Abstract
Intrinsic activity and β1-selectivity are important features of β-blockers in the treatment of patients with coronary syndromes and heart failure. In human myocardium, intrinsic activity and β1-selectivity of the novel β-adrenoceptor antagonist nebivolol have not yet been determined.
The study examines intrinsic activity, β-adrenoceptor-G-protein coupling and β1-selectivity of nebivolol and bisoprolol in human ventricular myocardium. Furthermore, intrinsic activity of both compounds is compared to the one of bucindolol, carvedilol and metoprolol in human atrial myocardium.
Radioligand binding studies ([125I]-lodocyanopindolol) were performed on membrane preparations of human failing and nonfailing myocardium and on COS-7 cells transfected with human β1- and β2-adrenoceptors, respectively. Functional experiments were carried out on isolated muscle preparations of human left ventricular and right atrial myocardium from failing and nonfailing hearts.
Radioligand binding studies reveal 3 – 4 fold β1-selectivity for nebivolol and 16 – 20 fold β1-selectivity for bisoprolol in human myocardium. In COS-7-cells, β1-selectivity is 3 fold for nebivolol and 15 fold for bisoprolol.
Neither the binding of nebivolol nor of bisoprolol is affected by the presence of guanylylimidodiphosphate (Gpp(NH)p).
Nebivolol and bisoprolol exert similar inverse agonist activity in human ventricular as well as atrial myocardium. In atrial myocardium, inverse agonism of both compounds is higher compared to bucindolol, equal to carvedilol and lower compared to metoprolol.
Favourable haemodynamic effects of nebivolol in humans are not due to β1-selectivity or partial agonist activity of this agent. Other mechanisms, i.e. the production of nitric oxide, may thus be responsible for its unique haemodynamic profile.
Keywords: β-adrenoceptor antagonists, β1-selectivity, inverse agonism, human myocardium, heart failure
Introduction
In patients with chronic heart failure, β-blockers have favourable effects on ventricular function and survival. These benefits are provided by second-generation β1-selective (bisoprolol, CIBIS II, 1999; metoprolol, MERIT-HF, 1999) as well as third-generation non-selective agents (carvedilol, Packer et al., 1996). In contrast, xamoterol, a partial agonist, has led to an increase of mortality (Nicholas, 1990). A recent study with bucindolol in patients with heart failure (BEST-trial) had to be terminated due to a lack of benefit (Bristow, 2000). It is currently discussed whether this negative result may be due to comparable high intrinsic activity of bucindolol that has been observed in animal models (Willette et al., 1998) and also human myocardium (Maack et al., 2000). These data indicate that intrinsic activity of β-blockers has to be considered for therapeutic reasons.
Nebivolol is a novel third-generation β-blocker, being a racemic mixture of equal amounts of d-(SRRR-) nebivolol and l-(RSSS-) nebivolol. The d-isomer is reported to be highly selective for β1-adrenoceptors in several animal and cell models (Pauwels et al., 1989; 1991), whereas the I-isomer accounts for vasodilatory effects that are mediated by endothelial generation of nitric oxide (Mangrella et al., 1998). A special feature of nebivolol is that, in contrast to classical β-blockers, its acute administration increases ejection fraction and maintains cardiac output despite lowering of blood pressure in patients with cardiovascular diseases (Stoleru et al., 1993). From these clinical results it cannot be excluded that besides vasodilation a positive inotropic or lack of negative inotropic effect of nebivolol may contribute to the acute increase of ejection fraction. Inotropic effects of β-adrenoceptor ligands are generally related to their intrinsic activity. To date, no determination of intrinsic activity of nebivolol in human myocardium has been performed.
In its unoccupied condition, the β-adrenoceptor exists in an equilibrium between its active (R*) and inactive conformation (R). The binding of an agonist induces a conformational change towards R*, whereas the binding of an inverse agonist stabilizes the inactive conformation of the receptor (Bond et al., 1995). The R* conformation allows the interaction of the receptor with the stimulatory G-protein (GS). Subsequent dissociation of the α-subunit from the βγ-subunits of GS leads to stimulation of adenylate cyclase. Due to the influence of receptor systems and experimental conditions (i.e. different stoichiometry of receptors/G-proteins, different sensitization state of receptors etc.), the same ligand may behave as an inverse agonist, a neutral antagonist or even a partial agonist (de Ligt et al., 2000; Chidiac et al., 1996). In the present study, β-adrenoceptor-G-protein interaction of the novel β-blocker nebivolol is evaluated in comparison to bisoprolol by radioligand binding experiments with [125I]-lodocyanopindolol in the absence and presence of guanylylimidodiphosphate (Gpp(NH)p). Furthermore, intrinsic activity of nebivolol is compared to bisoprolol, metoprolol, carvedilol and bucindolol by functional experiments on human myocardium.
In animal studies and cell systems, nebivolol was reported to be highly β1-selective (Pauwels et al., 1989; 1991). However, up to date, no direct assessment of binding characteristics of nebivolol to human myocardial β1- and β2-adrenoceptors has been performed. In the present study, affinity of nebivolol and bisoprolol to β1-and β2-adrenoceptors is determined by radioligand binding experiments in the absence and presence of ICI 118.551 and CGP 207.12A, respectively. Furthermore, the results of these experiments on human myocardium are confirmed by binding studies on COS-7-cells that have been transfected with human β1- and β2-adrenoceptors, respectively.
Methods
Myocardial tissue
Experiments were performed on human right atrial trabeculae obtained during open-heart bypass surgery and on left ventricular muscle strip preparations or cell membrane preparations from failing and nonfailing human hearts obtained during cardiac transplantations. Tissue was brought to the laboratory and prepared for functional experiments immediately. Tissue samples for binding experiments were stored at −80°C until assay. Failing hearts were taken from 17 patients with end-stage heart failure (New York Heart Association (NYHA) class IV), resulting from either idiopathic dilated (n=7) or ischemic (n=10) cardiomyopathy (15 male, two female; age, 55±1 years). Nonfailing hearts were taken from five organ donors whose hearts could not be used for transplantation (four male, one female; age 45±4 years). For the latter group, echocardiography revealed normal left ventricular contractility. Bypass patients (mean age, 67±2 years, 17 male, six female) had no signs of atrial dilation or arrhythmias or of left ventricular dysfunction. All patients gave written informed consent before surgery. Since nebivolol and bisoprolol were mostly compared in tissue samples from the same hearts (radioligand binding and functional experiments), there were no significant differences between these two groups concerning i.e. age, sex and medication. Tissue preparation for functional experiments and membrane preparation for binding experiments has been described elsewhere (Maack et al., 2000; Böhm et al., 1990b).
