Abstract
Several compounds, mainly opioid agonists such as methadone, are currently used for long term medication of heroin addicts. Nevertheless, these maintenance treatments have the disadvantage to induce a dependence to another opiate. As interactions between opioid and cannabinoid systems have been demonstrated, the ability of the CB1 antagonist, SR141716A to reduce morphine-induced addiction was investigated.
The effects of SR141716A on the rewarding responses of morphine were evaluated in the place conditioning paradigm. No significant conditioned preference or aversion were observed after repeated treatment with the CB1 antagonist alone. However, SR141716A was able to antagonize the acquisition of morphine-induced conditioned place preference.
SR141716A was co-administered with morphine for 5 days, and the withdrawal syndrome was precipitated by naloxone administration. A reduction in the incidence of two main signs of abstinence: wet dog shakes and jumping was observed while the other were not significantly modified. In contrast, an acute injection of the CB1 antagonist just before naloxone administration was unable to modify the incidence of the behavioural manifestations of the withdrawal, suggesting that only chronic blockade of CB1 receptors is able to reduce morphine-induced physical dependence.
Several biochemical mechanisms could explain the reduction of opioid dependence by CB1 antagonists. Whatever the hypotheses, this study supports the reported interaction between the endogenous cannabinoid and opioid systems, and suggests that SR 141716A warrants further investigations for a possible use in opioid addiction.
Keywords: Cannabinoid, morphine, dependence, κ opioid systems, mice
Introduction
Since drug addiction is often considered as a chronic, relapsing disorder, several therapeutical assays to reduce craving are under investigation. In the case of opiate addicts, long-term treatment with opioid agonists such as methadone or buprenorphine, are currently used (review in Kreek, 1997; Fischer et al., 1999; Ward et al., 1999). Nevertheless, there are several reasons why it should be of great interest to use non-opioid substances for maintenance treatments or detoxification of heroin abusers. Firstly, like heroin, methadone and buprenorphine used in maintenance program could generate a psychological dependence and can be diverted to illegal sales. Secondly, as an opioid agonist, methadone is able to trigger a withdrawal syndrome and even though it is less severe than that observed with heroin, it is longer lasting. Furthermore, physical dependence has also been reported to occur after chronic buprenorphine treatment (Kuhlman et al., 1998; Dyer et al., 1999).
Opioids and cannabinoids share several pharmacological properties (Dewey, 1986), both leading for example to analgesia (Lichtman & Martin, 1991), hypothermia (Anderson et al., 1975) and reduced locomotor activity at high doses (Anderson et al., 1975). Moreover, several lines of evidence support the occurrence of interactions between both systems (Welch et al., 1995; Tanda et al., 1997; Meng et al., 1998; Ambrosio et al., 1999; reviews in Manzanares et al., 1999; Piomelli et al., 2000). This has been recently confirmed by evaluating the pharmacological properties of morphine in central cannabinoid receptor (CB1) knockout mice (Ledent et al., 1999). Thus, the acute effects of morphine were unaffected in CB1 knockout mice as compared to wild type animals, whereas reinforcing properties of the alkaloid and severity of the withdrawal syndrome were found to be reduced, suggesting that long-term CB1 antagonist administration could be considered for preventing the development of dependence to opiates. However, frequent inconsistencies between observed and expected results are found in knockout mice, probably due to compensatory developmental mechanisms occurring during embryogenesis and early postnatal life (Zimmer et al., 1998; de Felipe et al., 1998). It was therefore of interest to investigate the effect resulting from the blockade of the CB1 receptors by an antagonist using wild type mice. The synthesis of the highly potent and selective CB1 antagonist, SR 141716A (N-(piperidin-1-yl)-5-(4-chlorophenyl) -1- (2,4-dichlorophenyl) -4-methyl-1H-pyrazole-3-carboxy-amide (Rinaldi-Carmona et al., 1994) affords the possibility to evaluate this hypothesis and to determine whether chronic blockade of CB1 receptors could be used to facilitate drug abstinence.
We have therefore studied the behavioural changes classically observed following chronic morphine treatment i.e. withdrawal syndrome and rewarding properties (review in Koob & Le Moal, 1997) in mice repeatedly treated with the combination morphine+SR 141716A. Moreover, as numerous studies have reported a link between both κ and cannabinoid systems (Smith et al., 1994; Welch, 1997) we have evaluated the effects of chronic SR 141716A treatment on the levels of κ endogenous opioid peptide, dynorphin, as well as κ opioid receptors. Indeed, stimulation of κ opioid receptors in the nucleus accumbens was shown to negatively modulate the rewarding properties of morphine. This occurs by a decrease in synaptic levels of dopamine induced by opioid activation of the mesolimbic dopaminergic pathway (Di Chiara & Imperato, 1988; Spanagel et al., 1992; Kuzmin et al., 1997).
