Abstract
The present study attempted to characterize the α1-adrenoceptor subtypes mediating vasoconstrictor responses to administered and nerve stimulation-evoked noradrenaline (NA) release in the isolated and perfused canine splenic artery.
A previous study demonstrated that periarterial electrical nerve stimulation (30 s trains of pulses at a frequency of 1, 4 or 10 Hz) induced a double peaked vasoconstriction consisting of an initial transient, predominantly P2X-purinoceptor-mediated constriction followed by a prolonged, mainly α1-adrenoceptor-mediated response in the canine splenic artery.
The effects of α1-adrenoceptor subtype antagonists on neuronally-mediated second peaked vasoconstrictions were analysed. BMY 7378 (10 – 100 nM), a selective α1D-adrenoceptor antagonist produced a dose-dependent inhibition of the second peak responses at all frequencies used. BMY 7378 (100 nM) reduced these responses by approximately 30%. Exposure of tissues to chloroethylclonidine (CEC, 60 μM), a selective α1B-adrenoceptor antagonist attenuated the second peak response by approximately 60%, even in the presence of BMY 7378 (100 nM). On the other hand, WB 4101 (100 nM), a selective α1A-adrenoceptor antagonist potentiated nerve-stimulation-evoked double peaked vasoconstrictions, especially at low frequencies (1 and 4 Hz).
Vasoconstrictor responses to administered NA were dose-dependently antagonized by WB 4101 (10 – 100 nM), but were not significantly affected by either BMY 7378 (10 – 100 nM) or by CEC (60 μM).
The present results indicate that NA released from sympathetic nerves may junctionally exert its vasoconstrictor effect via activation of postjunctional α1B- and in part α1D-adrenoceptors, whereas exogenous NA extrajunctionally activates α1A-adrenoceptors to produce its vascular action in canine splenic arteries.
Keywords: Neurojunctional transmission, α1-adrenoceptor subtype, BMY 7378, sympathetic nerve stimulation, canine splenic artery
Introduction
Several studies have addressed the issue of the heterogeneity of α1-adrenoceptor subtypes involved in the response to neurally released and exogenously applied noradrenaline (NA) (Mallard et al., 1992; Honner & Docherty, 1999). Previous results obtained using the rat vas deferens revealed that the α1B-adrenoceptor subtype mediated sympathetic nerve-induced adrenergic contraction to a single field stimulus, whereas the α1A-adrenoceptor subtype was involved in the response to exogenous NA (Mallard et al., 1992). Recent observations further confirmed these differences in the same preparation and indicated that the α1-adrenoceptor subtype involved in the nerve-stimulated response resembled the α1D-adrenoceptor subtype (Honner & Docherty, 1999). However, in rat blood vessel preparations, nerve-stimulated NA and exogenously applied NA may couple to the same subtype of α1-adrenoceptors, i.e., α1A-adrenoceptors for the rat mesenteric arterial bed (Kong et al., 1994; Williams & Clarke, 1995) and the vasculature of rat kidney (Blue et al., 1992), α1B-adrenoceptors for the rat irideal blood vessel (Gould & Hill, 1994). An α1-adrenoceptor has been identified as a functional subtype for vasoconstriction of the canine splenic artery to both neuronal and exogenous NA (Ren et al., 1994a,1994b; 1996). In the canine splenic artery, the vasoconstrictor response to longer trains of stimulation (30 s duration) appeared to be a double peaked constriction consisting of an initial transient, P2X-receptor-mediated response followed by a prolonged, α1-adrenoceptor-induced response (Yang & Chiba, 1998; 2000a). Furthermore, activation of neuropeptide Y (NPY) Y1-receptors by endogenous and exogenous NPY may potentiate the sympathetic nerve-stimulated α1-adrenoceptor vasoconstriction, whereas it did not affect the exogenous, NA-induced response (Yang & Chiba, 2000a,2000b). The reason for this difference may be that there is an α1-adrenoceptor subtype-specific role involved in mediating the canine splenic vasoconstrictor responses to neuronal and exogenous NA. In the present study, we used subtype selective antagonists to determine the specific α1-adrenoceptor subtypes activated by neurally released and exogenously applied NA in the canine splenic artery. Some of these data have been previously published in shortened form (Yang & Chiba, 2000c).
