Abstract
Recent results suggested that long-term treatment with a low dose of the organic nitrate pentaerythritol tetranitrate (PETN, 6 mg kg−1 per day) for 16 weeks slightly decreases aortic superoxide production in normal rabbits. We sought to determine if PETN can preserve endothelium dependent relaxation (EDR) in atherosclerotic rabbits.
Three groups of 9 – 10 New Zealand White rabbits received a cholesterol chow (0.75%) for 16 weeks. One group (CHOL16) served as control and two groups were fed for another 16 weeks a cholesterol-chow without (CHOL32) or with 6 mg PETN kg−1 per day (PETN32).
Isolated aortic rings of CHOL16 showed a typical impairment of EDR with a maximal relaxation at 1 μM acetylcholine of 28±16%. In CHOL32-rings EDR was completely impaired. In striking contrast, EDR in PETN32 (24±15%) was similar to that of CHOL16 indicating a protective effect of PETN on endothelial function. Vascular superoxide production measured with the lucigenin method was not different between the groups.
Aortic lesion formation in PETN32 was smaller than in CHOL32 (P<0.008). The onset of copper-induced LDL-oxidation (lag-time) after 16 weeks of cholesterol feeding (214±9 min) was reduced in CHOL32 (168±24 min, P=0.035) but not in PETN32 (220±21 min). This indicates prevention of increased LDL oxidation by PETN.
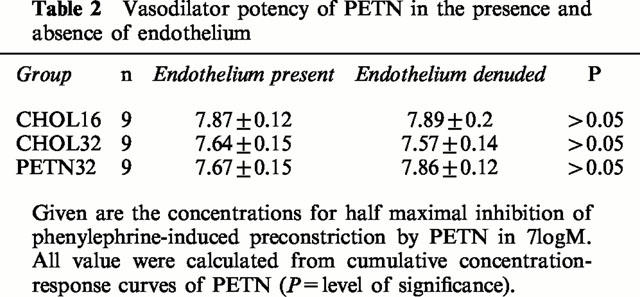
The halfmaximal effective vasodilator concentrations of PETN (in −logM) were identical in CHOL16 (7.9±0.1), CHOL32 (7.6±0.2) and PETN32 (7.7±0.2). Similar results were obtained with S-nitroso-N-acetyl-D,L-penicillamine.
These data suggest that PETN can reduce the progression of lesion formation, endothelial dysfunction and of LDL-oxidation in established atherosclerosis.
Keywords: Oxidized low density lipoprotein, endothelium-dependent vasorelaxation, organic nitrates, atherosclerosis, pentaerythritol tetranitrate
Introduction
In hypercholesterolemic rabbits and monkeys vasorelaxation to acetylcholine is almost absent or changed into vasoconstriction (Jayakody et al., 1985; Freiman et al., 1986). Similar observations were made in patients having coronary artery disease (Ludmer et al., 1986; Golino et al., 1991) or typical risk factors predisposing to this condition (Zeiher et al., 1993). Most likely, many other important endothelial functions to which the generation of •NO substantially contributes are also impaired. These include inhibition of platelet aggregation, adhesion molecule expression and smooth muscle proliferation and antioxidative effects (Busse & Fleming, 1996; Harrison, 1997). Therefore, •NO is probably an important antiatherogenic mediator in the vascular wall.
Organic nitrates such as glyceryl trinitrate are used for decades to treat coronary artery disease (Ahlner et al., 1991). These drugs act by the release of •NO in many different cell types including endothelial and vascular smooth muscle cells (Feelisch & Kelm, 1991; Salvemini et al., 1992). The organic nitrate pentaerythritol tetranitrate (PETN) was the most commonly used nitrovasodilator in former Eastern Germany. In vivo, PETN is metabolized to four denitrated products: pentaerythritol trinitrate, pentaerythritol dinitrate, pentaerythritol mononitrate and pentaerythritol as indicated by detection of these metabolites in plasma of man after oral application (Davidson et al., 1970; Weber et al., 1995). The nitrated metabolites are most likely pharmacologically active intermediates (Parker et al., 1975; Kojda et al., 1998a). Interestingly, several investigations showed that PETN has only a low tendency to produce typical nitrate tolerance (Kojda et al., 1998a; Dück & Richard, 1990; Kojda et al., 1995; Fink & Bassenge, 1997).