Isolated cardiac muscle strip preparation and measurement of force of contraction
Isometric force of contraction was determined on isolated, electrically driven muscle preparations as described previously (Böhm et al., 1990b). Bathing solution was maintained at 37°C, pH 7.4, and aerated with 95% O2 and 5% CO2. Muscles of uniform size (length, 5 – 6 mm; thickness, 1 – 2 mm) were stretched to the length at which force of contraction was maximal (5 – 8 mN). In experiments with inotropic prestimulation, muscle strips were pre-exposed to isoprenaline (1 μmol l−1, ≈percnt;EC90) for 30 min and to forskolin (0.3 μmol l−1, ≈percnt;EC50) for at least 45 min. The β-adrenoceptor antagonists nebivolol (1 – 10,000 nmol l−1), bisoprolol (1 – 10,000 nmol l−1), bucindolol (0.1 – 1000 nmol l−1), carvedilol (0.1 – 1000 nmol l−1) and metoprolol (1 – 100,000 nmol l−1) were applied cumulatively to the organ bath for 30 min at each concentration. When concentrations of β-blockers are indicated as Ki or 100×Ki (bar graphs), the results are also derived from these cumulative dose-response curves. In atrial myocardium, the normal decline of force of contraction was determined by a control group without β-blocker treatment. The decrease of contractility by the respective β-blocker was related to the decline in the control group. In ventricular myocardium, force of contraction in the control group remained constant.
Cell culture and transfection
COS-7 cells were cultured and transfected using the Transfectam reagent (Promega, Heidelberg, Germany) as described previously (Schnabel et al., 1993). Membrane preparations were performed as described (Schnabel et al., 1993), except that the pellet was resuspended in a buffer containing 50 mmol l−1 Tris/HCl, pH 7.4 and 10 mmol l−1 MgCl2.
β-adrenoceptor binding studies
β-adrenoceptors in cardiac tissue were investigated using [125I]-iodocyanopindolol (ICYP) as the radiolabelled ligand. Cold ligand binding affinity was measured by ligand-ICYP competition curves using 37.5 pmol l−1 of ICYP to maintain the radioligand concentration at approximate Kd. The assay was performed in a total volume of 250 μl. The total amount of protein used per assay was 20 – 30 μg. Protein concentrations were determined according to the method of Lowry et al. (1951). The incubation at 25°C for 60 min allowed complete equilibration of the β-adrenoceptors with the radioligand. The reaction was terminated by rapid vacuum filtration through Whatman GF/C filters (Whatman Inc., Clifton, NJ, U.S.A.). The filters were washed immediately three times with 6 ml of ice-cold incubation buffer. All experiments were performed in triplicate.
Statistical analysis
Regression analysis was performed with the computer program GraphPadPrism (GraphPad Software, San Diego, CA, U.S.A.). Competition curve slope (pseudo-Hill coefficient, nH), the concentration at which 50% of receptors are occupied (EC50) and the percentage of receptors in a high affinity (%RH) or low affinity (%RL) state were determined by nonlinear regression analysis, comparing the fitting of the curve to either one or two receptor states by F-test analysis. Cold ligand dissociation constants for a high affinity (KH) or a low affinity receptor state (KL) were calculated according to the method of Cheng & Prussoff (1973). Ki values were calculated from EC50 values that were determined by fitting the results of competition experiments with a nonlinear regression analysis assuming only one receptor state, regardless if they actually reflect one or two affinity states.
Unless indicated, the data shown are mean±s.e.mean. For multiple comparisons, ANOVA analysis was performed. Otherwise, statistical significance was analysed with the Mann-Whitney- or Wilcoxon-test. A value of P<0.05 was considered significant.
Materials
Nebivolol (a racemic mixture of equal parts of (±)-SRRR- and RSSS-nebivolol) was donated from Berlin Chemie, Berlin, Germany. (±)-Bisoprolol was purchased from Biotrend, Cologne, Germany. Carvedilol and bucindolol are from Smith Kline Beecham (King of Prussia, U.S.A). Metoprolol was provided from Astra Chemicals (Hamburg, Germany). [125I]-ICYP was produced by Amersham-Buchler (Freiburg i.Br., Germany). All other chemicals were of analytic grade or the best commercially available.
Results
Radioligand binding studies
Figure 1 shows representative results from competition experiments of the β-adrenoceptor ligands ICI 118.551, CGP 207.12A and isoprenaline on human nonfailing myocardium.
Figure 1.
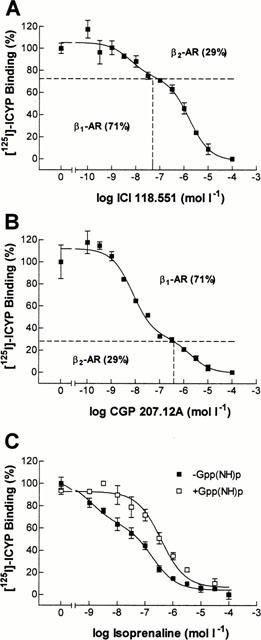
[125I]-ICYP competition curves in human left ventricular myocardium from a nonfailing heart. (A) ICYP-ICI 118.511 competition. The identification of two affinity sites allows the discrimination into 29% β2- and 71% β1-adrenoceptors. (B) ICYP-CGP 207.12A competition. 71% β1- and 29% β2-adrenoceptors are identified. The indicated concentrations (dashed lines) are used to determine binding properties of β-adrenoceptor ligands at β1- and β2-adrenoceptors specifically. (C) ICYP-isoprenaline competition in the absence and presence of 100 μmol l−1 of Gpp(NH)p. In the absence of Gpp(NH)p, 30% of adrenoceptors are in a high affinity state. This high affinity state is eliminated in the presence of Gpp(NH)p.
The highly selective ligands ICI 118.551 (β2-selective, Figure 1A) and CGP 207.12A (β1-selective, Figure 1B) exerted biphasic binding curves, allowing the discrimination of the total β-adrenoceptor population into approximately 70% β1- and 30% β2-adrenoceptors. When performing binding experiments in the presence of the indicated concentrations of ICI 118.551 and CGP 207.12A, respectively, binding characteristics of the studied ligand (nebivolol or bisoprolol) can be determined selectively on β1- and β2-adrenoceptors.
