Abstract
We aimed to study 5-HT4 receptors in canine stomach contractility both in vivo and in vitro.
In anaesthetized Beagle dogs, the selective 5-HT4 receptor agonist prucalopride (i.v.) induced dose-dependent tonic stomach contractions under isobaric conditions, an effect that was antagonized by the selective 5-HT4 receptor antagonist GR 125487 (10 μg kg−1, i.v.).
Electrical field stimulation (EFS) of corpus longitudinal muscle strips resulted in atropine- and tetrodotoxin-sensitive contractions (L-NOARG (0.1 mM) present in all organ bath solutions). Prucalopride increased these contractions (maximal response after single-dose addition (0.3 μM): 165% of initial value, or after cumulative addition: 188%). In the presence of methysergide (3 μM), 5-HT also increased EFS-contractions (after single-dose addition (0.3 μM): increase to 192%, after cumulative addition: 148%).
The selective 5-HT4 receptor antagonists GR 113808 (0.1 μM) or GR 125487 (10 nM) antagonized the prucalopride (0.3 μM)-induced contraction increments. When EFS-induced contractions were blocked by atropine or tetrodotoxin, prucalopride was ineffective. In the presence of methysergide (3 μM), the contraction increases to 5-HT (0.3 μM) were prevented by GR 113808 (0.1 μM).
The prucalopride curve (pEC50 7.9) was shifted in parallel to the right by GR 113808 3 nM (pA2 9.4). In the presence of methysergide (3 μM), the curve to 5-HT (pEC50 8.1) was competitively antagonized by GR 113808, yielding a Schild slope of 0.8±0.2 (pKB of 9.1 with unit Schild slope).
In corpus circular muscle strips, the prucalopride (0.3 μM)-induced augmentation of EFS-contractions (258%) was also prevented by GR 113808 (0.1 μM) (124%).
In conclusion, the effects of 5-HT4 receptor agonists on proximal stomach motor activity in vivo can be explained by an effect on 5-HT4 receptors on cholinergic nerves within the gastric muscle wall.
Keywords: Canine, 5-HT4 receptor, contractility, gastric, motility, cholinergic
Introduction
Generally, gastric motility consists of a tonic contraction of the proximal stomach wall (fundus and corpus) and phasic, short-lasting contractility in the distal stomach (antrum). Both the proximal tonic contraction and the distal contractility are stimulated by 5-HT4 receptor activation (see Hegde & Eglen, 1996).
In several animal species, 5-HT4 receptors involved in gastric motility have been demonstrated in vivo as well as in vitro. In conscious rats, it was shown that the selective 5-HT4 receptor antagonist GR 125487 completely blocked the gastrokinetic action of metoclopramide (Gale et al., 1994c). An in vitro study revealed the presence of 5-HT4 receptor of rat gastric cholinergic nerves (Amemiya et al., 1996). In the guinea-pig ex-vivo gastroduodenal preparation, cisapride, a 5-HT4 receptor agonist (Meulemans & Schuurkes, 1992), enhanced gastric peristaltic waves and improved antroduodenal coordination (Schuurkes et al., 1985). In guinea-pig stomach muscle strip studies, 5-HT4 receptor activation resulted in enhanced contractility after electrical field stimulation (EFS; Buchheit & Buhl, 1994), and increase in EFS-induced release of tritiated acetylcholine (Matsuyama et al., 1996). Further, it was demonstrated that in the antrum and corpus, but not the fundus of the guinea-pig stomach, the electrically evoked outflow of tritiated acetylcholine was stimulated after 5-HT4 receptor stimulation (Takada et al., 1999). In humans, cisapride stimulated antroduodenal motility and gastric emptying (Johnson, 1989; Lux et al., 1994). Additionally, excitatory 5-HT4 receptors located on cholinergic nerves mediated facilitation of cholinergic neurotransmission in muscle strips of human fundus, corpus and antrum resulting in enhanced contractility (Schuurkes et al., 1991). Recently, 5-HT4 receptors were histochemically demonstrated in muscle strips of human and guinea-pig gastric muscle strips (Sakurai-Yamashita et al., 1999). Taken together, in rodents and man, 5-HT4 receptors have been demonstrated in in vivo as well as in vitro experiments. Based on these studies, their stimulatory role on gastric motility was mainly ascribed to facilitation of cholinergic neurotransmission (Briejer et al., 1995). In the conscious dog, that is frequently used to study gastrointestinal motility, 5-HT4 receptors have been shown to mediate increased contraction (Bingham et al., 1995; Bermudez et al., 1990) as well as increased gastric emptying (Gullikson et al., 1993; Briejer et al., 1998b). As expected from the information available in rodents and man, these actions were ascribed to excitatory 5-HT4 receptors located on cholinergic nerves. However, there are no in vitro data available to substantiate this. Indeed, the only attempt to show 5-HT4 receptor in the dog stomach in vitro has failed, as the marked increase in EFS-evoked canine antral contractions by 5-HT and the 5-HT4 receptor agonist cisapride was unaffected by the 5-HT4 receptor antagonist tropisetron (de Ridder & Schuurkes, 1993). This controversy between the in vivo and in vitro data has led to hypotheses on 5-HT4 receptors linked to extragastric pathways, inducing the in vivo effects. Another possibility would be that 5-HT4 receptors are simply not present in the antrum, but rather in more proximal gastric areas.