Methods
Arterial preparations
Mongrel dogs of either sex, weighing 9 – 13 kg, were anaesthetized with sodium pentobarbitone (30 mg kg−1 i.v.). After treatment with sodium heparin (200 u kg−1 i.v.), the dogs were killed by rapid exsanguination from the right femoral artery. The arterial main branches of the splenic artery were isolated and side branches of the artery were tied with silk threads. Then, the artery (1 – 1.2 mm in an outer diameter) was cut into segments (15 – 20 mm in length). Four segments were obtained from each splenic artery. Each segment was cannulated and set up for perfusion as described previously (Hongo & Chiba, 1983; Tsuji & Chiba, 1984). Briefly, a stainless steel cannula was inserted into the arterial segment from the distal to the proximal end. A proximal portion of the segment was fixed to the distal portion of a needle-type cannula with silk threads. The cannula was 3 – 4 cm long and 0.8 – 1.0 mm in outer diameter with small side holes 5 mm from the distal sealed end. The cannulated arterial segment was placed in a cup-shaped glass bath and was perfused by a roller pump (Tokyo Rikakikai, Tokyo, Japan) with Krebs-Henseleit solution gassed with 95% O2 and 5% CO2. The solution contained (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 10. The flow rate was kept at approximately 2 ml min−1. The perfusion pressure was continuously measured with an electric manometer (MPU-0.5A, Nihon Kohden, Tokyo, Japan) and recorded with a rectigraph (WT-685G, Nihon Kohden, Tokyo, Japan). After a stabilization period of 1 h, the preparation was removed from the bath solution and fixed in a horizontal position. The preparation was perfused at a constant flow rate during the experiment. The basal perfusion pressure was 40 – 80 mmHg.
For electrical stimulation of the periarterial sympathetic nerve terminals, two platinum electrodes were placed on the extraluminal side of the arterial wall. Electrical stimulation was delivered by an electric stimulator (SEN-7203, Nihon Kohden) using 30 s trains of pulses at 10 V amplitude, 1 ms pulse duration, over a frequency range of 1, 4 and 10 Hz. The organ bath was sealed with plastic film to maintain the preparation at 37°C. Ten-minute intervals between electrical stimulation periods were needed to obtain reproducible response. The intervals between frequency-response curves were over 1 h. The preparations were incubated for 1 h with all antagonists used before the next response curves were made for electrical stimulation. α,β-Methylene ATP (αβ-m ATP) produced a transient increase in perfusion pressure and returned to its original level for 1 h. The other antagonists used in this study did not influence basal perfusion pressure at any concentration administered. NA was administered into the rubber tubing close to the cannula in a volume of 0.01 – 0.03 ml, using microinjectors (Terumo, Tokyo, Japan).
Drugs
Drugs used were α,β-methylene adenosine 5′-triphosphate lithium salt; dl-noradrenaline hydrochloride (Sigma, St. Louis, U.S.A.); chloroethylclonidine dihydrochloride; WB4101 (2-(2,6-dimethoxyphenoxyethyl)-aminomethyl-1,4-benzodioxane hydrochloride); BMY 7378 (8-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-8-azaspiro[4.5] decane-7,9-dione dihydrochloride) (Research Biochemicals Inc., Natick, MA, U.S.A.). All drugs were dissolved in distilled water. The stock solutions were kept at −20°C until used.
Statistical analysis
Vasoconstrictor responses to electrical stimulation are expressed as the maximal changes in perfusion pressure (mmHg) from their basal levels. The data are shown as mean±s.e.mean. An analysis of variance with Bonferroni's test was used for the statistical analysis of multiple comparisons of data. P values less than 0.05 were considered statistically significant.
