Abstract
Spinal cord slices and whole-cell patch clamp recordings were used to investigate the effects of serotonergic receptor ligands on dorsal root-evoked synaptic responses in deep dorsal horn (DDH) neurons of the neonatal rat at postnatal days (P) 3 – 6 and P10 – 14.
Bath applied 5-hydroxytryptamine (5-HT) potently depressed synaptic responses in most neurons. Similarly, the 5-HT1/7 receptor agonist, 5-carboxamidotryptamine (5-CT) depressed synaptic responses. This action was probably mediated by 5-HT1A receptor activation, since it occurred in the presence of the 5-HT7 receptor antagonist clozapine and was not observed in the presence of NAN-190, a 5-HT1A receptor antagonist. In the absence of any agonist, 5-HT1A receptor antagonists often facilitated synaptic responses, suggesting that there is sufficient endogenous 5-HT to tonically activate 5-HT1A receptors.
8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), the 5-HT1A/7 receptor agonist, facilitated synaptic responses, an action probably mediated by 5-HT7 receptors, since the facilitation could be reversed by subsequent application of the 5-HT7 receptor antagonist clozapine.
Agonists for the 5-HT1B, 5-HT2 and 5-HT3 receptors exerted only modest modulatory actions.
A pharmacological analysis of the depression evoked by 5-HT suggested an action partly mediated by 5-HT1A receptor activation, since antagonism of the 5-HT1A receptor with NAN-190 or WAY-100635 partly reversed 5-HT-evoked depression. In comparison, 5-HT7 receptor activation could account for much of the 5-HT-evoked facilitation.
We conclude that 5-HT is capable of modulating sensory input onto DDH neurons via several receptor subtypes, producing both facilitatory and depressant actions. Also, the actions of most receptor ligands on the evoked responses were similar within the first 2 postnatal weeks.
Keywords: Neuromodulation, nociception, sensory, afferent, 5-HT1A, 5-HT1B, 5-HT2, 5-HT3, 5-HT7
Introduction
The deep dorsal horn (DDH) region of the spinal cord is a primary site for the integration of somatosensory information, including nociception. Several endogenous systems within the CNS modulate the synaptic and cellular properties of DDH neurons (e.g. Basbaum & Fields, 1984; Hammond, 1986). One family of transmitters known to exert such actions comprises the brainstem monoamines that include several distinct descending serotonergic pathways. For example, serotonergic neurons of the raphe nuclei project widely to modulate spinal cord function via the dorsolateral and ventrolateral funiculi (Dahlström & Fuxe, 1965; for review see Basbaum & Fields, 1984; Fitzgerald, 1986; Hammond, 1986; Millan, 1995).
5-hydroxytryptamine (5-HT; serotonin) is released in the spinal cord following noxious input (e.g. Omote et al., 1998) and has been demonstrated to exert spinal antinociceptive actions (for review see Fitzgerald, 1986; Eide & Hole, 1993; Millan, 1995), implicating brainstem serotonergic systems in the control of spinal nociception. Exogenously applied 5-HT generally depresses, but can also facilitate primary afferent-evoked synaptic responses onto dorsal horn neurons (e.g. Randic & Yu, 1976; Headley et al., 1978; Jordan et al., 1979; Lopez-Garcia & King, 1996; Lopez-Garcia, 1998). The mechanisms underlying 5-HT-evoked modulation of synaptic properties of spinal neurons are not fully understood but it appears from most studies that the 5-HT1 receptors play an inhibitory, presumably antinociceptive role, while the 5-HT2 receptors play a facilitatory (pronociceptive) role (e.g. Eide & Hole, 1991; Hori et al., 1996; Lopez-Garcia & King, 1996).
Currently, there are seven families of 5-HT receptors (5- HT1 – 7), comprising at least 14 distinct receptor subtypes (for review see Hoyer et al., 1994; Barnes & Sharp, 1999). Several receptors, which include the 5-HT1, 5-HT2 and 5-HT3 receptors, have been identified in the spinal cord dorsal horn (e.g. Huang & Peroutka, 1987; Marlier et al., 1991; Kidd et al., 1993; Pompeiano et al., 1994). With the exception of the ionotropic 5-HT3 receptor, all serotonergic receptors are G protein-coupled receptors and hence capable of exerting a broad modulatory influence on network and cell behaviour, as most, if not all, ligand- and voltage-gated channels can be modulated by 5-HT (e.g. see reviews by Anwyl, 1990; Barnes & Sharp, 1999).
In this study, we used the spinal cord slice preparation and whole-cell patch recordings of sensory synaptic input onto DDH neurons to characterize and compare the modulatory actions of 5-HT to receptor subtype selective ligands with reported high affinity for 5-HT1A, 5-HT1B, 5-HT2, 5-HT3 and 5-HT7 receptors. While several investigators have examined the actions of 5-HT receptor ligands on the control of spinal cord function in vivo (e.g. Ali et al., 1994; 1996; Clarke et al., 1996; Gjerstad et al., 1996; 1997; Ogilvie et al., 1999), only a few studies have examined their actions at the spinal cellular level in vitro (e.g. Lopez-Garcia & King, 1996; Lopez-Garcia, 1998; Khasabov et al., 1999). In vitro studies offer the advantage of applying multiple ligands at known concentrations (e.g. Wallis et al., 1993a; Wallis & Wu, 1993) while intracellular recordings permit a cellular characterization of neuromodulatory mechanisms of action. We examined dorsal root-evoked synaptic responses in two age groups of neonatal rats (P3 – 6 and P10 – 14) to determine whether developmental alterations occur in serotonergic receptor modulation during the first 2 postnatal weeks. Our results suggest that primary afferent synapses are modulated by several 5-HT receptor subtypes and that most evoked responses can be depressed by 5-CT acting on the 5-HT1A receptor and facilitated by 8-OH-DPAT acting on the 5-HT7 receptor. These actions were independent of age range examined. Parts of this work have been published previously in abstract form (Hochman & Garraway, 1998).
Material
Preparation of spinal cord slices
Sprague-Dawley rats, postnatal days (P) 3 – 6 and P10 – 14, were used. The older animals (P10 – 14) were first anaesthetized with 10% urethane (2 mg kg−1 body weight i.p.), decapitated and spinal segments L2 – S1 were removed using cooled (<4°C) oxygenated (95%O2 – 5%CO2) high sucrose-containing artificial cerebrospinal fluid (aCSF) containing (in mM): sucrose, 250; KCl, 2.5; CaCl2, 1; MgCl2, 3; glucose, 25; NaH2PO4, 1.25; NaHCO3, 26; at a pH of 7.4. The younger animals (P3 – 6) were decapitated and spinal segments L2 – S1 were removed using cooled oxygenated normal aCSF containing (in mM): NaCl, 125; KCl, 2.5; CaCl2, 2; MgCl2, 1; glucose, 25; NaH2PO4, 1.25; NaHCO3, 26; at a pH of 7.4. The isolated spinal cord was embedded in Agar, 2.5% w v−1, (Type E, Sigma) and sliced on a vibrating blade microtome (Leica VT1000S or Pelco 101) to yield transverse slices (500 – 600 μm thick), incubated for at least 1 h prior to experimentation in normal oxygenated aCSF maintained at 32°C.
