Abstract
The role of PACAP receptor in nociceptive transmission was investigated in vitro using maxadilan, a PACAP receptor selective agonist and max.d.4, a PACAP receptor selective antagonist.
Potentials, from a ventral root (L3 – L5) of an isolated spinal cord preparation or a spinal cord – saphenous nerve – skin preparation from 0 – 3-day-old rats, were recorded extracellularly.
In the isolated spinal cord preparation, single shock stimulation of a dorsal root at C-fibre strength induced a slow depolarizing response lasting about 30 s (slow ventral root potential; slow VRP) in the ipsilateral ventral root of the same segment. Bath-application of max.d.4 (0.01 – 3 μM) inhibited the slow VRP in a concentration-dependent manner.
In the spinal cord – saphenous nerve – skin preparation, application of capsaicin (0.1 μM) to the skin evoked a depolarization of the ventral root. This response was also depressed by max.d.4 (1 μM).
Application of maxadilan evoked a long-lasting depolarization in a concentration-dependent manner in the spinal cord preparation. In the presence of max.d.4 (0.3 μM), the concentration response curve of maxadilan was shifted to the right.
Reverse transcription-polymerase chain reaction (RT – PCR) experiments demonstrated the existence of PACAP receptor and VPAC2 receptor in the neonatal rat spinal cord and [125I]-PACAP27 binding was displaced almost completely by maxadilan and max.d.4, but not by vasoactive intestinal peptide (VIP). These data indicate that PACAP receptor is dominantly distributed in the neonatal rat spinal cord.
The present study suggests that PACAP receptor may play an excitatory role in nociceptive transmission in the neonatal rat spinal cord.
Keywords: PACAP receptor, nociceptive transmission, spinal cord, neonatal rat, maxadilan, max.d.4
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a member of the secretin-glucagon-vasoactive intestinal peptide family and shows a 68% homology, of amino acids, with vasoactive intestinal peptide (VIP) at its N-terminal (Miyata et al., 1989; Arimura, 1992). PACAP has two amidated forms: PACAP38, a 38-amino-acid polypeptide, and PACAP27, a truncated form of PACAP38 containing 27 residues (Miyata et al., 1990). PACAP has been shown to have functions as a hypothalamic hormone, as a neurotrophic factor, as a neurotransmitter and as a neuromodulator (Arimura, 1998).
Cloning of the receptors for PACAP revealed three distinct subtypes; VPAC1, VPAC2 and PACAP receptor (Arimura, 1993). The PACAP receptor displays a much greater affinity for PACAP38 and PACAP27 than VIP, and the VPAC1 and VPAC2 receptors display no marked selectivity for any of them (Ishihara et al., 1992; Spengler et al., 1993). VPAC1 receptor mRNA appeared to be most widely expressed within the adult rat spinal cord, occurring in a moderate number of lamina II cells and also in laminae III – IV, and VPAC2 and PACAP receptor mRNAs were sparsely expressed through laminae II – IV of the dorsal horn (Dickinson et al., 1999). Distinct distribution of these receptors in the spinal cord suggested that they may have distinct functional roles in the spinal cord.
Morphological studies revealed that PACAP immunoreactive fibres were concentrated in the superficial layer of the dorsal horn of rat spinal cord (Moller et al., 1993; Dun et al., 1996). PACAP mRNA expression is localized primarily in small to medium-diameter dorsal root ganglion (DRG) neurons (Mulder et al., 1994). In addition, coexistence of PACAP with substance P and calcitonin gene-related peptide was shown in small DRG neurons (Moller et al., 1993; Mulder et al., 1994). This evidence strongly suggests that PACAP is involved in spinal nociceptive transmission. However, the exact role of PACAP in spinal nociceptive transmission is still unclear. It has been reported that intrathecal administration of either PACAP27 or PACAP38 reduced the instances of formalin-induced flinching behaviour (Yamamoto & Tatsuno, 1995; Zhang et al., 1996). Spinal application of PACAP27 and PACAP38 produced a significant and long-lasting suppression of the C-fibre evoked flexor reflex (Zhang et al., 1993). On the other hand, several groups showed that intrathecally-administered PACAP27 or PACAP38 facilitated nociceptive flexor reflex in the rat (Xu & Wiesenfeld-Hallin, 1996; Narita et al., 1996). These differences may be explained, at least partly, by the existence of receptor subtypes, such as PACAP receptor, VPAC1 receptor and VPAC2 receptor. However, the functional significance of receptor subtypes for PACAP in the spinal nociceptive neurotransmission could not be examined due to the lack of selective agonists and antagonists.