Results
Vasoconstrictor responses to periarterial electrical nerve stimulation
Figure 1A shows a typical double peaked vasoconstriction induced by periarterial electrical nerve stimulation in an isolated, perfused canine splenic artery. As demonstrated previously (Yang & Chiba, 1998; 2000d), the second peaked responses were mostly mediated by α1-adrenoceptors. Hence, we attempted to analyse and compare the effects of α1-adrenoceptor subtype antagonists on the second peaked constrictions. Figure 1 shows an original tracing of double peaked responses from typical experiments showing the effects of BMY 7378 and CEC. The summarized data for BMY 7378 and CEC are shown in Figure 2. As shown in Figure 2B, BMY 7378 (10 – 100 nM) produced a dose-dependent inhibition of the second peaked responses at all frequencies used. BMY 7378 (100 nM) reduced the second peaked responses by approximately 30%. A subsequent application of CEC (60 μM) exerted a further inhibition of these responses by approximately 70% at low frequencies (1 and 4 Hz) and by 50% at a high frequency (10 Hz). In addition, exposure to 1 μM CEC failed to affect the neuronally-mediated vasoconstrictions at all frequencies used but 60 μM CEC markedly depressed the second peaked responses by approximately 65% (Figure 3).
Figure 1.
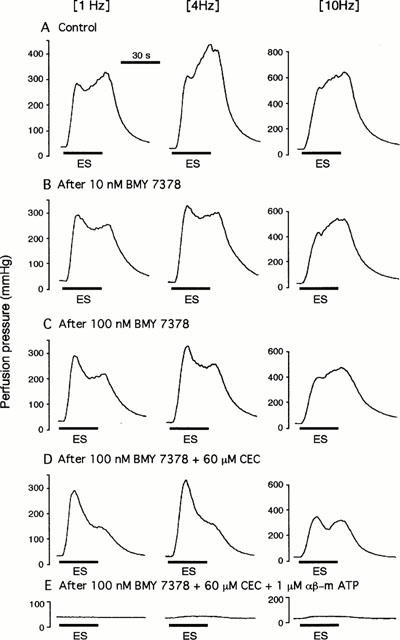
Double peaked vasoconstrictor responses to periarterial electrical nerve stimulation and the effects of BMY 7378, CEC and αβ-m ATP in an isolated, perfused canine splenic artery. The double peaked vasoconstrictions were induced by 30 s trains of pulses at 10 V amplitude and 1 ms pulse duration, with a frequency of 1, 4 or 10 Hz (A). At low frequences (1 and 4 Hz), pretreatment with BMY 7378 (10 – 100 nM) produce a dose-dependent inhibition on the second peaked responses without inhibition of the first peaked response. At a high frequency of 10 Hz, both the first and second peaks were reduced by BMY 7378 (B,C). A subsequent administration of CEC (60 μM) additionally inhibited those responses in the presence of 100 nM BMY 7378 (D). The remaining responses after treatment with BMY 7378 and CEC were abolished by 1 μM αβ-m ATP (E). (ES), Periarterial electrical nerve stimulation.
Figure 2.
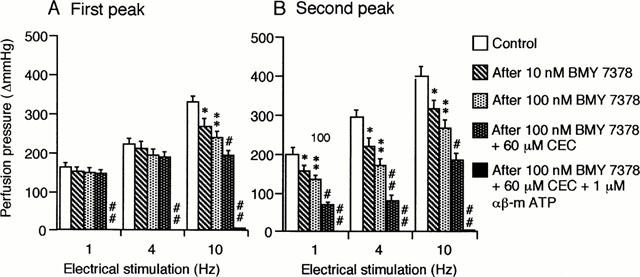
Effects of BMY 7378, CEC and αβ-m ATP on the first (A) and the second peak (B) of the vasoconstrictor responses to periarterial electrical nerve stimulation in the canine splenic arteries. The vessels were electrically stimulated by 30 s trains of pulses at 10 V amplitude and 1 ms pulse duration, with a frequency of 1, 4 or 10 Hz. Data are presented as mean±s.e.mean (n=8). *P<0.05; **P<0.01 as compared with the control group. #P<0.05; ##P<0.01 as compared with the preceding group.
Figure 3.