Electrophysiology
Spinal cord slices were affixed to a recording chamber for experimentation (Edwards et al., 1989). The transverse slice provides a reasonable outline of the spinal cord grey matter when viewed microscopically to permit reliable targeting of the DDH (King et al., 1988). Short dorsal rootlets remained attached to the spinal slices to allow for electrical stimulation of primary afferents. Patch electrodes were prepared from 1.5 mm outer diameter capillary tubes (Precision Instruments or Warner) using a two-stage puller (Narishige PP83) to produce resistance values ranging from 4 – 7 MΩ. The intracellular recording solution contained (in mM): K-gluconate, 140; EGTA, 0.2; HEPES, 10; Mg-ATP, 4; GTP, 1; pH 7.3. In most experiments, 2 mM QX-314 (RBI) was added to the recording solution to block voltage-dependent Na+ channels. The recording chamber was continuously superfused with oxygenated normal aCSF at a rate of ∼2 ml min−1.
The whole-cell ‘blind' patch clamp recording technique (Blanton et al., 1989) was undertaken at room temperature using the Axopatch 1D amplifier (Axon Instruments) filtered at 5 kHz (4-pole low-pass Bessel) to record from DDH neurons (laminae III – VI). Both voltage and current clamp data were acquired on computer with the pCLAMP acquisition software Clampex (v 6.0; Axon Instruments). Immediately following rupture of the cell membrane (in voltage clamp at −90 mV), the current clamp-recording configuration was used to determine resting membrane potential. Series resistance was subtracted in current clamp mode (bridge balance). Most experiments were conducted in the current clamp mode, although in a few cases, voltage clamp recordings were made. In voltage clamp experiments, series resistance remained uncompensated. For all neurons, resting membrane potential, leak conductance and bridge balance were monitored throughout to ensure recording stability. If recordings were unstable, the recording was discontinued and the data rejected. Mean electrode series resistance was 39±10 Mω.
Dorsal rootlets were electrically stimulated with bipolar tungsten electrodes at intensities that recruit the majority of afferent fibre types (typically ⩾500 μA, ⩾100 μs) (see Thompson et al., 1990). Stimulation-evoked excitatory postsynaptic potentials or currents (epsps or epscs) were collected at low frequencies (usually every 60 s in P3 – 6 and every 20 or 30 s in P10 – 14 rats). Membrane potential was held at −90 mV using injection of bias current for the entire duration of the recording.
Application of ligands
Drugs were prepared on the day of the experiment from frozen stock solutions. All drugs were dissolved in normal aCSF and bath applied using independent perfusion lines connected to a common output. Generally, following a 10 – 15 min period for collection of ‘control' evoked synaptic responses, each ligand was applied for a period of 10 – 15 min, during which time synaptic responses were continually recorded at baseline membrane potential and frequency. If membrane potential was altered by drug application, bias current was injected to return the membrane to −90 mV prior to continued collection of evoked synaptic responses. In order to compare the actions of several 5-HT receptor ligands on the evoked synaptic responses of a given neuron, we allowed a washout/recovery period of 10 – 20 min between subsequent drug applications.
Drugs used
5-HT was applied at 10 μM (in 100 μM ascorbic acid to prevent oxidation). The effective 5-HT receptor agonists used were: 5-carboxamidotryptamine (5-CT), 5-HT1/7 receptor agonist; 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), a 5-HT1A/7 receptor agonist; 7-trifluoromethyl-4-(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline (CGS), a 5-HT1B receptor agonist; 1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane (DOI), a 5-HT2 receptor agonist; and, 1-(m-chlorophenyl)-biguanide (CPBG), a 5-HT3 receptor agonist. The effective 5-HT receptor antagonists used were; NAN-190 or WAY-100635, 5-HT1A receptor antagonists; ketanserin, a 5-HT2A/2C receptor antagonist; and clozapine, a 5-HT7 receptor antagonist. 8-OH-DPAT (in 100 μm ascorbic acid), DOI, NAN-190, WAY-100635, ketanserin and clozapine were added at a concentration of 0.1 μM. 5-CT, CGS and CPBG were added at a concentration of 1 μM. All ligands were obtained from RBI/Sigma (Natick, MA, U.S.A.).
Analysis
Recordings were analysed using Clampfit (v 6.0, Axon Instruments). The maximum amplitude of the synaptic response of individual traces was measured. Comparison of evoked synaptic responses before and after application of 5-HT receptor ligands was made and an effect mediated by the applied ligands was considered modulatory if differences in amplitude of the evoked synaptic responses were ⩾10%. Because multiple drugs were added in some experiments and evoked responses did not often return to pre-drug baseline values, the change in synaptic amplitude was measured as the difference in the peak amplitude during drug application compared to the amplitude in the control period just prior to drug application.
Following analysis, graphs were constructed using Sigma Plot (SPSS) and imported into CorelDRAW (Corel) for final editing. All values in text are reported as mean±s.d. whilst figures are reported as mean+s.e.mean bars. Statistical comparisons were made using the Student's paired t-test.
Results
A total of 101 neurons from laminae III – VI were recorded, having a mean resting membrane potential of −55±9 mV. Since QX-314 was added to the recording solution in most neurons, threshold and firing properties were not systematically examined. However, we previously reported a detailed characterization of membrane properties of DDH neuron in these two age groups of rats (Hochman et al., 1997).
Effects of 5-HT on primary afferent-evoked synaptic responses
Bath applied 5-HT significantly depressed synaptic responses by 52±20% in 34/43 DDH neurons obtained from P3 – 6 animals (P<0.001) and by 49±20% in 22/28 neurons obtained from P10 – 14 animals (P<0.05). 5-HT also facilitated evoked responses in a minority of neurons from both age groups (3/43 in P3 – 6; 28±10%↑ and 4/28 in P10 – 14; 59±70%↑).
Effects of the 5-HT receptor ligands on primary afferent-evoked responses
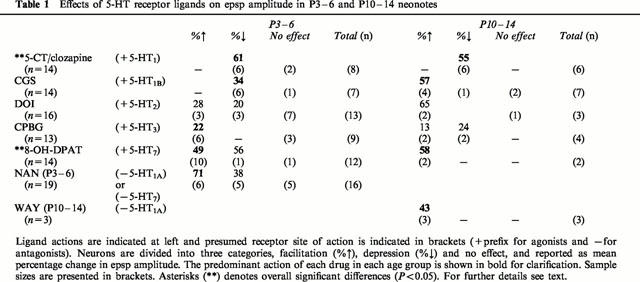
Table 1 summarizes the actions of receptor ligands in the two age groups examined and should be referred to in the following sections.
Table 1.
Effects of 5-HT receptor ligands on epsp amplitude in P3 – 6 and P10 – 14 neonotes
5-HT1 receptor mediated actions
Because 5-CT has relatively high affinity for both 5-HT1 and 5-HT7 receptors, we effectively activated only the 5-HT1 receptors by adding 5-CT in the presence of clozapine, a 5-HT7 receptor antagonist, which has a very low affinity for the 5-HT1 receptor. 5-CT/clozapine evoked a synaptic depression of 58±21% in 12 of 14 neurons obtained from both younger and older animals (P<0.001; Table 1). In the remaining two neurons (from younger animals), 5-CT/clozapine was without effect. Following washout of drugs, only a partial recovery of the evoked synaptic responses was observed (also see Lopez-Garcia, 1998), perhaps due to persistent alterations in G-protein mediated actions.
While it is possible that the actions of 5-CT were on receptors other than the 5-HT1A receptor, application of 5-CT in the presence of the 5-HT1A receptor antagonist NAN-190 prevented the depressant response (n=2) and instead a facilitatory action was observed presumably via activation of 5-HT7 receptors (Figure 1C). In addition, while 8-OH-DPAT, a 5-HT1A/7 receptor agonist generally facilitated synaptic actions presumably via agonist activity at the 5-HT7 receptor (see section below on 5-HT7 receptor mediated actions), 8-OH-DPAT was also capable of depressing the evoked response (Figure 1D).
Figure 1.