Recently, a novel PACAP receptor selective agonist and an antagonist were reported. Maxadilan, a vasodilator 61-amino acid peptide isolated from the sand fly Lutzomyia longipalpis, was shown to activate PACAP receptor with a high affinity and did not have any affinity for VPAC1 or VPAC2 receptors (Moro & Lerner, 1997). Max.d.4, which was developed by the deletion of 19 amino acids of maxadilan, was demonstrated to be a specific antagonist for PACAP receptor (Moro et al., 1999; Uchida et al., 1998). In the present study, we examined the role of PACAP receptor in the primary afferent fibre-evoked nociceptive reflex of the neonatal rat spinal cord using maxadilan, and max.d.4. We also examined whether maxadilan and max.d.4 shared binding sites with PACAP38 or PACAP27 in the neonatal rat spinal cord or not. In addition, we investigated which receptor subtypes for PACAP were expressed in the neonatal rat spinal cord by using a reverse transcription – polymerase chain reaction (RT – PCR) method.
Methods
The following investigations were performed under a protocol approved by the university's animal care committee. Neonatal Sprague-Dawley rats (0 – 3-day-old) were used in this experiment.
Preparations and electrophysiology
An isolated spinal cord preparation and a spinal cord-saphenous nerve-skin preparation were used. The isolated spinal cord preparation was prepared as described previously (Otsuka & Yanagisawa, 1988). Under ether anaesthesia, the spinal cord below the lower thoracic level together with spinal nerve roots (L3 – L5) was isolated. The spinal cord was hemisected, and placed in a recording chamber of 0.3 ml volume. The chamber was perfused with artificial cerebrospinal fluid (CSF) at a rate of 2.5 ml min−1. The composition of artificial CSF was as follows (mM): NaCl 138.6, KCl 3.35, NaHCO3 20.9, glucose 10.0, CaCl2 1.25 and MgCl2 1.15. The solution was equilibrated with a gas mixture of 95% O2 : 5% CO2 at room temperature (25 – 27°C). Tight-fitting suction electrodes were used for extracellular recording from a ventral root (L3 – L5) and for electrical stimulation of the dorsal root of the same segment. Intense electrical stimulation of a dorsal root at C-fibre strength (single shocks with a square pulses of 200 μs in duration and 20 – 30 V in amplitude) elicited a mono-synaptic reflex followed by a prolonged depolarization lasting about 30 s (hereafter referred to as the slow ventral root potential; slow VRP). A preparation that satisfied the following criteria was used; the amplitude of the dorsal root-evoked control monosynaptic reflex was higher than 6 mV and the level of the integrated area of the slow VRP was more than 0.3 mV min.
The isolated spinal cord-saphenous nerve-skin preparation consisted of a hemisected spinal cord that remained connected to the femoral and saphenous nerves and a piece of skin (approximately 5×5 mm) of the hind limb (Yanagisawa et al., 1992). The recording chamber consisted of two wells, which were independently perfused with artificial CSF at a rate of 2.5 ml min−1. The spinal cord was placed in one well (0.3 ml volume) and the skin was placed with the inside surface upwards in the neighbouring well (0.1 ml volume). The saphenous nerve was led through a break (0.5 mm width) in a thin septum (1 mm width) into the skin well. The break in the septum was sealed with Vaseline. Capsaicin (0.1 μM) was applied to the skin for 30 s by perfusing the skin well with solutions containing the drug at intervals of 45 min to minimize tachyphylaxis (Kurihara & Yoshioka, 1996). The evoked potentials were recorded extracellularly from the L3 ventral root.
In these preparations, potential changes of the ventral root were led to a d.c. amplifier and then to a pen recorder and a computer recording device (Axoscope version 7, Axon Instruments). The magnitude of depolarization was measured by computer software (Fetchan, Axon Instruments). The fast time course spinal reflex was stored in a transient memory device and then recorded on the pen recorded with an expanded time-scale.
To estimate the effect of drugs on the slow VRP, the integrated area (mV min) of the depolarization between immediately after the electrical simulation and the time when the level of ventral root potential decreased to 0.1 mV above the base-line level was calculated. Drugs were applied to the spinal cord in a cumulative manner. The magnitude of the depolarization by noxious skin stimulation was also estimated by the integrated area (mV min). In addition, we examined the effect of drugs on the ventral root potentials. In this case, the peak amplitude of depolarization of the ventral root was measured.