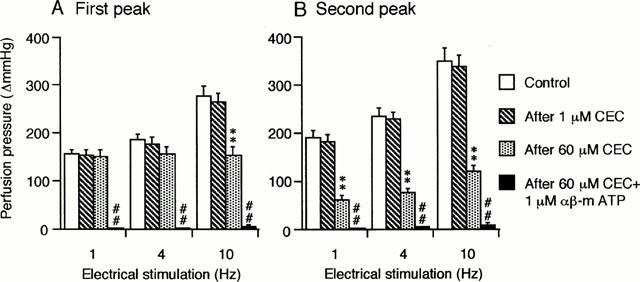
Effects of CEC and αβ-m ATP on the first (A) and the second peak (B) of the vasoconstrictor responses to periarterial electrical nerve stimulation in the canine splenic arteries. The vessels were electrically stimulated by 30 s trains of pulses at 10 V amplitude and 1 ms pulse duration, with a frequency of 1, 4 or 10 Hz. Data are presented as mean±s.e.mean (n=12). **P<0.01 as compared with the control group. ## P<0.01 as compared with the preceding group.
As shown in Figure 4, 100 nM WB 4101 significantly potentiated the double peaked vasoconstrictions, although it slightly but insignificantly enhanced the responses at high frequency (10 Hz). Subsequent treatment with CEC (60 μM) selectively antagonized the second peaked responses at all frequencies used without any significant influence on the first peaked responses (Figure 4).
Figure 4.
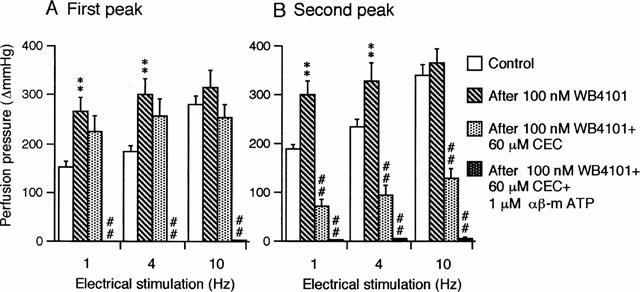
Effects of WB 4101, CEC and αβ-m ATP on the first peak (A) and the second peak (B) of the vasoconstrictor responses to periarterial electrical nerve stimulation in the canine splenic arteries. The vessels were electrically stimulated by 30 s trains of pulses at 10 V amplitude and 1 ms pulse duration, with a frequency of 1, 4 or 10 Hz. Data are presented as mean±s.e.mean (n=6). **P<0.01 as compared with the control group. ##P<0.01 as compared with the preceding group.
The remaining responses after exposure to BMY 7378 and CEC (Figures 1 and 2), or CEC (Figure 3) or to WB 4101 and CEC (Figure 4) were abolished by subsequent administration of α,β-m ATP (1 μM).
Vasoconstrictor response to administered NA
Increasing concentrations of WB 4101 (10 – 100 nM) produced a parallel rightward shift of the NA dose-response curves in this preparation (Figure 5A). The NA dose-response curve was not significantly influenced by CEC (60 μM) but was shifted to the right in parallel manner by subsequent administration of 100 nM WB 4101 (Figure 5B). The dose-response curve to NA was not significantly affected by BMY 7378 (10 – 100 nM) (Figure 5C).
Figure 5.
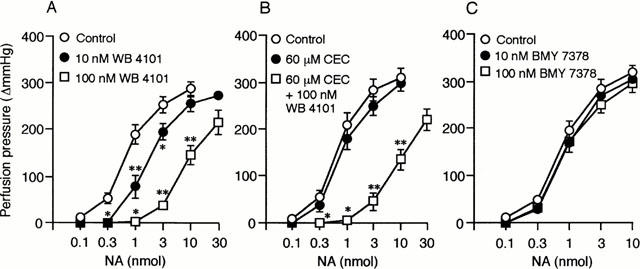
Effects of WB 4101, CEC and BMY 7378 on NA-induced vasoconstrictions in the canine splenic arteries. Data are presented as mean±s.e.mean. (A) Effects of WB 4101. *P<0.05; **P<0.01 as compared with the control group (n=6). (B) Effects of CEC and WB 4101. *P<0.05; **P<0.01 as compared with CEC-treated group (n=5). (C) Effects of BMY 7378 (n=6).
Discussion
The primary objective of this study was to characterize the functional roles of α1-adrenoceptor subtypes in the regulation of sympathetic vascular tone using an isolated and perfused canine splenic arterial preparation. Previous experiments have demonstrated that the α1-adrenoceptor is the main functional subtype in the canine splenic artery (Ren et al., 1994a,1994b; 1996; Yang & Chiba, 1998). In the present study, we obtained evidence that different subtypes of α1-adrenoceptor may be responsible for the vasoconstrictions of the canine splenic artery following neuronal stimulation and exogenous NA.