Therefore, we set out to resolve the controversy regarding the expression of gastric 5-HT4 receptors in the dog. First, to confirm 5-HT4 receptor-mediated effects on dog stomach in vivo, the effect of a selective 5-HT4 receptor antagonist was assessed against the effect of a selective 5-HT4 receptor agonist on dog stomach volume by use of a barostat. Second, as the barostat mainly measures contractility changes in the proximal stomach and in view of the negative in vitro study in the canine antrum, the influence of the available, selective, 5-HT4 receptor agonists and antagonists was tested on electrically stimulated muscle strips from the gastric corpus.
Methods
Barostat study in vivo
Eight female Beagle dogs (mean weight 11.0±0.7 kg; supplier Harlan, The Netherlands), that were allowed a minimum of 2 weeks recovery period from previous studies, were used. They were sedated by subcutaneous injection of Hypnorm® (10 ml, containing fentanyl citrate 3 mg and fluanison 100 mg), and after 30 min Nembutal® (pentobarbitone 15 mg kg−1 i.v.) was administered to induce general anaesthesia. During the experiment the dogs lay in supine position on a thermal blanket, kept at 37°C, and the depth of general anaesthesia was maintained with N2O, (400 ml min−1)+O2 (400 ml min−1) and Fluothane® (1 – 1.5%). The saphenous vein was cannulated for administration of drugs intravenously. After a median laparatomy, a 1-cm incision of mid-corpus right in the middle between the lesser and the greater curvature was made. An inflatable, non-compliant, bellows bag connected to a tube was inserted in the stomach. The incision was closed around this tube with a purse string to fix the bag in the stomach. The tube was connected to a barostat set-up (manufactured by Janssen Research Foundation) and, under a constant bag pressure of 6.0 mmHg, the dogs were left to stabilize to the conditions applied for 30 min. This yielded a mean volume of 467±31 ml in eight dogs (maximal bag volume: 800 ml). Then, saline (i.v.; solvent of GR 125487) or GR 125487 (10 μg kg−1 i.v.) was administered. Thirty minutes later, a concentration-response curve to prucalopride was constructed in a cumulative fashion while measuring volume changes. The dosing interval was 3 – 6 min, which was enough to establish a stable, tonic, reduction in volume at each concentration of prucalopride. Only one curve to prucalopride was made per dog. The experiment was terminated with an overdose of pentobarbitone (i.v.).
Muscle strip studies
Twenty-seven Beagle dogs of both sexes (weighing 5 – 14 kg) were used. Twenty-one of these dogs that had been previously used for gastrointestinal in vivo studies were allowed a minimum recovery period of 2 weeks, after which they were sacrificed on the day of the experiment by decerebration and successive bleeding through the carotid artery. The other six dogs had been used in cardiovascular in vivo studies (majority solvents tested) the day before the experiment. On the day preceding the experiment, they were anaesthetized with Nembutal® (30 mg kg−1 i.v.), and, subsequently, sacrificed with KCl (150 mg kg−1 i.v.). Those dogs were only used in case the compound tested for cardiovascular safety did not express relevant affinity (pKi>5) for 5-HT4 receptors, as judged from in-house radioligand binding studies. The gastric corpus of those dogs was excised and the omentum and mucosa were removed, after which the tissue was stored overnight at 4°C in fresh buffer to be used the next day. There were no differences in any responses observed between tissue from dogs sacrificed either the day before or on the day of the experiment.
Muscle strips of 2 – 3 mm width and 2 – 3 cm length were cut in the longitudinal or circular direction. In the case where longitudinal muscle was tested, much of the circular muscle was cut away taking care not to damage the myenteric plexus. In the case where circular muscle was used, no further dissection was performed. Preliminary experiments had revealed that longitudinal muscle displayed a stable basal muscle length throughout the experiment, whereas the length of circular muscle progressively increased during the experiment, finally stabilizing after more than 2 h. This allowed the rapid establishment of reproducible electrical field stimulation-evoked contractions in longitudinal muscle, whereas in circular muscle these contractions increased with increasing muscle length. In this manner, it required several hours to obtain reproducible responses in circular muscle. Therefore, we chose to perform the main part of the study with longitudinal muscle. The strips were anchored onto organ bath hooks equipped for electrical field stimulation with coaxially orientated platina-wire electrodes. The muscle strips were placed in an organ bath set-up for isotonic recording, and the organ baths (20 ml each) were filled with Krebs-Henseleit (containing (mM) glucose 11.1, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69 and NaCl 118), gassed with carbogen and kept at 37°C.
After a 30-min period of stabilization, the strips were contracted with carbachol (10 μM) to test their viability and responsiveness. After wash-out, we added L-NOARG (0.1 mM) to the organ bath solution in order to prevent relaxation due to nitric oxide (NO). NO is an important neurotransmitter in the stomach with a pronounced inhibitory effect on gastric tone. Preliminary experiments with dog gastric muscle strips showed that the NO-synthase inhibitor L-NOARG increased EFS-induced contractions and improved their reproducibility, thus confirming presence of a NO-mediated inhibitory component. Since the aim of the study was to demonstrate excitatory 5-HT4 receptor-mediated effects, we included L-NOARG in all muscle strip studies. At the same point in time, organ bath solutions with muscle strips that were to receive 5-HT as agonist, were supplied with methysergide (3 μM) to block interaction of 5-HT with 5-HT1, 5-HT2, 5-ht5, 5-HT6 and 5-HT7 receptors. After 30 min of incubation, the muscle strips were electrically stimulated (with initial parameters; 1-ms pulses in trains of 10 s, at an interval of 3 min, at 20 Hz and 20 V, current measured 0.9 – 2.0 V), and each pulse train resulting in a contraction. Preliminary experiments indicated that these stimulation parameters induced maximal contractions. The voltage was then reduced until reproducible contractions approximating 25 – 50% of the contraction observed at 20 V were obtained. This electrical field stimulation applied at reduced voltage is further denoted as EFS. This was allowed for at least 15 min, followed by addition of treatment (atropine 1 μM, tetrodotoxin 0.3 μM, GR 125487 10 nM or GR 113808 3, 10, 30 or 100 nM) or solvent, which, in turn, were left to incubate for 15 min, while EFS was continued. Then prucalopride or 5-HT were added in a single dose (0.3 μM) or in a log unit (5-HT) / semi-log unit (prucalopride) incrementing cumulative manner from 0.1 nM onwards under continued electrical pacing. In case concentration-response curves were established, the concentration increments of 5-HT were performed at a 6-min interval, whereas prucalopride was dosed at a 15-min interval. Each muscle strip was used to test the effect of one treatment on either a single-dose or a cumulative concentration-response curve to prucalopride or 5-HT.
