Abstract
We tested the effects of 11 commercially-available isoprostanes on platelet aggregation directly or when triggered by the thromboxane receptor agonist U46619 or collagen in healthy human citrated blood using a whole blood aggregometer.
None of the isoprostanes tested triggered aggregation alone, nor facilitated aggregation by a sub-threshold dose of U46619 or collagen. Five isoprostanes inhibited aggregation (rank order of potency 8-iso PGE1>8-iso PGE2>8-iso PGF2α>8-iso PGF3α>8-iso-13,14-dihydro-15-keto PGF2α).
Blood incubated with LPS to induce a gross inflammatory response exhibited a time dependent (2 – 12 h) reduction in aggregation to U46619 but maintained a consistent response to collagen. Under these conditions, as in control blood, none of the isoprostanes tested induced aggregation. In fact, the inhibitory actions of isoprostanes on U46619-induced aggregation were enhanced in blood treated with LPS.
L-NAME inhibited aggregation induced by U46619 in fresh blood and in blood treated with LPS. In the presence of L-NAME, (with or without LPS) none of the isoprostanes tested induced aggregation but retained their inhibitory action.
Thus, in human whole blood the action of 8-iso PGE1, 8-iso PGE2, 8-iso PGF2α, 8-iso PGF3α, and 8-iso-13,14-dihydro-15-keto PGF2α is antiaggregatory. Moreover, this inhibitory capacity is still apparent and may be enhanced in blood subjected to inflammatory stimulation.
Keywords: Isoprostanes, whole blood aggregometry, lipopolysaccharide
Introduction
Isoprostanes are a family of prostaglandin (PG) isomers generated by free radical-catalysed peroxidation and then rearrangement of arachidonic acid (Morrow et al., 1990; 1992). In addition, isoprostanes can be produced by cyclo-oxygenase (COX) dependent pathways (Pratico et al., 1995; Klein et al., 1997; Jourdan et al., 1997a; 1999). Several cell types have been shown to release increased levels of the F2 isoprostane, 8-iso PGF2α, when stimulated with inflammatory mediators or subjected to oxidative stress in vitro (Patrignani et al., 1996; Vacchiano et al., 1998; Jourdan et al., 1999). Furthermore, 8-iso PGF2α levels are elevated in rats in vivo when prooxidant conditions are induced experimentally (Morrow et al., 1992b; Dabbagh et al., 1994; Awad et al., 1994; Lynch et al., 1996). Similarly, elevated isoprostane production has been demonstrated in clinical conditions characterized by oxidant stress including diabetes (Davi et al., 1999), alcoholism (Alehnik et al., 1998), paraquat poisoning (Delanty et al., 1996), myocardial reperfusion (Reilly et al., 1997), critical illness (Carpenter et al., 1998), pre-eclampsia (Walsh et al., 2000) and smoking (Morrow et al., 1995). Consequently, the measurement of 8-iso PGF2α in plasma and urine has been proposed as a relevant and convenient method to monitor oxidant stress in man (Roberts & Morrow, 2000).
However, beyond their role as oxidant markers, isoprostanes have demonstrable biological actions in some tissues. 8-iso PGF2α causes contraction of isolated blood vessels, (Kromer & Tippins, 1996; Jourdan et al., 1997b; Gardan et al., 2000; Oliviera et al., 2000), lymphatics, (Sinzinger et al., 1997) and airway (Kawikova et al., 1996) and myometrial smooth muscle (Crankshaw, 1995). Where studied, smooth muscle contraction appears to be mediated in part via thromboxane TP receptors (Fukunaga et al., 1993; Kromer & Tippins, 1996; Jourdan et al., 1997b; Gardan et al., 2000; Oliviera et al., 2000). However, the effects of 8-iso PGF2α on smooth muscle function may be altered in pathophysiological settings. Thus, its vasoconstrictor effect is increased after ischaemia (Kromer & Tippins, 1996; 1999), endothelial damage and nitric oxide synthase (NOS) inhibition (Jourdan et al., 1997b; Sametz et al., 1999).
8-iso PGF2α also has actions on isolated platelets where, in contrast to smooth muscle preparations, it may not apparently fully activate platelet TP receptors to cause aggregation (Pratico et al., 1996; Habib et al., 1999) and instead inhibits the proaggregatory effects of the TP ligand U46619 (Morrow et al., 1992c; Yin et al., 1994). In addition, although the E2 isoprostane, 8-iso PGE2, causes aggregation of platelets in a sub-population of human volunteers, it also inhibits U46619-induced aggregation (Longmire et al., 1994). Whether or not pathophysiological events alter platelet responses to isoprostanes is not known.
In addition to 8-iso PGF2α and 8-iso PGE2, a range of synthetic isoprostanes is now available, the majority of which have not been tested in either smooth muscle or platelet preparations. The aims of this study were therefore firstly, to investigate the effects of a range of isoprostanes on aggregation of human whole blood and secondly, to assess the effects of a gross inflammatory stimulus induced by bacterial lipopolysaccharide (LPS), on the response of whole blood to isoprostanes.
Methods
The study protocol was approved by the Ethics Committee of the Royal Brompton and Harefield NHS Trust.
Measurement of aggregation in human whole blood
Venous blood was taken in glass tubes containing sodium citrate (1.05×10−1 M) from 20 healthy female and 20 male volunteers aged 20 – 40 years none of whom were taking medication. Volunteers who had taken any non-steroidal anti-inflammatory drug within 1 month were excluded from the study. Blood was rolled gently at 37°C whilst awaiting analysis. In preliminary experiments, aggregation induced by U46619 decreased significantly in blood maintained as above for more than 4 h and therefore samples were analysed within this time except where stated. Whole blood impedance aggregometry of 1 ml aliquots was performed in a CHRONO-LOG 560 aggregometer (Chronolog Corporation, Haverton, PA, U.S.A.) with a stir bar revolution rate of 8000 r.p.m. Aggregatory responses were monitored for 15 min after addition of stimuli and the maximum response in this time recorded.