α1-Adrenoceptors have been subclassified as α1A, α1B and α1D-subtypes in peripheral tissues (Hieble et al., 1995). α1A-Adrenoceptors have high affinity for 5-methyl-urapidil, WB 4101 whilst α1B-adrenoceptors are inactivated by the alkylating agent chloroethylclonidine (CEC) (Morrow & Creese, 1986; Han et al., 1987; Hieble et al., 1995). BMY 7378, a 5-hydroxytryptamine antagonist/partial agonist (Yocca et al., 1987) has recently been reported to be a highly selective α1D-adrenoceptor antagonist (Goetz et al., 1995; Kenny et al., 1995; Piascik et al., 1995). It has been demonstrated that CEC-sensitive α1-adrenoceptor subtype receives a sympathetic innervation in many peripheral tissues including canine carotid artery (Muramatsu, 1991), human saphenous vein (Racchi et al., 1999) and rat vas deferens (Mallard et al., 1992). The sympathetic vasoconstriction observed in our preparation was also mediated partially by a CEC-sensitive mechanism. In the rat vas deferens, the α1B-adrenoceptor subtype was initially hypothesized to mediate sympathetic nerve-evoked contraction, since CEC selectively inhibited the response without interfering with the effect of exogenous NA (Mallard et al., 1992). The latter evidence obtained in the same preparation showed an involvement of α1D-adrenoceptor subtype for nerve-evoked responses, since BMY 7378, a selective α1D-adrenoceptor antagonist, exhibited a relatively high potency against nerve-induced response (Honner & Docherty, 1999). Pharmacological evidence has demonstrated that CEC can inactivate both α1B- and α1D-adrenoceptors, although the former subtype is relatively more sensitive (Schwinn et al., 1995). Furthermore, the reduction of nerve-stimulated vasoconstriction by BMY 7378, and an additional inhibition by CEC, could be taken as good evidence of the functional role of both α1B- and α1D-adrenoceptors in mediating sympathetic vasoconstriction. However, one contradictory result encountered in this study was that both BMY 7378 and CEC significantly inhibited nerve-stimulated constriction, without affecting the exogenous NA-evoked response. The most probable explanation for this result is that the inhibition by BMY 7378 and CEC are mediated by prejunctional mechanisms. This suggestion is supported by the finding, obtained in the rat vas deferens, that nerve-induced release of NA and purinergic contractions are indeed blocked by CEC and that this effect of CEC is proposed to be mediated by prejunctional α2-adrenoceptors (Bültmann & Starke, 1993). It is generally accepted that an activation of prejunctional α2-adrenoceptors affects not only the release of NA but also that of ATP (von Kügelgen et al., 1994) as observed previously in the canine splenic artery (Yang & Chiba, 1999). Since CEC selectively inhibited sympathetic adrenergic vasoconstriction, but did not affect the purinergic response in this study, it is unlikely that CEC acts by stimulating prejunctional adrenoceptors. In addition, in the rat perfused kidney, CEC at a high concentration (100 μM) is unable to affect the nerve stimulation-induced release of NA (Blue et al., 1992). Thus, an alternative possibility is that neuronal and exogenous NA-induced responses might be mediated by different subtypes of α1-adrenoceptors. The fact that WB 4101 inhibited the exogenous NA-induced constriction suggests that extrajunctional α1A-adrenoceptor subtype may operate in the postjunctional blood vessel smooth muscle. Taking into account these results, the findings of the present study may support the hypothesis that postjunctional α1B- and α1D-adrenoceptors receive a sympathetic adrenergic innervation in the canine splenic artery, whereas α1A-adrenoceptors may be sited extrajunctionally and activated by humoral catecholamines. It is reasonable to hypothesize that α1B- and α1D-adrenoceptors may be located in the junctional region whereas α1A-adrenoceptors may reside in the extrajunctional region of the sympathetic neurovascular junction in the canine splenic artery.