For agonist/antagonist single-dose experiments, concentrations of antagonist were chosen on basis of their ability to produce a dose ratio of approximately 100 of 5-HT4 receptor-mediated responses. Therefore, for the selective 5-HT4 receptor antagonists GR 113808 (literature pKB estimates of 9.0 – 9.7; Gale et al., 1994b) and GR 125487 (literature pKB estimates around 9.7 – 10.4; Gale et al., 1994a), we chose 0.1 μM and 10 nM, respectively.
Data analysis
In vivo
The gastric volume measured prior to addition of solvent or GR 125487 (10 μg kg−1 i.v.) was taken as 100% and any effect on volume due to addition of prucalopride was related to this.
In vitro
The average contraction to five EFS pulses before addition of treatment or solvent was taken as 100% (called the initial value) and all contractions were related to this initial value. In experiments to assess the effect of single-dose agonist addition, repeated measures over time were analysed using PROC MIXED (SAS v PC 6.12) for data with an unbalanced covariance structure.
Concentration-response curves
Concentration-response curves to agonists were fitted iteratively to a three-parameter Hill-equation, obtaining estimates for mid-point location pEC50, upper asymptote (α) and Hill slope (nH). The effect of a single treatment on these parameters was tested by one-way ANOVA. In order to quantify antagonism, the Schild-equation was used to estimate a pA2 (Arunlakshana & Schild, 1959) or a pKB (Black et al., 1985). Antagonism was statistically analysed by one-way ANOVA, followed by a post-hoc Bonferroni's test (in case of multiple concentrations of antagonist). A level of P<0.05 was taken as significant and the number of dogs used was denoted by n.
Compounds
The following compounds were used (with their abbreviations, if any, in italics, and respective suppliers given in parentheses): 1-[2-[(methylsulphonyl)amino] ethyl]-4-piperidinyl-methyl 5-fluoro-2-methoxy-1H-indole-3-carboxylate (GR 125487; selective 5-HT4 receptor antagonist), [1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate (GR 113808; selective 5-HT4 receptor antagonist), 4-amino-5-chloro-2,3-dihydro-N-(1-[3-methoxypropyl]-4-piperidinyl)-7-benzofurancarboxamide HCl (prucalopride; R093877; selective 5-HT4 receptor agonist), Hypnorm® (Janssen Research Foundation, Belgium), carbachol, atropine sulphate (Janssen Chimica, Belgium), tetrodotoxin, 5-HT creatinine sulphate, (Serva, Germany), methysergide maleate (RBI, USA), Nembutal® (Sanofi, Belgium) and Fluothane® (Zeneca, Belgium).
All compounds were dissolved in 0.9% NaCl solution, except for GR 113808, which was dissolved in 0.9% NaCl acidified with tartaric acid. Dilutions were made using 0.9% NaCl. The solvents had no effect on the baseline gastric barostat bag volume, baseline muscle strip length or the effects of agonists.
Results
Barostat study
Prucalopride decreased gastric volume at doses from 0.75 μg kg−1 – 1 mg kg−1 i.v. The corresponding curve parameters were ED50 value 0.020±0.006 mg kg−1 i.v., maximal reduction 48±14% and Hill slope of 1.0±0.1 (Figure 1; n=4). GR 125487 (10 μg kg−1 i.v.) did not modify the gastric volume, but shifted the curve to prucalopride rightward (n=4). Further, no definite maximal response to prucalopride was obtained in the presence of GR 125487, therefore, individual curve fitting of data points from the experiments in the presence of GR 125487 was not feasible. Arithmetic means of each data point were calculated and used for a simple, single, curve fit. This procedure yielded a ED50 value of 0.86 mg kg−1 i.v. (dose ratio ∼43) and a maximal reduction of volume of 32%. Thus, GR 125487 shifted the curve to prucalopride to the right, and, in addition, appeared to depress the maximal response to prucalopride.
Figure 1.