Measurement of the proaggregatory effects of U46619, collagen or isoprostanes on aggregation in human blood
U46619 (10−8 – 10−5 M), collagen (1 – 10 μg ml−1) or isoprostanes (10−7 – 10−5 M) were added to blood samples in 5 μl aliquots and aggregation monitored as above. The vehicles used, ethanol or methylacetate at 0.125% (by volume) or less, had no effect on aggregation. In separate experiments a sub-threshold concentration of U46619, which was independently determined for each blood sample (range 1 – 4×10−7 M), was added 1 min after the addition of isoprostane and aggregation monitored as above.
Measurement of antiaggregatory effects of isoprostanes or the TP-receptor antagonists, SQ29458 on U46619 and collagen
To assess their inhibitory capacity, blood was incubated with isoprostanes (10−5 M) for 15 – 30 min before stimulation by U46619 (10−6 M) or collagen (5 μg ml−1). Where an inhibitory effect of isoprostanes (10−5 M) was observed further experiments were conducted using a concentration range of 10−9 – 10−5 M. In separate experiments, the TP receptor antagonist SQ29458, (10−9 – 10−7 M) was added to blood samples for 15 – 30 min, before the addition of U46619. Aggregation was then monitored for a further 15 min. The vehicles had no inhibitory effect on aggregation in any of the protocols used.
Effect of LPS treatment on whole blood aggregation responses to isoprostanes
Blood was incubated with or without LPS (10 μg ml−1) for 2, 4, 6, 8 and 12 h prior to stimulation with a sub-maximal concentration (5×10−7 M) of U46619 or collagen (5 μg ml−1). To verify that cellular components of the blood did not decrease significantly over the incubation period, samples were analysed for red cell, white cell and platelet numbers μl−1 before each experiment in a clinical blood cell counter (Hematology System Advia 120, Bayer, Newbury, Berkshire, U.K.). Aggregatory and inhibitory effects of isoprostanes (10−6 M) were re-examined in untreated and LPS-treated blood at a time (approximately 6 h) when LPS treatment induced maximal changes in blood aggregation.
Measurement of the effect of L-NAME on whole blood responses to isoprostanes
The effect of NOS inhibition on the ability of blood to aggregate in response to U46619 (5×10−7 M) was assessed using the inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) (10−4 – 10−2 M). Non-specific effects of this compound were determined by comparing its responses with those of the inactive analogue, Nω-nitro-D-arginine methyl ester (D-NAME) over the same concentration range. L-NAME and D-NAME were added 30 min before the addition of isoprostanes, to fresh whole blood or blood incubated for 6 h in the presence or absence of LPS.
Materials
Isoprostanes U46619 (11α, 9α, epoxymethano PGH2) and SQ29548 (1S-[1α,2β (5Z),3β,4α]-7-[3-2[[(phenylamino) carbonylhydrazino] methyl]-7-oxobicyclo [2.2.1]-hept-2-yl]-5-heptenoic acid) (Cayman Chemicals, Ann Arbor, MI, U.S.A.) were dissolved in ethanol (Sigma, Poole, Dorset, U.K.) or methylacetate (Fisher Scientific U.K., Loughborough, Leicestershire, U.K.) if provided in this solvent (8-iso PGF3a and 8-iso 13,14-dihydro-15-keto-PGF2α) and diluted with distilled water before experiments. Nω-nitro-L-arginine methyl ester, Nω-nitro-D-arginine methyl ester and lipopolysaccharide from E. coli 0127:B8 were dissolved in phosphate buffered saline (all from Sigma, Poole, Dorset, U.K.) Equine collagen solution was purchased from the Chronolog Corporation (Chronolog Corporation, Haverton, PA, U.S.A.)
Statistical tests
Results were analysed using a statistical package from GraphPAD Instat software and presented using GraphPAD PRISM (GraphPAD Software, San Antonio, CA, U.S.A.) The relevant test used is described in each figure legend. Results are presented as means±s.e.m. and P values <0.05 were considered to be statistically significant.
Results
Effects of U46619, collagen or isoprostanes on aggregation in human whole blood
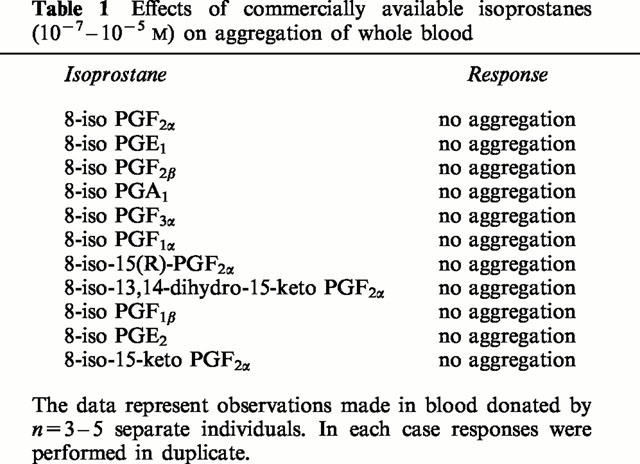
U46619 induced concentration-dependent aggregation of human whole blood with a threshold concentration of 10−7 M, an EC50 of approximately 3×10−7 M and a maximum response of 15.2±0.6 Ω (Figure 1). Collagen also induced aggregation with a maximal effect observed at 5 μg ml−1 of 21±0.8 Ω. By contrast, none of the isoprostanes tested over the concentration range 10−7 – 10−5 M induced detectable aggregation. In addition, when the isoprostanes (10−6 M) were added immediately before a sub-threshold concentration of U46619 no proaggregatory response was observed (Table 1).
Figure 1.