Analysis of the expression of PACAP/VIP family receptor
Binding assay
Binding assay was performed as described previously (Tatsuno et al., 1990; 1991). Briefly, spinal cords of neonatal Sprague-Dawley rats (1-day-old) were isolated as discribed above and homogenized using a glass Teflon homogenizer in ice-cold 50 mM Tris buffer (pH 7.4) containing 5 mM MgCl2, 0.5 mg ml−1 bacitracin and 0.5 mM p-amidinophenyl methanesulphonyl fluoride hydrochloride with a protease inhibitor cocktail (10 μg ml−1 of pepstatin A, 10 μg ml−1 of antipain, 10 μg ml−1 of chymostatin, 10 μg ml−1 of leupeptin) (membrane buffer). The homogenate was centrifuged at 250×g for 10 min. The supernatant was centrifuged at 50,000×g for 30 min. After removing the supernatant, the pellet was resuspended and centrifuged at 50,000×g for 30 min. Finally, the pellet was resuspended in the membrane buffer. All procedures were performed at 4°C, except as indicated. The binding assay was performed in a total volume of 300 μl of the membrane buffer containing 1% bovine serum albumin (binding buffer) at 22°C. [125I]-PACAP27 membrane preparations and various concentrations of peptides were incubated. The assay was terminated by rapid filtration, through Whatman GF/B glass paper filters which were pre-soaked in 0.5% polyethylenimine, using a cell harvester (Brandel Biomed. Res. & Dev. Labs., Gaithersburg, MD, U.S.A.). Each filter was counted for radioactivity by an automatic gamma counter. The binding data were analysed with a computer program for Scatchard plot analysis called ‘Ligand'. The program was kindly provided by Dr Peter J. Munson (National Institute of Health, Bethesda, MD, U.S.A.).
RT – PCR to analyse mRNA expression of PACAP/VIP family receptors
Total RNA was isolated by the guanidinium isothiocyanate/CsCl procedure from neonatal rat spinal cord (1-day-old). RT – PCR was performed using RNA PVR Kit (TaKaRa, Ohtsu, Japan). Three micrograms of RNA was used for each RT – PCR. The template produced from the RT reaction was amplified using one of three sets of primers dependent upon the PACAP/VIP receptor as reported by Rawlings et al. (1995). For PACAP receptor, the two primers used were PACAP-FL(5′-TTTCATCGGCATCATCATCATCATCCTT-3′) and PACAP-VK(5′-CCTTCCAGCTCCTCCATTTCCTCTT-3′), which would be expected to produce PCR product sizes of 280 base pairs (bp) for the short receptor, 364 bp for a single cassette insert (hip, hop1 or hop2), and 448 bp for a double insert (hiphop1 or hiphop2) (Propato-Mussafiri et al., 1992). For VPAC1 receptor, the primers used were VIP1-AI (5′-GCCCCCATCCTCCTCTCCATC-3′) and VIP1-EL(5′-TCCGCCTGCACCTCACCATTG-3′), which should give a PCR product of 299 bp. The VPAC2 primers used were VIP2-AE (5′-ATGGATAGCAACTCGCCTTTCTTTAG-3′) and VIP2-QL (5′-GGAAGGAACCAACACATAACTCAAACAG-3′), yielding a predicted PCR product 325 bp in length.
Drugs
The substances used in this study were obtained from the following sources: maxadilan and max.d.4 (donated by Shiseido Co., Tokyo, Japan); PACAP27, PACAP38, PACAP(6-38) (PACAP/VPAC2 receptor antagonist; Dickinson et al., 1997) and VIP (Peptide Institute Inc., Osaka, Japan); tetrodotoxin (TTX; Sankyo Co., Tokyo, Japan); capsaicin (Sigma Chemicals, St. Louis, MO, U.S.A.). Capsaicin was dissolved in dimethyl sulphoxide to form a stock solution of 10 mM. Other drugs were dissolved in water and diluted in artificical CSF to various concentrations. Iodination of PACAP27 was performed by the lactoperoxidase method as previously described (Tatsuno et al., 1990; 1991).
Statistical analysis
Experimental data are expressed as mean values±s.e.mean and were analysed with one-way or two-way analysis of variance (ANOVA). For multiple comparisons, Fisher's Protected Least Significant test was used. P<0.05 was considered as being significant.
Results
Electrophysiological experiments
Bath-application of max.d.4 (0.1 – 3 μM) to the spinal cord exerted a depressant effect on the dorsal root-evoked slow VRP in a concentration-dependent manner (n=5, P<0.05, by one way ANOVA, Figures 1B and 2) with no effect on the baseline ventral root potential. On the other hand, max.d.4 did not affect the monosynaptic reflexes at any concentration examined in this study (n=5, P>0.5, by one-way ANOVA, Figure 1A). PACAP(6-38) also depressed the dorsal root-evoked slow VRP in a concentration-dependent manner (0.1 – 3 μM, n=5, P<0.05, by one-way ANOVA, Figure 2) without affecting the monosynaptic reflexes (P>0.5, by one-way ANOVA).
Figure 1.