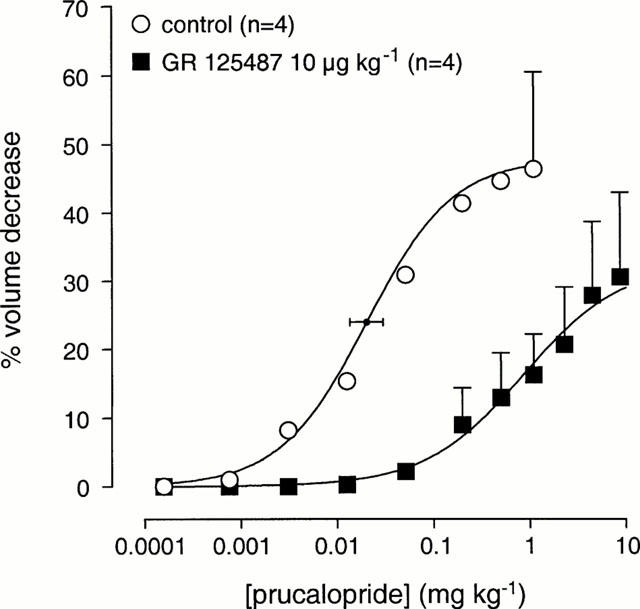
The effect of a 30-min pre-treatment with GR 125487 (10 μg kg−1 i.v.) on the effect of cumulative dosing of prucalopride (i.v.) on gastric barostat bag volume in anaesthetized Beagle dogs (n denotes number of dogs used). The control curve shown superimposed on vertically averaged experimental data points is a simulation using the Hill equation and the parameters for upper asymptote, Hill slope and mid-point location, that were obtained from the iterative curve fitting procedure. The GR 125487-treatment curve shown superimposed on vertically averaged experimental data points is a simulation with the logistic parameters from the fitting procedure using arithmic mean data points, as individual curve fitting was not feasible.
Muscle strips study
Throughout the experiment, the corpus longitudinal muscle strips were not spontaneously active, whereas the circular muscle strips displayed subordinate contractility (approximately 5% of the contraction to carbachol 10 μM). Carbachol induced a stable contraction that returned to baseline after washout.
Longitudinal muscle
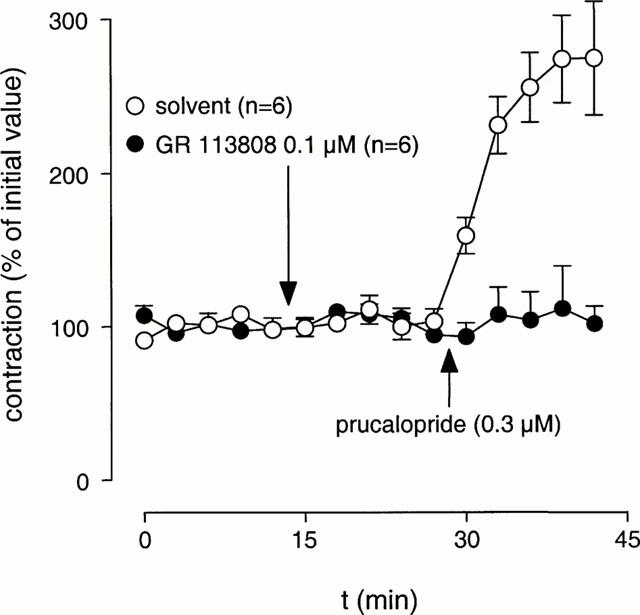
In the presence of L-NOARG (0.1 mM), electrical field stimulation resulted in reproducible contractions that were blocked by atropine (1 μM) or tetrodotoxin (0.3 μM), thus suggesting that these contractions were induced by acetylcholine released from cholinergic nerves. In the presence of atropine (1 μM) or tetrodotoxin (0.3 μM), addition of prucalopride (0.3 μM) or 5-HT (0.3 μM; tested in the presence of methysergide 3 μM) was without effect (Figures 2 and 3).
Figure 2.
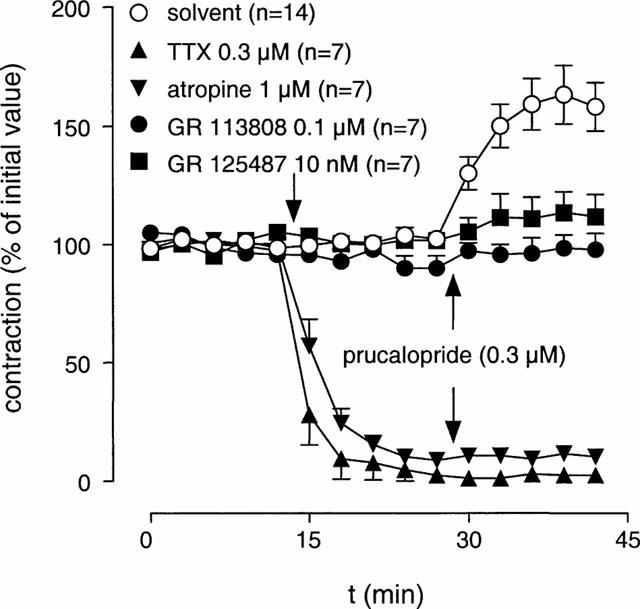
The effect of pre-treatment of tetrodotoxin (TTX), atropine, GR 113808 or GR 125487 on the stimulant effect of prucalopride on EFS-induced contractions of canine gastric corpus longitudinal muscle strips. Experiments were carried out in the presence of L-NOARG (0.1 mM). Data is presented as mean±s.e.mean expressed as percentage of initial value.
Figure 3.