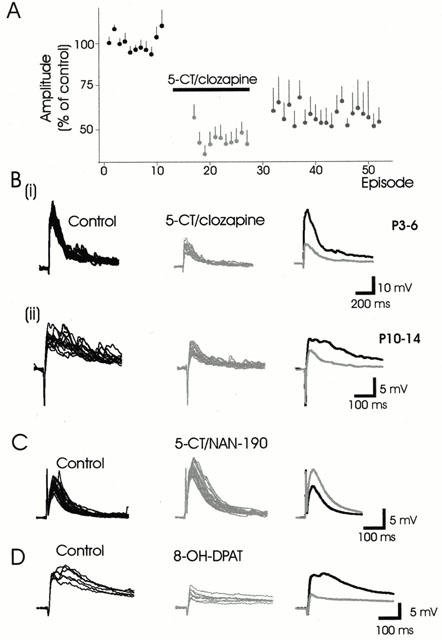
5-HT1A receptor activation depresses naïve synaptic responses. (A) Normalized data showing the effect of 5-CT/clozapine on the amplitude of evoked synaptic responses in both older and younger animals combined (n=12). The horizontal bar indicates the timing of the drug application. Note that the amplitudes of the synaptic responses are only partially reversed following washout of 5-CT/clozapine. The values for normalized data are presented as the mean+s.e.mean. (B) Examples showing the 5-CT/clozapine-induced depression of epsps observed in neurons obtained from P3 – 6 (63%) and P10 – 14 (50%) animals respectively. (C) Application of the 5-HT1A/7 receptor agonist 5-CT in the presence of the 5-HT1A receptor antagonist NAN-190 facilitated e.ps.p amplitude (52%). (D) Example of a cell where the 5-HT1A receptor agonist 8-OH-DPAT depressed the evoked e.p.sp. In this and the following similar figures, the individual raw traces of the control synaptic responses are superimposed in black, while the raw traces in the presence of the ligands are superimposed in grey. Averaged synaptic actions are presented to the right.
Interestingly, in the absence of any applied agonist, application of either 5-HT1A receptor antagonist, NAN-190 or WAY-100635, produced synaptic facilitation (62±42%) in nine of 19 neurons. This action suggests that 5-HT1A receptors are tonically active and inhibiting evoked responses in the spinal slice, presumably via persistent endogenous 5-HT release. In addition, in 5/19 neurons, application of NAN-190 produced a synaptic depression (38±21%). We interpreted this as suggesting that in some neurons, NAN-190 may be acting at another receptor subtype (e.g. 5-HT7) to block a tonic facilitating action of 5-HT (see Discussion). Figure 2 illustrates the facilitatory and inhibitory effects of these antagonists.
Figure 2.
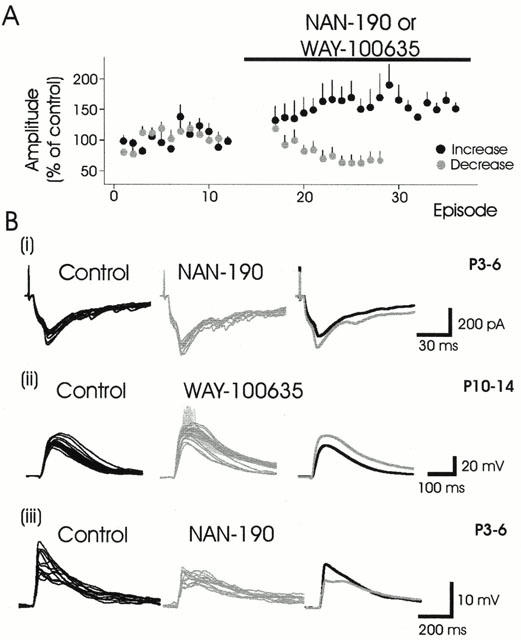
In the absence of agonist, 5-HT1A receptor antagonists can facilitate or depress evoked synaptic responses. (A) Normalized data showing the effects of NAN-190 or WAY-100635 on evoked synaptic responses. The antagonists could facilitate (black circles) or depress (grey circles) evoked synaptic responses. (B) Examples showing 5-HT1A receptor antagonist-induced synaptic facilitation in younger (i) and older (ii) animals. In addition to synaptic facilitation spikes were recruited in the presence of WAY-100635 in (ii) and they are presented as lighter shaded events in the raw traces. These events were not included in the analysis of the average epsp amplitude increase at right. Note traces in B(i) are epscs, voltage clamp recording. B(iii) Example of synaptic depression produced by NAN-190. Percentage change in peak synaptic response in the presence of the ligands were 27%↑, 29%↑ and 37%↓, respectively.
Actions of 5-HT1B, 5-HT2 and 5-HT3 receptor agonists
The 5-HT1B receptor agonist CGS depressed the evoked synaptic responses in 6/7 neurons from younger animals (P<0.05), but caused an insignificant facilitation in 4/7 neurons in the older animals (Table 1). Figure 3A(i) demonstrates the effects of CGS on evoked synaptic responses. Part of the increased synaptic response in older animals may be attributed to an observed increase in input resistance (Figure 3A(ii)).
Figure 3.
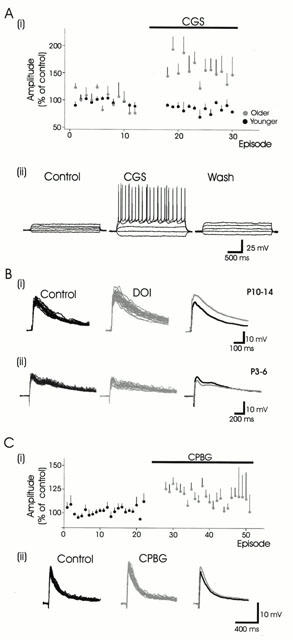
Actions of 5-HT1B, 5-HT2 and 5-HT3 receptor agonists. (A) The 5-HT1B receptor agonist CGS produces both depression and facilitation of synaptic responses in DDH neurons. (i) Normalized data showing a modest CGS-induced epsp depression in younger animals (black circles; n=6), while facilitating epsps in older animals (grey circles; n=4). (ii). The CGS-induced epsp amplitude increase is associated with a reversible increase in neuronal input resistance (160 MΩ in control; 330 MΩ in CGS) and a decrease in rheobase (70 pA in control; 50 pA in CGS). Responses presented arise from 30 pA depolarizing and hyperpolarizing current steps. (B) The 5-HT2 receptor agonist DOI modestly facilitates (18%) (i) or depresses (20%) evoked synaptic responses in DDH neurons (ii). (C) The 5-HT3 receptor agonist CPBG facilitates synaptic responses in the younger animals, but produces both facilitation and depression in neurons obtained from older animals. (i). Normalized data showing the modest facilitatory actions of CPBG on neurons obtained from both younger (n=6) and older (n=2) animals. (ii). Example showing synaptic facilitation (15%) obtained in a neuron obtained from an older animal.
The 5-HT2 receptor agonist DOI facilitated responses in 5/16 neurons (44±43%), and only modestly depressed responses in three neurons (20±10%) (Figure 3B). In half of the neurons (n=8) DOI was without effect. In five neurons, application of the 5-HT2A/2C receptor antagonist ketanserin alone did not modify synaptic responses, suggesting that, unlike the 5-HT1A receptor, 5-HT2 receptors are not tonically activated by endogenous 5-HT release in the slice preparation.
The 5-HT3 receptor agonist CPBG had modest actions, facilitating synaptic responses in eight of 13 neurons (19±7%) (Figure 3C), while depressing responses in only two neurons obtained from older animals.