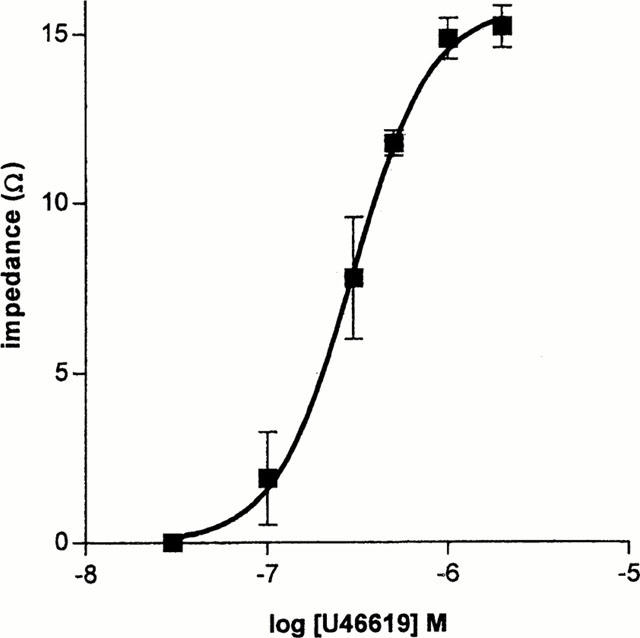
Concentration-aggregation response curve of whole blood aggregation to U46619 in fresh blood of n=9 donors. Each determination was performed in duplicate.
Table 1.
Effects of commercially available isoprostanes (10−7 – 10−5 M) on aggregation of whole blood
Apparent role of TP receptors in aggregation induced by U46619 or collagen
The proaggregatory effects of U46619 (10−6 M) were completely blocked by pretreatment with SQ29548 (10−7 M). By contrast, the proaggregatory effects of collagen (5 μg ml−1) were reduced (by 50%±10%; n=5), but not blocked, by SQ29548.
Effect of isoprostanes on aggregation in human whole blood induced by U46619 or collagen
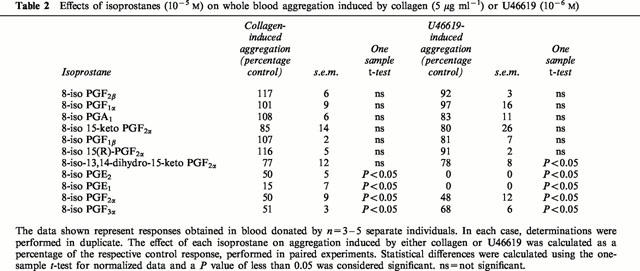
8-iso PGF2α, 8-iso PGE1, 8-iso PGE2 and 8-iso PGF3α induced consistent, concentration-dependent inhibition of U46619 (10−6 M) (Figure 2). In addition to a concentration inhibition of aggregation induced by U46619 these compounds also inhibited collagen (5 μg ml−1) induced aggregation (Table 2). By contrast, 8-iso-13,14-dihydro-15-keto PGF2α produced weak inhibition of aggregation induced by U46619 or collagen and caused no reduction in aggregation in two out of five donors. No other isoprostane tested produced significant inhibition of aggregation (Table 2).
Figure 2.
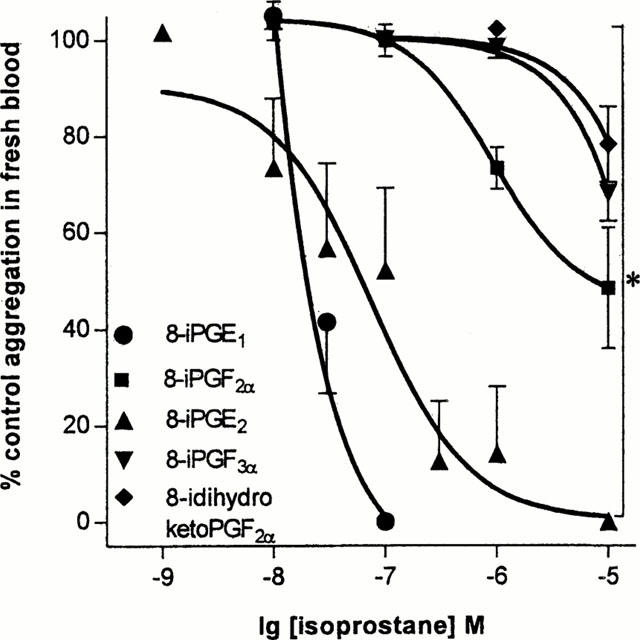
Effects of isoprostanes on aggregation induced by U46619 (10−6 M). The data represents single responses in blood donated by n of five to nine separate individuals. *Illustrates a statistical difference between the effects of either 8-iso (8-i) PGE1 or 8-iso (8-i) PGE2 and the other isoprostanes shown, calculated by two-way ANOVA. A P value of less than 0.05 was taken to be significant.
Table 2.
Effects of isoprostanes (10−5 M) on whole blood aggregation induced by collagen (5 μg ml−1) or U46619 (10−6 M)
Effect of LPS on aggregatory responses to U46619 or collagen in human whole blood
Erythrocyte (μl−1), white cell (μl−1) and platelet counts (μl−1) were not significantly affected by incubation at 37°C over 12 h with or without LPS (10 μg ml−1) (one way ANOVA P>0.05). Equally there was no difference between cell counts in control blood and blood incubated with LPS at 2, 4, 6, 8 and 12 h (two-way ANOVA P>0.05; n=5 donors; single experiments; data not shown). However, when blood was incubated at 37°C there was a time dependent (2 – 12 h) reduction in the proaggregatory responses induced by U46619 (10−6 M), an effect that was enhanced by LPS (10 μg ml−1) (Figure 3). The greatest effect on aggregation induced by LPS was observed at 6 h of incubation. By contrast, the proaggregatory effects of collagen (5 μg ml−1) were not modified by time of incubation (up to 12 h), either in the presence or absence of LPS.
Figure 3.
