG were purchased from ICN Pharmaceuticals, Inc. (Aurora, Ohio, U.S.A.). The anti-P450scc antibody and anti-StAR antibody were kindly provided by Dr B.C. Chung (Hu et al., 1991) and Dr D. Stocco (Lin et al., 1998b), respectively.
Isolation and culture of granulosa cells
Immature female Sprague Dawley rats were housed in a temperature-controlled room (22±1°C) with 14 h of artificial illumination daily (0600 to 2000 h) and were given food and water ad libitum. The preparation of granulosa cells was modified from the method described elsewhere (Hwang et al., 1996; Tsai et al., 1999). The immature female rats 22 – 25 days of age were injected subcutaneously with PMSG (15 iu rat−1). Forty-eight hours later, rats were killed by cervical dislocation. Ovaries were excised and transferred into the sterile DMEM/F12 (1 : 1) medium, containing 0.1% BSA, 20 mM HEPES, 100 iu ml−1 penicillin-G, 50 μg ml−1 streptomycin sulphate. After trimming free fat and connective tissues, the large and medium sized follicles were punctured with a 26-gauge needle to release granulosa cells. The harvested cells were pelleted and resuspended in growth medium (DMEM/F12 containing 10% foetal calf serum, 2 μg ml−1 insulin, 100 iu ml−1 penicillin, and 100 μg ml−1 streptomycin sulphate). Cell viability was greater than 90% as determined using a haemocytometer and trypan blue method. Granulosa cells were aliquoted in 24-well plates at approximately 1×105 cells per well and incubated at 37°C with 5% CO2 – 95% air for 2 days. Morphologically the cultured granulosa cells maintained a characteristic round (or polygonal) shape, throughout our culture conditions.
Effects of digoxin, digitoxin, and ouabain on the release of progesterone by rat granulosa cells
To ascertain the dose-dependent effects of ouabain (10−8 M∼10−5 M), digoxin (10−8 M∼10−5 M), and digitoxin (10−8 M∼10−5 M), in the presence or absence of hCG (0.5 iu ml−1), the granulosa cells were washed and incubated with 500 μl aliquots of serum-free BSA-M199 medium (M199 without phenol red, 0.3% BSA, 25 mM HEPES, 4 mM L-glutamine) containing different doses of ouabain, digoxin, digitoxin with or without hCG at 37°C for 2 h. The medium was collected and stored at −20°C until further analysis for progesterone by radioimmunoassay (RIA).
Effects of digoxin, digitoxin, and ouabain on the adenylyl cyclase activity in granulosa cells
Granulosa cells were incubated with medium containing ouabain (10−5 M), digoxin (10−5 M), and digitoxin (10−5 M) for 2 h in the presence or absence of forskolin (an adenylyl cyclase activator, 10−7∼10−5 M). Two hours later, medium was collected and stored at −20°C until analysed for progesterone by RIA.
Effects of digoxin, digitoxin, and ouabain on the cyclic AMP action in granulosa cells
Granulosa cells were incubated with medium containing ouabain (10−5 M), digoxin (10−5 M), or digitoxin (10−5 M) for 2 h in the presence or absence of 8-Br-cAMP (a membrane permeable analogue of cyclic AMP, 10−5∼10−3 M). Two hours later, medium was collected and stored at −20°C until analysed for progesterone by RIA.
Effects of digoxin, digitoxin, and ouabain on the activities of steroidgenic enzymes (cytochrome P450scc enzyme and 3β-HSD)
Granulosa cells were incubated with medium containing ouabain (10−5 M), digoxin (10−5 M), or digitoxin (10−5 M) for 2 h in the presence or absence of steroidogenic precursors including 25-OH-cholesterol (10−8∼10−6 M) and pregnenolone (10−8∼10−6 M). Two hours later, medium was collected and stored at −20°C until analysed for progesterone and pregnenolone by RIA.
Effects of digoxin, digitoxin, and ouabain on the expression of P450scc and StAR protein
Granulosa cells were incubated with medium containing ouabain (10−5 M), digoxin (10−5 M), and digitoxin (10−5 M) for 2 h. Two hours later, cells were washed twice with saline and detached by trypsinization (1.25 mg ml−1). The cells were collected and extracted in homogenization buffer (pH 8.0) containing 1.5% Na-lauroylsacrosine, 2.5×10−3 M Tris-base, 1×10−3 M EDTA, 0.68% PMSF, and 2% proteinase inhibitor cocktail, and then disrupted by ultrasonic sonicator (Heat Systems, Farmingdale, NY, U.S.A.) in an ice-bath. Cell extracts were centrifuged at 13,500×g for 10 min (Kau et al., 1999). The supernatant fluid was collected and the protein concentration was determined by a colorimetric method of the protein assay according to Bradford (1976).