In a previous study we found that continuous treatment with a low dose of PETN (6 mg kg−1 per day) prevents the development of endothelial dysfunction in hypercholesterolemic rabbits (Kojda et al., 1995). These findings are in contrast to another study investigating the effect of molsidomine, a structurally different NO-donor (Bult et al., 1995), while another more experimentally used NO-Donor ((N-nitratopivaloyl-S-(N′-acetylalanyl)-cysteineethylester, SPM5185) showed beneficial effects in rat carotid artery intimal injury (Guo et al., 1994). Thus, it seems questionable that prolonged in vivo treatment with NO-donors can initiate a protective effect in atherosclerosis. We sought to determine if a long-term treatment with PETN can preserve endothelium dependent relaxation (EDR) and stop the progression of atherosclerosis after initiation of lesion development. As a model of atherosclerosis we used rabbits fed for 32 weeks with cholesterol and added PETN from week 17 onward.
Methods
Animal preparation
In this study, 30 New Zealand White rabbits were fed a cholesterol chow with or without PETN. The mean body weight was 2109±21 g, the age was 10 – 12 weeks. All animals were housed individually in stainless steel cages at a temperature of 18 – 20°C, a humidity of 50 – 60% and a day-night-rhythm of 12 h and received water ad libitum. Rabbits were randomly divided in three groups of 10 rabbits and were fed 40 g kg−1 per day a cholesterol-enriched (0.75%) rabbit chow for 16 weeks. One group served as control (CHOL16) and two groups were fed for another 16 weeks a cholesterol-chow without (CHOL32) or with 6 mg PETN kg−1 per day (PETN). During the experiment the body weight was determined weekly and the animals were supervised by a veterinarian. In CHOL32 and in PETN32 one rabbit died at week 20 of the feeding period. The autopsy showed massive cholesterol overloading of several organs and pulmonary oedema. These animals were not included in the experimental procedure and the statistic evaluation of the study. The concentration of plasma cholesterol was monitored using standard methods (Nägele et al., 1984). On the last day of the feeding period the acute experiments were performed after a 24-h fast.
Permission for this study was provided by the regional government (AZ 23.05-230-3-54/95) and the experiments were performed according to the guidelines for the use of experimental animals as given by ‘Deutsches Tierschutzgesetz' and to the ‘Guide for the care and use of laboratory animals' of the US National Institutes of Health.
Vasorelaxation studies
Rabbits were anaesthetized by injection of a mixture of xylazine (5 mg kg−1) and ketamine (25 mg kg−1) into the tibialis muscle. Blood samples for determination of plasma cholesterol and isolation of low density lipoprotein (LDL) were obtained from the central ear artery. The animals were killed by exsanguination in deep anaesthesia and the entire thoracic and abdominal aorta was dissected free and rapidly immersed in cold oxygenated (95% O2+5% CO2) Krebs-Henseleit solution (pH 7.4) of the following composition (mM): Na+ 143.07, K+ 5.87, Ca2+ 1.6, Mg2+ 1.18, Cl− 125.96, HCO3− 25.00, H2PO4− 1.18, SO42− 1.18 and glucose 5.05. Four ring segments (5 mm width) of thoracic aorta of each animal were mounted between stainless steel triangles in a water jacketed organ bath (37°C) for measurement of tension-development as described previously (Kojda et al., 1991). Two of these rings were gently rubbed on their intimal surface with a small wooden stick in order to remove the endothelium. Experiments with KCl (10 – 80 mM) in normal rabbit aorta revealed an optimal resting tension of 2 g for development of contractile function in the vessels (Kojda et al., 1995). After equilibration (1 h), contractile function of aortic segments was tested by application of KCl (80 mM). This was followed by a cumulative application of phenylephrine (0.01 – 10 μM), which resulted in a maximal tension (see Results). Function of endothelium was then examined by cumulative addition of acetylcholine (0.01 – 10 μM) following submaximal precontraction with 0.1 μM phenylephrine. Thereafter the aortic rings were divided in subgroups and the vasorelaxations to different type of •NO-donors such as S-nitroso-N-acetyl-D,L-penicillamine (SNAP, 1 nM – 10 μM) and PETN (0.1 nM – 10 μM) were studied by cumulative application following precontraction with phenylephrine (0.2 μM). KCl, acetylcholine, phenylephrine and each •NO-donor was studied in two endothelium intact and in two endothelium denuded thoracic rings from each animal.