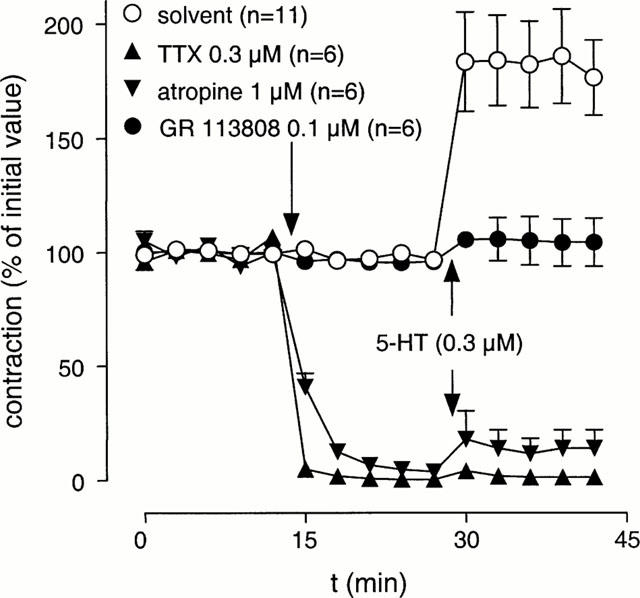
The effect of pre-treatment with tetrodotoxin (TTX), atropine or GR 113808 on the stimulant effect of 5-HT on EFS-induced contractions of canine gastric corpus longitudinal muscle strips. Experiments were carried out in the presence of L-NOARG (0.1 mM) and methysergide (3 μM). Data is presented as mean±s.e.mean expressed as percentage of initial value.
Prucalopride (single dose of 0.3 μM; n=14) induced an increase in EFS-induced contractions to 165±13% (Figure 2). Pre-incubation with the selective 5-HT4 receptor antagonists GR 113808 (0.1 μM) or GR 125487 (10 nM) blocked the enhancement to single-dose addition of prucalopride (0.3 μM). When dosed cumulatively, prucalopride produced a maximal enhancement to 188±20% with a pEC50 of 7.9±0.3 (n=6; Figure 4). The effect of GR 113808 (3 nM) was tested against the cumulative concentration-response curve to prucalopride, yielding a pA2 estimate of 9.4±0.2 (Figure 4). In muscle strips that were not electrically stimulated, prucalopride (0.3 μM) did not modify the concentration-contraction curve to acetylcholine (n=2, data not shown).
Figure 4.
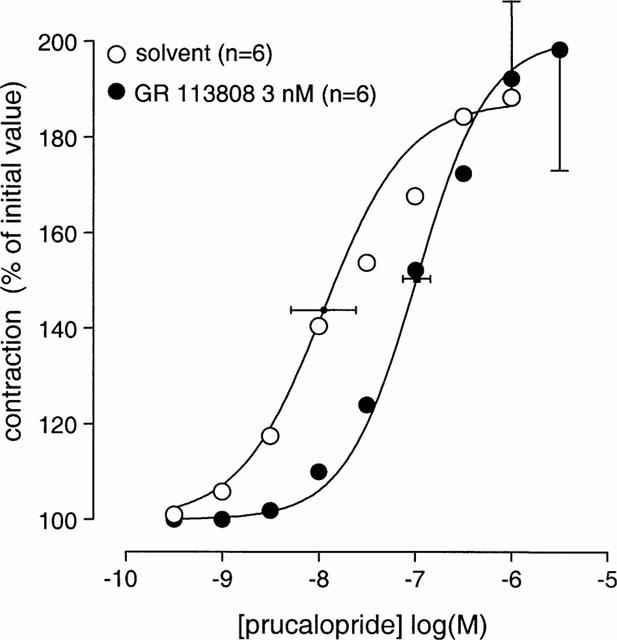
The effect of GR 113808 on the stimulation of EFS-induced contractions by cumulative dosing of prucalopride in canine gastric corpus longitudinal muscle strips. Curves shown superimposed on vertically averaged experimental data points are simulations using the Hill equation and the parameters for upper asymptote, Hill slope and mid-point location, that were obtained from the iterative curve fitting procedure.
In the presence of methysergide (3 μM), to block interactions of 5-HT with 5-HT1, 5-HT2, 5-ht5, 5-HT6 or 5-HT7 receptors, 5-HT induced an increase in EFS-evoked contractions to 192±21% (single-dose of 0.3 μM; Figure 3) and to 148±7.6% (cumulatively dosed; pEC50 8.1±0.1, Figure 5). Pre-incubation with GR 113808 (0.1 μM) antagonized the response to 5-HT (0.3 μM). GR 113808 (3, 10 and 30 nM) behaved as a competitive antagonist of 5-HT-induced contraction increments (Figure 5). The Schild slope obtained (0.8±0.2) was not significantly different from unity, and, after constraining the Schild slope to unity, a pKB estimate of 9.1±0.1 was obtained. The pA2 of GR 113808 obtained against prucalopride was not significantly different from the pKB obtained with 5-HT as agonist.
Figure 5.
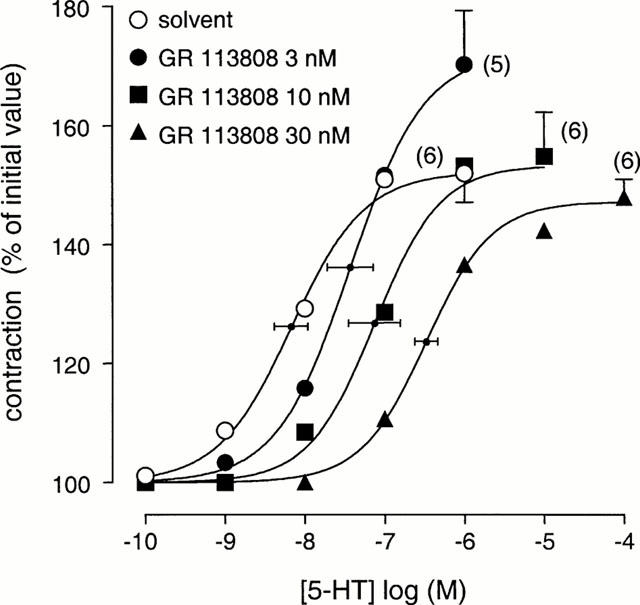
The effect of various concentrations of GR 113808 on the enhancement of EFS-induced contractions to 5-HT in canine gastric corpus longitudinal muscle strips. Experiments were carried out in the presence of L-NOARG (0.1 mM) and methysergide (3 μM). Curves shown superimposed on vertically averaged experimental data points are simulations using the Hill equation and the parameters for upper asymptote, Hill slope and mid-point location, that were obtained from the iterative curve fitting procedure. The number between parentheses represents the number of dogs used.