Methods
Animals
Male CD-1 mice (Charles River, France) weighing 20 – 22 g at the start of the experiments, were housed in groups of 20 at least 2 days before the chronic treatments, and maintained at a controlled temperature of 21±1°C.
Chemicals
Δ9-THC (THC) (Sigma, France) was dissolved into a final concentration of 10% ethanol, 10% cremophor EL (Sigma) and 80% distilled water and injected via the intraperitoneal (i.p.) route. The CB1 receptor antagonist SR 141716A (generously provided by Sanofi Recherche, France) was dissolved in a solution of 5% ethanol, 5% cremophor EL and 90% distilled water and injected i.p. Morphine (Francopia, France) and naloxone (Sigma, France) were dissolved in saline (0.9% sodium chloride in water) and injected i.p. and via the subcutaneous (s.c.) route, respectively. Volumes of injection were 0.1 ml per 10 g of body weight. (3H) CI-977 (51 Ci mmol−1) was purchased from Amersham (France) and the dynorphin radioimmunoassay kit from Phoenix Pharmaceuticals, Inc.
Place conditioning paradigm
To evaluate the effects of SR 141716A on the rewarding properties of morphine, an unbiased place conditioning methodology was used. Tests were performed as previously reported (Valverde et al., 1996a) except for the conditioning time (20 min) and the size of the compartments which were adapted for studies with mice (15×15×15 cm). Briefly, the protocol consisted of three phases. (1) The preconditioning phase, where drug naive mice had free access to both compartments of the conditioning apparatus for 20 min with the time spent in each compartment recorded. (2) The conditioning phase, during which mice were treated for 6 consecutive days with alternate injections of drug or vehicle, and immediately placed individually in one of the conditioning environments for 20 min. Mice received the drug on days 1, 3 and 5, and vehicle on days 2, 4 and 6. Control animals received vehicle every day. (3) The postconditioning or testing phase, was conducted exactly as the preconditioning phase (free acces to each compartment for 20 min) 24 h after the last conditioning session. Separate groups of mice (n=8 – 10 per group) received morphine (3 mg kg−1, i.p.), SR 141716A (1, 5 or 10 mg kg−1, i.p), morphine+SR 141716A (used at the different doses previously mentioned) or vehicle injection. Data were compared using Newman-Keuls's test, after main effect of ANOVA on score (calculated as the difference between postconditioning and preconditioning time spent in the compartment associated with the drug).
Measurement of withdrawal syndrome
Experiment 1
Four groups of mice (n=7 – 9 per group) were injected i.p. twice daily for 5 days with morphine, SR 141716A (10 mg kg−1), morphine+SR 141716A, or vehicle. The morphine dose was progressively increased as follows: day 1, 20 mg kg−1; day 2, 40 mg kg−1; day 3, 60 mg kg−1; day 4, 80 mg kg−1; day 5, 100 mg kg−1. On day 6, the mice only received one injection in the morning (100 mg kg−1), and 2 h later received a s.c. injection of naloxone (1 mg kg−1) to precipitate the withdrawal syndrome. The mice were observed for 40 min, and the following signs were measured: wet dog shakes, paw tremor, sniffing, body tremor, ptosis and jumping.
Experiment 2
Four groups of mice (n=7 per group) were injected i.p. twice daily for 5 days with THC (20 mg kg−1), SR 141716A (10 mg kg−1), THC+SR 141716A, or vehicle. On day 6, the mice only received one injection in the morning, and 4 h later received an injection of SR 141716A (10 mg kg−1, i.p.) to precipitate the withdrawal syndrome, the signs of which were observed for 40 min (writhing, sniffing, wet-dog shakes, paw to face, ataxia, body tremor, ptosis, and piloerection).
Experiment 3
Four groups of mice (n=10 per group) were injected i.p. twice daily for 5 days with morphine (two groups) or saline (two groups). The morphine dose was progressively increased as described in experiment 1. On day 6, the mice only received one injection in the morning of morphine (100 mg kg−1) or saline, alone or in combination with SR 141716A (10 mg kg−1, i.p.). Two hours later mice received a s.c. injection of naloxone (1 mg kg−1) to precipitate the withdrawal syndrome measured as in experiment 1.
Statistical analysis
Somatic signs of withdrawal were compared by using a one-way ANOVA (between subjects) using post hoc Newman-Keuls's test.