Gel electrophoresis and Western blotting for P450scc and StAR protein expression
Extract proteins were denatured by boiling for 5 min in SDS buffer (0.125 M Tris-base, 4% SDS, 0.001% bromophenol blue, 12% sucrose, and 0.15 M dithiothreitol) (Hu et al., 1991; Kau et al., 1999). The proteins (10 μg) in the samples were separated on 12% SDS-polyacrylamide gel electrophoresis (SDS – PAGE) at 75 V for 15 min and then at 150 V for 40 min using a running buffer. The proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (NEN Life Science Products, Inc., Boston, MA, U.S.A.) using a Trans-Blot SD semi-dry transfer cell (170-3490, Bio-Rad, Hercules, CA, U.S.A.) at 64 mA (for 8 mm×10 mm membrane) for 45 min in a blotting solution. The membranes were washed in TBS-T buffer (0.8% NaCl, 0.02 M Tris-base, and 0.3% Tween-20, pH 7.6) for 5 min and then blocked by a 120-min incubation in blocking buffer (TBS-T buffer containing 5% nonfat dry milk). Then the membranes were incubated with anti-P450scc antibodies (1 : 2000), anti-StAR protein antibodies (1 : 1000), and β-actin antibodies (1 : 2000) in 5% nonfat dry milk of TBS-T buffer overnight at 4°C. After one wash for 15 min and three washes for 5 min each time with TBS-T buffer, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (1 : 6000 dilution) and horseradish peroxidase-conjugated goat anti-mouse IgG (1 : 8000 dilution) in 5% nonfat dry milk of TBS-T buffer. The membranes were washed four times with TBS-T buffer, and then the bands for P450scc and StAR were visualized by chemiluminescence (ECL, Western blotting detection reagents, Amersham International, U.K.).
Analysis of chemiluminescence Western blot data
Quantification of chemiluminescence signal data on X-ray film was performed as follows: chemiluminescence pseudo-autogradiograms were scanned using a scanner (Personal Densitometer, Molecular Dynamics, Sunyale, CA, U.S.A.). Quantification of scanned images was performed according to the user manual of the ImageQuaNT program (Molecular Dynamics, Sunyale, CA, U.S.A.). The P450scc and StAR protein signals are normalized to the β-actin signal.
RIAs of progesterone and pregnenolone
The concentration of progesterone in the medium was determined by RIA as described elsewhere (Chen et al., 1997; Lu et al., 1996). With anti-progesterone serum No. W5, the sensitivity of the progesterone RIA was 5 pg per assay tube. Intra- and interassay coefficients of variation (CV) were 4.8% (n=5) and 9.5% (n=4), respectively.
The concentration of pregnenolone in the medium was determined by RIA. Anti-pregnenolone antiserum was diluted with 0.1% gelatin-PBS. The cross-reactivities of anti-pregnenolone were 67% with pregnen-3 β-20-ONE sulphate, 19% with progesterone, and less than 3% with 17α-hydroxypregnenolone, cholesterol, 17α-OH-progesterone, 20α-diOH-progesterone, cortisol, desoxycorticosterone, corticosterone, aldosterone, androstenedione, testosterone, dihydrotestosterone, etiocholanolone, estradiol, estrone, and estriol. In this RIA system, a known amount of unlabelled pregnenolone or an aliquot of rat granulosa cell medium was adjusted to a total volume of 0.3 ml by a buffer solution (0.1% gelatin-phosphate-buffered saline (PBS), pH 7.5), and incubated with 0.1 ml of pregnenolone antiserum (1 : 200) diluted with 0.1% gelatin-PBS and [3H]-pregnenolone (8000 c.p.m., Amersham International plc, Buckinghamshire, U.K.) at 4°C for 24 h. Duplicate standard curves of pregnenolone were prepared in each assay. An adequate amount (0.1 ml) of dextran-coated charcoal (0.5%) was added and further incubated in an ice bath for 15 min. After incubation, the assay tubes were centrifuged at 1500×g for 40 min. The supernatant fluid was mixed with 3 ml liquid scintillation fluid (Ready Safe, Beckman, Fullerton, CA, U.S.A.) before the radioactivity was counted in an automatic beta counter (Wallac 1449, Pharmacia, Turku, Finland). The sensitivity of the pregnenolone RIA was 16 pg per assay tube. The inhibition curves produced by granulosa cell medium samples were parallel to that produced by pregnenolone. The intra- and interassay coefficients of variation were 2.3% (n=6) and 3.7% (n=4), respectively.
Statistic analysis
All data were expressed as mean±s.e.mean. Treatment means were tested for homogeneity using the analysis of variance (ANOVA), and the differences between the specific means were tested for the significance by Duncan's multiple range test (Steel & Torrie, 1960). The level of significance chosen was P<0.05.
Results
Effects of digoxin, digitoxin, and ouabain on the release of progesterone in rat granulosa cells
During a 2 h incubation, digoxin and digitoxin at 10−6 and 10−5 M elicited a dose-dependent inhibition of progesterone release by rat granulosa cells (digoxin, 387.73±41.25 & 217.44±25.47 pg 105 cells−1 2 h−1, n=8, versus vehicle 509±74.29 pg 105 cells−1 2 h−1, n=8, P<0.05 or P<0.01; digitoxin, 337.49±28.56 & 239.29±32.79 pg 105 cells−1 2 h−1, n=8, versus vehicle 491.4±65.14 pg 105 cells−1 2 h−1, n=8, P<0.05 or P<0.01) (Figure 1). Incubation of granulosa cells with hCG (0.5 iu ml−1) for 2 h increased the level of progesterone secretion. Combination of hCG with digoxin or digitoxin of 10−6 and 10−5 M resulted in a significant inhibition of the hCG-stimulated release of progesterone (digoxin, 937.58±84.11 & 304.12±30.45 pg 105 cells−1 2 h−1, n=8, versus hCG-treated group 1457.77±175.14 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 754±71.65 and 376.11±34.15 pg 105 cells−1 2 h−1, n=8, versus hCG-treated group 1360.11±137.99 pg 105 cells−1 2 h−1, n=8, P<0.05 or P<0.01) (Figure 1). Ouabain at the same doses did not affect the basal and hCG-stimulated production of progesterone by rat granulosa cells.