5-HT7 receptor mediated actions
8-OH-DPAT has high affinity for both the 5-HT1A and 5-HT7 receptors. In the presence of 8-OH-DPAT alone, synaptic facilitation (50±43%) was observed in the majority of neurons tested (12/14) (Figure 4A,B(i)). Addition of the 5-HT7 receptor antagonist clozapine was capable of partially reversing the 8-OH-DPAT-induced facilitation in two neurons tested (Figure 4B(ii)), suggesting that the facilitatory action of 8-OH-DPAT is due to activation of the 5-HT7 and not the 5-HT1A receptor. We also tested the ability of the 5-HT1/7 receptor agonist 5-CT to activate 5-HT7 receptors by adding 5-CT in the presence of the 5-HT1A receptor antagonist NAN-190 in two neurons. We observed a synaptic facilitation of similar magnitude (52%) to that produced by 8-OH-DPAT (Figure 1C), suggesting that the facilitatory actions of 5-CT and 8-OH-DPAT are mediated by 5-HT7 and not 5-HT1A receptor activation.
Figure 4.
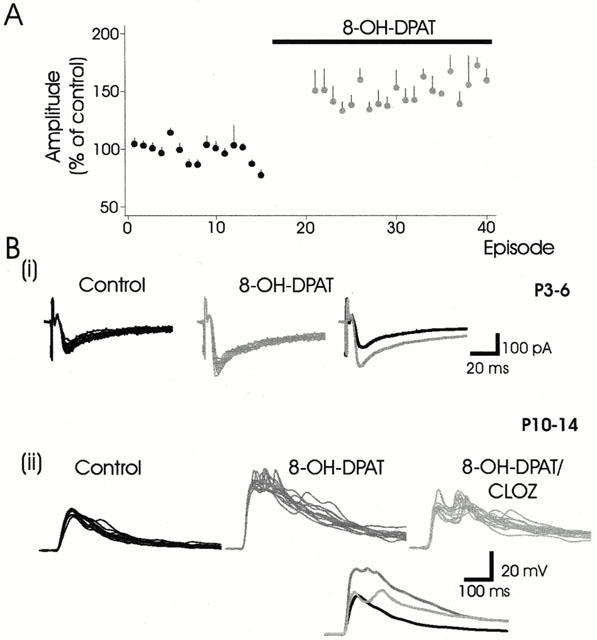
5-HT7 receptor activation mediates synaptic facilitation. (A) Normalized data demonstrating synaptic facilitation (n=12) evoked by 8-OH-DPAT in neurons obtained from both younger and older animals. (B(i)) An example of 8-OH-DPAT-induced facilitation (58%) of evoked epsc. (ii). The facilitation of the early epsp peak produced by 8-OH-DPAT (62%) is largely reversed by clozapine, the 5-HT7 receptor antagonist.
Receptors mediating 5-HT-evoked depression and facilitation
The 5-HT1 receptor agonist 5-CT depressed evoked synaptic responses at least partly via activation of the 5-HT1A receptor (Figure 1). Therefore, we tested the possibility that the 5-HT1A class of receptor mediates the depression evoked by 5-HT. In some neurons, we added 5-HT to produce synaptic depression, then added the 5-HT1A receptor antagonists NAN-190 or WAY-100635. In three of eight neurons, the depression induced by 5-HT was partly reversed in the presence of these antagonists (Figure 5A). However, in other neurons (n=5), the antagonists had minimal effects on 5-HT-evoked depression, while drug washout reversed 5-HT's depressant actions (Figure 5B). These results suggest that additional receptors contribute to mediating the 5-HT-evoked depression. Interestingly, in one neuron, neither CGS, 8-OH-DPAT, WAY-100635 nor clozapine altered the amplitude of the evoked response, while 5-HT caused a large depression even following co-application with NAN-190, the 5-HT1A receptor antagonist (Figure 5C), suggesting that there are serotonergic receptors other than 5-HT1A or 5-HT1B receptors that can mediate the 5-HT-evoked depression. We did not explore the possibility of a contribution from 5-HT3 receptors to the 5-HT induced depression because the actions of the 5-HT3 receptor agonist CPBG (at 1 μM) were only modest and usually facilitatory (see Discussion).
Figure 5.
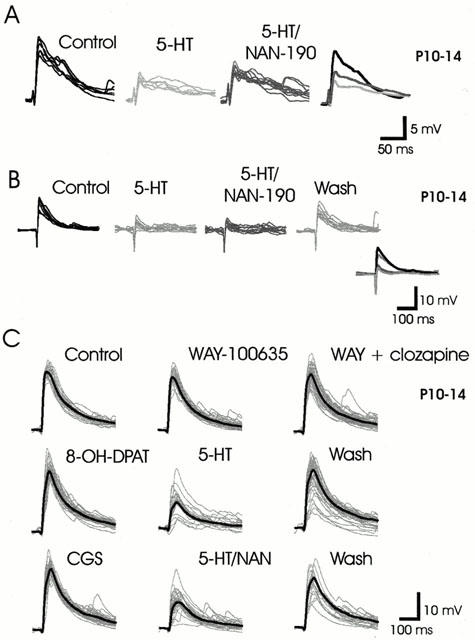
Unidentified receptors mediate part of the depressant actions of 5-HT. (A) 5-HT-evoked epsp depression is partly reversed in the presence of the 5-HT1A receptor antagonist NAN-190. (B) NAN-190 has no effect on 5-HT-evoked depression while washout of 5-HT leads to a near complete recovery of synaptic amplitude. (C) WAY-100635, clozapine, 8-OH-DPAT, or CGS has no effect on the evoked synaptic responses. However, 5-HT produces potent depression of the epsp, which is unchanged by NAN-190, suggesting that 5-HT receptors other than 5-HT1A, 5-HT1B or 5-HT7 are mediating the depressant action of 5-HT. In C only, the individual traces are presented superimposed in grey, while the average response is presented overlaid as a thick-black trace.
Since 5-HT7 receptor activation facilitated synaptic responses, we tested whether the 5-HT-evoked facilitation observed in a small proportion of neurons could be 5-HT7 receptor mediated. In these experiments, the 5-HT7 receptor antagonist clozapine was added subsequent to 5-HT. Clozapine reversed the 5-HT-induced facilitation in two of four neurons (Figure 6A), but had no effect on three neurons where 5-HT produced a synaptic depression (Figure 6B). Thus, 5-HT7 receptors appear to play a role in mediating 5-HT-evoked facilitation but not 5-HT-evoked depression. The contribution of 5-HT2 receptors to a 5-HT induced synaptic facilitation was not examined but may have been responsible for the 5-HT induced facilitation in neurons that were insensitive to clozapine.
Figure 6.
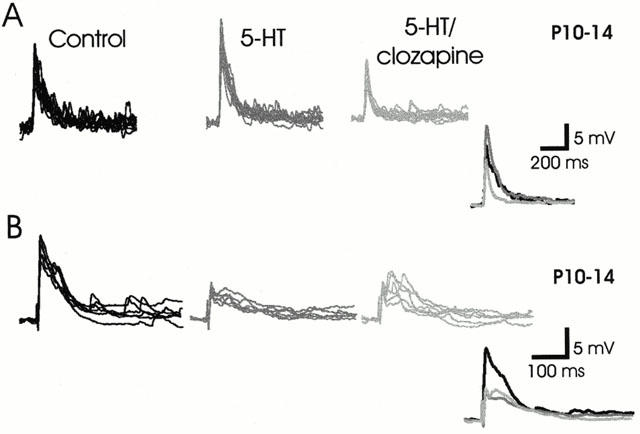
5-HT7 receptor antagonist partly reverses 5-HT-evoked facilitation, but not 5-HT-evoked depression of epsps. (A) The 5-HT-induced facilitation (35%) of epsp amplitude in a neuron obtained from an older animal is blocked by clozapine, allowing 5-HT to produce synaptic depression, presumably by actions on other receptor subtypes. (B) Clozapine does not remove the synaptic depression evoked by 5-HT.