Binding on brain homogenates
Mice were chronically treated in the same conditions used to evaluate the withdrawal syndrome after administration of naloxone. On day 6, mice were killed by decapitation. Whole brains minus cerebellum were dissected, homogenized in Tris-HCl buffer 50 mM, EDTA 1 mM (pH 7.4), and centrifuged at 35,000 r.p.m. for 30 min at 4°C. The pellet was homogenized in 5 ml ice-cold 50 mM Tris-HCl, 1 mM EDTA. The binding was performed using 100 μg of total brain membrane proteins incubated in 50 mM Tris-HCl pH 7.4, 1 mM EDTA at 25°C for 1 h with (3H)CI-977 ([5R-(5,7,8β)]-N-methyl-N-[7-(1-pyrrolidinyl)1-oxaspiro [4,5]dec-8-yl] -4-benzofuranacetamide) at concentrations of 0.01 – 1.28 nM. Naloxone was used at a 2 μM concentration to determine non-specific binding. Experiments were performed in triplicate using at least three distinct membrane preparations. Binding data were analysed using the EBDA-LIGAND program (Biosoft).
Dynorphin A radioimmunoassay (RIA)
The RIA was carried out using a radioimmunoassay kit for dynorphin A from the Phoenix Pharmaceuticals Inc. (Belmont, CA, U.S.A.), following the supplier's instructions.
Results
Place preference paradigm
The effects of the different doses of SR 141716A (1, 5 and 10 mg kg−1) on the rewarding properties of morphine were investigated. As shown in Figure 1, morphine administration (3 mg kg−1, i.p.) induced a clear conditioned place preference, indicated by a significant increase in the time spent in the drug-associated compartment during the testing phase, whereas no place preference was observed in the vehicle group. At the lowest dose of SR 141716A used (1 mg kg−1) no modification of the rewarding properties of morphine were observed (Figure 1a), whereas at higher doses (5 mg kg−1 and 10 mg kg−1), the reinforcing properties of the alkaloid were totally abolished (Figure 1b,c).
Figure 1.
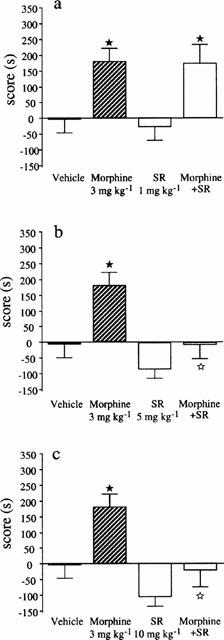
Conditioned place preference induced by morphine (3 mg kg−1, s.c.), SR 141716A (at different doses: 1, 5 and 10 mg kg−1, i.p.) and combination of morphine+SR 141716A (at different doses). Data are expressed as scores (means±s.e.mean) calculated as the difference between postconditioning and preconditioning time spent in the compartment associated with the drug. n=10 per group. ★P<0.05 vs vehicle group; ⋆P<0.05 vs morphine group.
Physical dependence
Experiment 1
In this experiment, mice were chronically treated with morphine (5 days) alone or in association with the CB1 antagonist SR 141716A. Then, the withdrawal syndrome was precipitated by administration of the opioid antagonist naloxone. A decrease in the incidence of two main signs of physical dependence to opiates, i.e. wet dog shakes and jumping was observed in mice chronically treated with morphine+SR 1451716A as compared to morphine-treated mice, whereas other signs (paw tremor, ptosis, sniffing, body tremor) of naloxone precipitated withdrawal were not different between both groups (Figure 2).
Figure 2.
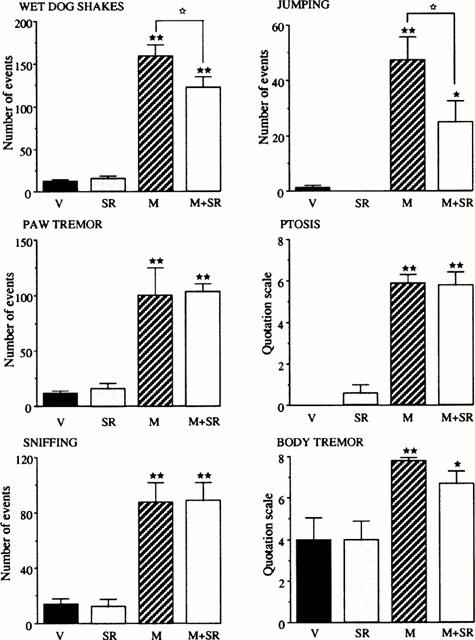
Effects of naloxone administration (1 mg kg−1, s.c.) on physical withdrawal signs in mice (n=7 – 9 per group) chronically treated for 5 days with morphine (M) (from 20 to 100 mg kg−1, i.p.), SR 141716A (SR) (10 mg kg−1, i.p.), morphine+SR 141716A (M+SR) (20 – 100 mg kg−1+10 mg kg−1, i.p., respectively) or vehicle (V). Each animal received two injections daily. The results are expressed as means±s.e.mean of the number of events counted during the 40 min period of observation immediately after naloxone injection. ★P<0.05, ★★P<0.01 vs vehicle group; ⋆P<0.05 vs morphine group.