Figure 1.
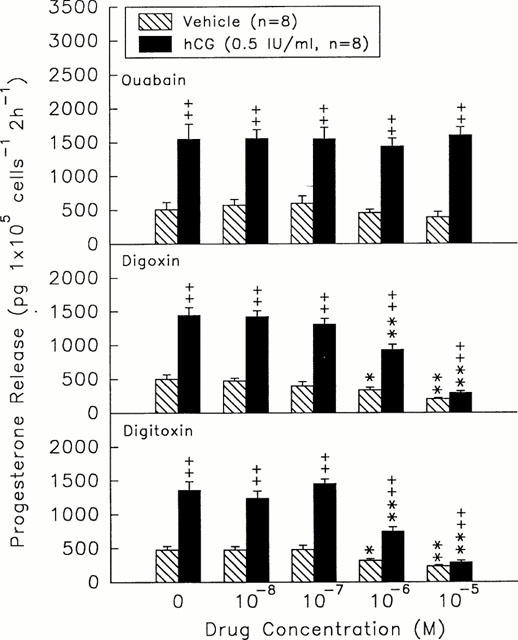
Effects of different doses of ouabain (top), digoxin (centre), and digitoxin (bottom) on the release of progesterone in the presence (solid columns) or absence (hatch columns) of hCG (0.5 iu ml−1). *P<0.05, **P<0.01 compared with the value at drug=0 M, respectively. ++P<0.01 compared with vehicle-treated group. Each column represents mean±s.e.mean.
Effects of digoxin, digitoxin, and ouabain on the adenylyl cyclase activity in granulosa cells
Forskolin (10−7∼10−5 M) dose-dependently stimulated the release of progesterone by rat granulosa cells (1146.40±90.47 – 3311.52±385.52 pg 105 cells−1 2 h−1, n=8, versus forskolin=0 M, 365.82±82.34 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 2). Digoxin or digitoxin at 10−5 M markedly decreased both basal and forskolin-stimulated release of progesterone by granulosa cells (digoxin, 81.87±12.11 – 292.49±28.44 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 52.91±14.27 – 300.55±16.44 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 2). Ouabain at 10−5 M did not alter the stimulatory effects caused by 10−7 and 10−6 forskolin, but diminished (P<0.01) the enhanced release of progesterone induced by 10−5 M forskolin.
Figure 2.
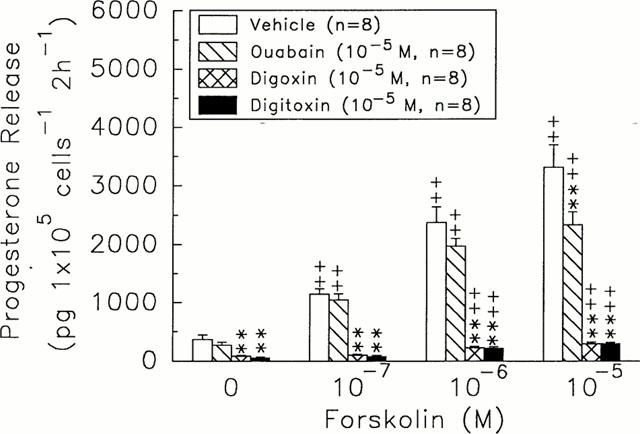
Effects of ouabain (hatch columns), digoxin (crosshatch columns), and digitoxin (solid columns) on the release of progesterone in rat granulosa cells in the response to different doses of forskolin. **P<0.01 compared with vehicle group. +P<0.05, ++P<0.01 compared with 0 M of forskolin, respectively. Each column represents mean±s.e.mean.
Effects of digoxin, digitoxin, and ouabain on the cyclic AMP function in granulosa cells
8-Br-cAMP at 10−4 and 10−3 M stimulated the release of progesterone (600.15±38.30 & 1072.30±82.08 pg 105 cells−1 2 h−1, n=8, versus 8-Br-cAMP=0 M, 383.57±17.16 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 3), but did not fully reverse the inhibitory effects of digoxin (10−5 M) and digitoxin (10−5 M) (digoxin, 58.10±9.23 and 135.65±29.95 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 80.19±16.44 and 119.44±16.92 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 3). Ouabain at 10−5 M did not alter the stimulatory effects caused by low dose (10−4 M) of 8-Br-cAMP, but enhanced that elicited by high dose (10−3 M) of 8-Br-cAMP (1349.50±102.36 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 3).
Figure 3.