On myocardial membranes of the same heart, the agonist isoprenaline exerted a biphasic binding curve with the identification of a high- and a low-affinity binding site (Figure 1C). In the presence of guanylylimidodiphosphate (Gpp(NH)p), a non-hydrolyzable guanine nucleotide, binding became monophasic with the detection of only one (low-affinity) binding site. As a consequence, the slope factor (nH) steepened from 0.43 to 0.67. This indicates that in the examined heart, a fraction of 30% of β-adrenoceptors were in a high affinity state and thus sensitive for the detection of eventual agonist-like binding properties of the examined β-blockers. The average amount of β-adrenoceptors in a high affinity state was 16±5% (not shown).
In Figure 2, binding characteristics of nebivolol (left panels) and bisoprolol (right panels) are compared on a representative nonfailing heart. In Table 1, the cumulative results are listed. In the majority of experiments (11 of 16), nebivolol modelled for a one-site binding curve (Figure 2A). The slope factor of 0.90±0.07 approached unity, indicating similar dissociation constants of nebivolol for β1- and β2-adrenoceptors. In the presence of Gpp(NH)p, in two experiments the two-affinity site binding was converted into monophasic curves (Table 1). However, neither the slope factor nor the pKi-value were significantly affected by the presence of Gpp(NH)p. Table 2 indicates that there were no differences in the binding of nebivolol to β-adrenoceptors on nonfailing and failing myocardium.
Figure 2.
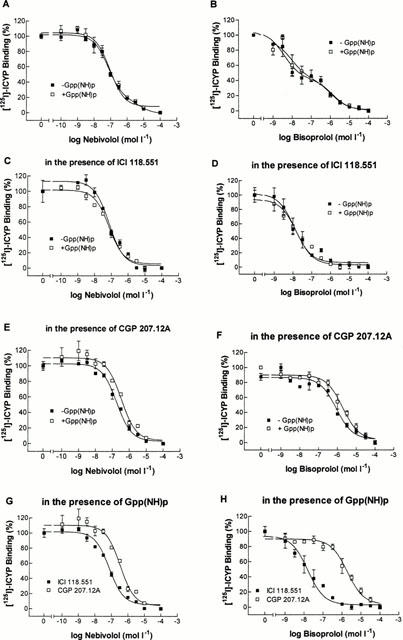
[125I]-ICYP competition curves in human left ventricular myocardium from a nonfailing heart. (A, C, E) ICYP-nebivolol competition in the absence and presence of 100 μmol l−1 of Gpp(NH)p. (B, D, F) ICYP-bisoprolol competition in the absence and presence of Gpp(NH)p. (A, B) competition experiments in a mixed β1- and β2-adrenoceptor population. (C, D) Experiments in the presence of ICI 118.551 (50 nmol l−1), determining the affinity of the respective β-blocker to β1-adrenoceptors. (E, F) Experiments in the presence of CGP 207.12A (300 nmol l−1), determining the affinity of the respective β-blocker to β2-adrenoceptors. (G, H) All experiments in the presence of Gpp(NH)p, in the presence of ICI 118.551 or CGP 207.12A, respectively, indicating β1-selectivity of the β-blockers.
Table 1.
Radioligand binding experiments in human ventricular myocardium
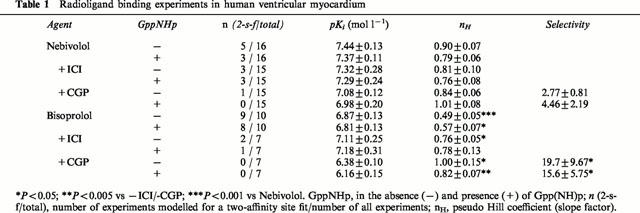
Table 2.
Radioligand binding experiments in failing and nonfailing myocardium
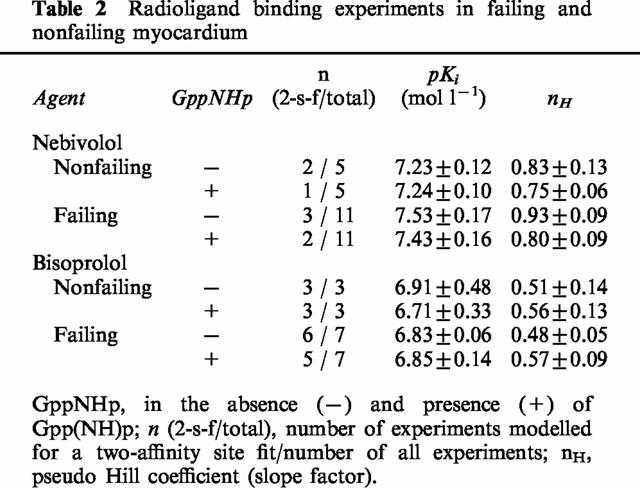
In contrast to nebivolol, bisoprolol modelled for two distinct affinity sites in nine out of 10 experiments in the absence and in eight out of 10 experiments in the presence of Gpp(NH)p (Figure 2B, Table 1). The slope factors in both cases were significantly different from unity and also from the slope factors of nebivolol. Neither pKi-values nor slope factors of bisoprolol were affected in the presence of Gpp(NH)p. Also, binding characteristics did not differ between nonfailing and failing myocardium (Table 2).
In the presence of ICI 118.551 and CGP 207.12A, respectively, binding curves revealed slightly higher pKi-values for nebivolol on β1- compared to β2-adrenoceptors (Figure 2C,E, Table 1). The calculated β2/β1-ratio, expressing β1-selectivity, was 2.8±0.8 in the absence and 4.5±2.2 in the presence of Gpp(NH)p (Table 1). Slope factors were not affected by the presence of ICI 118.551 or CGP 207.12A, respectively. In contrast, in the majority of the experiments with bisoprolol in the presence of ICI 118.551 or CGP 207.12A, respectively, binding curves were converted into monophasic curves. The slope factors steepened significantly towards unity and the pKi-values were significantly different between β1- and β2-adrenoceptors (Table 1). The calculated β2/β1-ratio was 19.7±9.7 in the absence and 15.6±5.8 in the presence of Gpp(NH)p and significantly higher compared to nebivolol. In the presence of ICI 118.551 or CGP 207.12A, respectively, Gpp(NH)p neither affected binding characteristics of nebivolol nor of bisoprolol.