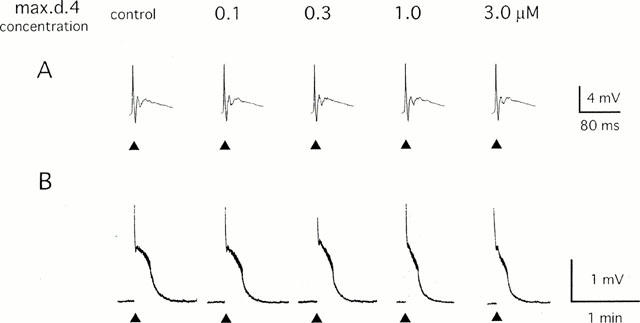
Effect of max.d.4 on the primary afferent evoked potential in the neonatal rat spinal cord. A single-shock stimulus (200 μs 20 V) was given to the dorsal root (L4) at the time indicated by ▴ and the potential was recorded extracellularly from the ispilateral ventral root. Max.d.4 was applied to the spinal cord in a cumulative manner. (A) Sample records of the fast time course monosynaptic and polysynaptic reflexes that were stored in a transient memory device and recorded by a pen recorder with a 500 fold expansion of the time base. (B) Sample records of slow ventral root potential (VRP) recorded by a pen recorder.
Figure 2.
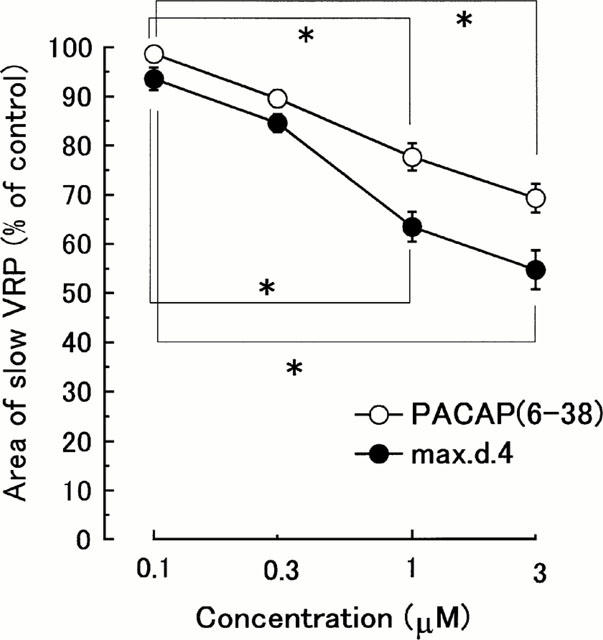
Concentration-inhibition curves showing the effects of max.d.4 and PACAP(6-38) on the slow VRP. Ordinate scale: integrated area of slow VRP expressed as a percentage of the averaged three consecutive control responses just before the drug application. Abscissa scale: logarithmic concentration of max.d.4 and PACAP(6-38). Each point and vertical bar represent mean±s.e.mean (n=5) *P<0.05.
In the spinal cord – saphenous nerve – skin preparation, application of capsaicin (0.1 μM) to the skin for 30 s produced a depolarizing response of the L3 ventral root of a slow time course (0.64±0.16 mV min). This depolarization was significantly depressed by pre-application of max.d.4 (1 μM) for 15 min to the spinal cord (to 0.37±0.19 mV min) (n=5, P<0.05, by one way ANOVA, Figure 3). The capsaicin evoked-depolarization almost completely recovered after a 45 min washout (to 0.62±0.21 mV min).
Figure 3.
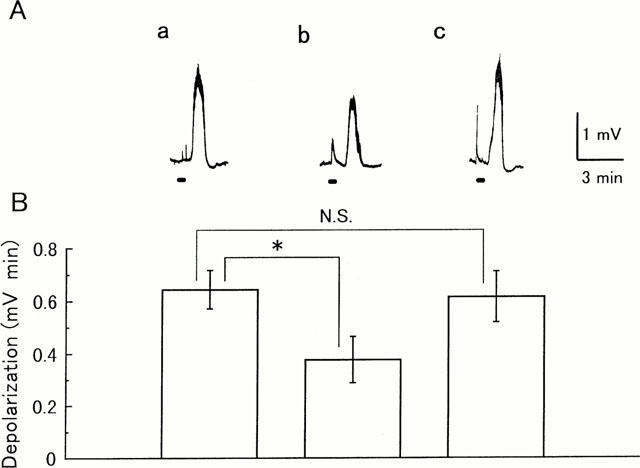
Effect of max.d.4 on the ventral root depolarization evoked by noxious skin stimulation. Potentials were recorded extracellularly from the L3 ventral root of a spinal cord-saphenous nerve-skin preparation prepared from a 1-day-old rat. Capsaicin (0.1 μM) was applied to the skin during the period (30 s) indicated by the bars. (A) Sample records of capsaicin-induced depolarization. (a) Control response. (b) After pretreatment of max.d.4 (1 μM) to the medium superfusing the spinal cord for 15 min. (c) Forty-five minutes after the removal of max.d.4. (B) Each column and vertical bar represent mean±s.e.mean of the magnitude of capsaicin-induced depolarization (n=5) *P<0.05.