Preliminary experiments revealed that the maximal achieved concentration of dimethylsulphoxide (0.05%), which was necessary to dissolve SNAP and PETN, exhibited no influence on aortic contractile function. In another set of preliminary experiments using rabbit aorta we confirmed that SNAP released •NO largely extracellularly (Kojda et al., 1998b). Preliminary measurements of aortic pressure, cardiac output, vascular resistance and left ventricular enddiastolic pressure in anaesthetized rabbits showed no effect of 0.63 mg PETN kg−1 and 2.8 mg PETN kg−1 when applicated as an aqueous suspension directly into the stomach, while 0.3 μg glyceryl trinitrate kg−1 per min effectively reduced these parameters.
Determination of aortic superoxide production
Aortic superoxide production was determined as described previously (Kojda et al., 1998c). Briefly, equilibrated segments of thoracic aorta were incubated at 37°C in albumin-buffer (pH 7.4) of the following composition (in mM): Na+ 144.93, K+ 7.23, Cl− 138.77, H2PO4− 4.55, HPO42− 8.03, glucose 5.55 and bovine serum albumine (0.1%, weight volume−1). This buffer was enriched with lucigenin (0.5 mM) and superoxide production was calculated from chemiluminescence measurements (Packard Luminometer Analyzer, Picolite A6112, Packard, Downers Grove, IL, U.S.A.).
Copper-induced LDL-oxidation
LDL-oxidation was performed according to Esterbauer et al. (1989). Briefly, fresh blood samples obtained from the central ear artery were mixed with sodium EDTA to a final concentration of 0.1%. The supernatant of a 1000×g spin (10°C, 10 min) was centrifuged again, supplemented with saccharose to 600 mg/ml and frozen (−80°C). LDL was prepared by KBr dense gradient centrifugation of thawed and precentrifuged (10,000×g, 10 min, 10°C) plasma samples supplemented with KBr to 1.22 g/l (20 h, 10°C, 120,000×g, Beckmann SW41 Ti, 40,000 r.p.m.). The easily visible yellow LDL-fraction was isolated and desalted (Econo-Pac, Biorad, München, Germany). Total protein was determined (Bradford, 1976) and the aqueous oxidation mix (3 ml) containing 1.7 μM CuSO4 and 150 μg LDL protein was continuously monitored at 234 nm for 11 h. In order to limit spontaneous oxidation of LDL, the desalting procedure was done at 4°C within 45 min after LDL isolation.
Determination of atherosclerotic lesions
The aortic arch, the aortic segments used for the tension studies and the abdominal aorta were fixed in formol solution (10 ml formaldehyde solution 37%, 90 ml double distilled water, 2.5 g calcium acetate, pH 6.8) and stored after repeated changing of the fixative at 4°C. The tissues were stained for 2 – 3 min by placing in different solutions as follows: distilled water, ethanol 70%, staining solution (composition: Sudan IV 0.1 g, acetone 50 ml, ethanol 70% 50 ml), ethanol 70%, distilled water. The percentage of the intimal lesion area as related to the total intimal surface was determined in each segment separately by a computer-scanning apparatus (HP ScanJet 6100 C) and calculated with a computer program (Paint Shop Pro, version 3.11).
Substances and solutions
SNAP was synthesized in our laboratory according to Field et al. (1978) as described previously (Kojda et al., 1996). PETN was provided by ISIS-Pharma, Zwickau, Germany. All other chemicals were obtained from Merck, Darmstadt, Germany or from Sigma, Deisenhofen, Germany, in analytical grade.
The stock solutions of acetylcholine (10 mM) and phenylephrine (10 mM) were prepared in distilled water. Solutions of SNAP (200 mM) and PETN 10 mM were prepared in dimethylsulphoxide. All stock solutions were prepared daily, diluted with Krebs-buffer as required, kept on ice and protected from daylight until use. All concentrations indicated in the text and figures are expressed as final bath concentrations.
Statistics
The concentrations of the half-maximal vasorelaxant effect of the nitrates (pD2-values) were calculated from the individual concentration-effect-curves as proposed previously (Hafner et al., 1977). Vasocontractile responses are expressed as percentage of the maximal vasoconstriction to phenylephrine and vasorelaxant responses are expressed as percentage of the contractile response achieved with 0.2 μM phenylephrine at the beginning of the experiments.
All data were analysed by a standard computer program (GraphPad Prism PC Software, Version 2.0, Analysis of Variance, ANOVA) and are expressed as mean values and standard error of the mean (s.e.m.) or as median values. Significant differences were evaluated using either unpaired two-tailed Students t-test or Newman-Keuls Multiple Comparison Test (ANOVA). A P-value below 0.05 was considered as significant.