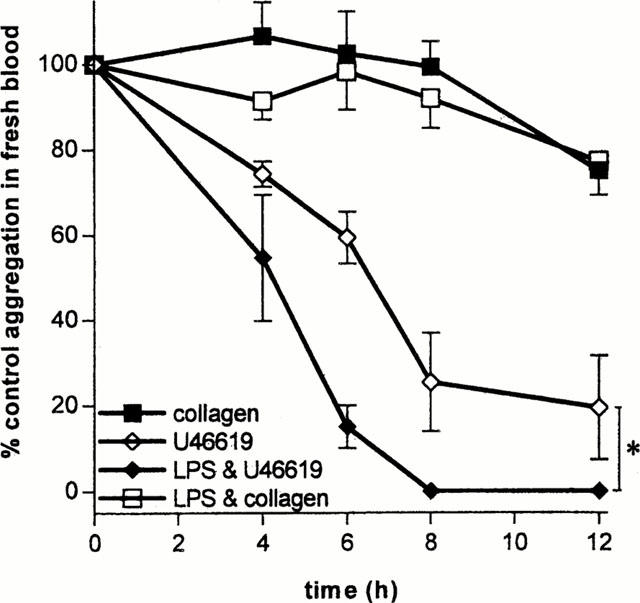
Effects of incubation time (2 – 12 h) with LPS on aggregation of whole blood induced by collagen (5 μg ml−1) or U46619 (5×10−7 M). The data shows responses, performed in parallel, of blood incubated with or without LPS and then stimulated with aggregatory agent. Each set of single responses was performed using blood donated by n of five separate individuals. *Illustrates a statistical difference in the responses of blood treated with LPS compared to control calculated using two-way ANOVA. A P value of less than 0.05 was taken to be significant.
Effect of isoprostanes on aggregation in human whole blood stimulated with LPS
After 6 h incubation with LPS, none of the isoprostanes were proaggregatory in whole blood, nor did they enhance the proaggregatory effects of a sub-maximal concentration of U46619 (n=3 – 5 donors; duplicate determinations; data not shown). In fact, under these conditions, as with non-LPS-stimulated blood, 8-iso PGF2α, (10−6 M) (Figure 4) as well as 8-iso PGE1, 8-iso PGE2, 8-iso PGF3α, and 8-iso-13,14-dihydro-15-keto PGF2α inhibited aggregation induced by U46619 (n=3 – 5 donors; single determinations paired with non-LPS-stimulated blood; data not shown). Interestingly, the inhibitory effect of 8-iso PGF2α (10−6 M) (Figure 4), and the other isoprostanes (data not shown) was enhanced by stimulation of blood with LPS.
Figure 4.
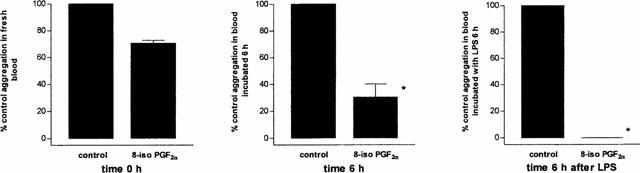
Effect of LPS (10 μg ml−1) on the antiaggregatory responses of 8-iso PGF2α (10−6 M) in U46619 (5×10−7 M) stimulated whole blood. Responses are shown in untreated blood after (a) collection without storage or (b) after storage for 6 h. For comparison, responses are shown in (c) in blood stimulated with LPS for 6 h. The data is represented as percentage of control response obtained in each condition in blood stimulated with U46619 alone. Each data point comprises data from n of five separate donors. Experiments illustrated in (a), (b) or (c) were performed in parallel. *Illustrates a significant difference between aggregation in comparison to control response in non-LPS-treated blood using two-way ANOVA. A P value of less than 0.05 was taken to be significant.
Effect of L-NAME on human whole blood
L-NAME (10−4 – 10−2 M) had an inhibitory effect on aggregation induced by U46619 (5×10−7 M) in fresh whole blood and blood incubated with LPS (10 μg ml−1) (data not shown). D-NAME was also inhibitory at 10−2 M, but inhibition was specific to L-NAME at concentrations between 10−4 and 10−3 M. Further, neither 8-iso PGF2α, 8-iso PGE1, 8-iso PGE2, 8-iso PGF3α, nor 8-iso-13,14-dihydro-15-keto PGF2α (10−6 M), induced aggregation in the presence or absence of a sub-threshold concentration of U46619 in blood containing L-NAME (10−3 M) nor in blood incubated with LPS (10 μg ml−1) and then treated with L-NAME (10−3 M) (data not shown).
Discussion
The isoprostanes are commonly described as inert markers of oxidative damage. However, their PG-like structure clearly implicates them as potential biological mediators of inflammatory responses. We have shown that a number of structurally distinct isoprostanes inhibit human whole blood aggregation, an effect enhanced under proinflammatory conditions.
In the current study 8-iso PGF2α inhibited whole blood aggregation stimulated by U46619 or collagen. This observation is in agreement with others showing that 8-iso PGF2α inhibits platelet aggregation stimulated with TP-agonists (Yin et al., 1994; Morrow et al., 1992c). Indeed, there is a growing body of evidence that illustrates interactions between 8-iso PGF2α and TP receptors. In human platelets 8-iso PGF2α binds to TP receptors, although its interaction at these sites appears complex and has not been fully defined (Pratico et al., 1996; Minuz et al., 1998; Habib et al., 1999). Using platelet-rich plasma, Pratico et al. (1996) have shown that 8-iso PGF2α can activate human platelets to induce shape change and facilitate the proaggregatory actions of other full agonists but cannot itself induce full aggregation. Initially, this phenomenon could not be attributed to TP receptor activation. However, 8-iso PGF2α was subsequently (Habib et al., 1999) shown to bind a specific site on TPα receptors and to trigger signal transduction distinct from thromboxane (Takahara et al., 1990). Using whole blood, we were not able to reliably determine effects on shape change. Furthermore, in our study 8-iso PGF2α did not potentiate the proaggregatory effects of either U46619 or collagen. This apparent discrepancy between platelet stimulation in isolated preparations and whole blood may be attributable to proaggregatory alterations in platelets and plasma during separation (Nicholson et al., 1998; Cox, 1998; Kulkarni et al., 2000) and the removal of components of whole blood that inhibit platelet aggregation (Zatta et al., 1990).