Circular muscle
Prucalopride (0.3 μM) enhanced circular muscle contractions due to EFS to 285±30% (Figure 6). Similar to that observed in longitudinal muscle, GR 113808 (0.1 μM) blocked the prucalopride-evoked effect in circular muscle (124±30%). Prucalopride was without effect in the presence of tetrodotoxin or atropine (data not shown).
Figure 6.
The effect of pre-incubation of GR 113808 on the increase in EFS-evoked contractions to prucalopride in canine corpus circular muscle. Data is presented as mean±s.e.mean expressed as percentage of initial value, where n denotes the number of dogs used.
Discussion
The data presented here confirm that the contraction of canine stomach observed in vivo after administering prucalopride is attributable to 5-HT4 receptor activation. Furthermore, the corpus muscle wall, whose contraction is primarily responsible for a reduction in gastric barostat bag volume, exhibits excitatory 5-HT4 receptors on cholinergic nerves, mediating facilitation of contractility. Given that gastric tone is highly dependent on vagal drive, it is conceivable that the 5-HT4 receptor-mediated facilitation of cholinergic neurotransmission shown in vitro may provide an explanation for the 5-HT4 receptor-mediated increase in gastric tone in vivo.
Prucalopride dose-dependently decreased gastric barostat bag volume, pointing to an increase in tonic contraction of the stomach. There are two primary reasons to attribute this prucalopride-induced effect to 5-HT4 receptor stimulation. First, prucalopride is the most selective and specific 5-HT4 receptor agonist available to date, with a high oral bioavailability (Janssen Research Foundation, data on file). In this manner, prucalopride 0.31 mg kg−1 has been reported to stimulate canine colonic motility due to 5-HT4 receptor activation to a similar extent when dosed either p.o. or i.v. (Briejer et al., 1998c). Prucalopride at 0.31 mg kg−1 (inducing a nearly maximal contraction in our study dosed i.v.) can, therefore, be used as a tool to demonstrate 5-HT4 receptors in dogs in vivo. Second, the effect of prucalopride was antagonized by the selective 5-HT4 receptor antagonist GR 125487 (10 μg kg−1 i.v.). GR 125487 is a metabolically stable compound that has been shown to selectively antagonize 5-HT4 receptor-mediated responses at 0.25 – 1 μg kg−1 i.v. in anaesthetized piglets in vivo (Gale et al., 1994c). Exhibiting 10,000 fold specificity for 5-HT4 receptors (Gale et al., 1994a), GR 125487 at 10 μg kg−1 is expected to selectively antagonize responses to 5-HT4 receptor agonists, such as prucalopride, as was indeed the case in our study. The additional observation that the maximal response to prucalopride appeared depressed by GR 125487 is not uncommon for this compound. GR 125487 depressed the maximal responses to 5-HT4 receptor agonists in an in vitro bioassay (Gale et al., 1994a), and in in vivo models (Gale et al., 1994c). Thus, the observed antagonism by GR 125487 of the prucalopride-induced contraction of dog stomach indicates that 5-HT4 receptor stimulation in dogs in vivo results in stomach contraction. This is in full agreement with other studies demonstrating 5-HT4 receptor-mediated contraction of canine stomach (Bingham et al., 1995; Bermudez et al., 1990).
Gastric barostat bag volume reduction is caused by a tonic contraction of the stomach in vivo. Gastric tonic contraction in dogs is maintained primarily by cholinergic drive of the proximal gastric muscle wall (Azpiroz & Malagelada, 1987). Therefore, it could be expected that if the 5-HT4 receptor-mediated contractile response by prucalopride (as observed in vivo) is mediated by 5-HT4 receptors located in the gastric muscle wall, these receptors should be experimentally detectable in contraction studies with isolated gastric muscle strips. Accordingly, in our study corpus muscle strips responded with enhanced contraction due to EFS after addition of prucalopride at a 5-HT4 receptor-selective concentration (0.3 μM). The antagonism of the effect to this single-dose addition of prucalopride by the selective 5-HT4 receptor antagonists GR 125487 and GR 113808 confirmed this to be true. This point was also corroborated by the observation that the increment in contractions after 5-HT was blocked by GR 113808. GR 125487 and GR 113808 share high affinity for human 5-HT4 receptors (human colon: pKB 10.1 and 9.4, respectively; Prins et al., 2000) and canine 5-HT4 receptors (canine rectum: pKB 9.7 and 9.1, respectively; Prins et al., 1999), and at the concentrations used in this study, these compounds do not significantly bind to other than 5-HT4 receptors (Gale et al., 1994a,1994b). In the current study, the GR 113808-induced antagonism was associated with pKB/pA2 estimates of 9.1 and 9.4. This unambiguously emphasized that the effect of 5-HT or prucalopride under the applied conditions is fully attributable to 5-HT4 receptor agonism. In an additional experiment to test whether 5-HT4 receptor-mediated stimulation is also detectable in circular muscle, prucalopride induced a marked increase in EFS-induced contractions, which was blocked by pre-incubation with GR 113808 (0.1 μM). Therefore, 5-HT4 receptor-mediated stimulation of EFS-induced contraction affects the circular as well as the longitudinal muscle layer. This stresses the impact of 5-HT4 receptor activation on mid-gastric tone.