Results
Plasma cholesterol
The cholesterol chow induced a marked increase of plasma cholesterol concentration reaching a maximum of 2184±132 mg dl−1 (CHOL16), 2287±127 mg dl−1 (CHOL32) and 2168±162 mg dl−1 (PETN32) after 4 weeks. These values gradually declined to 1663±112 mg dl−1 (CHOL16), 2070±169 mg dl−1 (CHOL32) and 1740±147 mg dl−1 (PETN32) after 16 weeks. A further reduction to 1703±183 mg dl−1 (CHOL32) and 1467±170 mg dl−1 (PETN32) was observed at the last measurement. There was no significant difference between the groups at 16 weeks (one-way ANOVA, P=0.1258). Furthermore, analysis by two-way ANOVA showed no significant differences for time and plasma-cholesterol at weeks 16 and 32 between CHOL32 and PETN32. Variations of the plasma cholesterol concentrations within the groups had no significant influence on the severity of aortic intimal lesions or the sensitivity of LDL to copper induced oxidation (Table 1).
Table 1.
Correlation coefficients for linear relationships
Atherosclerotic lesions
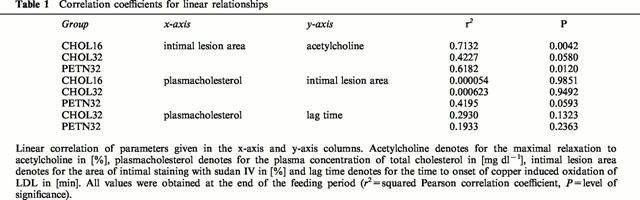
The readily visible atherosclerotic lesions were more pronounced in the aortic arch than in the thoracic and the abdominal aortic segments (Figure 1A). In CHOL32 the percentage of the total intimal area covered with sudan IV visualized lesions was somewhat greater than in CHOL16 but the difference did not reach statistical significance (Figure 1B). In contrast, the intimal lesion area detected in PETN32 was significantly smaller compared to CHOL32 suggesting that treatment with PETN slightly inhibited the progression of intimal aortic lesions.
Figure 1.
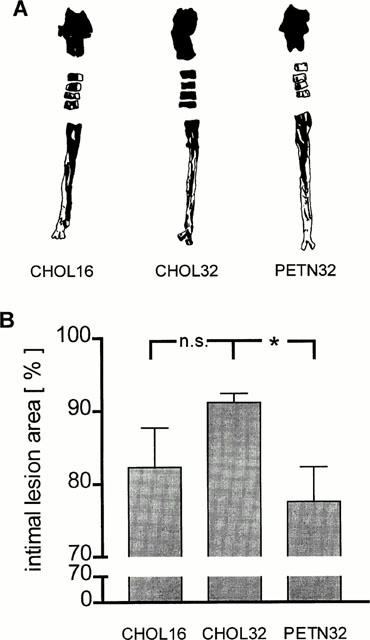
Area of intimal lesions in the aortic arch, four ring segments of the thoracic aorta and the abdominal aorta of CHOL16, CHOL32 and PETN32 stained with sudan IV (black areas). Given are (A) representative examples of stained aortas and (B) mean±s.e.mean of the stained intimal areas (n.s.=not significant; *, P=0.008).
Endothelium-dependent vasorelaxation
In CHOL16 relaxation of aortic rings induced by acetylcholine was concentration dependent but showed a maximum of only 28±16% (Figure 2) indicating severe impairment of endothelial function. Extension of the cholesterol feeding for another 16 weeks (CHOL32) further aggravated endothelial function as evidenced by the complete absence of vasorelaxation induced by acetylcholine. In striking contrast, endothelium-dependent vasorelaxation in PETN32 and CHOL16 was identical (Figure 2) suggesting that treatment with PETN protected against the progression of the impairment of endothelial function in hypercholesterolemia. In accordance, there was a significant linear relationship between the maximal relaxation to acetylcholine and the severity of aortic intimal lesion area in CHOL16 and PETN32 but not in CHOL32 (Table 1). Comparative analysis of the correlations by ANOVA showed no significant difference (P=0.0879) between the groups suggesting that the relationship between endothelium-dependent vasorelaxation and the severity of lesion formation was independent of the PETN-treatment. In endothelium denuded aortic rings acetylcholine evoked vasoconstrictions only.
Figure 2.