Another way of calculating β1-selectivity of a compound is to determine the dissociation constants of the high- and the low-affinity site in two-site fitted binding curves. Table 3 gives the results discriminated into experiments with the detection of two and experiments with the detection of only one affinity site. According to the calculation of β1-selectivity from the high- and low-affinity binding site, the β1-selectivity was substantially higher for both compounds compared to the calculation in Table 1. Again, bisoprolol displayed higher β1-selectivity in the absence (143 vs 76) as well as in the presence of Gpp(NH)p (242 vs 66) compared to nebivolol. However, due to the limited number of experiments in the nebivolol group, this difference was statistically not significant. Also, it has to be considered that nebivolol modelled for two-site binding in the minority and bisoprolol in the majority of experiments. Thus, these selectivity data do not represent the cumulative results of all experiments.
Table 3.
Radioligand binding experiments – subgroup analysis
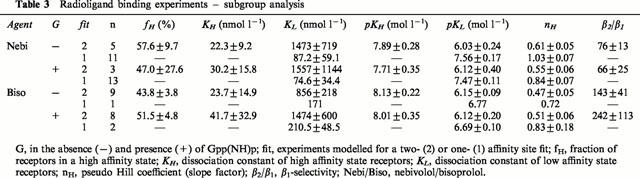
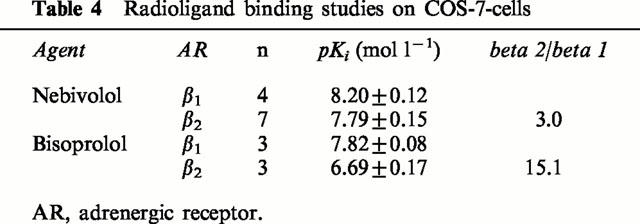
Table 4 displays the pKi-values from binding experiments in COS-7-cells transfected with human β1- and β2-adrenoceptors, respectively. The β2/β1-ratios for nebivolol (3.0) and bisoprolol (15.1) were in agreement with the selectivity ratios derived from experiments on human myocardium (Table 1).
Table 4.
Radioligand binding studies on COS-7-cells
Functional studies
In order to estimate the implications of β-adrenoceptor binding properties of nebivolol and bisoprolol on myocardial contractile function, experiments on left ventricular and right atrial muscle strips from failing and nonfailing hearts were performed.
In contraction experiments, muscles were stretched to the length at which force of contraction was maximal. In all experiments on ventricular as well as atrial myocardium, diastolic tension did not differ between different treatment groups (not shown). In human ventricular myocardium, the application of 1 μmol l−1 of isoprenaline enhanced basal force of contraction by 123±16% (n=11). After equilibration, cumulative concentrations of nebivolol or bisoprolol were added to the organ bath. There were no significant differences among the three groups in basal as well as in isoprenaline-enhanced force of contraction (Table 5). At concentrations occupying 50% (Ki) and 100% (100×Ki) of β-adrenoceptors, both β-blockers significantly antagonized isoprenaline enhanced force of contraction (Figure 3A,B).
Table 5.
Force of contraction of ventricular myocardium at baseline and after treatment
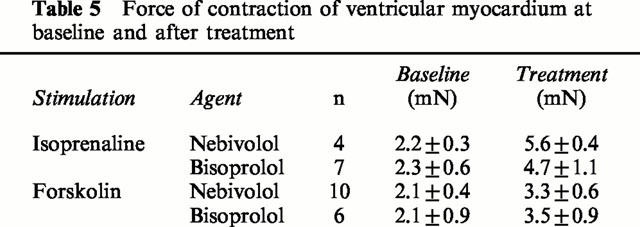
Figure 3.
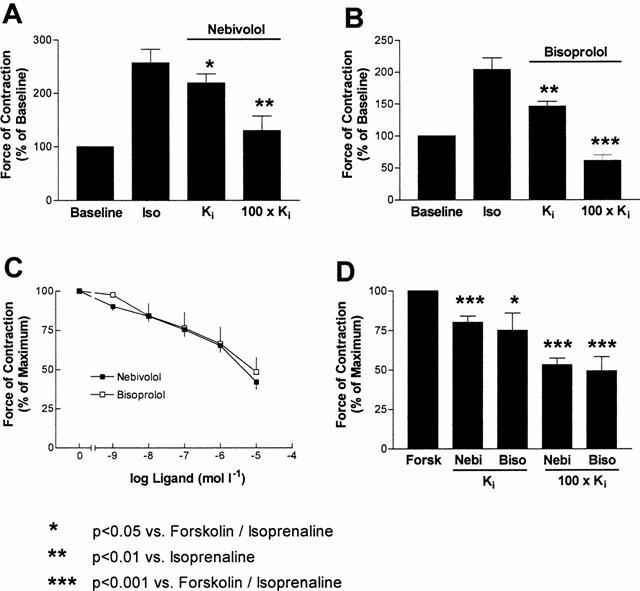
(A, B) Effects of nebivolol (n=4) and bisoprolol (n=7) on isoprenaline (1 μmol l−1) prestimulated force of contraction in human ventricular myocardium. Both β-blockers significantly inhibit the isoprenaline induced increase in force of contraction. (C) Cumulative dose-response curves of nebivolol (n=10) and bisoprolol (n=6) in the presence of forskolin (0.3 μmol l−1). (D) Inotropic effect of nebivolol and bisoprolol at concentrations occupying 50% (Ki) and 100% (100×Ki) of β-adrenoceptors. Data are derived from cumulative dose-response curves (C), related to the respective dissociation constants determined in radioligand binding experiments (Table 1). Concentrations are as follows: nebivolol, 0.03 (Ki) and 3 μmol l−1 (100×Ki); bisoprolol, 0.1 (Ki) and 10 μmol l−1 (100×Ki).
In order to determine the intrinsic activity of both agents, the coupling of β-adrenoceptors to adenylate cyclase was facilitated by forskolin (0.3 μmol l−1). By this method, both partial agonist (Böhm et al., 1990b) as well as inverse agonist activity (Mewes et al., 1993) of ligands can be detected. Forskolin increased force of contraction by 81±20% (n=16). Nebivolol and bisoprolol induced similar decreases of contractility at the cumulative concentrations (Figure 3C). Accordingly, when relating the decrease of contractility to the respective Ki-values, the decrease of contractility was not different between both agents (Figure 3D). Also in myocardium not pretreated with forskolin or isoprenaline, force of contraction was significantly reduced by both β-blockers (Figure 4).