Of the 11 commercially-available isoprostanes tested in this study, as has been found with 8-iso PGF2α (Yin et al., 1994; Morrow et al., 1992c), we found that, 8-iso PGE1, 8-iso PGE2, 8-iso PGF3α, and 8-iso-13,14-dihydro-15-keto PGF2α all inhibited whole blood aggregation. However, others have suggested, again using isolated platelet preparations, that 8-iso PGE2 can act as a full agonist in a small proportion of individuals and induce irreversible aggregation (Longmire et al., 1994; Leitinger et al., 1997; Kobzar et al., 1997). The proaggregatory effects of 8-iso PGE2 on platelets are thought to be, like those of 8-iso PGF2α, mediated by interactions with TP receptors (Longmire et al., 1994). The absence of this effect in whole blood may again be due to the factors mentioned above or related to the population of donors used in this study.
The ability of 8-iso PGF2α to inhibit whole blood aggregation in response to U46619 or collagen may be related to its effects as a partial or false agonist for TP receptors on platelets (Yin et al., 1994). As with 8-iso PGF2α, we found that 8-iso PGE2 inhibited the proaggregatory effects of U46619 and collagen. This observation is in agreement with others using isolated platelets, where the inhibitory effect of low concentrations of 8-iso PGE2 were attributed to antagonism at the TP receptor (Longmire et al., 1994). To our knowledge, we have shown here for the first time that 8-iso PGE1 also inhibits TP-receptor and collagen-mediated platelet aggregation in whole blood. In fact, the rank order of potency for inhibition of U46619-induced aggregation was 8-iso PGE1>8-iso PGE2>8-iso PGF2α>8-iso PGF3α>8-iso-13,14-dihydro-15-keto PGF2α. Furthermore, 8-iso PGE1 was able to inhibit completely collagen-induced aggregation, an effect not demonstrable by the potent thromboxane receptor antagonist SQ29548, nor any of the other isoprostanes tested. This suggests that 8-iso PGE1 inhibits platelet aggregation, at least in part, independently of its actions on TP receptors. The mechanism of the additional effects of 8-iso PGE1 on platelet aggregation in whole blood remains unclear. An action at IP receptors has been proposed (Leitinger et al., 1997) but EP, DP or novel isoprostane receptors are other possible effector sites (Armstrong, 1996).
Inflammatory states are commonly associated with platelet abnormalities and both inappropriate aggregation and inadequate aggregation are features of clinical sepsis (Lundahl et al., 1996; Mammen, 1998). Endotoxaemia results in increased production of reactive oxygen species (Blackwell et al., 1996) and LPS induces isoprostane production in vivo (Mcadam et al., 2000). As blood vessels alter their responses to 8-iso PGF2α after exposure to oxidative (Kromer & Tippins, 1996) or inflammatory (Jourdan et al., 1998) stress we hypothesized that the effect of isoprostanes on platelet aggregation might also change in blood stimulated by LPS. LPS alone induced a time-dependent depression in the aggregatory response to U46619 but not to collagen. This observation is in agreement with other researchers (Sheu et al., 1999) who have shown similar effects in isolated platelets stimulated with LPS. However, under the same conditions, in contrast to the reported effects on blood vessels, we did not reveal any enhancement of TP receptor-mediated effects by isoprostanes. In fact, the efficacy of the five inhibitory isoprostanes increased in part possibly through a rise in endogenous generation of isoprostanes in this setting.
However, it is interesting to note that the inhibitory effect of LPS on washed human platelets is affected by the presence of calcium-chelating citrate ions in the suspending medium (Baba, 1994). We cannot be sure that this is not relevant to our experiments. The role of raised cytoplasmic calcium in platelet activation is undisputed. However the relative importance to activation of the intracellular or extracellular mobilization of calcium ions is dependent on the agonist, with some weak agonists being more dependent on extracellular concentrations. Other strong agonists including collagen, are capable of inducing platelet aggregation by several mechanisms including thromboxane-independent paths, and dependence on extracellular calcium is less than for weak agonists (Holmsen, 1994). Nevertheless a significant proportion of the aggregatory effect of collagen is dependent on induced platelet thromboxane production (Blackmans et al., 1995). Our experiments indicate that collagen-initiated aggregation remains wholly intact in blood incubated with LPS but incubation with LPS may have produced extracellular calcium dependence in U46619-initiated aggregation. However it is clear that a gross inflammatory insult does not reveal any masked proaggregatory function of the isoprostanes tested in a system where thromboxane-mediated responses are still apparent.
The inhibitory effect of endothelially-derived NO on platelet adhesion and aggregation is well established (Radomski & Moncada, 1991) and there is evidence that NO formed in blood may inhibit platelet function as well (Amin et al., 1995; Chen & Mehta, 1998; Wallerath et al., 1997; Freedman et al., 1998; Queen et al., 2000). In blood vessels, impaired endothelially-derived NO production or NOS inhibition is involved in revealing isoprostane-induced TP receptor activation after inflammatory and oxidative stress. We therefore assessed the effects of isoprostanes on aggregation of whole blood treated with L-NAME. However, paradoxically, we found that L-NAME inhibited aggregation induced by U46619 and collagen. Moreover, in the presence of L-NAME, the five inhibitory isoprostanes tested, remained inhibitory. We cannot, therefore implicate NO in the augmented isoprostane activity detectable in LPS-stimulated blood. The apparently contradictory inhibitory action of L-NAME in this setting joins other conflicting observations of the effect of NOS inhibition on the formation of thrombus (Albert et al., 1999), clot (Dambisya & Lee, 1996) and platelet aggregates (Queen et al., 2000). However, NO synthesized during platelet activation affects COX activity (Naseem et al., 2000) which may be relevant to the data presented here.
In conclusion, we have been unable to demonstrate any proaggregatory effects of the commercially available isoprostanes in human blood under control or inflammatory conditions. However, we have demonstrated that five isoprostanes have biological activity and inhibit platelet aggregation in citrated whole blood. Although previous research has focused on the effect 8-iso PGF2α on isolated platelets, we have found that 8-iso PGE2, which is also produced in vivo after oxidative stress (Morrow et al., 1993), and 8-iso PGE1 are more potent inhibitors of aggregation in whole blood. Isoprostanes may therefore have biological actions in blood under the pathological conditions that are associated with their production.