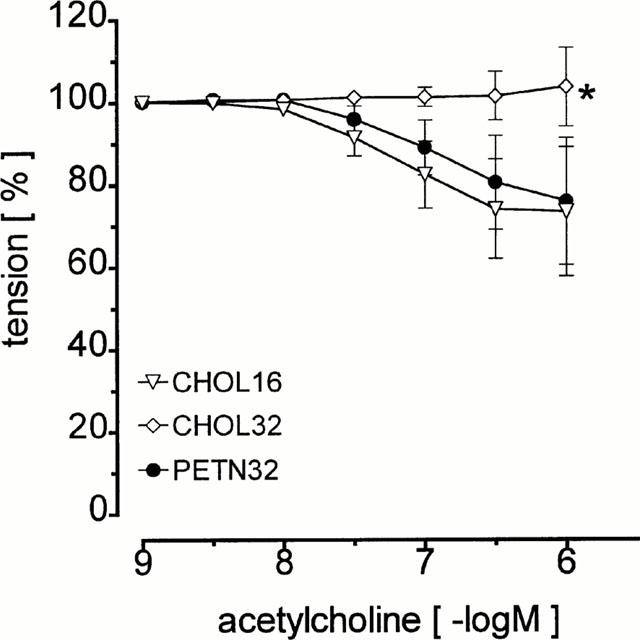
Endothelium-dependent vasorelaxation in the ring segments of the thoracic aorta of CHOL16, CHOL32 and PETN32 in the presence of endothelium (*, P<0.05 vs CHOL16 and PETN32).
Vasorelaxation to •NO-donors
Increasing the time of the cholesterol feeding from 16 to 32 weeks had no effect on vasorelaxation induced by the •NO-donors PETN (Figure 3A) and SNAP (Figure 3B). In particular, there was no difference in the relaxant response to PETN of aortic rings of CHOL32 and PETN32 (Figure 3A) indicating that continuous oral treatment with 6 mg kg−1 per day PETN for 16 weeks did not change the vasorelaxant response to this organic nitrate.
Figure 3.
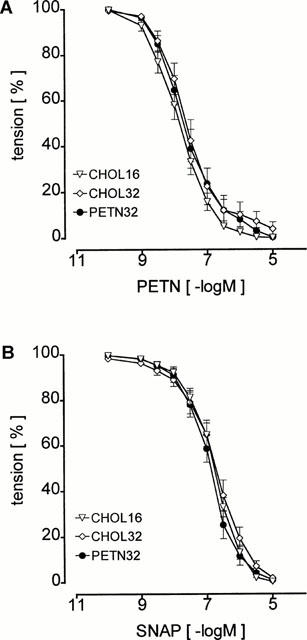
Identical vasorelaxation in ring segments of the thoracic aorta of CHOL16, CHOL32 and PETN32 induced by the •NO-donors PETN (A) and SNAP (B) indicating absence of nitrate tolerance or changes in •NO-sensitivity of the aortic smooth muscle.
Aortic superoxide production
Production of superoxide by aortic rings as measured by the lucigenin chemiluminescence method was identical in the three groups of rabbits. The median values (25 and 75% Percentile) were 1050 (851 – 1225) counts mg−1 min−1 for CHOL16, 909 (739 – 1173) counts mg−1 min−1 for CHOL32 and 985 (713 – 1316) counts mg−1 per min for PETN32.
Oxidation of LDL
The sensitivity of LDL to copper induced oxidation was determined after 16 weeks of cholesterol feeding and at the end of the experiment in CHOL32 and PETN32. The measured lag-times correspond to the onset of oxidation of LDL and a short lag-time indicates a greater susceptibility of LDL to oxidation. After 32 weeks of cholesterol feeding the lag-time of CHOL32 significantly (P=0.035) decreased while that of PETN32 remained (Figure 4). The difference between CHOL32 and PETN32 was not significant (P=0.058). These data suggest that treatment with PETN appears to prevent the increase in LDL-susceptibility to copper induced oxidation.
Figure 4.