Experiment 2
Most of the withdrawal signs observed after administration of the CB1 antagonist (10 mg kg−1) in mice chronically treated with THC (wet dog shakes, paw to face, piloerection, ptosis, writhing and sniffing) were totally abolished in animals chronically treated with THC+SR 141716A. The only exceptions were ataxia and body tremor (Figure 3).
Figure 3.
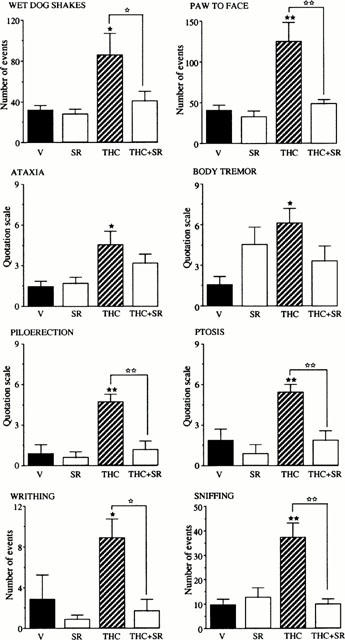
Effects of SR 141716A administration (10 mg kg−1, i.p.) on physical withdrawal signs in mice (n=7 – 9 per group) chronically treated for 5 days with THC (20 mg kg−1, i.p.), SR 141716A (SR) (10 mg kg−1, i.p.), THC+SR 141716A (THC+SR) (20 mg kg−1+10 mg kg−1, i.p., respectively) or vehicle (V). Each animal received two injections daily. The results are expressed as means±s.e.mean of the number of events counted during the 40 min period of observation immediately after SR 141716A injection. ★P<0.05, ★★P<0.01 vs vehicle group; ⋆P<0.05, ⋆⋆P<0.01 vs THC group.
Experiment 3
Mice chronically treated with morphine for 5 days received on day 6 a last injection of morphine alone or in association with SR 141716A, and 2 h later the withdrawal syndrome was precipitated by naloxone administration. The results show that acute injection of the CB1 antagonist with the last morphine treatment did not modify the behavioural manifestations of withdrawal triggered by naloxone as compared to mice which received only morphine on day 6 (Figure 4).
Figure 4.
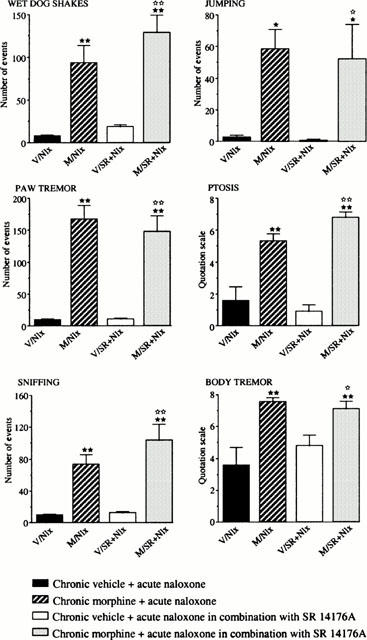
Effects of naloxone (Nlx) administration (1 mg kg−1, s.c.) alone or in combination with SR 141716A (10 mg kg−1 i.p., administered 2 h before naloxone injection) on physical withdrawal signs in mice (n=10 per group) chronically treated for 5 days with morphine (M) (from 20 to 100 mg kg−1, i.p.), or vehicle (V). Each animal received two injections daily. The results are expressed as means±s.e.mean of the number of events counted during the 40 min period of observation immediately after naloxone injection. ★P<0.05, ★★P<0.01 vs V/Nlx group; ⋆P<0.05, ⋆⋆P<0.01 vs V/SR+Nlx group.