Discussion
Summary
This study characterized the actions of 5-HT and several reported receptor-effective ligands on the regulation of sensory synaptic input onto DDH neurons of the neonatal rat. With the exception of the 5-HT1B receptor agonist CGS, the general effects of 5-HT receptor ligands in this study were similar within the first 2 weeks of postnatal development, and also compare to actions reported from earlier studies conducted in adult rats (e.g. Xu et al., 1994; Ali et al., 1996; Gjerstad et al., 1996; 1997), suggesting that the function of these 5-HT receptor subtypes in the DDH remains relatively unchanged during postnatal development.
Synaptic depression
Consistent with previous studies, primary afferent-evoked synaptic responses were usually depressed in the presence of the 5-HT1/7 receptor agonist 5-CT (also see, Lopez-Garcia & King, 1996; Lopez-Garcia, 1998; Khasabov et al., 1999). Our studies strengthen the notion that the 5-CT-evoked depression is via 5-HT1 receptor activation since the depressant action of 5-CT occurred in the presence of the 5-HT7 receptor antagonist clozapine. Antagonism of the 5-HT1A receptor with NAN-190 or WAY-100635 partly reversed a 5-HT-evoked depression in some neurons, demonstrating that 5-HT1A receptors depress synaptic responses. In the younger animals only, we observed that the 5-HT1B receptor agonist, CGS also produced synaptic depression, albeit less potent and widespread than that observed for 5-HT1 receptor activation with 5-CT.
The 5-HT1A and 5-HT1B receptors, which are present on primary afferent terminals and dorsal horn neurons (Daval et al., 1987; Marlier et al., 1991; Ridet et al., 1994; Laporte et al., 1995) may constitute dominant receptor subtypes in the dorsal horn of the rat (see Huang & Peroutka, 1987; Marlier et al., 1991). Interestingly, Huang & Peroutka (1987) reported that at least 33% of the total 5-HT binding sites in the rat spinal cord are distinct from 5-HT1A, 5-HT1B or 5-HT2C. Since like 5-HT1A&B receptors, the 5-HT1D, 5-HT1E, and 5-HT1F receptors are negatively coupled to adenylate cyclase (Barnes & Sharp, 1999), they are also likely to produce synaptic depression (see Manuel et al., 1995). Though not presently investigated, these receptors may also contribute to the widespread depressant action of 5-HT.
In this study, passive membrane properties of DDH neurons were generally unchanged (except for CGS in P10 – 14 group; discussed later), suggesting that modulatory actions occurred at the glutamatergic synapse itself, though a pre- and/or postsynaptic locus could not be determined from our studies. Both pre- and post-synaptic spinal actions of 5-HT have been demonstrated previously (e.g. Sillar & Simmers, 1994; Hori et al., 1996; Travagli & Williams, 1996; El Manira et al., 1997; Murase et al., 1990; Lopez-Garcia, 1998).
Synaptic facilitation
An interesting observation in this study is the facilitation of evoked synaptic responses by 8-OH-DPAT, the 5-HT1A/7 receptor agonist (Kennett, 1998). The additional observations that the 5-HT7 receptor antagonist clozapine partly reversed the actions of 8-OH-DPAT and that facilitation of a similar magnitude was observed using 5-CT (5-HT1/7 receptor agonist) in the presence of 5-HT1A receptor antagonists NAN-190 or WAY-100635, further suggest that these actions are mediated at the 5-HT7 receptor. Moreover clozapine could block the 5-HT induced facilitation observed in some neurons. Thus, it is likely that 5-HT7 receptors contribute to the facilitatory actions of 5-HT observed in some DDH neurons. Although the 5-HT7 receptor has been identified in several CNS regions (e.g. Gustafson et al., 1996; also see Eglen et al., 1997), its distribution in the spinal cord has not been examined.
Our observation that 8-OH-DPAT preferably activates the 5-HT7 receptor is somewhat surprising given the comparatively higher affinity reported for the 5-HT1A receptor (see Hoyer et al., 1994), which generally produces synaptic depression. However, this observation could explain reported inconsistencies between the actions of 5-CT and 8-OH-DPAT. For example, the enhancement of spinal reflexes in the rabbit by 8-OH-DPAT reported by Clarke et al. (1997) and Ogilvie et al. (1999) was reported to be due to a combined action mediated at the 5-HT1A and 5-HT7 receptors.
Activation of the 5-HT2 receptors with DOI facilitated evoked responses in only 5/16 neurons tested. This low frequency may be explained in relation to the relatively small percentage of 5-HT2A/2C receptors present in the dorsal horn of the rat (Pompeiano et al., 1994; Maeshima et al., 1998; Cornea-Hebert et al., 1999). The facilitation observed here is consistent with a facilitation of evoked excitatory synaptic responses in nearly 50% of dorsal horn neurons tested with higher concentrations of DOI (10 μM) reported by Hori et al. (1996). 5-HT2 receptor activation has been previously implicated in pronociceptive actions (e.g. Eide & Hole, 1991).
In addition to the 5-HT7 and 5-HT2 receptors, other receptor subtypes, which were not investigated in this study, may also contribute to the facilitatory actions of 5-HT. For instance, the 5-HT4 and 5-HT6 are positively coupled to adenylate cyclase and may also tend to have facilitatory actions. These receptors are localized in various CNS regions (see Verge & Calas, 2000), but only 5-HT6 receptor expression has been examined in the spinal cord, and its expression is weak (Gerard et al., 1997).
Unlike the metabotropic 5-HT receptors, the 5-HT3 receptor is ionotropic and leads to the opening of a monovalent cation permeable channel. Reports from previous studies on the events mediated by 5-HT3 receptor activation are conflicting. For instance, in relation to nociception, both pronociceptive (Ali et al., 1996; Oyama et al., 1996) and antinociceptive effects (Alhaider et al., 1991; Bardin et al., 1997; 2000; Giordano, 1997) have been reported. Here, we observed that the high affinity 5-HT3 receptor agonist CPBG (applied at 1 μM), produced facilitation of naïve synaptic response in most neurons, though of small magnitude, an observation consistent with an increase in number of evoked spikes in dorsal horn neurons of adult rats (Ali et al., 1996). This however opposes the attenuation of afferent-evoked neurotransmission observed by Khasabov et al. (1999) in similarly aged animals when CPBG was applied at much higher concentrations (10 – 50 μM). Since the vast majority of 5-HT3 receptors present in the spinal cord dorsal horn are found on primary afferent terminals (Hamon et al., 1989; Kidd et al., 1993; Laporte et al., 1995) where they can mediate a primary afferent depolarization (Khasabov et al., 1999), synaptic depression via presynaptic inhibitory mechanisms is expected. Even though 5-HT3 receptors are also present on some dorsal horn neurons including GABAergic (Morales et al., 1998) and enkephalinergic interneurons (Tsuchiya et al., 1999), we found no evidence to suggest that CPBG mediated direct excitatory actions on the DDH neurons. We suggest that the lower concentrations of CPBG used here preferentially activates receptors other than the 5-HT3 receptor, and so our results using CPBG should be interpreted cautiously. For example, although CPBG is a potent high affinity 5-HT3 receptor agonist (Kilpatrick et al., 1990), it has also been shown to interact with the catecholamines, facilitating the release of noradrenaline (Schlicker et al., 1994) and inhibiting the reuptake of dopamine (Campbell et al., 1995).