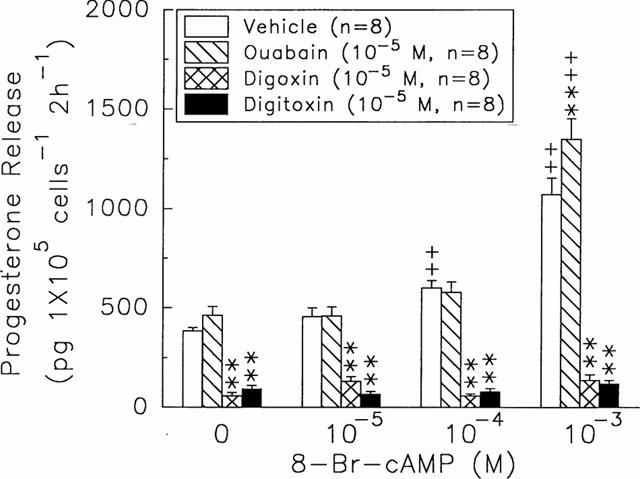
Effects of ouabain (hatch columns), digoxin (crosshatch columns), and digitoxin (solid columns) on the release of progesterone in rat granulosa cells in response to different doses of 8-Br-cAMP. **P<0.01 compared with vehicle group, respectively. +P<0.05, ++P<0.01 compared with 8-Br-cAMP=0 M, respectively. Each column represents mean±s.e.mean.
Effects of digoxin, digitoxin, and ouabain on the activities of steroidogenic enzymes (cytochrome P450scc and 3-β-HSD)
Administration of 25-OH-cholesterol (10−8∼10−6 M) and pregnenolone (10−8∼10−6 M) dose-dependently increased progesterone release (25-OH-cholesterol, 380.60±45.21 – 912.89±68.90 pg 105 cells−1 2 h−1, n=8, versus 25-OH-cholesterol=0 M, 411.05±41.94 pg 105 cells−1 2 h−1, n=8, P<0.05 or P<0.01; pregnenolone, 1271.44±79.12 – 5643.21±657.11 pg 105 cells−1 2 h−1, n=8, versus pregnenolone=0 M, 483.31±70.12 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 4). Digoxin (10−5 M) and digitoxin (10−5 M) decreased not only the basal release of progesterone but also the progesterone response to the 25-OH-cholesterol (10−8 M∼10−6 M) (digoxin, 218.08±62.13 – 161.70±44.04 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 116.82±32.84 – 110.14±14.40 pg 105 cells−1 2 h−1, n=8, P<0.01) or to the pregnenolone (10−8 M) (digoxin, 825.44±57.44 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 875.61±74.12 pg 105 cells−1 2 h−1, n=8, P<0.01) (Figure 4). Pregnenolone, rather than 25-OH-cholesterol, at the range of 10−6 M reversed the inhibitory effect of digoxin and digitoxin on progesterone release (digoxin, 4985.88±219.47 pg 105 cells−1 2 h−1, n=8; digitoxin, 4899.81±221.13 pg 105 cells−1 2 h−1, n=8) (Figure 4).
Figure 4.
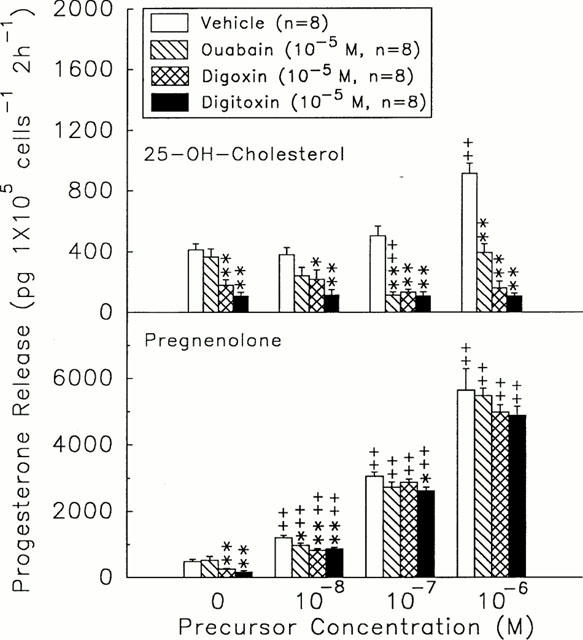
Effects of ouabain (hatch columns), digoxin (crosshatch columns), and digitoxin (solid columns) on the cytochrome P450scc enzyme and 3β-HSD activities in rat granulosa cells after incubation with different doses of 25-OH-cholesterol (top) or pregnenolone (bottom) for 2 h. *P<0.05, **P<0.01 compared with vehicle group, respectively. ++P<0.01 compared with precursor=0 M. Each column represents mean±s.e.mean.
In the range of 10−7∼10−5 M, digoxin and digitoxin caused a dose-dependent inhibition of pregnenolone release by granulosa cells either in the presence (digoxin, 1527.21±148.11 to 263.44±112.91 pg 105 cells−1 2 h−1, n=8, versus digoxin=0 M, 1764.88±155.78 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 1081.35±138.95 to 367.85±77.55 pg 105 cells−1 2 h−1, n=8, versus digitoxin=0 M, 1389.72±167.12 pg 105 cells−1 2 h−1, n=8, P<0.01) or absence (digoxin, 965.12±107.57 to 426.36±97.41 pg 105 cells−1 2 h−1, n=8, versus digoxin=0 M, 1097.24±115.65 pg 105 cells−1 2 h−1, n=8, P<0.01; digitoxin, 1087.84±169.12 to 313.55±67.68 pg 105 cells−1 2 h−1, n=8, versus digitoxin=0 M, 1198.41±137.54 pg 105 cells−1 2 h−1, n=8, P<0.01) of 25-OH-cholesterol (Figure 5). Ouabain did not alter the release of pregnenolone in the presence or absence of precursor.