Biochemical experiments
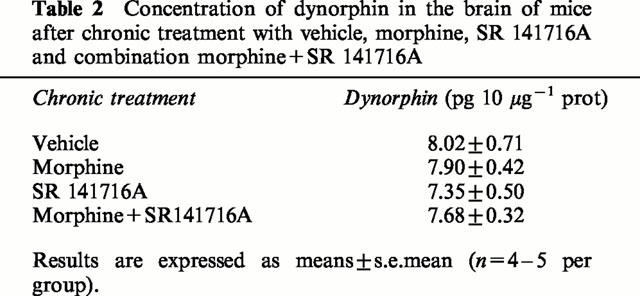
As reported in Table 1, similar KD and Bmax values were obtained for κ opioid receptors on whole brain membranes using a specific radioligand (3H)CI-977 in the four different groups of mice chronically treated with vehicle, morphine, SR 141716A (10 mg kg−1) or morphine+SR 141716A combination. Moreover, no modification in the dynorphin levels were observed in the different groups of animals, determined using a radioimmunoassay (Table 2) performed on whole brain.
Table 1.
Binding parameters (KD and Bmax) obtained with [3H]-CI977 in brain membranes of mice
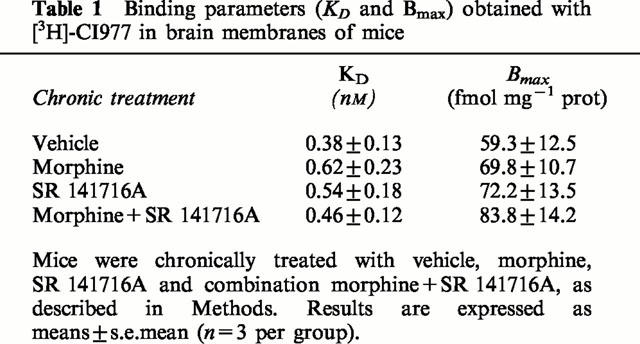
Table 2.
Concentration of dynorphin in the brain of mice after chronic treatment with vehicle, morphine, SR 141716A and combination morphine+SR 141716A
Discussion
The aim of this study was to investigate the effects of repetitive treatment with the cannabinoid CB1 antagonist SR 141716A on physical and psychological dependencies induced by chronic morphine administration. Regarding physical dependence, the opioid antagonist naloxone produced withdrawal signs which were not found to be very different in mice chronically treated with morphine alone or with the combination morphine+SR 141716A except for the two main withdrawal signs (wet dog shakes and jumping) which were significantly reduced (Figure 2). The observed SR 141716A-evoked withdrawal signs in mice chronically treated with THC, were abolished in animals repeatedly treated with the combination THC+SR 141716A, suggesting that the dose of SR 141716A (10 mg kg−1) used during chronic morphine treatment was sufficient to block the CB1 receptors. The present results indicate that only long term CB1 antagonist administration mimicking the situation encountered in CB1 receptor deleted mice, reduces development of morphine physical dependence. According to this hypothesis, an acute administration of SR 141716A was unable to modify the naloxone-precipitated withdrawal signs in chronically morphine-treated mice. There is some disparity between the effects observed in the present study and in CB1 receptor knock-out mice, as in the latter (Ledent et al., 1999), seven of the nine signs of naloxone-precipitated morphine withdrawal evaluated were found significantly reduced. This observation, taken with the present results, indicates that the duration of the CB1 receptor blockade could modulate the intensity of the morphine withdrawal syndrome expression. This hypothesis suggests that results obtained in knock-out mice should be carefully considered before any extrapolation and require additional experiments with wild type animals and selective antagonists blocking reversibly the receptor.