Figure 4.
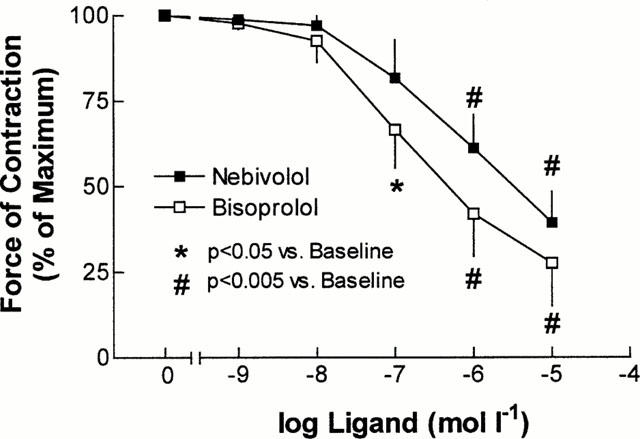
Effect of cumulative doses of nebivolol (n=10) and bisoprolol (n=6) on force of contraction in human atrial myocardium.
When examining intrinsic activity of β-adrenoceptor antagonists, it is of importance to compare various compounds within the same system, since intrinsic activity of ligands is strongly dependent on the underlying tissue type (de Ligt et al., 2000). Thus, we compared the intrinsic activity of nebivolol and bisoprolol to the one of bucindolol, carvedilol and metoprolol in human atrial myocardium. In Figure 5, the effects of all agents on contractility are compared at concentrations occupying 50% (Figure 5A) and 100% (Figure 5B) of β-adrenoceptors. At both conditions, bisoprolol and nebivolol displayed significantly higher amounts of inverse agonism compared to bucindolol and lower amounts compared to metoprolol. Carvedilol exerted slightly lower inverse agonism compared to nebivolol and bisoprolol. In general, β-blockers with Gpp(NH)p-modulatable binding properties, like bucindolol and carvedilol (Yoshikawa et al., 1996; Maack et al., 2000), displayed less inverse agonist activity than agents without these properties. A typical feature of inverse agonists is that their negative inotropic effect can be antagonized by a ligand with lower inverse agonist activity (ideally a neutral antagonist). In Figure 6 it is illustrated that in human myocardium pretreated with forskolin, the negative inotropic effect of nebivolol could be antagonized in the presence of bucindolol.
Figure 5.
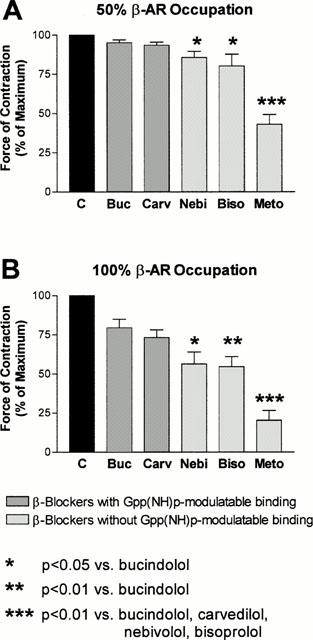
Inotropic effects of bucindolol (n=15), carvedilol (n=8), nebivolol (n=16), bisoprolol (n=12) and metoprolol (n=6) on human atrial myocardium in the presence of forskolin (0.3 μmol l−1) at concentrations occupying 50% (Ki, panel A) and 100% (100×Ki, panel B) of β-adrenoceptors. Ki-values are derived from radioligand binding studies of this study (Table 1, nebivolol and bisoprolol, see also legend to Figure 3) and from Maack et al. (2000) for bucindolol (0.01 (Ki) and 1 μmol l−1 (100×Ki)), carvedilol (0.001 (Ki) and 0.1 μmol l−1 (100×Ki)) and metoprolol (1 (Ki) and 100 μmol l−1 (100×Ki)). Force of contraction is related to the respective vehicle-control groups.
Figure 6.
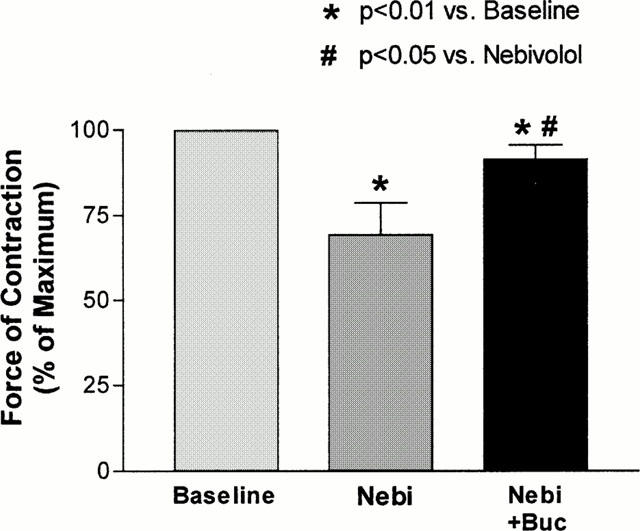
The negative inotropic effect of nebivolol (Nebi, 1 μmol l−1) in human atrial myocardium was antagonized in the presence of bucindolol (Buc, 0.1 μmol l−1). Experiments (n=5) were carried out in the presence of forskolin (0.3 μmol l−1).
Discussion
The main findings of the present study are that in human myocardium, nebivolol and bisoprolol behave as inverse agonists. The rank order of inverse agonism in human myocardium is metoprolol >>bisoprolol=nebivolol ⩾ carvedilol >bucindolol. Furthermore, nebivolol is nonselective for myocardial β1- and β2-adrenoceptors, while bisoprolol is 16 – 20 fold β1-selective.
Intrinsic activity
Determination of intrinsic activity in intact human myocardium is complicated by several mechanisms. In myocardium from patients with heart failure, β-adrenoceptor density is reduced (Bristow et al., 1982), and remaining receptors are uncoupled from GS (Hausdorff et al., 1990). In addition, increased membrane concentrations of inhibitory G-protein α-subunits (Böhm et al., 1990a) might also reduce partial agonist or inverse agonist responses. Therefore, contraction experiments were performed in the presence of forskolin, a diterpene that facilitates the coupling of GSα to adenylate cyclase. By this method, effects of partial agonists and also inverse agonists become detectable (Böhm et al., 1990b).