In the present experiments, WB 4101 inhibited the exogenous NA-induced response but enhanced the sympathetic nerve-stimulated double peaked vasoconstrictions. WB 4101 has been shown to have a high affinity for α1D-adrenoceptors in addition to the α1A-adrenoceptor subtype (Minneman & Esbenshade, 1994). However, the α1D-adrenoceptor mechanism is not an important factor involved in the effect of WB 4101, since BMY 7378, a selective α1D-adrenoceptor antagonist, slightly but significantly reduced the nerve-stimulated response without inhibition of the NA-induced response. It should nevertheless be emphasized that the blockade of α1A-adrenoceptors accounts for all of the effects of WB 4101. Furthermore, an additional treatment with CEC blocked WB 4101-induced potentiation of the second peaked response whereas this failed to affect that of the first responses. The remaining responses after treatment with WB 4101 and CEC were abolished by a subsequent P2X-receptor desensitization with αβ-m ATP. Hence, it is proposed that activation of α1A-adrenoceptors may exert an inhibitory modulation on both adrenergic and purinergic transmission of the canine splenic artery. Since the vasoconstrictor effect of administered NA is indeed blocked by WB 4101, a postjunctional mechanism for WB 4101-induced potentiation is at least excluded. The precise explanation for these results remains to be established.
It has been suggested that the synergism between NPY Y1-receptors and α1-adrenoceptors is an important mechanism in the regulation of peripheral sympathetic mesenteric vascular tone in the rat (Donoso et al., 1997; Cortés et al., 1999). Recently, we also obtained similar results in the isolated and perfused canine splenic artery (Yang & Chiba, 2000a,2000b). However, we found that activation of NPY Y1-receptors can enhance sympathetic α1-adrenocepor-mediated vasoconstriction, whereas it was unable to influence exogenous NA-evoked response (Yang & Chiba, 2000a,2000b). These results indicate that the NPY Y1-receptor is likely coupled to the specific subtype of α1-adrenoceptors responsible for the NPY-mediated cooperation of sympathetic adrenergic response. The present study provides evidence that the specific subtype of α1-adrenoceptor involved in the response to neuronal stimulation seems to be the α1B-adrenoceptor and in part α1D-adrenoceptor subtypes rather than the α1A-adrenoceptor subtype. Thus, we can postulate that NPY enhances sympathetic α1-adrenoceptor-mediated vasoconstriction of canine splenic artery primarily through the synergism between NPY Y1-receptor and specific subtypes of α1B-adrenoceptor and in part α1D-adrenoceptor.
In conclusion, the present results indicate that there may be different α1-adrenoceptor subtypes in the sympathetic neurovascular junction and extrajunctional region of the canine splenic artery. It is therefore hypothesized that the adrenergic transmitter and hormonal catecholamines modulate the peripheral vascular tone via acting at different subtypes of α1-adrenoceptors.
Abbreviations
- BMY 7378
8-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride
- CEC
chloroethylclonidine
- ES
periarterial electrical nerve stimulation
- αβ-m ATP
α,β-methylene ATP
- NA
noradrenaline
- WB 4101
2-(2,6-dimethoxyphenoxyethyl)-aminomethyl-1,4-benzodioxane
References
- BLUE D.R., VIMONT R.L., JR, CLARKE D.E. Evidence for a noradrenergic innervation to α1A-adrenoceptors in rat kidney. Br. J. Pharmacol. 1992;107:414–417. doi: 10.1111/j.1476-5381.1992.tb12760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜLTMANN R., STARKE K. Chloroethylclonidine: an irreversible agonist at prejunctional α2-adrenoceptors in rat vas deferens. Br. J. Pharmacol. 1993;108:336–341. doi: 10.1111/j.1476-5381.1993.tb12806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTÉS V., DONOSO M.V., BROWN N., FANJUL R., LÓPEZ C., FOURNIER A., HUIDOBRO-TORO J.P. Synergism between neuropeptide Y and norepinephrine highlights sympathetic cotransmission: Studies in rat arterial mesenteric bed with neuropeptide Y, analogs, and BIBP 3226. J. Pharmacol. Exp. Ther. 1999;289:1313–1322. [PubMed] [Google Scholar]