On the other hand, the results show that SR 141716A (5 mg kg−1) antagonized the acquisition of morphine-induced conditioned place preference. This result is in agreement with the experiments of Ledent et al. (1999) showing that the number of nose pokes leading to morphine administration was much lower in CB1 receptor knockout mice as compared to wild type animals. In the present study a possible trend towards conditioned place aversion was observed though it was not significant after three drug paired conditioning periods with the highest doses, 5 and 10 mg kg−1. This could be due to side-effects, such as paw tremors, head shakes or ataxia, observed after administration of high doses of the CB1 antagonist (Cook et al., 1998). It is interesting to notice that invalidation of the CB1 receptor was also reported to generate mice with problems of ataxia and hypolocomotion (Zimmer et al., 1999). Another hypothesis to explain the results obtained is the existence of an endogenous cannabinoid tone, as several authors observed that CB1 agonists are able to induce a place preference (Lepore et al., 1995; Valjent & Maldonado, 2000). However, it is important to note that the rewarding effects of cannabinoids in the different animals models currently used are controversial and may strongly depend in part of the species and treatments used (Harris et al., 1974; Mansbach et al., 1994). Thus, cannabinoid agonists failed to induce self-administration behaviour in monkeys or rats (Leite & Carlini, 1974), but are self-administered in mice (Martellota et al., 1998). Several hypotheses could be proposed to explain the present results. The well-known dysphoric effects of THC at high concentrations may likely reduce the rewarding effects of opioids (Hutcheson et al., 1998; Valjent & Maldonado, 2000). Furthermore, as it is well established that κ agonists have aversive properties, it could be speculated that the lack of morphine reinforcing effects in mice chronically treated with the combination morphine+SR 141716A results from an alteration of the κ opioid system, which may counteract the rewarding effects of the alkaloid (Spanagel et al., 1992). However, this hypothesis appears unlikely as no modification in the level of dynorphin (the endogenous ligand of the κ opioid receptor) and in the binding parameters of κ opioid receptors were observed in morphine+SR 141716A-treated mice as compared to morphine-, SR 141716A- or vehicle-treated mice. Other hypotheses could be suggested to account for the reduction of morphine-induced dependence in mice concomitantly treated with the alkaloid and the CB1 antagonist. One of them involves a modulation in the release of hypothalamic corticotropin releasing factor (CRF), well known to be involved in determining propensity to develop drug addiction (Shaham et al., 1998; Rouge-Pont et al., 1998). Accordingly CRF-containing neurons and CB1 receptors were found co-localized in hypothalamic and limbic areas such as amygdala complex, the medial prefrontal cortex or the hippocampus (Swanson et al., 1983; Herkenham et al., 1991). As in a previous study it has been shown that the endogenous ligand of CB1 receptors, anandamide parallels THC in activation of hypothalamo-pituitary-adrenal (HPA) axis via mediation of a central mechanism which involves the secretion of CRF (Weidenfeld et al., 1994), it could be speculated that blockade of CB1 receptor with SR 141716A reduces the endogenous activity of the HPA axis, leading to a reduction of morphine-induced rewarding effects. On the other hand, the CB1 receptor may exist in different states, an inactive state precoupled (Vasquez & Lewis, 1999) or not (Bouaboula et al., 1997) to Gi/o in its GDP-bound form and an active R* state coupled to Gi/o in its GTP-bound form. SR 141716A may contribute to stabilize the inactive R-GDP state of the receptor due to its inverse agonist properties (Bouaboula et al., 1997), sequestering a pool of Gi/o -proteins, making them unavailable to couple to other receptors, such as mu opioid receptors, almost exclusively involved in morphine addiction (Matthes et al., 1996). Thus, long term adaptation in intracellular messenger proteins along the cyclic AMP pathway which is a mechanism likely to be involved in opiate addiction (Nestler et al., 1993) may be less marked in presence of the CB1 antagonist. As there is now evidence for a role of the upregulated cyclic AMP pathway in opiate dependence (Maldonado et al., 1995; Valverde et al., 1996b; Punch et al., 1997), this hypothesis could explain the reduction in development of physical dependence and acquisition of morphine-induced reinforcement in the conditioning place preference paradigm. This is reinforced by the presence of both opioid and CB1 receptors in the same brain areas (Navarro et al., 1998), including the limbic system, well-known to play a major role in rewarding processes (review in Koob & Le Moal, 1997).
In conclusion, the present study shows that the CB1 antagonist SR 141716A co-administered with morphine is able to abolish the rewarding effects of the alkaloid, investigated in the place preference paradigm and to diminish the naloxone-precipitated withdrawal syndrome in mice. These results support an interaction between the endogenous cannabinoid and opioid systems. Whilst the results obtained could be predicted on the basis of the observation that the morphine effects were diminished in CB1 receptor knock-out mice (Ledent et al., 1999), it was essential to reproduce the effects by the pharmacological administration of an antagonist in wild-type animals. Indeed, adaptative compensatory mechanisms may produce unexpected results, as gene deletion is operative during embryogenesis (Zimmer et al., 1998; de Felipe et al., 1998). The reduction in rewarding responses induced by SR 141716A in mice chronically treated with morphine supports the use of the CB1 antagonist to prevent withdrawal in heroin addicts. This could be an inconvenience for maintenance treatment with methadone (or buprenorphine) since SR 141716A blocks the rewarding effects of the opiate substitutes. However, well chosen doses of the CB1 antagonist and methadone could gradually reduce the psychological dependence to the latter, reducing its often reported drawback (Steinpreis et al., 1996). This hypothesis warrants further investigations with other CB1 antagonists devoid of the inverse agonist properties of SR 141716A (Bouaboula et al., 1997; Landsman et al., 1997).
Acknowledgments
This work was supported by grants from SANOFI Recherche (AED no. 0199B056).