Abbreviations
- COX
cyclo-oxygenase
- DP
prostaglandin D
- EP
prostaglandin E
- LPS
lipopolysaccharide
- NO
nitric oxide
- NOS
nitric oxide synthase
- PG
prostaglandin
- TP
thromboxane
References
- ALBERT J., WALL N.H., LI N., FROSTELL C., HJEMDAL P. Neither endogenous nor inhaled nitric oxide influences the function of circulating platelets in healthy volunteers. Clin. Sci. (Colch.) 1999;97:345–353. [PubMed] [Google Scholar]
- ALEHNIK S., LEO M., ALEYNIK M., LIEBER C. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol. Clin. Exp. Res. 1998;22:192–196. [PubMed] [Google Scholar]
- AMIN A.R., ATTUR M., VYAS P., LESZCZYNSKA-PIZIAK J., LEVARTOVSKY D., REDISKE J., CLANCY R.M., VORA K.A., ABRAMSON S.B. Expression of nitric oxide synthase in peripheral blood mononuclear cells and neutrophils. J. Inflamm. 1995;47:190–205. [PubMed] [Google Scholar]
- ARMSTRONG R.A. Platelet prostanoid receptors. Pharmacol. Ther. 1996;27:171–191. doi: 10.1016/s0163-7258(96)00103-9. [DOI] [PubMed] [Google Scholar]
- AWAD J., BURK R., ROBERTS L. Effect of selenium deficiency and glutathione modulating agents on diquat toxicity and lipid peroxidation in rats. J. Pharmacol. Exp. Ther. 1994;270:858–864. [PubMed] [Google Scholar]
- BABA K. Effects of E. Coli LPS on human platelet aggregation. Masui. 1994;43:339–345. [PubMed] [Google Scholar]
- BLACKWELL T.S., BLACKWELL T.R., HOLDEN E.P., CHRISTMAN B.W., CHRISTMAN J.W. In vivo antioxidant treatment suppresses nuclear factor-κB activation and neutrophilic lung inflammation. J. Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- BLOCKMANS D., DECKMYN H, , & VERMYLEN J. Platelet activation. Blood Rev. 1995;9:143–156. doi: 10.1016/0268-960x(95)90020-9. [DOI] [PubMed] [Google Scholar]
- CARPENTER C., PRICE P., CHRISTMAN B. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest. 1998;114:1653–1659. doi: 10.1378/chest.114.6.1653. [DOI] [PubMed] [Google Scholar]
- CHEN L.Y., MEHTA J.L. Evidence for the presence of L-arginine-nitric oxide pathway on human red blood cells: relevance in the effects of red blood cells on platelet function. J. Cardiovasc. Pharmacol. 1998;32:57–61. doi: 10.1097/00005344-199807000-00009. [DOI] [PubMed] [Google Scholar]
- COX D. Methods of monitoring platelet function. Am. Heart. J. 1998;135:S160–S169. doi: 10.1016/s0002-8703(98)70244-3. [DOI] [PubMed] [Google Scholar]
- CRANKSHAW D. Effects of the isoprostane 8-epi-prostaglandin isoprostane 8-epi-prostaglandin F2α on the contractility of the human myometrium. Eur. J. Pharmacol. 1995;285:151–158. doi: 10.1016/0014-2999(95)00398-5. [DOI] [PubMed] [Google Scholar]
- DABBAGH A., MANNION T., LYNCH S., FREI B. The effect of iron overload on rat plasma and liver oxidant stress in vivo. Biochem. J. 1994;300:799–803. doi: 10.1042/bj3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMBISYA Y.M., LEE T.L. A thromboelastographic study on the in vitro effects of L-arginine and L-NG-nitro arginine methyl ester on human whole blood coagulation and fibrinolysis. Blood Coagul. Fibrinolysis. 1996;7:678–683. doi: 10.1097/00001721-199610000-00003. [DOI] [PubMed] [Google Scholar]
- DAVI G., CIABATTONI G., CONSOLI A., MEZZETTI A., FALCO A., SANTARONE S., PENNESE E., VITACOLONNA E., BUCCIARELLI T., CONSTANTINI F., CAPANI F., PATRONO C. In vivo formation of 8-iso prostaglandin F2α and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- DELANTY N., REILLY M., PRATICO D., FITZGERALD D., LAWSON J., FITZGERALD G. 8 epi-PGF2α: specific analysis of an isoeicosanoid as an index of oxidant stress in vivo. Br. J. Clin. Pharmacol. 1996;42:15–19. doi: 10.1046/j.1365-2125.1996.03804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEDMAN J.E., TING B., HANKIN B., LOSCALZO J., KEANEY J.F., VITA J.A. Impaired platelet production of nitric oxide predicts presence of acute coronary syndromes. Circulation. 1998;98:1481–1486. doi: 10.1161/01.cir.98.15.1481. [DOI] [PubMed] [Google Scholar]
- FUKUNAGA M., TAKAHASHI K., BADR K.F. Vascular smooth muscle actions and receptor interactions of 8-iso-prostaglandin E2 an E2 isoprostane. Biochem. Biophys. Res. Commun. 1993;195:507–515. doi: 10.1006/bbrc.1993.2075. [DOI] [PubMed] [Google Scholar]
- GARDAN B., CRACOWSKI J., SESSA C., HUNT M., STANKE-LABESQUE F., DEVILLIER P., BESSARD G. Vasoconstrictor effects of isoprostaglandin F2α type-III on human saphenous veins. J. Cardiovasc. Pharmacol. 2000;35:729–734. doi: 10.1097/00005344-200005000-00008. [DOI] [PubMed] [Google Scholar]
- HABIB A., FITZGERALD G., MACLOUFF J. Phosphorylation of the thromboxane receptor α, the predominant isoform expressed in human platelets. J. Biol. Chem. 1999;274:2645–2651. doi: 10.1074/jbc.274.5.2645. [DOI] [PubMed] [Google Scholar]
- HOLMSEN H. Significance of testing platelet function in vitro. Eur. J. Clin. Invest. 1994;24 suppl 1:3–8. doi: 10.1111/j.1365-2362.1994.tb02418.x. [DOI] [PubMed] [Google Scholar]