The involvement of cholinergic nerves in the 5-HT4 receptor-mediated responses was demonstrated by the blockade of EFS-induced contractions by atropine and tetrodotoxin. In the presence of these agents, neither prucalopride nor 5-HT were able to evoke any effect. Prucalopride (0.3 μM) failed to affect the curve to exogenously administered acetylcholine in non-electrically stimulated longitudinal muscle strips, thus ruling out post-junctional potentiation mechanism or an acetylcholine-esterase inhibition property at the concentration tested. Therefore, 5-HT4 receptors mediating enhanced gastric contractility are most likely pre-junctional 5-HT4 receptors, located on cholinergic nerves within the gastric muscle wall.
Earlier, this phenomenon was evidently demonstrated for guinea-pig gastric tissue, in which 5-HT4 receptor agonists increased EFS-induced contraction as well as EFS-induced acetylcholine release in a 5-HT4 receptor antagonist-sensitive manner (Matsuyama et al., 1996; Takada et al., 1999). From those studies and the clear 5-HT4 receptor-mediated stimulation of canine gastric motility observed in vivo (Bermudez et al., 1990; Gullikson et al., 1993), it could be anticipated that the dog gastric muscle wall was also endowed with 5-HT4 receptors located on cholinergic nerves. However, it was previously demonstrated that the 5-HT-induced increment in EFS-induced contractions of dog antral longitudinal muscle was not antagonized by the selective 5-HT2A receptor antagonist ketanserin, the 5-HT1, 5-HT2 and 5-HT7 receptor antagonist methiothepin or the mixed 5-HT3 and 5-HT4 receptor antagonist tropisetron (de Ridder & Schuurkes, 1993). Thus, the 5-HT-induced increase in dog antral contractility could not be explained by 5-HT4 receptors within the gastric muscle wall. That study has been utilized to suggest that the stimulation of gastric motility by 5-HT4 receptor agonists in dogs may be differently organized in comparison with other animal species. It even fostered the suggestion that 5-HT4 receptors might be located on extragastric neuronal pathways. The current study unambiguously characterized 5-HT4 receptors in the dog proximal stomach muscle wall, and thus demonstrates that the presence of these receptors mediating facilitation of cholinergic neurotransmission is actually similar across species assessed (rat, guinea-pig, dog and man). What 5-HT receptor population mediates the 5-HT-evoked excitation of antral longitudinal muscle remains to be elucidated.
The maximal response to 5-HT in the cumulative dosing protocol was remarkably lower than the response to a single dose of 5-HT (0.3 μM). This may suggest that during cumulative dosing of 5-HT, 5-HT is progressively broken down, or desensitization of 5-HT4 receptors occurs. However, the efficacy of prucalopride was similar in both dosing protocols, therefore, desensitization is not the likely cause of the observed difference. Although we do not have a clear explanation for this observation, this phenomenon appears to be related rather to disposition of agonist than to 5-HT4 receptor stimulation in its own right.
Our results indicate that excitatory 5-HT4 receptors are located on cholinergic nerves within the canine proximal gastric muscle wall. The longitudinal as well as the circular muscle layer are stimulated by a 5-HT4 receptor agonist, indicative of the impact on gastric muscle tone under cholinergic control. Facilitation of cholinergic neurotransmission by 5-HT4 receptors on cholinergic fibres within the gastric muscle wall may explain the tonic stomach contraction in dogs.
Abbreviations
- EFS
electrical field stimulation
- L-NOARG
NG-Nitro-L-arginine
- NO
nitric oxide
- TTX
tetrodotoxin
References
- AMEMIYA N., HATTA S., TAKEMURA H., OHSHIKA H. Characterization of the contractile response induced by 5-methoxytryptamine in rat stomach fundus strips. Eur. J. Pharmacol. 1996;318:403–409. doi: 10.1016/s0014-2999(96)00777-7. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–54. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZPIROZ F., MALAGELADA J.R. Importance of vagal input in maintaining gastric tone in the dog. J. Physiol. 1987;384:511–524. doi: 10.1113/jphysiol.1987.sp016467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMUDEZ J., DUNBAR A., SANGER G.J., TURNER D.H. Stimulation of canine gastric motility by BRL 24924, a new gastric prokinetic agent. J. Gastrointest. Motility. 1990;2:281–286. [Google Scholar]
- BINGHAM S., KING B.F., RUSHANT B., SMITH M.I., GASTER L., SANGER G.J. Antagonism by SB 204070 of 5-HT-evoked contractions in the dog stomach: an in-vivo model of 5-HT4 receptor function. J. Pharm. Pharmacol. 1995;47:219–222. doi: 10.1111/j.2042-7158.1995.tb05782.x. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br. J. Pharmacol. 1985;86:581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIEJER M.R., AKKERMANS L.M.A., SCHUURKES J.A.J. Gastrointestinal prokinetic benzamides: the pharmacology underlying stimulation of motility. Pharmacol. Rev. 1995;47:631–651. [PubMed] [Google Scholar]