Abbreviations
- CB
cannabinoid
- cyclic AMP
cyclic adenosine monophosphate
- HPA
hypothalamo-pituitary adrenal
- THC
Δ 9-tetrahydrocannabinol
References
- AMBROSIO E., MARTIN S., GARCIA-LECUMBERRI C., CRESPO J.A. The neurobiology of cannabinoid dependence: sex differences and potential interactions between cannabinoid and opioid systems. Life Sci. 1999;65:687–694. doi: 10.1016/s0024-3205(99)00291-x. [DOI] [PubMed] [Google Scholar]
- ANDERSON P.F., JACKSON D.M., CHESHER G.B., MALOR R. Tolerance to the effects of delta-9-tetrahydrocannabinol in mice on intestinal motility, temperature and locomotor activity. Psychopharmacology. 1975;48:31–36. doi: 10.1007/BF00437611. [DOI] [PubMed] [Google Scholar]
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.P., LE FUR G., CASELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- COOK S.A., LOWE J.A., MARTIN B.R. CB1 receptor antagonist precipitates withdrawal in mice exposed to Δ9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 1998;285:1150–1156. [PubMed] [Google Scholar]
- DE FELIPE C., HERRERO J.F., O'BRIEN J.A., PALMER J.A., DOYLE C.A., SMITH A.J.H., LAIRD J.M.A., BELMONTE C., CERVERO F., HUNT S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- DEWEY W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- DI CHIARA G., IMPERATO A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- DYER K.R., FOSTER D.J., WHITE J.M., SOMOGYI A.A., MENELAOU A., BOCHNER F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin. Pharmacol. Ther. 1999;65:685–694. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- FISCHER G., GOMBAS W., EDER H., JAGSCH R., PETERNELL A., STUHLINGER G., PEZAWAS L., ASCHAUER H.N., KASPER S. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- HARRIS R.T., WATERS W., MCLENDON D. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia. 1974;37:23–29. doi: 10.1007/BF00426679. [DOI] [PubMed] [Google Scholar]
- HERKENHAM M., GROEN B.G.S., LYNN A.B., DE COSTA B.R., RICHFIELD E.K. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res. 1991;552:301–310. doi: 10.1016/0006-8993(91)90096-e. [DOI] [PubMed] [Google Scholar]
- HUTCHESON D.M., TZAVARA E.T., SMADJA C., VALJENT E., ROQUES B.P., HANOUNE J., MALDONADO R. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with Δ-9-tetrahydrocannabinol. Br. J. Pharmacol. 1998;125:1567–1577. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB G.F., LE MOAL M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- KREEK M.J. Opiate and cocaine addictions: challenge for pharmacotherapies. Pharmacol. Biochem. Behav. 1997;57:551–569. doi: 10.1016/s0091-3057(96)00440-6. [DOI] [PubMed] [Google Scholar]
- KUHLMAN J.J., JR, LEVINE B., JOHNSON R.E., FUDALA P.J., CONE E.J. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93:549–559. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- KUZMIN A.V., SEMENOVA S., GERRITS M.A., ZVARTAU E.E., VAN REE J.M. Kappa-opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur. J. Pharmacol. 1997;321:265–271. doi: 10.1016/s0014-2999(96)00961-2. [DOI] [PubMed] [Google Scholar]
- LANDSMAN R.S., BURKEY T.H., CONSROE P., ROESKE W.R., YAMAMURA H.I. SR 141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur. J. Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.F., BESLOT F., BÖHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LEITE J.R., CARLINI E.A. Failure to obtain “cannabis-directed behavior” and abstinence syndrome in rats chronically treated with Cannabis sativa extracts. Psychopharmacology. 1974;36:133–145. doi: 10.1007/BF00421785. [DOI] [PubMed] [Google Scholar]
- LEPORE M., VOREL S.R., LOWINSON J., GARDNER E.L. Conditioned place preference induced by Δ-9-tetrahydrocannabinol comparison with cocaine, morphine and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., MARTIN B.R. Spinal and supraspinal components of cannabinoid-induced antinociception. J. Pharmacol. Exp. Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- MALDONADO R., VALVERDE O., GARBAY C., ROQUES B.P. Protein kinases in the locus coeruleus and periaqueductal gray matter are involved in the expression of opiate withdrawal. Naunyn-Schmiedberg's Arch. Pharmacol. 1995;352:565–575. doi: 10.1007/BF00169392. [DOI] [PubMed] [Google Scholar]
- MANSBACH R.S., NICHOLSON K.L., MARTIN B.R., BALSTER R.L. Failure to Δ-9-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav. Pharmacol. 1994;5:219–225. doi: 10.1097/00008877-199404000-00014. [DOI] [PubMed] [Google Scholar]