- JOURDAN K., EVANS T., CURZEN N., MITCHELL J. Evidence for a dilator function of 8-prostaglandin F2 alpha in rat pulmonary artery. Br. J. Pharmacol. 1997b;120:1280–1285. doi: 10.1038/sj.bjp.0701052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOURDAN K., EVANS T., GOLDSTRAW P., MITCHELL J. Isoprostanes and PGE2 production in human isolated pulmonary artery smooth muscle cells: concomitant and differential release. FASEB. J. 1999;13:1025–1030. doi: 10.1096/fasebj.13.9.1025. [DOI] [PubMed] [Google Scholar]
- JOURDAN K., MITCHELL J., EVANS T. Release of isoprostanes by human pulmonary artery in organ culture: a cyclo-oxygenase and nitric oxide dependent pathway. Biochem. Biophys. Res. Com.#. 1997a;233:668–672. doi: 10.1006/bbrc.1997.6523. [DOI] [PubMed] [Google Scholar]
- KAWIKOVA I., BARNES P., TAKAHASHI T., TADJKARIMI S., YACOUB M., BELVISI M. 8-epi-PGF2α, a novel noncyclooxygenase-derived prostaglandin, constricts airways in vitro. Am. J. Respir. Crit. Care Med. 1996;153:590–596. doi: 10.1164/ajrccm.153.2.8564103. [DOI] [PubMed] [Google Scholar]
- KLEIN T., REUTTER F., SCHWEER H., SEYBERTH H., NUSING R. Generation of the isoprostane 8-epi-prostaglandin F2α in vitro and in vivo via the cyclooxygenases. J. Pharmacol. Exp. Ther. 1997;282:1658–1665. [PubMed] [Google Scholar]
- KOBZAR G., MARDLA V., JARVING I., SAMEL N., LOHMUS M. Modulatory effect of 8-iso-PGE2 on platelets. Gen. Pharmacol. 1997;28:317–321. doi: 10.1016/s0306-3623(96)00224-8. [DOI] [PubMed] [Google Scholar]
- KROMER B., TIPPINS J. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2α. Br. J. Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROMER B., TIPPINS J. The vasoconstrictor effect of 8-epi prostaglandin F2α in the hypoxic rat heart. Br. J. Pharmacol. 1999;126:1171–1174. doi: 10.1038/sj.bjp.0702433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKARNI S., DOPHEIDE S.M., YAP C.L., RAVANAT C., FREUND M., MANGIN P., HEEL K.A., STREET A., HARPER I.S., LANZA F., JACKSON S.P. A revised model of platelet aggregation. J. Clin. Invest. 2000;105:783–791. doi: 10.1172/JCI7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITINGER N., BLAZEK I., SINZINGER H. The influence of isoprostanes on ADP-induced platelet aggregation and cyclic AMP-generation in human platelets. Thromb. Res. 1997;86:337–342. doi: 10.1016/s0049-3848(97)00077-7. [DOI] [PubMed] [Google Scholar]
- LONGMIRE A., ROBERTS L., MORROW J. Actions of the E2 isoprostane, 8-iso-PGE2 on the platelet thromboxane/endoperoxide receptor in humans and rats: additional evidence for the existence of a unique isoprostane receptor. Prostaglandins. 1994;48:247–256. doi: 10.1016/0090-6980(94)90011-6. [DOI] [PubMed] [Google Scholar]
- LUNDAHL T.H., LUNDAHL T.L., FAGERBERG I.H., EGBERG N., BUNESCU A., LARSSON A. Activated platelets and impaired platelet function in intensive care patients analyzed by flow cytometry. Blood Coagul. Fibrinolysis. 1996;7:218–220. doi: 10.1097/00001721-199603000-00027. [DOI] [PubMed] [Google Scholar]
- LYNCH S., MORROW J., ROBERTS L., FREI B. Increased plasma isoprostanes in rats fed a copper-deficient diet. Circulation. 1996;94:4133. [Google Scholar]
- MAMMEN E.F. The haematological manifestations of sepsis. J. Antimicrobial Chem. 1998;41 suppl. A:17–24. doi: 10.1093/jac/41.suppl_1.17. [DOI] [PubMed] [Google Scholar]
- MCADAM B., MARDINI I., HABIB A., BURKE A., LAWSON J., KAPOOR S., FITZGERALD G. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J. Clin. Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINUZ P., ANDRIOLI G., DEGAN M., GAINO S., ORTOLANI R., TOMMASOLI R., ZULIANI V., LECHI A., LECHI C. The F2-isoprostane 8-epiprostaglandin F2α increases platelet adhesion and reduces the antiadhesive and antiaggregatory effects of NO. Arterioscler. Thromb. Vasc. Biol. 1998;18:1248–1256. doi: 10.1161/01.atv.18.8.1248. [DOI] [PubMed] [Google Scholar]
- MORROW J., AWAD J., BOSS H., BLAIR I., ROBERTS L. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J., AWAD J., KATO T., TAKAHASHI K., BADR K., ROBERTS L., BURK R. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J. Clin. Invest. 1992b;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J., FREI B., LONGMIRE A., GAZIANO J., LYNCH S., STAUSS W., OATES W., ROBERTS L., II Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers: Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- MORROW J., HILL K., BURK R., NAMMOUR T., BADR K., ROBERTS L. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclo-oxygenase, free-radical catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J., MINTON T., ROBERTS L. The F2-isoprostane, 8-epiprostaglandin F2α, a potent agonist of the vascular thromboxane/endoperoxide receptor is a platelet thromboxane/endoperoxide antagonist. Prostaglandins. 1992c;44:155–163. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., MINTON A.M., MUKUNDAN C.R., CAMPBELL M.D., ZACKERT W.E,. , DANIEL V.C., BADR K.F., BLAIR I.A., ROBERTS L.J. Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1993;269:4317–4326. [PubMed] [Google Scholar]