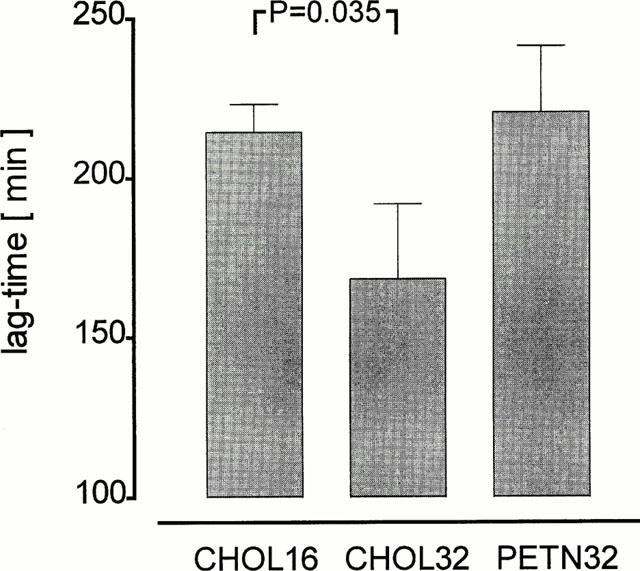
Sensitivity of LDL to copper induced oxidation. Given are the observed lag-times which correspond to the onset of oxidation of LDL as measured by continuous monitoring of absorption changes at 234 nm (diene absorption maximum). Treatment with PETN prevented the decrease in lag-time observed from weeks 16 to 32 of cholesterol feeding.
Discussion
Impairment of endothelium-dependent vasorelaxation by hypercholesterolemia is a well known phenomenon occurring not only in rabbits (Jayakody et al., 1985) but also in primates and man (Freiman et al., 1986; Ludmer et al., 1986). Most likely, other important biological functions of endogenous •NO-production such as inhibition of platelet aggregation, adhesion molecule expression and smooth muscle proliferation are impaired as well (Busse & Fleming, 1996; Harrison, 1997; Kojda & Harrison, 1999). In our study, the impairment of endothelium-dependent vasorelaxation was a function of time. Earlier investigations have shown that aging from 16 weeks to 32 weeks has no effect on the complete vasorelaxation induced by acetylcholine (Kojda et al., 1998a). After 16 weeks of cholesterol chow (CHOL16) we still observed a significant degree of endothelium dependent vasorelaxation to acetylcholine, while after 32 weeks (CHOL32) this relaxation was completely lost. This observation is consistent with other reports showing that endothelial dysfunction progresses as the duration of cholesterol feeding is prolonged (Busse & Fleming, 1996). Here we show for the first time that the long acting •NO-donor PETN is able to prevent this progression.
In rabbits, pentaerythritol dinitrate and pentaerythritol mononitrate have been detected in plasma samples taken after a 18 – 24 h washout period from animals treated with 6 mg PETN kg−1 per day (Kojda et al., 1995). It is likely that these metabolites are involved in the therapeutic efficacy of PETN since both of them have been shown to be active in vitro and in vivo (Parker et al., 1975; Kojda et al., 1998a; Müllenheim et al., 2000). These data are consistent with our finding that PETN is a typical organic nitrate that is metabolized to the denitrated derivatives and •NO (Kojda et al., 1998a). Taken together, these data demonstrate that the pharmacological effects of PETN in vivo are most likely mediated by •NO. •NO has several potentially anti-atherosclerotic vascular activities. Of these, anti-aggregative, anti-adhesive and antioxidative effects are believed to be particularly important (Busse & Fleming, 1996; Kojda & Harrison, 1999). Recent data on the development of vascular damage (intimal hyperplasia) following angioplasty in mice lacking the endothelial •NO-synthase gene have shown that endogenous •NO is vasoprotective in the setting of non-atherosclerotic vascular damage (Moroi et al., 1998). Since PETN has been shown to be an •NO-donor, the results of our study are consistent with vasoprotective effects of •NO in atherosclerosis.
Other investigations have failed to demonstrate a protective effect of •NO-donors on atherosclerosis. Our previous finding that isosorbide mononitrate had no anti-atherosclerotic effect does not necessarily speak against anti-atherosclerotic activity of organic nitrates or other •NO-donors, since the difference between PETN and isosorbide mononitrate might be related to experimental conditions such as an unsufficient dosage or pharmacokinetic differences between these drugs (Kojda et al., 1995). In contrast, a study with molsidomine showed that area, thickness, weight and cholesterylester content of the lesions were augmented by the •NO donor molsidomine (Bult et al., 1995). It seems possible that these effects might have been induced by peroxynitrite rather than •NO. In vitro, SIN-1, the bioactive metabolite of molsidomine, releases both NO and superoxide (Feelisch et al., 1989) and generation of peroxynitrite can be easily proved by measuring oxidation of dihydrorhodamine (Crow, 1997). In vivo, superoxide generated by SIN-1 is most likely converted to hydrogen peroxide by either conventional Cu/Zn- or the extracellular superoxide dismutase. Nevertheless, generation of peroxynitrite may also occur in vivo, since the reaction between superoxide and •NO is three times faster than that between superoxide and superoxide dismutase (Kojda & Harrison, 1999). Thus, the negative outcome of the study by Bult et al. (1995) might reflect a proatherosclerotic effect of peroxynitrite rather than •NO.