Bath-application of maxadilan for 60 s to the spinal cord evoked a long-lasting depolarization of the ventral root (Figure 4). The amplitude of depolarization became maximum within 30 s after the application and the depolarization decreased gradually. The peak amplitude of depolarization evoked by maxadilan increased in a concentration-dependent manner between 0.01 and 1 μM (n=4, P<0.001 by two-way ANOVA, Figure 5). In the presence of TTX (0.3 μM), which completely blocked the spinal reflexes evoked by dorsal root stimulation (Kurihara & Yoshioka, 1996), the depolarization induced by maxadilan (0.03 – 0.3 μM) was markedly depressed (n=3, P<0.001 by two-way ANOVA, Figure 5).
Figure 4.
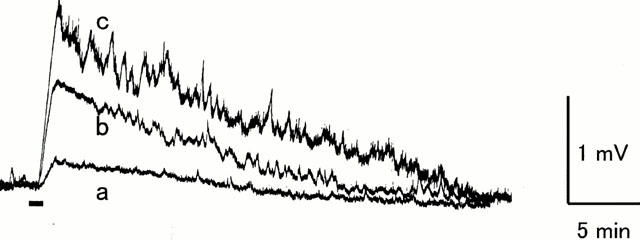
Ventral root depolarization evoked by maxadilan. Potential was recorded extracellularly from the L4 ventral root of an isolated spinal cord from a 1-day-old rat. Maxadilan was applied to the medium superfusing the spinal cord during the period (60 s) indicated by the bar. (a) 0.01 μM (b) 0.1 μM (c) 1 μM.
Figure 5.
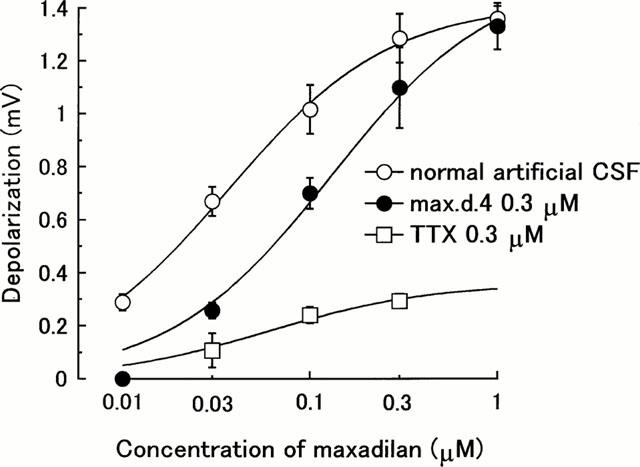
Effects of max.d.4 (0.3 μM) and tetrodotoxin (TTX 0.3 μM) on the concentration-response curve for maxadilan. Maxadilan was applied to the medium superfusing the spinal cord for 60 s. Max.d.4 and TTX were pre-applied for 15 min before the application of maxadilan. Ordinate scale: peak amplitude (mV) of ventral root depolarization. Abscissa scale: logarithmic concentration of maxadilan. Each point and vertical bar represent mean±s.e.mean (n=3 – 4) *P<0.05.
Pre-application of max.d.4 (0.3 μM) depressed the maxadilan (0.01 – 0.1 μM) induced-depolarization and significantly shifted the concentration-response curve for maxadilan to the right (n=4, P<0.001, by two-way ANOVA, Figure 5).
Expression of PACAP/VIP family receptors in the spinal cord
Binding assay
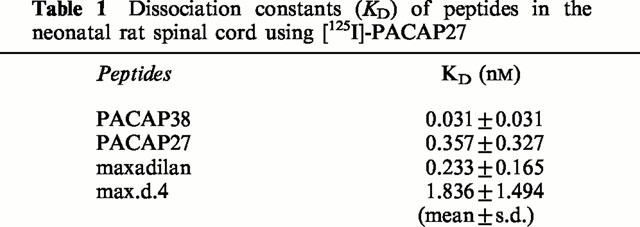
In neonatal rat spinal cord, [125I]-PACAP27 binding was displaced by 100 nM of PACAP27 and PACAP38, but not VIP, indicating that PACAP receptor, and not VPAC1 nor VPAC2 receptor, is dominantly distributed in the spinal cord. The indication of dominant distribution of PACAP receptor was further supported by data showing that both maxadilan, a specific agonist of PACAP receptor, and max.d.4, its specific antagonist, displaced the [125I]-PACAP27 binding in the neonatal rat spinal cord (Figure 6). The Scatchard analysis of the displacement of [125I]-PACAP27 binding indicated the existence of a single class of binding sites (data not shown) and the dissociation constants (KD) were calculated (Table 1). The affinities of maxadilan, PACAP38 and their related peptides were as follows: PACAP38>maxadilan=PACAP27>max.d.4>>VIP. The maximum binding capacity in neonatal rat spinal cord was 5.2±1.5 pmol mg−1 protein.
Figure 6.