With this approach, in a recent study we determined intrinsic activity of bucindolol, carvedilol and metoprolol (Maack et al., 2000). In the mentioned study, metoprolol behaved as a strong inverse agonist, while bucindolol and carvedilol displayed Gpp(NH)p-modulatable binding and significantly lesser amounts of inverse agonist activity compared to metoprolol. In a part of the experiments, bucindolol even behaved as a partial agonist. Since in some studies (Willette et al., 1998; 1999; Trochu et al., 1999; Maack et al., 2000) bucindolol had revealed partial agonist effects, yet not in other studies (Hershberger et al., 1990; Bristow et al., 1992), intrinsic activity of β-blockers can be system-dependent.
For nebivolol, in a study on reserpinized dogs and spontaneously hypertensive rats, no partial agonist activity could be detected (Janssens et al., 1989). In the pithed rat model of normal and heart failure rats, bisoprolol also had no partial agonist activity (Willette et al., 1999). In the present study, contraction experiments in human myocardium were performed in the presence of forskolin. Stimulation of adenylate cyclase by forskolin is dependent on the presence of GSα (Darfler et al., 1982), but the dissociation of GSα from the βγ subunits of the G-protein only occurs when the receptor is in an activated conformation (R*). The negative inotropic actions of nebivolol and bisoprolol in the presence of forskolin may reflect a reduced GS-mediated stimulation of adenylate cyclase and thus indicate that both β-blockers are inverse agonists that primarily stabilize the inactive conformation of the β-adrenoceptor (R). Accordingly, neither nebivolol nor bisoprolol induce a dissociation of GSα from the βγ-subunits as estimated by radioligand binding experiments in the absence and presence of Gpp(NH)p.
When examining inverse agonist activity of β-blockers in intact tissue, it is of importance to rule out that the negative inotropic effects of the compounds are due to the presence of contaminating endogenous catecholamines. This can be done by performing contraction experiments in the presence of a rather weak inverse agonist (ideally a ‘neutral antagonist'). In our experiments, the negative inotropic effect of nebivolol could be specifically antagonized in the presence of bucindolol. This further indicates that the negative inotropic effect of nebivolol can be related to its action on β-adrenoceptors and is not due to a decrease of myofilamental calcium sensitivity (Zeitz et al., 2000) or non-specific effects.
The activation state of β-adrenoceptors has an impact on receptor regulation. Benovic et al. (1988) observed a correlation of the intrinsic activity of partial and full agonists with the degree of receptor phosphorylation by β-adrenergic receptor kinase (β-ARK), which is a prerequisite for receptor desensitization and downregulation (Hausdorff et al., 1990). In mice transfected with a constitutively active mutant of the β2-adrenoceptor, treatment with the inverse agonist ICI 118.551 led to a 50 fold upregulation of myocardial β-adrenoceptor density (Samana et al., 1997). According to these data, it may be hypothesized that in patients with heart failure, phosphorylation of the β-adrenoceptor by β-ARK and subsequent desensitization or even down-regulation might be effectively prevented by nebivolol and bisoprolol. In fact, in patients undergoing coronary artery bypass grafting, chronic treatment with bisoprolol increased myocardial β1-adrenoceptor density (Motomura et al., 1990). For nebivolol, no data concerning its effects on β-adrenoceptor regulation exist.
β1-selectivity
Another important feature of β-blockers is selectivity for β1-adrenoceptors. Nebivolol is frequently termed a β1-selective compound. In fact, in a study of van de Water et al. (1988) nebivolol revealed a 293 fold β1-selectivity. However, in that study dissociation constants were determined by Schild plot analysis of inhibitory effects of nebivolol on β-adrenergic responsiveness of right atrial (β1) vs tracheal (β2) tissue in guinea-pigs. Pauwels et al. (1989) performed radioligand binding studies on rabbit lung (β1-) and rat lung (β2-) adrenoceptors and observed a 48 – 55 fold β1-selectivity of nebivolol. In CHO-cells transfected with human β1- and β2-adrenoceptors, the same group (Pauwels et al., 1991) found only a 10 fold β1-selectivity of nebivolol assessed by radioligand binding studies. In the present study, in human ventricular myocardium β1-selectivity of nebivolol was 2 – 4 fold, which is more or less non-selective. Also in COS-7 cells transfected with human β1- and β2-adrenoceptors, selectivity was only 3 fold. In contrast, the β1-selectivity of bisoprolol was 16 – 20 fold in human myocardium and 15 fold in COS-7 cells.
The importance of β1-selectivity in the treatment of patients with heart failure is controversial. Both the β1-selective agents bisoprolol (CIBIS II, 1999) and metoprolol (MERIT-HF, 1999) as well as the non-selective compound carvedilol (Packer et al., 1996) have improved survival in heart failure. However, since carvedilol has also α-blocking (Bristow et al., 1992) and antioxidative effects (Yue et al., 1992), it is difficult to discriminate which pharmacological properties lead to its beneficial effects in the treatment of patients with heart failure. Accumulated evidence from experimental in vivo and in vitro studies indicate that a blockade of all adrenergic signal transduction pathways may be of particular importance in the treatment of heart failure (Bristow, 2000). In two meta-analyses of the effects of β-blockers on mortality in patients after myocardial infarction, both selective and non-selective compounds reduced mortality (Soriano et al., 1997; Freemantle et al., 1999). In contrast, agents with intrinsic sympathomimetic activity (ISA) were associated with a lower risk-reduction compared to agents without ISA. These data indicate that among different features of β-blockers, especially intrinsic activity, but not β1-selectivity is important in terms of prognosis in cardiovascular diseases.
Besides β-adrenoceptor antagonistic effects, nebivolol has vasodilatory effects mediated by the endothelial release of nitric oxide (Bowman et al., 1994; Cockcroft et al., 1995). A recent study indicates that these effects are at least in part due to active metabolites of nebivolol that may act via the stimulation of endothelial β2-adrenergic receptors (Broeders et al., 2000). Thus, in vivo effects of nebivolol may be slightly different compared to the in vitro effects. However, the present study investigates intrinsic activity of β-blockers independent of haemodynamically confounding effects on the vasculature. In this respect, these experiments might possess some advantage compared with in vivo experiments.