Figure 5.
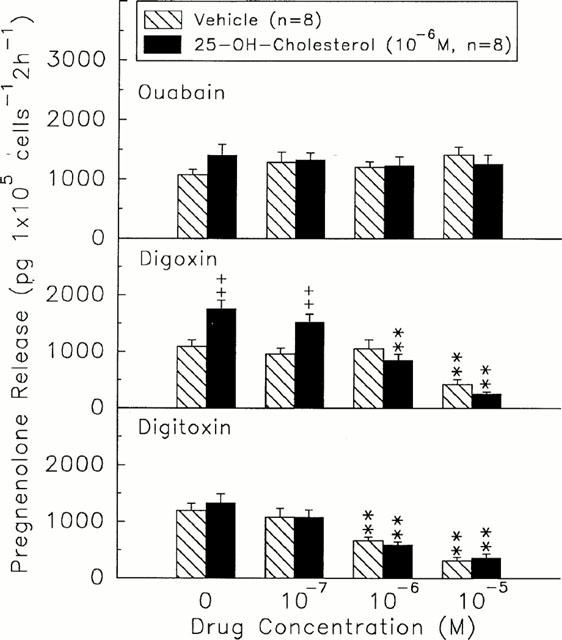
Effects of different doses of ouabain (top), digoxin (centre), or digitoxin (bottom) on the release of pregnenolone in the presence (solid columns) or absence (hatch columns) of 25-OH-cholesterol (1×10−6 M). **P<0.01 compared with the value at drug=0 M. ++P<0.01 compared with vehicle-treated group. Each column represents mean±s.e.mean.
Effects of digoxin, digitoxin, and ouabain on the expression of cytochrome P450scc and StAR protein
Based on the ratio of inner standard, β-actin, the expressions of P450scc and StAR protein were reduced by 41 and 37%, respectively, after the administration of digoxin (10−5 M) (P450scc, 15.89 versus digoxin=0 M, 26.80, n=4, P<0.05; StAR, 14.77 versus digoxin=0 M, 23.44, n=4, P<0.05) and 36 and 36%, respectively, after the treatment of digitoxin (10−5 M) (P450scc, 17.09 versus digitoxin=0 M, 26.80, n=4, P<0.05; StAR, 14.96 versus digitoxin=0 M, 23.44, n=4, P<0.05) following 2 h incubation with rat granulosa cells (Figure 6). Neither P450scc nor StAR protein expression was altered by the administration of ouabain.
Figure 6.
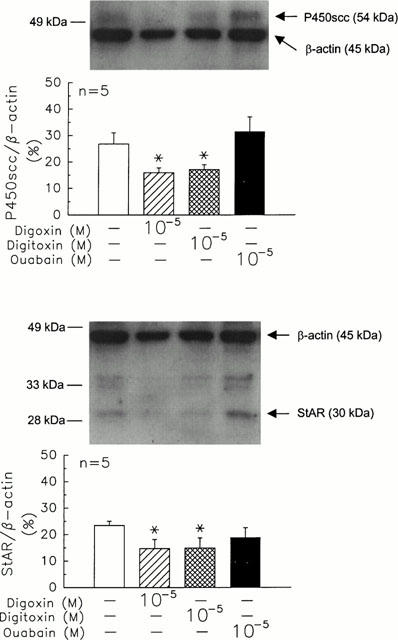
Effects of ouabain, digoxin, and digitoxin on the protein expression of cytochrome P450scc (top) and StAR (bottom) protein in rat granulosa cells. Rat granulosa cells were incubated with ouabain (10−5 M), digoxin (10−5 M), or digitoxin (10−5 M) at 37°C for 2 h. Then, the cells were collected and analysed by Western blotting. Each lane was loaded with 10 μg protein of sample. *P<0.05 compared with the value at drug=0 M. Each column represents mean±s.e.mean.
Discussion
The present results demonstrate that both digoxin and digitoxin, but not ouabain, inhibit the spontaneous and hCG-stimulated secretion of progesterone and decrease the activity of P450scc by acting directly on rat granulosa cells. The expression of P450scc and StAR protein was reduced by digoxin and digitoxin. Furthermore, our data suggest that the inhibitory effects of digoxin and digitoxin on progesterone release are mediated distal to the formation of cyclic AMP by Na+, K+-ATPase-independent pathway which involves attenuation of P450scc and StAR function in granulosa cells.
It has been reported that administration of digoxin for 2 years decreases the concentrations of plasma testosterone and LH in male patients with cardiac function capacity in late class II and early class III stages (Neri et al., 1987; Stoffer et al., 1973). Recently, we have demonstrated that digoxin inhibits production of testosterone both in vivo and in vitro through the mechanisms involving a decrease of the basal and hCG-stimulated testosterone release, and an attenuation of the activities of cytochrome P450scc enzyme and 3β-HSD in rat testicular interstitial cells (Lin et al., 1998a).