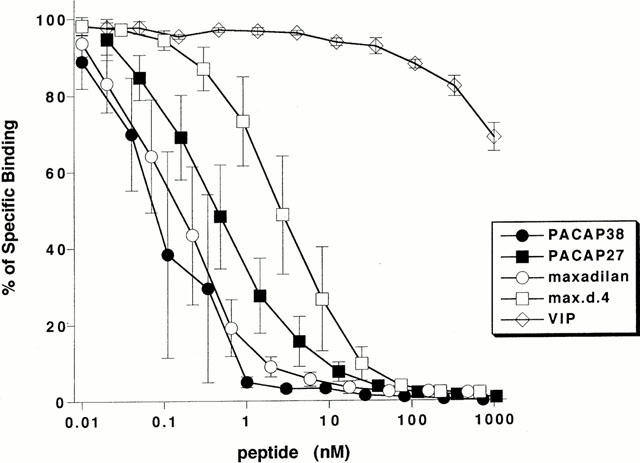
Displacement curves of [125I]-PACAP27 binding to neonatal spinal cord preparations by related peptides. Ordinate scale: [125I]-PACAP27 binding expressed as a percentage of control. Abscissa scale: logarithmic concentration of peptides. Each point and vertical bar represent mean±s.e.mean (n=3).
Table 1.
Dissociation constants (KD) of peptides in the neonatal rat spinal cord using [125I]-PACAP27
mRNA expression of PACAP/VIP family receptors analysed by RT – PCR in spinal cords
The mRNA expression of PACAP/VIP family receptors in rat spinal cords was analysed by the RT – PCR method (Figure 7) as previously reported by others (Rawlings et al., 1995). The PCR products for PACAP receptor at both 280 bp of the short form receptor and 364 bp of a single cassette insert (hip, hop1 or hop2) were equally present. In addition, the PCR products for VPAC2 receptor were also detected. In contrast, the PCR product for VPAC1 receptor was not detected. However, the number of VPAC2 receptors seemed to be very restricted from the data of binding assay.
Figure 7.
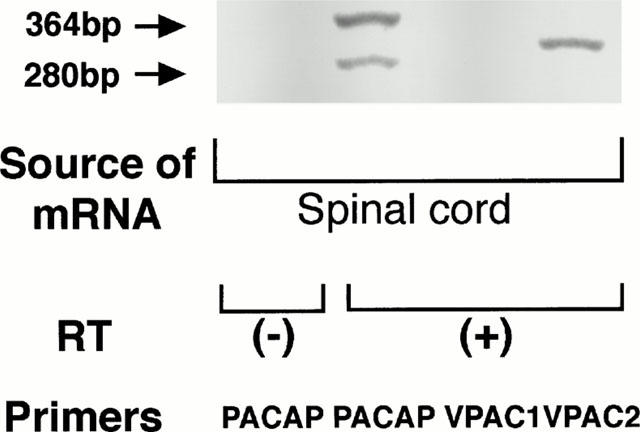
The mRNA expression of PACAP/VIP family receptors in neonatal rat spinal cord using RT – PCR. Total RNA was isolated by the guanidinium isothiocyanate/CsCl procedure from neonatal rat spinal cord (1-day-old). RT – PCR was performed using RNA PVR Kit (TaKaRa, Ohtsu, Japan) as described in ‘Methods'.
Discussion
In the present study, we clearly demonstrated that spinally-applied maxadilan, a PACAP receptor selective agonist, induced ventral root depolarization in a concentration-dependent manner and this effect was antagonized by max.d.4, a PACAP receptor selective antagonist. This indicated that the activation of spinal PACAP receptor resulted in the ventral root depolarization. Furthermore, the maxadilan-induced depolarization was markedly reduced in the presence of TTX, suggesting that a major component of the depolarization resulted from a summation of the trans-synaptic action through spinal interneurons and the contribution of direct action on motoneurons appeared to be relatively small.
Spinally applied max.d.4 had no effect on the fast reflex response, but it depressed slow VRP, evoked by the activation of primary afferents at C-fibre strength, in a concentration-dependent manner. In addition, PACAP(6-38), a putative PACAP/VPAC2 receptor antagonist, also depressed slow VRP evoked by the activation of primary afferents in a concentration-dependent manner. Furthermore, in the spinal cord – saphenous nerve – skin preparation, depolarization induced by the application of capsaicin to the skin was significantly depressed by max.d.4. These data strongly suggest that, when C-fibres are stimulated, PACAP receptor is activated by endogenous PACAP, thereafter generating slow VRP.
Of course, PACAP activates not only PACAP receptor, but also VPAC1 and VPAC2 receptors. The RT – PCR study showed that the mRNA expression of PACAP receptor and of VPAC2 receptor, but not of VPAC1 receptor, were demonstrated in the neonatal rat spinal cord. However, it is supposed that PACAP receptor is dominantly expressed, and that there may be only a small number of VPAC2 receptors in the neonatal rat spinal cord because the binding data showed that [125I]-PACAP27 binding was almost completely displaced by maxadilan, a specific agonist of PACAP receptor, and that [125I]-PACAP27 binding was displaced by VIP only 20% of control binding even at the highest concentration. Thus, it is not unreasonable to suppose that endogenous PACAP acts mostly through the activation of PACAP receptor.