In an attempt to assess the extent of oxidant stress in atherosclerosis we measured the sensitivity of LDL to copper induced oxidation. We found that extension of the cholesterol feeding from 16 to 32 weeks induced a significant reduction of the LDL oxidation resistance. This reduction was absent in the group treated with cholesterol and PETN suggesting that PETN increased LDL resistance to oxidation. These data may warrant the conclusion that treatment with the •NO-donor PETN is associated with antiatherosclerotic effects mediated by the release of nitric oxide from this drug. According to previously reported actions of •NO in the vasculature, the effects of PETN might be related to the antioxiative, anti-adhesive, antiproliferative, anti-aggregative or anti-apoptotic effects of •NO (Kojda & Harrison, 1999; Bult et al., 1999). Oxidized LDL is believed to play a causative role in mediating vascular dysfunction in vivo and has been shown to be correlated with the severity of cardiovascular disease (Steinberg, 1995; Cox & Cohen, 1996; Esterbauer et al., 1997). In accordance, prevention of LDL oxidation would be most likely associated with vascular protection against atherosclerotic damage. It is therefore tempting to speculate that antioxidative effects of •NO derived from PETN contributed to the prevention of endothelial dysfunction.
In general, prevention of LDL-oxidation in vivo might reflect either reduced vascular superoxide production and/or increased protection of LDL from oxidation. In a previous study we reported that long-term treatment of rabbits with PETN in the absence of atherosclerosis slightly reduced aortic superoxide generation (Kojda et al., 1998a). In this study we found that PETN treatment in the setting of hypercholesterolemia has no significant effect on aortic superoxide generation (see Results). These data do not provide a direct link between aortic superoxide production and the reduction of LDL-oxidation (Figure 4). However, oxidation of LDL in vivo is not necessarily dependent on aortic superoxide production but may be taken as a more general reflection of vascular oxidant stress (Cox & Cohen, 1996; Kojda & Harrison, 1999). Furthermore, the prevention by PETN of the cholesterol-induced impairment of endothelium-dependent vasodilation (Figure 2) is also not matched by a reduction of aortic superoxide production, although both parameters were measured in directly adjacent aortic ring segments. One possible explanation might be that the lucigenin method does not detect small increases in aortic superoxide production which may account for the aggravation of endothelium-dependent vasodilation by prolongation of hypercholesterolemia from 16 to 32 weeks. On the other hand, treatment with the •NO-donor PETN may reduce the deleterious actions of superoxide in vivo by favourably shifting the vascular superoxide-•NO-balance without having a direct effect on vascular superoxide generation. It has been shown that •NO can react with peroxynitrite, a highly toxic reaction product of •NO and superoxide, to form the untoxic products N2O and NO2− (Miles et al., 1996).
Continuous treatment with organic nitrates is known to produce nitrate tolerance. According to a recently developed hypothesis, nitrate tolerance induced by a glyceryl trinitrate patch is associated with an increase of vascular superoxide production that reduces the bioavailability of •NO and may have proatherosclerotic effects (Münzel & Harrison, 1997). In contrast, continuous treatment with PETN did not induce typical nitrate tolerance in this study since there was no change of the vasodilator activity of PETN between the different groups. To further confirm this unexpected finding, another set of rabbits were treated for 3 weeks with either 6 mg PETN kg−1 per day or 100 mg PETN kg−1 per day and compared to control rabbits receiving normal chow. Neither acetylcholine-induced nor PETN-induced aortic relaxations or aortic superoxide production were different between the groups (data not shown). This is in accord with previous studies in animals (Kojda et al., 1995; 1998a; Fink & Bassenge, 1997) and man (Dück & Richard, 1990) suggesting that PETN induces only a minor degree of nitrate tolerance in rabbits and dogs. In addition, the oral dose of 6 mg PETN kg−1 per day applied in our study does not change haemodynamics in rabbits (see Methods) (Müllenheim et al., 2000). Thus, the lack of nitrate tolerance may be an important prerequisite for the protective action of long acting nitrates.