- NASEEM K.M., LOW S.Y., SABETKAR M., BRADLEY N.J, , KHAN J., JACOBS M., BRUCKDORFER K.R. The nitration of platelet cytosolic proteins during agonist-induced activation of platelets. FEBS Lett. 2000;473:119–122. doi: 10.1016/s0014-5793(00)01490-3. [DOI] [PubMed] [Google Scholar]
- NICHOLSON N.S., PANZER-KNODLE S.G., HAAS N.F., TAITE B.B., SZALONY J.A., PAGE J.D., FEIGEN L.P., LANSKY D.M., SALYERS A.K. Assessment of platelet function assays. Am. Heart. J. 1998;135:S170–S178. doi: 10.1016/s0002-8703(98)70245-5. [DOI] [PubMed] [Google Scholar]
- OLIVIERA L., STALLWOOD N., CRANKSHAW D. Effects of some isoprostanes on the human umbilical artery in vitro. Br. J. Pharmacol. 2000;129:509–514. doi: 10.1038/sj.bjp.0703083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRIGNANI P., SANTINI G., PANARA M., SCIULLI M., GRECO A., ROTONDO M., GIAMBERARDINO M., MACLOUF J., CIABATTONI G., PATRONO C. Induction of prostaglandin endoperoxide synthase-2 in human monocytes associated with cyclo-oxygenase dependent F2-isoprostane formation. Br. J. Pharmacol. 1996;118:1285–1293. doi: 10.1111/j.1476-5381.1996.tb15535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRATICO D., LAWSON J., FITZGERALD G. Cyclooxygenase-dependent formation of the isoprostane, 8-epi prostaglandin F2α. J. Biol. Chem. 1995;17:9800–9808. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- PRATICO D., SMYTH E.M., VIOLI F., FITZGERALD G. Local amplification of platelet function by 8-epi prostaglandin F2α is not mediated by thromboxane receptor isoforms. J. Biol. Chem. 1996;271:14916–14924. doi: 10.1074/jbc.271.25.14916. [DOI] [PubMed] [Google Scholar]
- QUEEN L.R., XU B., HORINOUCHI K., FISHER I., FERRO A. Beta(2)-adrenoceptors activate nitric oxide synthase in human platelets. Circ. Res. 2000;87:39–44. doi: 10.1161/01.res.87.1.39. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., MONCADA S.Biological role of nitric oxide in platelet function Clinical relevance of nitric oxide in the cardiovascular system 1991Madrid: Edicomplet; 45–56.ed. Moncada S., Higgs, E. A. & Barrazueta, J. R. pp [Google Scholar]
- REILLY M., DELANTY N., ROY L., ROKACH J., O'CALLAGHAN P., CREAN P., LAWSON J., FITZGERALD G. Increased formation of the isoprostanes IPF2α-I and 8-epiprostaglandin F2α in acute coronary angioplasty. Circulation. 1997;96:3314–3320. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- ROBERTS L., MORROW J. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Rad. Biol. Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- SAMETZ W., PRASTHOFER S., WINTERSTEIGER R., JUAN H. Vascular effects of isoprostanes after endothelial damage. Prostaglandins Leukot. Essent. Fatty Acids. 1999;61:369–372. doi: 10.1054/plef.1999.0113. [DOI] [PubMed] [Google Scholar]
- SHEU J.R., HUNG W.C., SU C.H., LIN C.H., LEE L.W., LEEY M., YEN M.H. The antiplatelet activity of Escherichia coli lipopolysaccharide mediated through a nitric oxide/cyclic GMP pathway. Eur. J. Haematol. 1999;62:317–326. doi: 10.1111/j.1600-0609.1999.tb01909.x. [DOI] [PubMed] [Google Scholar]
- SINZINGER H., OGUOGHO A., KALIMAN J. Isoprostane 8-epi-prostaglandin F2 alpha is a potent contractor of human peripheral lymphatics. Lymphology. 1997;30:155–159. [PubMed] [Google Scholar]
- TAKAHARA K., MURRAY R., FITZGERALD G.A., FITZGERALD D.J. The response to thromboxane A2 analogues in human platelets. Discrimination of two binding sites linked to distinct effector systems. J. Biol. Chem. 1990;265:6836–6844. [PubMed] [Google Scholar]
- VACCHIANO C.A., OSBORNE G.R., TEMPEL G.E. 8-iso-PGF2alpha production by alveolar macrophages exposed to hyperoxia. Shock. 1998;9:266–273. doi: 10.1097/00024382-199804000-00006. [DOI] [PubMed] [Google Scholar]
- WALLERATH T., GATH I., AULITZKY W.E., POLLOCK J.S., KLEINERT H., FORSTERMANN U. Identification of the NO synthase isoforms expressed in human neutrophil granulocytes, megakaryocytes and platelets. Thromb. Haemost. 1997;77:163–167. [PubMed] [Google Scholar]
- WALSH S.W., VAUGHAN J.E., WANG Y., ROBERTS L.J., II Placental isoprostane is significantly increased in pre-eclampsia. FASEB J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- YIN K., HALUSHKA P.V., YAN Y-T., WONG P.Y.K. Antiaggregatory activity of 8-epi-prostaglandin F2α and other F-series prostanoids and their binding to thromboxane A2/prostaglandin H2 receptors in human platelets. J. Pharmacol. Exp. Ther. 1994;270:1192–1196. [PubMed] [Google Scholar]
- ZATTA A., PROSDOCIMI M., BERTELE V., BAZZONI G., DEL MASCHIO A. Inhibition of platelet function by polymorphonuclear leukocytes. J. Lab. Clin. Med. 1990;116:651–660. [PubMed] [Google Scholar]