It has been reported that PACAP(6-38) inhibited sustained neuronal firing of the wide dynamic range (WDR) neurons of adult rat spinal cord induced by topical application of mustard oil to the skin (Dickinson et al., 1997). PACAP(6-38) also demonstrates inhibition of sustained neuronal firing of the WDR neurons induced by 5°C cold stimulation in the rats with an experimental peripheral mononeuropathy (Dickinson et al., 1999). As mentioned above, PACAP(6-38) interacts with both PACAP receptor and VPAC2 receptor (Dickinson et al., 1997) and the data of the study using PACAP(6-38) did not reveal which receptor, PACAP or VPAC2, is important for the transmission of nociceptive information in the spinal cord. Maxadilan and max.d.4 are the first reported totally specific PACAP receptor agonist and antagonist, respectively, and our present study has now provided the first physiological evidence that PACAP receptor itself plays an important role in the nociceptive transmission in the spinal cord.
As mentioned in the introduction, VPAC1 receptor mRNA is most widely expressed and VPAC2 and PACAP receptor mRNAs are sparsely expressed in the adult rat spinal cord (Dickinson et al., 1999). On the other hand, the data indicate that, in the neonatal rat spinal cord, VPAC1 receptor mRNA is not expressed and PACAP receptor is the most abundant receptor among the receptors for PACAP. It is possible that the role of PACAP receptor in the nociceptive transmission in the neonatal rat spinal cord might be different from that in the adult rat spinal cord.
In conclusion, it was found that the PACAP receptor agonist evoked ventral root depolarization and this response was depressed by the PACAP receptor antagonist. Furthermore, the PACAP receptor antagonist depressed the primary afferent fibre-evoked nociceptive transmission in neonatal rat spinal cord. Although the role of PACAP receptor in the nociceptive transmission in the neonatal rat spinal cord may be different from that in the adult rat spinal cord, these findings indicate a possibility that PACAP receptor plays an excitatory role in nociceptive pathway in the neonatal rat spinal cord.
Acknowledgments
We thank Professor Takashi Nishino, Department of Anesthesiology, School of Medicine Chiba University for his generous support of our study. We are also grateful to Drs O. Moro and M. Tajima, Shiseido Research Center, Shiseido Co., Japan for the gift of maxadilan and max.d.4.
Abbreviations
- ANOVA
analysis of variance
- CSF
cerebrospinal fluid
- DRG
dorsal root ganglion
- KD
dissociation constant
- PACAP
pituitary adenylate cyclase-activating polypeptide
- RT – PCR
reverse transcription – polymerase chain reaction
- TTX
tetrodotoxin
- VIP
vasoactive intestinal peptide
- VRP
ventral root potential
- WDR
wide dynamic range
References
- ARIMURA A. Pituitary adenylate cyclase-activating polypeptide (PACAP): Discovery and current status of research. Reg. Peptides. 1992;37:287–303. [PubMed] [Google Scholar]
- ARIMURA A. Receptors for pituitary adenylate cyclase activating polypeptide: comparison with vasoactive intestinal peptide receptors. Trends Endocrinol. Metab. 1993;3:288–294. doi: 10.1016/1043-2760(92)90139-r. [DOI] [PubMed] [Google Scholar]
- ARIMURA A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- DICKINSON T., FLEETWOOD-WALKER S.M., MITCHELL R., LUTZ E.M. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides. 1997;31:175–185. doi: 10.1016/s0143-4179(97)90087-1. [DOI] [PubMed] [Google Scholar]
- DICKINSON T., MITCHELL R., ROBBERECHT P., FLEETWOOD-WALKER S.M. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology. 1999;38:167–180. doi: 10.1016/s0028-3908(98)00171-3. [DOI] [PubMed] [Google Scholar]
- DUN N.J., MIYAZAKI H., TANG H., DUN E.C. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and automatic functions. Neuroscience. 1996;73:677–680. doi: 10.1016/0306-4522(96)00057-7. [DOI] [PubMed] [Google Scholar]