The present data for the first time characterize the β-adrenergic effects of nebivolol in human myocardium. The lack of G-protein interaction as well as inverse agonist activity are desirable effects of a β-blocker in the treatment of heart failure. The observation that in human myocardium, nebivolol is non-selective sheds new light on the pharmacological actions of this agent. Further studies are needed to clearify the special haemodynamic profile of nebivolol.
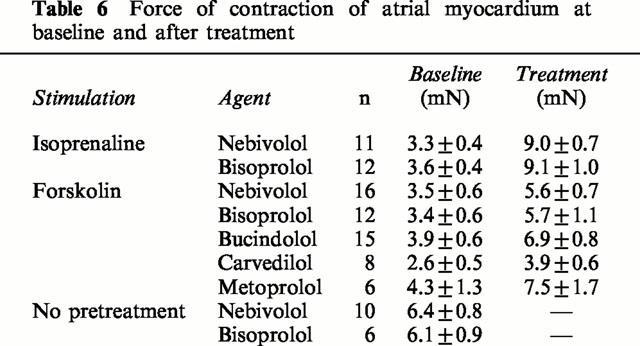
Table 6.
Force of contraction of atrial myocardium at baseline and after treatment
Acknowledgments
Experimental work was supported by the Deutsche Forschungsgemeinschaft (to M. Böhm) and by Berlin Chemie, Berlin, Germany. This work contains parts of the Doctoral Thesis of S. Tyroller (University of Cologne).
Abbreviations
- β-AR
β-adrenergic receptor
- EC50
concentration achieving 50% of maximum effect
- FOC
force of contraction
- Gpp(NH)p
guanylylimidodiphosphate
- GS(α)
stimulatory G-protein (α-subunit)
- ICYP
[125I]-Iodocyanopindolol
- ISA
intrinsic sympathomimetic activity
- Kd, Ki
dissociation constant
- NF
nonfailing
- NO
nitric oxide
- nH
pseudo Hill coefficient (slope factor)
- NYHA
New York Heart Association
- RH
receptors in a high-affinity state
- KH, KL
dissociation constant of receptors in a high (KH) and low affinity (KL) state
References
- BENOVIC J.L., STANISZEWSKI C., MAYOR F., CARON M.G., LEFKOWITZ R.J. β-Adrenergic receptor kinase. J. Biol. Chem. 1988;263:3893–3897. [PubMed] [Google Scholar]
- BÖHM M., GIERSCHIK P., JAKOBS K.-H., PIESKE B., SCHNABEL P., UNGERER M., ERDMANN E. Increase of Giα in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990a;82:1249–1265. doi: 10.1161/01.cir.82.4.1249. [DOI] [PubMed] [Google Scholar]
- BÖHM M., MITTMANN C., SCHWINGER R.H.G., ERDMANN E. Effects of xamoterol on inotropic and lusitropic properties of the human myocardium and on adenylate cyclase activity. Am. Heart J. 1990b;120:1381–1392. doi: 10.1016/0002-8703(90)90252-s. [DOI] [PubMed] [Google Scholar]
- BOND R.A., LEFF P., JOHNSON T.D., MILANO C.A., ROCKMAN H.A., MCMINN T.R., APPARSUNDARAM S., HYEK M.F., KENAKIN T.P., ALLEN L.F., LEFKOWITZ R.J. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- BOWMAN A.J., CHEN C.P., FORD G.A. Nitric oxide mediated venodilator effects of nebivolol. Br. J. Clin. Pharmacol. 1994;38:199–204. doi: 10.1111/j.1365-2125.1994.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRISTOW M.R. β-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., GINSBURG R., MINOBE W., CUBICIOTTI R.S., SAGEMAN W.S., LURIE K., BILLINGHAM M.E., HARRISON D.E., STINSON E.B. Decreased catecholamine sensitivity and β-adrenergic receptor density in failing human hearts. N. Engl. J. Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., LARRABEE P., MINOBE W., RODEN R., SKERL L., KLEIN J., HANDWERGER D., PORT J.D., MÜLLER-BECKMANN B. Receptor pharmacology of carvedilol in the human heart. J. Cardiovasc. Pharmacol. 1992;19 Suppl. 1:S68–S80. doi: 10.1097/00005344-199219001-00014. [DOI] [PubMed] [Google Scholar]
- BROEDERS M.A.W., DOEVENDANS P.A., BEKKERS B.C.A.M., BRONSAER R., VAN GORSEL E., HEEMSKERK J.W.M., OUDE EGBRINK M.G.A., VAN BREDA E., RENEMAN R.S., VAN DER ZEE R. Nebivolol: A third-generation β-blocker that augments vascular nitric oxide release. Circulation. 2000;102:677–684. doi: 10.1161/01.cir.102.6.677. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzyme reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIDIAC P., NOUET S., BOUVIER M. Agonist-induced modulation of inverse agonist efficacy at the β2-adrenergic receptor. Mol. Pharmacol. 1996;50:662–669. [PubMed] [Google Scholar]
- CIBIS II INVESTIGATORS AND COMMITTEES The Cardiac Insufficiency Bisoprolol Study (CIBIS-II): a randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- COCKCROFT J.R., CHOWIENCZYK P.J., BRETT S.E., CHEN C.P., DUPONT A.G., VAN NUETEN L., WOODING S.J., RITTER J.M. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J. Pharmacol. Exp. Ther. 1995;274:1067–1071. [PubMed] [Google Scholar]
- DARFLER F.J., MAHAN L.C., KOACHMAN A.M., INSEL P.A. Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J. Biol. Chem. 1982;257:11901–11907. [PubMed] [Google Scholar]
- DE LIGT R.A.F., KOUROUNAKIS A.P., IJZERMAN A.P. Inverse agonism at G-protein-coupled receptors: (patho-)physiological relevance and implications for drug discovery. Br. J. Pharmacol. 2000;130:1–12. doi: 10.1038/sj.bjp.0703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMANTLE N., CLELAND J., YOUNG P., MASON J., HARRISON J. β-Blockade after myocardial infarction: systematic review and meta regression analysis. Br. J. Med. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSDORFF W.P., CARON M.G., LEFKOWITZ R.J. Turning off the signal: Desensitization of β-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- HERSHBERGER R.E., WYNN J.R., SUNDBERG L., BRISTOW M.R. Mechanisms of action of bucindolol in human ventricular myocardium. J. Cardiovasc. Pharmacol. 1990;15:959–967. doi: 10.1097/00005344-199006000-00014. [DOI] [PubMed] [Google Scholar]