Endogenous release of 5-HT in the spinal slice
In the absence of applied agonists, the 5-HT1A receptor antagonists NAN-190 and WAY-100635 typically produced modest facilitation of the evoked responses. Since there appears to be no serotonergic neurons intrinsic to the spinal cord, this observation suggests that even in the spinal cord slice preparation, the endogenous 5-HT derived from brainstem nuclei can tonically depress primary afferent input to DDH neurons at least partly via 5-HT1A receptor activation (c.f. Wallis et al., 1993a,1993b). 5-HT has very high affinity for the 5-HT1A receptor (Zifa & Fillion, 1992) and Hadjiconstantinou et al. (1984) demonstrated that endogenous 5-HT remains in the spinal cord for more than a week following spinal transection. It is possible that low levels of endogenously released 5-HT serve to tonically regulate synaptic gain of primary afferent input.
Unexpectedly, in a few cases, NAN-190 alone instead produced a depression of the evoked response. We presume this action is mediated by blockade of tonic activation at the 5-HT7 receptor, which binds NAN-190 with moderate affinity (see Hoyer et al., 1994). Interestingly, Zhuo & Gebhart (1991) found that the descending serotonergic facilitation of nociception, abolished by bilateral transection of the dorsolateral funiculus, was not attenuated by the 5-HT2 or 5-HT3 receptor antagonists, but by methysergide, a non-specific 5-HT receptor antagonist (including block of 5-HT7 receptors), concluding that the facilitation is mediated by a 5-HT1 receptor. Rather, our findings indicate that tonic descending facilitation may be mediated by 5-HT7 receptor activation, while tonic descending inhibition is mediated via the 5-HT1A receptor.
Unlike the 5-HT1A/7 receptors, there was no evidence to support tonic endogenous activation of the 5-HT2 receptor, since ketanserin applied alone had no effect on naïve synaptic responses. These results are consistent with the higher affinity of 5-HT for 5-HT1A and 5-HT7 receptors over 5-HT2 receptors (see Zifa & Fillion, 1992; Hoyer et al., 1994) and also the low expression of 5-HT2A/2C receptors in the dorsal horn (see references above).
Interpreting actions of 5-HT receptor subtypes and ligands based on G-protein coupling
There is considerable evidence to suggest that metabotropic 5-HT receptors that activate G-proteins positively coupled to signal transduction pathways (e.g. Gs, Gq) facilitate synaptic responses, while those activating G-proteins that inhibit signalling pathways (e.g. Gi, Go) exert synaptic depression (for review see Anwyl, 1990; Uphouse, 1997). Accordingly, the facilitatory action of 8-OH-DPAT is consistent with 5-HT7 receptor activation, which activates Gs. Conversely, the depression evoked using 5-CT is consistent with activation of 5-HT1A receptors, which activates Gi/o. The actions of DOI were predominantly facilitatory, consistent with 5-HT2 receptor activation of Gq leading to a protein kinase C (PKC) mediated facilitation of glutamatergic responses, as reported previously in dorsal horn neurons (Hori et al., 1996). The CGS-mediated synaptic depression in the young animals is consistent with 5-HT1B receptor activation of Gi/o, but the facilitation in the older neonates would not be predicted to be 5-HT1B receptor mediated. Since our CGS-mediated facilitatory actions were due partly to postsynaptic mechanisms (increased cell resistance) that conflict with observations that 5-HT1B receptors only occur presynaptically on axon terminals (Verge & Calas, 2000; see also Wu et al., 1991), it is likely that CGS had its dominant action at a receptor other than 5-HT1B in older animals. Previous studies that have demonstrated inhibitory actions of 5-HT1B receptor agonists in the mature rat did not use CGS as the effective 5-HT1B receptor agonist (e.g. Murphy & Zemlan, 1990; Ali et al., 1994; Xu et al., 1994; Gjerstad et al., 1997).
Limitations of the experimental approach
There are several limitations to our experimental approach. First, bath applied 5-HT will activate all 5-HT receptors simultaneously, whereas physiologically, it is probable that there is some degree of preferential activation of receptor subtypes by separate descending serotonergic systems (Wei et al., 1999). Hence, physiological conditions may exist where synaptic facilitation dominates over the strong depression observed presently (see below). Second, this study used high-intensity electrical stimulation to recruit the majority of afferents within the stimulated dorsal root, and this could mask weaker opposing actions occurring in some specific afferent fibre populations. For example, Jankowska and co-workers have demonstrated that 5-HT and raphe-spinal stimulation can exert a differential control over primary afferent-evoked responses of different modalities (e.g. Riddell et al., 1993; Jankowska et al., 1997; 2000). A third limitation to this study is that the DDH is functionally heterogeneous, yet all dorsal horn neurons were treated here as a single population. Jankowska and co-workers have demonstrated that the actions of 5-HT may depend on the functional identity of the spinal neuron studied (Jankowska et al., 1997; 2000). These limitations notwithstanding, the present observations demonstrate that the 5-HT1A and 5-HT7 receptor subtypes have rather consistent actions in all neurons examined, and suggest that the serotonergic systems that modify sensory input by these receptors do so in a diffuse and general matter.
Relevance
Although 5-HT is implicated in the descending control of nociception, its potential analgesic benefit is limited by its widespread actions and complex pharmacology with different receptor subtypes capable of producing opposing modulatory actions. Our results demonstrate that despite the functional heterogeneity of 5-HT receptor subtypes and neurons within the DDH, pharmacological strategies may be employed to activate selective receptor subtypes with a broad neuromodulatory influence on spinal cord sensory function.