It has been well established that hCG stimulates progesterone secretion by granulosa cells and increases granulosa cyclic AMP content (Sokka et al., 1996). In the present study, we found that the basal and hCG-stimulated production of progesterone by granulosa cells are diminished by digoxin and digitoxin, but not by ouabain (Figure 1), a selective Na+-K+-ATPase inhibitor (Matsumoto et al., 2000). These data show that digoxin and digitoxin decreases the progesterone release by granulosa cells via a Na+, K+-ATPase-independent pathway. In comparison to the clinically effective serum concentrations of digoxin (0.5 – 2.5 ng ml−1, ≈0.6 – 3.2×10−9 M) and digitoxin (15 – 30 ng ml−1, ≈19.6 – 39.2×10−9 M) (Clark et al., 1992), the concentrations of digitalis used in our study (10−8∼10−5 M) were high but, nonetheless, they are indicators of the acute effects of digitalis on the female endocrine system. Administration of forskolin, an adenylyl cyclase activator, did not fully reverse the inhibitory effects of digoxin and digitoxin (Figure 2). These observations indicate that the activity of adenylyl cyclase is not altered by digoxin or digitoxin. Apparently, the inhibition of progesterone release by digoxin or digitoxin is independent of adenylyl cyclase activity.
Although the administration of 8-Br-cAMP treatment increased progesterone release by granulosa cells, it did not prevent the inhibitory effects of digoxin and digitoxin (Figure 3). These observations suggest that the inhibitory effects of digoxin and digitoxin on progesterone production in rat granulosa cells are associated with a post-cyclic AMP pathway.
It has been demonstrated that 25-OH-cholesterol at 2∼2.5×10−5 M stimulates testosterone release by the Leydig cells in rat testes (Chen et al., 1996), and progesterone release in rat adrenocortical cell culture (Doris et al., 1996). In the present study, the attenuation of stimulatory effect of 25-OH-cholesterol on the release of progesterone by digoxin and digitoxin suggests an inhibition of digoxin and digitoxin on the activity of cytochrome P450scc enzyme (Figure 5), the rate limiting enzyme for the conversion of cholesterol to pregnenolone in progesterone biosynthesis. The decreased production of pregnenolone, the product of P450scc, by digoxin and digitoxin confirmed a reduction of cytochrome P450scc activity in rat granulosa cells. Based on Western blot analysis, the protein expressions of cytochrome P450scc and StAR protein were decreased by the administration of digoxin and digitoxin. The reduction of P450scc function was in part due to the decline of P450scc expression. These results suggest that the activity and content of cytochrome P450scc enzyme in rat granulosa cells are reduced by the action of digoxin and digitoxin. In addition to the reduced activity of P450scc, the decreased expression of StAR protein which transfers cholesterol to mitochondria for P450scc utilization (Reinhart et al., 1999) may be another reason for the decline of progesterone production in the digoxin- and digitoxin-treated groups compared with control group.
The final step in progesterone biosynthesis is the conversion of pregnenolone to progesterone under the catalyzation of the microsomal enzyme 3β-HSD (Hadley, 1995). Inasmuch as administration of a higher dose of pregnenolone fully reverse the inhibitory effect of digoxin and digitoxin on progesterone production (Figure 5), we suggest that digoxin and digitoxin did not inhibit the 3β-HSD enzyme activity.
In summary, the present results demonstrate that both digoxin and digitoxin inhibit progesterone production by acting directly on rat granulosa cells. Since ouabain even at the effective doses of digoxin and digitoxin failed to affect progesterone production, we suggest that the inhibition of digitalis on progesterone production is independent of the action of Na+,K+-ATPase. Taken together, the present data suggest that digoxin and digitoxin decrease the progesterone release by granulosa cells via a Na+,K+-ATPase-independent mechanism involving the inhibition of post-cyclic AMP pathway, cytochrome P450scc and StAR functions.
Acknowledgments
This study was supported by the grant NSC89-2320-B-182-036 from the National Science Council and awards from the Medical Research and Advancement Foundation in the memory of Dr Chi-Shuen Tsou, ROC to P.S. Wang. The anti-P450scc antibody was kindly provided by Dr B.C. Chung, Institute of Molecular Biology, Academia Sinica, Republic of China. The anti-StAR antibody was kindly provided by Dr D.M. Stocco, Department of Cell Biology and Biochemistry, Texas Tech University Health Science Center, Lubbock, Texas, U.S.A.