- ISHIHARA T., SHIGEMOTO R., MORI K., TAKAHASHI K., NAGATA S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- KURIHARA T., YOSHIOKA K. The excitatory and inhibitory modulation of primary afferent fibre evoked responses of ventral roots in the neonatal rat spinal cord exerted by nitric oxide. Br. J. Pharmacol. 1996;118:1743–1753. doi: 10.1111/j.1476-5381.1996.tb15600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYATA A., ARIMURA A., DAHL R.R., MINAMINO N., UEHARA A., JIANG L., CULLER M.D., COY D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- MIYATA A., JIANG L., DAHL R.D., KITADA C., KUBO K., FUJINO M., MINAMINO N., ARIMURA A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the Pituitary adenylate cyclase activating peptide with 38 residues (PACAP38) Biochem. Biophys. Res. Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- MOLLER K., ZHANG Y.Z., HAKANSON R., LUTS A., SJOLUND B., UDDMAN R., SUNDLER F. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- MORO O., LERNER E.A. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type 1 receptor agonist. J. Biol. Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- MORO O., WAKITA K., OHNUMA M., DENDA S., LERNER E.A., TAJIMA M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J. Biol. Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- MULDER H., UDDMAN R., MOLLER K., ZHANG Y.Z., EKBLAD E., ALUMETS J., SUNDLER F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63:307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- NARITA M., DUN S.L., DUN N.J., TSENG L.F. Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord. Eur. J. Pharmacol. 1996;311:121–126. doi: 10.1016/0014-2999(96)00359-7. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., YANAGISAWA M. Effect of a tachykinin antagonist on a nociceptive reflex in the isolated spinal cord-tail preparation of the newborn rat. J. Physiol. 1988;395:255–270. doi: 10.1113/jphysiol.1988.sp016917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROPATO-MUSSAFIRI R., KANSE S.M., GHATEI M.A., BLOOM S.R. Pituitary adenylate cyclase-activating polypeptide releases 7B2, adrenocorticotrophin, growth hormone and prolactin from the mouse and rat clonal pituitary cell lines AtT-20 and GH3. J. Endocrinol. 1992;132:107–113. doi: 10.1677/joe.0.1320107. [DOI] [PubMed] [Google Scholar]
- RAWLINGS S.R., PIUZ I., SCHLEGEL W., BOCKAERT J., JOURNOT L. Differential expression of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptor subtypes in clonal pituitary somatotrophs and gonadotrophs. Endocrinology. 1995;136:2088–2098. doi: 10.1210/endo.136.5.7720658. [DOI] [PubMed] [Google Scholar]
- SPENGLER D., WAEBER C., PANTALONI C., HOLSBOER F., BOCKAERT J., SEEBURG F.H., JOURNOT L. Differential signal tranduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- TATSUNO I., GOTTSCHALL P.E., KOVES K., ARIMURA A. Demonstration of specific binding sites for pituitary adenylate cyclase activating polypeptide (PACAP) in rat astrocytes. Biochem. Biophys. Res. Commun. 1990;168:1027–1033. doi: 10.1016/0006-291x(90)91132-c. [DOI] [PubMed] [Google Scholar]
- TATSUNO I., GOTTSCHALL P.E., ARIMURA A. Specific binding sites for pituitary adenylate cyclase activating polypeptide (PACAP) in rat cultured astrocytes: molecular identification and interaction with vasoactive intestinal peptide (VIP) Peptides. 1991;12:617–621. doi: 10.1016/0196-9781(91)90110-b. [DOI] [PubMed] [Google Scholar]
- UCHIDA D., TATSUNO I., TANAKA T., HIRAI A., SAITO Y., MORO O., TAJIMA M. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP Type 1 receptor. Ann. NY Acad. Sci. 1998;865:253–258. doi: 10.1111/j.1749-6632.1998.tb11185.x. [DOI] [PubMed] [Google Scholar]
- XU X.-J., WIESENFELD-HALLIN Z. Intrathecal pituitary adenylate cyclase activating polypeptide facilitates the spinal nociceptive flexor reflex in the rat. Neuroscience. 1996;72:801–804. doi: 10.1016/0306-4522(96)00006-1. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., TATSUNO I. Antinociceptive effect of intrathecally administered pituitary adenylate cyclase activating polypeptide (PACAP) on the rat formalin test. Neurosci. Lett. 1995;184:32–35. doi: 10.1016/0304-3940(94)11161-b. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., HOSOKI R., OTSUKA M. The isolated spinal cord-skin preparation of the newborn rat and effects of some algogenic and analgesic substances. Eur. J. Pharmacol. 1992;220:111–117. doi: 10.1016/0014-2999(92)90737-o. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., MALMBERG A.B., SJÖLUND B., YAKSH T.L. The effect of pituitary adenylate cyclase activating polypeptide (PACAP) on the nociceptive formalin test. Neurosci. Lett. 1996;207:187–190. doi: 10.1016/0304-3940(96)12516-7. [DOI] [PubMed] [Google Scholar]
- ZHANG Y.Z., SJÖLUND B., MOLLER K., HÅKANSON R., SUNDLER F. Pituitary adenylate cyclase activating polypeptide produces a marked and long-lasting depression of a C-fiber-evoked flexion reflex. Neuroscience. 1993;57:733–737. doi: 10.1016/0306-4522(93)90019-c. [DOI] [PubMed] [Google Scholar]