Acknowledgments
We thank Carolyn Gibbs for expert technical assistance, David Machacek and Barbara Shay for critically reviewing this manuscript. S. Hochman was supported by a grant from the Christopher Reeve Paralysis Foundation. S.M. Garraway obtained studentship salary support from the Rick Hansen Institute.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- 5-CT
5-carboxamidotryptamine
- CPBG
1-(m-chlorophenyl)-biguanide
- DDH
deep dorsal horn
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane
- epsc
excitatory postsynaptic current
- epsp
excitatory postsynaptic potential
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino) tetralin
- 5-HT
5-hydroxytryptamine
- PKA
protein kinase A
- PKC
protein kinase C
- CGS
7-trifluoromethyl-4-(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline
References
- ALHAIDER A.A., LEI S.Z., WILCOX G.L. Spinal 5-HT3 receptor-mediated antinociception: Possible release of GABA. J. Neurosci. 1991;11:1881–1888. doi: 10.1523/JNEUROSCI.11-07-01881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALI Z., WU G., KOZLOV A., BARASI S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Brain Res. 1994;661:83–90. doi: 10.1016/0006-8993(94)91184-3. [DOI] [PubMed] [Google Scholar]
- ALI Z., WU G., KOZLOV A., BARASI S. The role of 5HT3 in nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Neurosci. Lett. 1996;208:203–207. doi: 10.1016/0304-3940(95)12600-7. [DOI] [PubMed] [Google Scholar]
- ANWYL R. Neurophysiological actions of 5-hydroxytryptamine in the vertebrate nervous system. Prog. Neurobiol. 1990;35:451–468. doi: 10.1016/0301-0082(90)90031-b. [DOI] [PubMed] [Google Scholar]
- BARDIN L., BARDIN M., LAVARENNE J., ESCHALIER A. Effect of intrathecal serotonin on nociception in rats: influence of the pain test used. Exp. Brain Res. 1997;113:81–87. doi: 10.1007/BF02454144. [DOI] [PubMed] [Google Scholar]
- BARDIN L., LAVARENNE J., ESCHALIER A. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain. 2000;86:11–18. doi: 10.1016/s0304-3959(99)00307-3. [DOI] [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BASBAUM A.I., FIELDS H.L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- BLANTON M.G., LO TURCO J.J., KRIEGSTEIN A.R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J. Neurosci. Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A.D., WOMER D.E., SIMON J.R. The 5-HT3 receptor agonist 1-(m-chlorophenyl)-biguanide interacts with the dopamine transporter in rat brain synaptosomes. Eur. J. Pharmacol. 1995;290:157–162. doi: 10.1016/0922-4106(95)90029-2. [DOI] [PubMed] [Google Scholar]
- CLARKE R.W., HARRIS J., HOUGHTON A.K. Spinal 5-HT-receptors and tonic modulation of transmission through a withdrawal reflex pathway in the decerebrated rabbit. Br. J. Pharmacol. 1996;119:1167–1176. doi: 10.1111/j.1476-5381.1996.tb16019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE R.W., OGILVIE J., HOUGHTON A.K. Enhancement and depression of spinal reflexes by 8-hydroxy-2-(di-n-propylamino) tetralin in the decerebrated and spinalized rabbit: involvement of 5-HT1A- and non-5-HT1A-receptors. Br. J. Pharmacol. 1997;122:631–638. doi: 10.1038/sj.bjp.0701430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNEA-HEBERT V., RIAD M., WU C., SINGH S.K., DESCARRIES L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- DAHLSTRÖM A., FUXE K. Evidence for the existence of monoamine neurons in the central nervous system. Acta. Physiol. Scand. 1965;64 suppl:1–36. [PubMed] [Google Scholar]
- DAVAL G., VERGE D., BASBAUM A.I., BOURGOIN S., HAMON M. Autoradiographic evidence of serotonin1 binding sites on primary afferent fibres in the dorsal horn of the rat spinal cord. Neurosci. Lett. 1987;83:71–76. doi: 10.1016/0304-3940(87)90218-7. [DOI] [PubMed] [Google Scholar]
- EDWARDS E.A., KONNERTH A., SAKMANN B., TAKAHASHI T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. Trends Pharmacol. Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- EIDE P.K., HOLE K. Different role of 5-HT1A and 5-HT2 receptors in spinal cord in the control of nociceptive responsiveness. Neuropharmacology. 1991;30:727–731. doi: 10.1016/0028-3908(91)90180-j. [DOI] [PubMed] [Google Scholar]
- EIDE P.K., HOLE K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85. doi: 10.1046/j.1468-2982.1993.1302075.x. [DOI] [PubMed] [Google Scholar]
- EL MANIRA A., ZHANG W.Q., SVENSSON E., BUSSIÈRES N. 5-HT inhibits calcium current and synaptic transmission from sensory neurons in lamprey. J. Neurosci. 1997;17:1786–1794. doi: 10.1523/JNEUROSCI.17-05-01786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD M. Monoamines and descending control of nociception. Trends Neurosci. 1986;9:51–52. [Google Scholar]
- GERARD C., MARTRES M.P., LEFEVRE K., MIQUEL M.C., VERGE D., LANFUMEY L., DOUCET E., HAMON M., EL MESTIKAWY S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–219. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- GIORDANO J. Antinociceptive effects of intrathecally administered 2-methylserotonin in developing rats. Brain Res. Dev. Brain Res. 1997;98:142–144. doi: 10.1016/s0165-3806(96)00186-1. [DOI] [PubMed] [Google Scholar]
- GJERSTAD J., TJOLSEN A., HOLE K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1996;318:315–321. doi: 10.1016/s0014-2999(96)00819-9. [DOI] [PubMed] [Google Scholar]
- GJERSTAD J., TJOLSEN A., HOLE K. A dual effect of 5-HT1B receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1997;335:127–132. doi: 10.1016/s0014-2999(97)01183-7. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON E.L., DURKIN M.M., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADJICONSTANTINOU M., PANULA P., LACKOVIC Z., NEFF N.H. Spinal cord serotonin: a biochemical and immunohistochemical study following transection. Brain Res. 1984;322:245–254. doi: 10.1016/0006-8993(84)90114-8. [DOI] [PubMed] [Google Scholar]
- HAMMOND D.L.Control systems for nociceptive afferent processing: The descending inhibitory pathways Spinal Afferent Processing 1986Plenum; 363–390.ed. Yaksh, T [Google Scholar]
- HAMON M., GALLISSOT M.C., MENARD F., GOZLAN H., BOURGOIN S., VERGE D. 5-HT3 receptor binding sites are on capsaicin-sensitive fibres in the rat spinal cord. Eur. J. Pharmacol. 1989;164:315–322. doi: 10.1016/0014-2999(89)90472-x. [DOI] [PubMed] [Google Scholar]
- HEADLEY P.M., DUGGAN A.W., GRIERSMITH B.T. Selective reduction by noradrenaline and 5-hydroxytryptamine of nociceptive responses of cat dorsal horn neurones. Brain Res. 1978;145:185–189. doi: 10.1016/0006-8993(78)90809-0. [DOI] [PubMed] [Google Scholar]
- HOCHMAN S., GARRAWAY S.M. Modulation of primary-afferent evoked responses in rat deep dorsal horn neurons with serotonin receptor ligands. Soc. Neurosci. Abstr. 1998;24 154.2:392. [Google Scholar]
- HOCHMAN S., GARRAWAY S.M., POCKETT S. Membrane properties of deep dorsal horn neurons from neonatal rat spinal cord in vitro. Brain Res. 1997;767:214–219. doi: 10.1016/s0006-8993(97)00578-7. [DOI] [PubMed] [Google Scholar]
- HORI Y., ENDO K., TAKAHASHI T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J. Physiol. (Lond) 1996;492:867–876. doi: 10.1113/jphysiol.1996.sp021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HUANG J.C., PEROUTKA S.J. Identification of 5-hydroxytryptamine binding site subtypes in rat spinal cord. Brain Res. 1987;436:173–176. doi: 10.1016/0006-8993(87)91572-1. [DOI] [PubMed] [Google Scholar]
- JANKOWSKA E., HAMMAR I., CHOJNICKA B., HEDEN C.H. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur. J. Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- JANKOWSKA E., HAMMAR I., DJOUHRI L., HEDEN C., SZABO L.Z., YIN X.K. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur. J. Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- JORDAN L.M., KENSHALO D.R., JR, MARTIN R.F., HABER L.H., WILLIS W.D. Two populations of spinothalamic tract neurons with opposite responses to 5-hydroxytryptamine. Brain Res. 1979;164:342–346. doi: 10.1016/0006-8993(79)90034-9. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A. Serotonin receptors and their function. TOCRIS. 1998.