- DONOSO M.V., BROWN N., CARRASCO C., CORTÉS V., FOURNIER A., HUIDOBRO-TORO J.P. Stimulation of the sympathetic perimesenteric arterial nerves releases neuropeptide Y potentiating the vasomotor activity of noradrenaline: Involvement of neuropeptide Y-Y1 receptors. J. Neurochem. 1997;69:1048–1059. doi: 10.1046/j.1471-4159.1997.69031048.x. [DOI] [PubMed] [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D.C., TRUE T.A., RIMELE T.J., SAUSSY D.L., JR BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur. J. Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- GOULD D.J., HILL C.E. Alpha1B-receptors and intracellular calcium mediate sympathetic nerve induced constriction of rat irideal blood vessels. J. Auton. Nerv. Sys. 1994;50:139–150. doi: 10.1016/0165-1838(94)90004-3. [DOI] [PubMed] [Google Scholar]
- HAN C., ABEI P.W., MINNEMAN K.P. α1-Adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature (Lond.) 1987;329:333–335. doi: 10.1038/329333a0. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R., JR Internatinal Union of Pharmacology. X. Recommendation for nomenclature of α1-adrenoceptor: consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HONGO K., CHIBA S. A new method for measuring vascular responsiveness of relatively larger arteries of dogs. J. Pharmacol. Methods. 1983;9:83–91. doi: 10.1016/0160-5402(83)90054-2. [DOI] [PubMed] [Google Scholar]
- HONNER V., DOCHERTY J.R. Investigation of the subtypes of α1-adrenoceptor mediating contractions of rat vas deferens. Br. J. Pharmacol. 1999;128:1323–1331. doi: 10.1038/sj.bjp.0702913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNY B.A., CHALMERS D.H., PHILPOTT P.C., NAYLOR A.M. Characterization of an α1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br. J. Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG J.Q., TAYLOR D.A., FLEMING W.W. Functional distribution and role of alpha-1 adrenoceptor subtypes in the mesenteric vasculature of the rat. J. Pharmacol. Exp. Ther. 1994;268:1153–1159. [PubMed] [Google Scholar]
- MALLARD N.J., MARSHALL R.W., SITHERS A.J., SPRIGGS T.L. Separation of putative α1A- and α1B-adrenoceptor mediated components in the tension response of the rat vas deferens to electrical field stimulation. Br. J. Pharmacol. 1992;105:727–731. doi: 10.1111/j.1476-5381.1992.tb09046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINNEMAN K.P., ESBENSHADE T.A. α1-Adrenergic receptor subtypes. Ann. Rev. Pharmacol. Toxicol. 1994;34:117–133. doi: 10.1146/annurev.pa.34.040194.001001. [DOI] [PubMed] [Google Scholar]
- MORROW A.L., CREESE I. Characterization of α1-adrenergic receptor subtypes in rat brain: A reevaluation of [3H] WB4101 and [3H] prazosin binding. Mol. Pharmacol. 1986;29:321–330. [PubMed] [Google Scholar]
- MURAMATSU I. Relation between adrenergic neurogenic contraction and α1-adrenoceptor subtypes in dog mesenteric and carotid arteries and rabbit carotid arteries. Br. J. Pharmacol. 1991;102:210–214. doi: 10.1111/j.1476-5381.1991.tb12155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIASCIK M.T., GUARINO R.D., SMITH M.S., SOLTIS E.E., SAUSSY D.L., PEREZ D.M. The specific contribution of the novel α1D-adrenoceptor to the contraction of vascular smooth muscle. J. Pharmacol. Exp. Ther. 1995;275:1583–1589. [PubMed] [Google Scholar]
- RACCHI H., IRARRÁZABAL M.J., HOWARD M., MORÁN S., ZALAQUETT R., HUIDOBRO-TORO J.P. Adenosine 5′-triphosphate and neuropeptide Y are co-transmitters in conjunction with noradrenaline in the human saphenous vein. Br. J. Pharmacol. 1999;126:1175–1185. doi: 10.1038/sj.bjp.0702396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN L.-M., NAKANE T., CHIBA S. Characteristics of the responses of isolated and perfused canine splenic arteries to vasoactive substances and to periarterially electrical stimulation. Jpn. J. Pharmacol. 1994a;64:19–25. doi: 10.1254/jjp.64.19. [DOI] [PubMed] [Google Scholar]