- JANSSENS W.J., VAN DE WATER A., XHONNEUX R., RENEMAN R.S., VAN NUETEN J.M., JANSSEN P.A. Nebivolol is devoid of intrinsic sympathomimetic activity. Eur. J. Pharmacol. 1989;159:89–95. doi: 10.1016/0014-2999(89)90047-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951. pp. 265–275. [PubMed]
- MAACK C., CREMERS B., FLESCH M., HÖPER A., SÜDKAMP M., BÖHM M. Different intrinsic activities of bucindolol, carvedilol and metoprolol in human failing myocardium. Br. J. Pharmacol. 2000;130:1131–1139. doi: 10.1038/sj.bjp.0703400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANGRELLA M., ROSSI F., FICI F., ROSSI F. Pharmacology of nebivolol. Pharmacol. Res. 1998;38:419–431. doi: 10.1006/phrs.1998.0387. [DOI] [PubMed] [Google Scholar]
- MERIT-HF STUDY GROUP Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- MEWES T., DUTZ S., RAVENS U., JAKOBS K.H. Activation of calcium currents in cardiac myocytes by empty beta-adrenoceptors. Circulation. 1993;88:2916–2922. doi: 10.1161/01.cir.88.6.2916. [DOI] [PubMed] [Google Scholar]
- MOTOMURA S., DEIGHTON N.M., ZERKOWSKI H.R., KHAMSSI M., BRODDE O.E. Differential regulation of human cardiac beta-adrenergic and muscarinic receptors by chronic beta-adrenoceptor antagonist treatment. Br. J. Clin. Pharmacol. 1990;30 Suppl. 1:112S–114S. doi: 10.1111/j.1365-2125.1990.tb05478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLAS G., OAKLEY C., POULEUR H., ROUSSEAU M.F., RYDÉN L.E., WELLENS H. , for The Xamoterol In Heart Failure Study Group Xamoterol in severe heart failure. Lancet. 1990;336:1–6. [Google Scholar]
- PACKER M., BRISTOW M.R., COHN J.N., COLUCCI W., FOWLER M.B., GILBERT E.M., SHUSTERMAN N.H. , for the US Carvedilol Heart Failure Study Group The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., GOMMEREN W., VAN LOMMEN G., JANSSEN P.A.J., LEYSEN J.E. The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various β-adrenergic blockers. Mol. Pharmacol. 1989;34:843–851. [PubMed] [Google Scholar]
- PAUWELS P.J., VAN GOMPEL P., LEYSEN J.E. Human β1- and β2-adrenergic receptor binding and mediated accumulation of cAMP in transfected chinese hamster ovary cells. Biochem. Pharmacol. 1991;42:1683–1689. doi: 10.1016/0006-2952(91)90502-v. [DOI] [PubMed] [Google Scholar]
- SAMANA P., COTECCHIA S., COSTA T., LEFKOWITZ R.J. A mutation-induced activated state of the β2-adrenergic receptor. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- SCHNABEL P., CAMPS M., CAROZZI A., PARKER P.J., GIERSCHIK P. Mutational analysis of phospholipase C-β 2: Identification of regions required for membrane association and stimulation by G-protein βγ subunits. Eur. J. Biochem. 1993;217:1109–1115. doi: 10.1111/j.1432-1033.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- SORIANO J.B., HOES A.W., MEEMS L., GROBBEE D.E. Increased survival with β-blockers: importance of ancillary properties. Prog. Cardiovasc. Dis. 1997;39:445–456. doi: 10.1016/s0033-0620(97)80039-4. [DOI] [PubMed] [Google Scholar]
- STOLERU L., WIJNS W., VAN EYLL C., BOUVY T., VAN NUETEN L., POULEUR H. Effects of d-nebivolol and I-nebivolol on left ventricular systolic and diastolic function: comparison with d-l-nebivolol and atenolol. J. Cardiovasc. Pharmacol. 1993;22:183–190. doi: 10.1097/00005344-199308000-00002. [DOI] [PubMed] [Google Scholar]
- TROCHU J.N., ERFANIAN M., KHANDOUDI N., BARON O., BRIL A., GAUTHIER C.Carvedilol produces contractile effects different from those of bucindolol in human atria Circulation 19991001–439.(abstract) [Google Scholar]
- VAN DE WATER A., JANSSENS W., VAN NEUTEN J., XHONNEUX R., DE CREE J., VERHAEGEN H., RENEMAN R.S., JANSSEN P.A. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective β1-adrenergic receptor antagonist. J. Cardiovasc. Pharmacol. 1988;11:552–563. doi: 10.1097/00005344-198805000-00007. [DOI] [PubMed] [Google Scholar]
- WILLETTE R.N., AIYAR N., YUE T.L., MITCHELL M.P., DISA J., STORER B.L., NASELSKY D.P., STADEL J.M., OHLSTEIN E.H., RUFFOLO R.R., Jr In vitro and in vivo characterization of intrinsic sympathomimetic activity in normal and heart failure rats. J. Pharmacol. Exper. Therap. 1999;289:48–53. [PubMed] [Google Scholar]
- WILLETTE R.N., MITCHELL M.P., OHLSTEIN E.H., LUKAS M.A., RUFFOLO R.R., JR Evaluation of intrinsic sympathomimetic activity of bucindolol and carvedilol in rat heart. Pharmacology. 1998;56:30–36. doi: 10.1159/000028179. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA T., PORT J.O., ASANO K., CHIDIAC P., BOUVIER M. , DUTCHER D., RODEN R.L., MINABE W., TREMMEL K.O., BRISTOW M.R. Cardiac adrenergic receptor effects of carvedilol. Eur. Heart J. 1996;17 Suppl. B:8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]
- YUE T.L., CHENG H.Y., LYSKO P.G., MCKENNA P.J., FEUERSTEIN R., GU J.L., LYSKO K.A., DAVIS L.L., FEUERSTEIN G. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J. Pharmacol. Exp. Ther. 1992;263:92–98. [PubMed] [Google Scholar]
- ZEITZ O., RAHMAN A., HASENFUSS G., JANSSEN P.M. Impact of beta-adrenoceptor antagonists on myofilament calcium sensitivity of rabbit and human myocardium. J. Cardiovasc. Pharmacol. 2000;36:126–131. doi: 10.1097/00005344-200007000-00017. [DOI] [PubMed] [Google Scholar]