Abbreviations
- 17β-HSD
17β-hydroxysteroid dehydrogenase
- 25-OH-Cholesterol
25-hydroxy-cholesterol
- 3β-HSD
3β-hydro-xysteroid dehydrogenase
- 8-Br-cAMP
8-bromo-adenosine 3′ : 5′-cyclic monophosphate
- BSA
bovine serum albumin
- hCG
human chorionic gonadotropin
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- LH
luteinizing hormone
- P450arom
cytochrome P450 aromatase
- P450scc
cytochrome P450 side-chain cleavage enzyme
- PMSG
pregnant mares serum gonadotropin
- StAR protein
steroidogenic acute regulatory protein
References
- ANTMAN E.M., SMITH T.W. Digitalis toxicity. Annun. Rev. Med. 1985;36:357–367. doi: 10.1146/annurev.me.36.020185.002041. [DOI] [PubMed] [Google Scholar]
- BLANCO G., MERCER R.W. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRITSCH C.J., AZAR H.A. Estrogen effect in exfoliated vaginal cells following treatment with digitalis. Am. J. Obstet. Gynecol. 1963;85:989–993. doi: 10.1016/s0002-9378(16)35634-4. [DOI] [PubMed] [Google Scholar]
- BURCKHARDT D., VERA C.A., LADUE J.S. Effect of digitalis on urinary pituitary gonadotropin excretion: a study in postmenopausal women. Ann. Intern. Med. 1968;68:1069–1071. doi: 10.7326/0003-4819-68-5-1069. [DOI] [PubMed] [Google Scholar]
- CAPELLER D., COPCLAND G.D., STERN T.N. Digitalis intoxication: a clinical report of 148 cases. Ann. Intern. Med. 1959;50:869–878. doi: 10.7326/0003-4819-50-4-869. [DOI] [PubMed] [Google Scholar]
- CHEN T.S., DOONG M.L., WANG S.W., TSAI S.C., LU C.C., SHIH H.C., CHEN Y.H., CHANG F.Y., LEE S.D., WANG P.S. Gastric emptying and gastrointestinal transit during lactation in rat. Am. J. Physiol. 1997;272:G626–G631. doi: 10.1152/ajpgi.1997.272.3.G626. [DOI] [PubMed] [Google Scholar]
- CHEN H., HUHTANIEMI I., ZIRKIN B.R. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- CLARK W.G., BRATER D.C., JOHNSON A.R. Goth's Medical Pharmacology. Mosby Year Book, Inc; 1992. Digitalis; pp. 408–420. [Google Scholar]
- DENNING-KENDALL P.A., WATHES D.C. Acute effect of prostaglandin F2α, luteinizing hormone, and estradiol on second messenger systems and on the secretion of oxytocin and progesterone from granulosa and early luteal cells of the ewe. Biol. Reprod. 1994;50:765–773. doi: 10.1095/biolreprod50.4.765. [DOI] [PubMed] [Google Scholar]
- DIRAMI G., COOKE B.A. Effect of a dopamine agonist on luteinizing hormone receptors, cyclic AMP production and steroidogenesis in rat Leydig cells. Toxicol. App. Pharmacol. 1998;150:393–401. doi: 10.1006/taap.1998.8429. [DOI] [PubMed] [Google Scholar]
- DORIS A., HAYWARD-LESTER A., BOURNE D., STOCCO D.M. Ouabain production by cultured adrenal cells. Endocrinology. 1996;137:533–539. doi: 10.1210/endo.137.2.8593799. [DOI] [PubMed] [Google Scholar]
- GONZÁLEZ REYES J., SANTANA P., GONZÁLEZ ROBAINA I., CABRERA OLIVA J., ESTÉVEZ F., HERNÁNDEZ I., LÓPEZ BLANCO F., QUINTANA AGUIAR J., FANJUL L.F., RUIZ DE GALARRETA C.M. Effect of the protein phosphatase inhibitor okadaic acid on FSH-induced granulosa cell steroidogenesis. J. Endocrinol. 1997;152:131–139. doi: 10.1677/joe.0.1520131. [DOI] [PubMed] [Google Scholar]
- HADLEY M.E. Endocrinology. ed. Hadley M.E. Prentice-Hall, Inc; 1995. Hormones and female reproductive physiology; pp. 476–504. [Google Scholar]
- HU M.C., GUO I.C., LIN J.H., CHUNG B.C. Regulated expression of cytochrome P-450scc (cholesterol-side-chain cleavage enzyme) in cultured cell lines detected by antibody against bacterially expressed human protein. Biochem. J. 1991;274:813–817. doi: 10.1042/bj2740813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG J.J., LIN S.W., TENG C.H., KE F.C., LEE M.T. Relaxin modulates the ovulatory process and increases secretion of different gelatinases from granulosa and theca-interstitital cells in rats. Biol. Reprod. 1996;55:1276–1283. doi: 10.1095/biolreprod55.6.1276. [DOI] [PubMed] [Google Scholar]
- KAU M.M., CHEN J.J., WANG S.W., CHO W.L., WANG P.S. Age-related impairment of aldosterone secretion in zona glomerulosa cells ovariectomized rats. J. Investig. Med. 1999;47:425–432. [PubMed] [Google Scholar]
- LAHAV M., GARMEY J.C., VELDHUIS J.D. Paradoxical effect of 3-isobutyl-1-methylxanthine on cytochrome P450 cholesterol side-chain cleavage mRNA accumulation in porcine granulosa cells. Mol. Cell. Endocrinol. 1996;117:203–210. doi: 10.1016/0303-7207(95)03748-9. [DOI] [PubMed] [Google Scholar]