- REN L.-M., NAKANE T., CHIBA S. Differential effects of ω-conotoxin GVIA and tetrodotoxin on vasoconstrictions evoked by electrical stimulation and nicotinic receptor stimulation in canine isolated, perfused splenic arteries. Br. J. Pharmacol. 1994b;111:1321–1327. doi: 10.1111/j.1476-5381.1994.tb14889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN L.-M., NAKANE T., CHIBA S. Purinergic and adrenergic transmission and presynaptic modulation in canine isolated perfused splenic arteries. Eur. J. Pharmacol. 1996;295:61–68. doi: 10.1016/0014-2999(95)00654-0. [DOI] [PubMed] [Google Scholar]
- SCHWINN D.A., JOHNSON G.I., PAGE S.O., MOSLEY M.J., WILSON K.A., WORMAN N.P., CAMPBELL B., BAILRY D.S. Cloning and pharmacological characterization of human α1-adrenergic receptors: Sequence corrections and direct comparison with other species homologues. J. Pharmacol. Exp. Ther. 1995;272:134–142. [PubMed] [Google Scholar]
- TSUJI T., CHIBA S. Potentiating effects of methysergide on norepinephrine-induced constriction of the internal carotid artery of the dog. Jpn. J. Pharmacol. 1984;34:95–100. doi: 10.1254/jjp.34.95. [DOI] [PubMed] [Google Scholar]
- VON KÜGELGEN I., KURZ K., BÜLTMANN R., DRIESSEN B., STARKE K. Presynaptic modulation of the release of the co-transmitters noradrenaline and ATP. Fundam. Clin. Pharmacol. 1994;8:207–213. doi: 10.1111/j.1472-8206.1994.tb00800.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., CLARKE D.E. Characterization of α1-adrenoceptors mediating vasoconstriction to noradrenaline and nerve stimulation in the isolated perfused mesentery of rat. Br. J. Pharmacol. 1995;114:531–536. doi: 10.1111/j.1476-5381.1995.tb13259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG X.-P., CHIBA S. Pharmacological analysis for double peaked vasoconstrictor responses to periarterial electric stimulation. J. Auton. Pharmacol. 1998;18:343–347. doi: 10.1046/j.1365-2680.1998.1860343.x. [DOI] [PubMed] [Google Scholar]
- YANG X.-P., CHIBA S. Adrenergic-purinergic interactions on vasoconstrictor responses to periarterial electric nerve stimulation in canine splenic arteries. J. Auton. Pharmacol. 1999;19:139–144. doi: 10.1046/j.1365-2680.1999.00126.x. [DOI] [PubMed] [Google Scholar]
- YANG X.-P., CHIBA S. Effects of a selective neuropeptide Y Y1 receptor antagonist BIBP 3226 on double peaked vasoconstrictor responses to periarterial nerve stimulation in canine splenic arteries. Br. J. Pharmacol. 2000a;130:1699–1705. doi: 10.1038/sj.bjp.0703484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG X.-P., CHIBA S. Dissociation of potentiation of Leu31 Pro34 neuropeptide Y on adrenergic and on purinergic transmission in isolated canine splenic artery. Jpn. J. Pharmacol. 2000b;83:197–205. doi: 10.1254/jjp.83.197. [DOI] [PubMed] [Google Scholar]
- YANG X.-P., CHIBA S. Periarterial electrical nerve stimulation-induced adrenergic vasoconstriction inhibited by adrenergic α1B-receptor blockade but not by α1A-blockade. Jpn. J. Pharmacol. 2000c;84:360–362. doi: 10.1254/jjp.84.360. [DOI] [PubMed] [Google Scholar]
- YANG X.-P., CHIBA S. Effects of ω-conotoxin GVIA and diltiazem on double peaked vasoconstrictor responses to periarterial electric nerve stimulation in isolated canine splenic artery. Br. J. Pharmacol. 2000d;129:47–52. doi: 10.1038/sj.bjp.0702989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOCCA F.D., HYSLOP D.K., SMITH D.W., MAAYANI S. BMY 7378, a buspirone analog with high affinity, selectivity and low intrinsic activity at the 5-HT1A receptor in rat and guinea pig hippocampal membranes. Eur. J. Pharmacol. 1987;137:293–294. doi: 10.1016/0014-2999(87)90241-x. [DOI] [PubMed] [Google Scholar]