- MANZANARES J., CORCHERO J., ROMERO J., FERNANDEZ-RUIZ J.J., RAMOS J.A., FUENTES J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- MARTELLOTA M.C., COSSU G., GESSA G.L., FRATTA W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85:327–330. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- MATTHES H.W.D., MALDONADO R., SIMONIN F., VALVERDE O., SLOWE S., KITCHEN I., BEFORT K., DIERICH A., LE MEUR M., DOLLE P., TZAVARA E., HANOUNE J., ROQUES B.P., KEIFFER B.L. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- MENG I.D., MANNING B.H., MARTIN W.J., FIELDS H.L. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- NAVARRO M., CHOWEN J., ROCIO A., CARRERA M., DEL ARCO I., VILLANUA M.A., MARTIN Y., ROBERTS A.J., KOOB G.F., DE FONSECA F.R. CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport. 1998;26:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- NESTLER E.J., HOPE B.T., WIDNELL K.L. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D., GIUFFRIDA A., CALIGNANO A., RODRIGUEZ DE FONSECA F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- PUNCH L.J., SELF D.W., NESTLER E.J., TAYLOR J.N. Opposite modulation of opiate withdrawal behaviors on microinfusion of a protein kinase A inhibitor versus activator into the locus coeruleus or periaqueductal gray. J. Neurosci. 1997;17:8520–8527. doi: 10.1523/JNEUROSCI.17-21-08520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR G. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- ROUGE-PONT F., DEROCHE V., LE MOAL M., PIAZZA P.V. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur. J. Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- SHAHAM Y., ERB S., LEUNG S., BUCZEK Y., STEWART J. CP-154,546, a selective, non-peptide antagonist of the corticotropin-releasing factor 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- SMITH P.B., WELCH S.P., MARTIN B.R. Interactions between Δ9-tetrahydrocannabinol and Kappa opioids in mice. J. Pharmacol. Exp. Ther. 1994;268:1381–1386. [PubMed] [Google Scholar]
- SPANAGEL R., HERZ A., SHIPPENBERG T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINPREIS R.E., RUTELL A.L., PARRETT F.A. Methadone produces conditioned place preference in the rat. Pharmacol. Biochem. Behav. 1996;54:339–341. doi: 10.1016/0091-3057(95)02141-8. [DOI] [PubMed] [Google Scholar]
- SWANSON L.W., SAWCHENKO P.E., RIVIER J., VALE W.W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- TANDA G., PONTIERI F.E., DI CHIARA G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mul opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- VALJENT E., MALDONADO R. A behavioral model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology. 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- VALVERDE O., FOURNIE-ZALUSKI M.C., ROQUES B.P., MALDONADO R. The CCK-B antagonist PD-134,308 facilitates rewarding effects of endogenous enkephalins but does not induce place preference in rats. Psychopharmacology. 1996a;123:119–126. doi: 10.1007/BF02246168. [DOI] [PubMed] [Google Scholar]
- VALVERDE O., TZAVARA E., HANOUNE J., ROQUES B.P., MALDONADO R. Protein kinases in the rat nucleus accumbens are involved in the aversive component of opiate withdrawal. Eur. J. Pharmacol. 1996b;8:2671–2678. doi: 10.1111/j.1460-9568.1996.tb01562.x. [DOI] [PubMed] [Google Scholar]
- VASQUEZ C., LEWIS D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD J., HALL W., MATTICK R.P. Role of maintenance treatment in opioid dependence. Lancet. 1999;353:221–226. doi: 10.1016/S0140-6736(98)05356-2. [DOI] [PubMed] [Google Scholar]
- WEIDENFELD J., FELDMAN S., MECHOULAM R. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;59:110–112. doi: 10.1159/000126646. [DOI] [PubMed] [Google Scholar]
- WELCH S.P. Characterization of anandamide-induced tolerance: comparison to Δ9-THC-induced interactions with dynorphinergic systems. Drug Alcohol Dep. 1997;45:39–45. doi: 10.1016/s0376-8716(97)01342-2. [DOI] [PubMed] [Google Scholar]
- WELCH S.P., THOMAS C., PATRICK S. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J. Pharmacol. Exp. Ther. 1995;272:310–321. [PubMed] [Google Scholar]
- ZIMMER A., ZIMMER A.M., BAFFI J., USDIN T., REYNOLDS K., KÖNIG M., PALKOVITS M., MEZEY E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMER A., ZIMMER A.M., HOHMANN A.G., HERKENHAM M., BONNER T.I. Increased mortality, hypoactivity, and hypoalgesia in cannbinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