- KHASABOV S.G., LOPEZ-GARCIA J.A., ASGHAR A.U., KING A.E. Modulation of afferent-evoked neurotransmission by 5-HT3 receptors in young rat dorsal horn neurones in vitro: a putative mechanism of 5-HT3 induced anti-nociception. Br. J. Pharmacol. 1999;127:843–852. doi: 10.1038/sj.bjp.0702592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDD E.J., LAPORTE A.M., LANGLOIS X., FATTACCINI C.M., DOYEN C., LOMBARD M.C., GOZLAN H., HAMON M. 5-HT3 receptors in the rat central nervous system are mainly located on nerve fibres and terminals. Brain Res. 1993;612:289–298. doi: 10.1016/0006-8993(93)91674-h. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., BUTLER A., BURRIDGE J., OXFORD A.W. 1-(m-chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. Eur. J. Pharmacol. 1990;182:193–197. doi: 10.1016/0014-2999(90)90513-6. [DOI] [PubMed] [Google Scholar]
- KING A.E., THOMPSON S.W.N., URBAN L., WOOLF C.J. The responses recorded in vitro of deep dorsal horn neurones to direct and orthodromic stimulation in the young rat spinal cord. Neuroscience. 1988;27:231–242. doi: 10.1016/0306-4522(88)90233-3. [DOI] [PubMed] [Google Scholar]
- LAPORTE A.M., FATTACCINI C.M., LOMBARD M.C., CHAUVEAU J., HAMON M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B, and 5-HT3 receptors in the rat spinal cord. J. Neural Transm. Gen. Sect. 1995;100:207–223. doi: 10.1007/BF01276459. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A. Serotonergic modulation of the responses to excitatory amino acids of rat dorsal horn neurons in vitro: implications for somatosensory transmission. Eur. J. Neurosci. 1998;10:1341–1349. doi: 10.1046/j.1460-9568.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A., KING A.E. Pre- and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: Implications for somatosensory transmission. Eur. J. Neurosci. 1996;8:2188–2197. doi: 10.1111/j.1460-9568.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- MAESHIMA T., ITO R., HAMADA S., SENZAKI K., HAMAGUCHI-HAMADA K., SHUTOH F., OKADO N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res. 1998;797:118–124. doi: 10.1016/s0006-8993(98)00360-6. [DOI] [PubMed] [Google Scholar]
- MANUEL N.A., WALLIS D.I., CRICK H. Ketanserin-sensitive depressant actions of 5-HT receptor agonists in the neonatal rat spinal cord. Br. J. Pharmacol. 1995;116:2647–2654. doi: 10.1111/j.1476-5381.1995.tb17221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARLIER L., TEILHAC J.-R., CERRUTI C., PRIVAT A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J. Serotonin (5-HT) and pain: a reappraisal of its role in the light of receptor multiplicity. Sem. Neurosci. 1995;7:409–419. [Google Scholar]
- MORALES R., BATTENBERG E., BLOOM F.E. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J. Comp. Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- MURASE K., RANDIC M., SHIRASAKI T., NAKAGAWA T., AKAIKE N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- MURPHY R.M., ZEMLAN F.P. Selective serotonin1A/1B agonists differentially affect spinal nociceptive reflexes. Neuropharmacology. 1990;29:463–468. doi: 10.1016/0028-3908(90)90168-q. [DOI] [PubMed] [Google Scholar]
- OGILVIE J., WIGGLESWORTH M., APPLEBY L., KINGSTON T.O., CLARKE R.W. On the role of 5-HT1B/1D receptors in modulating transmission in a spinal reflex pathway in the decerebrated rabbit. Br. J. Pharmacol. 1999;128:781–787. doi: 10.1038/sj.bjp.0702832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMOTE K., KAWAMATA T., KAWAMATA M., NAMIKI A. Formalin-induced nociception activates a monoaminergic descending inhibitory system. Brain Res. 1998;814:194–198. doi: 10.1016/s0006-8993(98)01086-5. [DOI] [PubMed] [Google Scholar]
- OYAMA T., UEDA M., KURAISHI Y., AKAIKE A., SATOH M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci. Res. 1996;25:129–135. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- POMPEIANO M., PALACIOS J.M., MENGOD G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- RANDIC M., YU H.H. Effects of 5-hydroxytryptamine and bradykinin in cat dorsal horn neurones activated by noxious stimuli. Brain Res. 1976;111:197–203. doi: 10.1016/0006-8993(76)91063-5. [DOI] [PubMed] [Google Scholar]
- RIDDELL J.S., JANKOWSKA E., EIDE E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J. Physiol. (Lond.) 1993;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDET J.-L., TAMIR H., PRIVAT A. Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: A light and electron microscopic study using an anti-idiotypic antiserum. J. Neurosci. Res. 1994;38:109–121. doi: 10.1002/jnr.490380114. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., KATHMANN M., EXNER H.J., DETZNER M., GOTHERT M. The 5-HT3 receptor agonist 1-(m-chlorophenyl)-biguanide facilitates noradrenaline release by blockade of alpha 2-adrenoceptors in the mouse brain cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:20–24. doi: 10.1007/BF00178201. [DOI] [PubMed] [Google Scholar]
- SILLAR K.T., SIMMERS A.J. Presynaptic inhibition of primary afferent transmitter release by 5-hydroxytryptamine at a mechanosensory synapse in the vertebrate spinal cord. J. Neurosci. 1994;14:2636–2647. doi: 10.1523/JNEUROSCI.14-05-02636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON S.W.N., KING A.E., WOOLF C.J. Activity-dependent changes in rat ventral horn neurons in vitro summation of prolonged afferent evoked postsynaptic depolarizations produce a D-2-amino-5-phosphonovaleric acid sensitive windup. Eur. J. Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- TRAVAGLI R.A., WILLIAMS J.T. Endogenous monoamines inhibit glutamate transmission in the spinal trigeminal nucleus of the guinea-pig. J. Physiol. (Lond.) 1996;491:177–185. doi: 10.1113/jphysiol.1996.sp021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIYA M., YAMAZAKI H., HORI Y. Enkephalinergic neurons express 5-HT3 receptors in the spinal cord dorsal horn: single cell RT–PCR analysis. Neuroreport. 1999;10:2749–2753. doi: 10.1097/00001756-199909090-00010. [DOI] [PubMed] [Google Scholar]
- UPHOUSE L. Multiple serotonin receptors: too many, not enough, or just the right number. Neurosci. Biobehav. Rev. 1997;21:679–698. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- VERGE D., CALAS A. Serotoninergic neurons and serotonin receptors: gains from cytochemical approaches. J. Chem. Neuroanat. 2000;18:41–56. doi: 10.1016/s0891-0618(99)00050-2. [DOI] [PubMed] [Google Scholar]
- WALLIS D.I., WU J. The pharmacology of descending responses evoked by thoracic stimulation in the neonatal rat spinal cord in vitro. Naunyn Schmiedebergs Arch. Pharmacol. 1993;347:643–651. doi: 10.1007/BF00166948. [DOI] [PubMed] [Google Scholar]
- WALLIS D.I., WU J., WANG X.C. Is 5-hydroxytryptamine mediating descending inhibition in the neonatal rat spinal cord through different receptor subtypes. Eur. J. Pharmacol. 1993a;250:371–377. doi: 10.1016/0014-2999(93)90023-b. [DOI] [PubMed] [Google Scholar]
- WALLIS D.I., WU J., WANG X. Descending inhibition in the neonate rat spinal cord is mediated by 5-hydroxytryptamine. Neuropharmacology. 1993b;32:73–83. doi: 10.1016/0028-3908(93)90132-m. [DOI] [PubMed] [Google Scholar]
- WEI F., DUBNER R., REN K. Nucleus reticularis gigantocellularis and nucleus raphe magnus in the brain stem exert opposite effects on behavioral hyperalgesia and spinal Fos protein expression after peripheral inflammation. Pain. 1999;80:127–141. doi: 10.1016/s0304-3959(98)00212-7. [DOI] [PubMed] [Google Scholar]
- WU S.Y., WANG M.Y., DUN N.J. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991;554:111–121. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- XU W., QIU X.C., HAN J.S. Serotonin receptor subtypes in spinal antinociception in the rat. J. Pharmacol. Exp. Ther. 1994;269:1182–1189. [PubMed] [Google Scholar]
- ZHUO M., GEBHART G.F. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- ZIFA E., FILLION G. 5-Hydroxytryptamine receptors. Pharmacol. Rev. 1992;44:401–458. [PubMed] [Google Scholar]