- LAUBER M.E., PICTON H.M., BEGEOT M., MOMOI K., WATERMAN M.R., SIMPSON E.R. Regulation of CYP11A gene expression in bovine ovarian granulosa cells in primary culture by cAMP and phorbol esters is conferred by a common cis-acting element. Mol. Cell. Endocrinol. 1993;94:235–242. doi: 10.1016/0303-7207(93)90172-g. [DOI] [PubMed] [Google Scholar]
- LCWINN E.B. Gynecomastia during digitalis therapy. Report of eight additional cases with liver function studies. N. Engl. J. Med. 1953;248:316–320. doi: 10.1056/NEJM195302192480802. [DOI] [PubMed] [Google Scholar]
- LIN H., WANG S.W., TSAI S.C., CHEN J.J., CHIAO Y.C., LU C.C., HUANG W.J., WANG G.J., CHEN C.F., WANG P.S. Inhibitory effect of digoxin on testosterone secretion through mechanisms involving decreases of cyclic AMP production and cytochrome P450scc activity in rat testicular interstitial cells. Brit. J. Pharmacol. 1998a;125:1635–1640. doi: 10.1038/sj.bjp.0702229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN T., HU J., WANG D., STOCCO D.M. Interferon-gamma inhibited the steroidogenic acute regulatory protein messenger ribonucleic acid expression and protein levels in primary cultures of rat Leydig cells. Endocrinology. 1998b;139:2217–2222. doi: 10.1210/endo.139.5.6006. [DOI] [PubMed] [Google Scholar]
- LIU Y.X., HSUEH A.J.W. Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: studies on the two-cell, two-gonadotropin hypothesis using steroid antisera. Biol. Reprod. 1986;35:27–36. doi: 10.1095/biolreprod35.1.27. [DOI] [PubMed] [Google Scholar]
- LU S.S., LAU C.P., TUNG Y.F., HUANG S.W., CHEN Y.H., SHIH H.C., TSAI S.C., LU C.C., WANG S.W., CHEN J.J., CHIEN E.J., CHIEN C.H., WANG P.S. Lactate stimulates progesterone secretion via an increase in cAMP production in exercised female rats. Am. J. Physiol. 1996;271:E910–E915. doi: 10.1152/ajpendo.1996.271.5.E910. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S., TAKAHASHI T., TANIMOTO T., SAIKI C., TAKEDA M. Effects of ouabain and flecainide on CO2-induced slowly adapting pulmonary stretch receptor inhibition in the rabbit. Life Sci. 2000;66:441–448. doi: 10.1016/s0024-3205(99)00610-4. [DOI] [PubMed] [Google Scholar]
- NAVAB A., KOSS L.G., LADUE J.S. Estrogen-like activity of digitalis: its effect on the squamous epithelium of the female genital tract. JAMA. 1965;194:30–32. doi: 10.1001/jama.194.1.30. [DOI] [PubMed] [Google Scholar]
- NERI A., AYGEN M., ZEKERMAN Z., BAHARY C. Subjective assessment of sexual dysfunction of patients on long-term administration of digoxin. Arch. Sex Behav. 1980;9:343–347. doi: 10.1007/BF01541359. [DOI] [PubMed] [Google Scholar]
- NERI A., ZUKERMAN Z., AYGEN M., LIDOR Y., KAUFMAN H. The effect of long-term administration of digoxin on plasma androgens and sexual dysfunction. J. Sex. Marital. Ther. 1987;13:58–63. doi: 10.1080/00926238708403879. [DOI] [PubMed] [Google Scholar]
- REINHART A.J., WILLIAMS S.C., STOCCO D.M. Transcriptional regulation of the StAR gene. Mol. Cell. Endocrinol. 1999;151:161–169. doi: 10.1016/s0303-7207(98)00257-3. [DOI] [PubMed] [Google Scholar]
- SOKKA T.A., HAMALAINEN T.M., KAIPIA A., WARREN D.W., HUHTANIEMI I. Development of luteinizing hormone action in the perinatal rat ovary. Biol. Reprod. 1996;55:663–670. doi: 10.1095/biolreprod55.3.663. [DOI] [PubMed] [Google Scholar]
- STEEL R.D., TORRIE J.H. Principles and Procedures of Statistics. McGraw-Hill, New York; 1960. [Google Scholar]
- STOFFER S.S., HYNES K.M., JIANY N.S., RYAN R.J. Digoxin and abnormal serum hormone levels. J. Am. Med. 1973;225:1643–1644. [PubMed] [Google Scholar]
- TOO C.K.L., BRYANT-GREENWOOD G.D., GREENWOOD F.C. Relaxin increases the release of plasminogen activator, collagenase, and proteoglycanase from rat granulosa cells in vitro. Endocrinology. 1984;115:1043–1050. doi: 10.1210/endo-115-3-1043. [DOI] [PubMed] [Google Scholar]
- TSAI S.C., LU C.C., CHEN J.J., CHIAO Y.C., WANG S.W., HWANG J.J., WANG P.S. Inhibition of salmon calcitonin on secretion of progesterone and GnRH-stimulated pituitary luteinizing hormone. Am. J. Physiol. 1999;277:E49–E55. doi: 10.1152/ajpendo.1999.277.1.E49. [DOI] [PubMed] [Google Scholar]
- WATERMAN M.R., SIMPSON E.R. Regulation of the biosynthesis of cytochrome P450 involved in steroid hormone synthesis. Mol. Cell. Endocrinol. 1985;39:81–89. doi: 10.1016/0303-7207(85)90123-6. [DOI] [PubMed] [Google Scholar]