- BRIEJER M.R., GHOOS E., EELEN J., SCHUURKES J.A.J. Serotonin 5-HT4 receptors mediate the R093877-induced changes in contractile patterns in the canine colon. Gastroenterology. 1998a;112:A705. [Google Scholar]
- BRIEJER M.R., MEULEMANS A.L., WELLENS A., SCHUURKES J.A.J. R093877 dose-dependently accelerates delayed gastric emptying in conscious dogs. Gastroenterology. 1998b;112:A705. [Google Scholar]
- BRIEJER M.R., VAN DAELE P., BOSMANS J.-P., GHOOS E., EELEN J., SCHUURKES J.A.J. Dose-dependent effects after oral and intravenous administration of R093877 on colonic motility in conscious dogs. Gastroenterology. 1998c;112:A705. [Google Scholar]
- BUCHHEIT K.H., BUHL T. Stimulant effects of 5-hydroxytryptamine on guinea pig stomach preparations in vitro. Eur. J. Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- DE RIDDER W.J., SCHUURKES J.A.J. Cisapride and 5-hydroxytryptamine enhance motility in the canine antrum via separate pathways, not involving 5-hydroxytryptamine1,2,3,4 receptors. J. Pharmacol. Exp. Ther. 1993;264:79–88. [PubMed] [Google Scholar]
- GALE J.D., GREEN A., DARTON J., SARGENT R.S., CLAYTON N.M., BUNCE K.T. GR125487: A 5-HT4 receptor antagonist with a long duration of action in vivo. Br. J. Pharmacol. 1994c;113 Suppl.:119P. [Google Scholar]
- GALE J.D., GROSSMAN C., DARTON J., BUNCE K.T., WHITEHEAD J.W.F., KNIGHT J., PARKHOUSE T.J., OXFORD A.W., HUMPHREY P.P.A. GR125487: A selective and high affinity 5-HT4 receptor antagonist. Br. J. Pharmacol. 1994a;113 Suppl.:120P. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994b;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GULLIKSON G.W., VIRINA M.A., LOEFFLER R.F., YANG D.C., GOLDSTIN B., WANG S.X., MOUMMI C., FLYNN D.L., ZABROWSKI D.L. SC-49518 enhances gastric emptying of solid and liquid meals and stimulates gastrointestinal motility in dogs by a 5-hydroxytryptamine4 receptor mechanism. J. Pharmacol. Exp. Ther. 1993;264:240–248. [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- JOHNSON A.G. The effects of cisapride on antroduodenal co-ordination and gastric emptying. Scand. J. Gastroent. Supp. 1989;165:36–43. doi: 10.3109/00365528909091229. [DOI] [PubMed] [Google Scholar]
- LUX G., KATSCHINSKI M., LUDWIG S., LEDERER P., ELLERMAN A &, DOMSCHKE W. The effect of cisapride and metoclopramide on human digestive and interdigestive antroduodenal motility. Scand. J. Gastroent. 1994;29:1105–1110. doi: 10.3109/00365529409094895. [DOI] [PubMed] [Google Scholar]
- MATSUYAMA S., SAKIYAMA H., NEI K., TANAKA C. Identification of putative 5-hydroxytryptamine(4) (5-HT4) receptors in guinea pig stomach: The effect of TKS159, a novel agonist, on gastric motility and acetylcholine release. J. Pharmacol. Exp. Ther. 1996;276:989–995. [PubMed] [Google Scholar]
- MEULEMANS A.L., SCHUURKES J.A. Is the action of cisapride on the guinea-pig ileum mediated via 5-HT4 receptors. Eur. J. Pharmacol. 1992;212:51–59. doi: 10.1016/0014-2999(92)90071-b. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., SHANKLEY N.P., WELSH N.J., BRIEJER M.R., LEFEBVRE R.A., AKKERMANS L.M.A., SCHUURKES J.A.J. An improved in vitro bioassay for the study of 5-HT4 receptors in the human isolated large intestinal circular muscle. Br. J. Pharmacol. 2000;129:1601–1608. doi: 10.1038/sj.bjp.0703254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., VAN HASELEN J.F.W.R., LEFEBVRE R.A., BRIEJER M.R., AKKERMANS L.M.A., SCHUURKES J.A.J. Pharmacological characterization of 5-HT4 receptor mediating relaxation of canine isolated rectum circular smooth muscle. Br. J. Pharmacol. 1999;127:1431–1437. doi: 10.1038/sj.bjp.0702665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI-YAMASHITA Y., TAKADA K., TAKEMURA K., YAMASHITA K., ENJOJI A., KANEMATSU T., TANIYAMA K. Ability of mosapride to bind to 5-HT4 receptors in the human stomach. Jap. J. Pharmacol. 1999;79:493–496. doi: 10.1254/jjp.79.493. [DOI] [PubMed] [Google Scholar]
- SCHUURKES J.A., VAN NUETEN J.M., VAN DAELE P.G., REYNTJENS A.J., JANSSEN P.A. Motor-stimulating properties of cisapride on isolated gastrointestinal preparations of the guinea pig. J. Pharmacol. Exp. Ther. 1985;234:775–783. [PubMed] [Google Scholar]
- SCHUURKES J.A.J., MEULEMANS A.L., OBERTOP H., AKKERMANS L.M.A. 5-HT4 receptors on the human stomach. J. Gastrointest. Mot. 1991;3:199. [Google Scholar]
- TAKADA K., SAKURAI-YAMASHITA Y., YAMASHITA K., KAIBARA M., HAMADA Y., NAKANE Y., HIOKI K., TANIYAMA K. Regional difference in correlation of 5-HT4 receptor distribution with cholinergic transmission in the guinea pig stomach. Eur. J. Pharmacol. 1999;374:489–494. doi: 10.1016/s0014-2999(99)00321-0. [DOI] [PubMed] [Google Scholar]