We have used the cholesterol fed rabbit as a model of atherosclerosis. Earlier reports suggested that rabbit atherosclerotic lesions consists primarily of macrophage derived foam cells and poorly match human lesions which consists primarily of smooth muscle cells (Wissler, 1992). This estimation is based mainly on results from studies using a short term (2 – 3 months) high cholesterol (1 – 2%) feeding protocol. In contrast, recent studies have shown that feeding rabbits for a longer time (⩾6 months) with a lower cholesterol content in the diet (0.2 – 0.5%) results in the formation of advanced lesions which closely resemble those in humans (Daley et al., 1994a,1994b). In keeping with these findings, our results obtained in this animal model might be of considerable relevance for clinical settings.
In summary, our current findings are compatible with an anti-atherosclerotic and vasoprotective effect of NO. It is likely that the continuous release of NO from the PETN metabolites PEDN and PEMN, which had no effect on haemodynamics and did not induce typical nitrate tolerance, can inhibit several aspects of the atherosclerotic process.Table 2
Table 2.
Vasodilator potency of PETN in the presence and absence of endothelium
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft; Projekt: K0155713-2.
Abbreviations
- CHOL16
rabbits receiving a cholesterol diet for 16 weeks
- CHOL32
rabbits receiving a cholesterol diet for 32 weeks
- EDR
endothelium dependent relaxation
- LDL
low density lipoprotein
- •NO
nitric oxide
- PETN
pentaerythritol tetranitrate
- PETN32
rabbits receiving a cholesterol diet for 16 weeks and a cholesterol diet enriched with PETN for another 16 weeks
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
References
- AHLNER J., ANDERSSON R.G.G., TORFGÅRD K., AXELSSON K.L. Organic nitrate esters: Clinical use and mechanisms of actions. Pharmacol. Rev. 1991;43:351–423. [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BULT H., DE MEYER G.R.Y., HERMAN A.G. Influence of chronic treatment with a nitric oxide donor on fatty streak development and reactivity of the rabbit aorta. Br. J. Pharmacol. 1995;114:1371–1382. doi: 10.1111/j.1476-5381.1995.tb13358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULT H., HERMAN A.G., MATTHYS K.E. Antiatherosclerotic activity of drugs in relation to nitric oxide function. Eur. J. Pharmacol. 1999;375:157–176. doi: 10.1016/s0014-2999(99)00328-3. [DOI] [PubMed] [Google Scholar]
- BUSSE R., FLEMING I. Endothelial dysfunction in atherosclerosis. J. Vasc. Res. 1996;33:181–194. doi: 10.1159/000159147. [DOI] [PubMed] [Google Scholar]
- COX D.A., COHEN M.L. Effects of Oxidized Low-Densitiy Lipoprotein on Vascular Contraction and Relaxation: Clinical and Pharmacological Implications in Atherosclerosis. Pharmacol. Rev. 1996;48:3–19. [PubMed] [Google Scholar]
- CROW J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric. Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- DALEY S.J., HERDERICK E.E., CORNHILL J.F., ROGERS K.A. Cholesterol-fed and casein-fed rabbit models of atherosclerosis: Part 1: Differing lesion area and volume despite equal plasma cholesterol levels. Arterioscler. Thromb. 1994a;14:95–104. doi: 10.1161/01.atv.14.1.95. [DOI] [PubMed] [Google Scholar]
- DALEY S.J., KLEMP K.F., GUYTON J.R., ROGERS K.A. Cholesterol-fed and casein-fed rabbit models of atherosclerosis: Part 2: Differing morphological severity of atherogenesis despite matched plasma cholesterol levels. Arterioscler. Thromb. 1994b;14:105–114. doi: 10.1161/01.atv.14.1.105. [DOI] [PubMed] [Google Scholar]
- DAVIDSON I.E.F., MILLER H.S., DICARLO F.J. Absorption, excretion and metabolism of pentaerythritol tetranitrate in humans. J. Pharmacol. Exp. Ther. 1970;175:42–50. [PubMed] [Google Scholar]
- DÜCK K.D., RICHARD F. Langzeittherapie bei koronarer Herzkrankheit. Wirkungsverlust durch Toleranzentwicklung. Z. Ges. Inn. Med. 1990;45:736–741. [PubMed] [Google Scholar]
- ESTERBAUER H., SCHMIDT R., HAYN M.Relationship among Oxidation of Low-Density Lipoprotein, Antioxidant Protection, and Atherosclerosis Antioxidants in disease mechanisms and therapy 1997San Diego, California, U.S.A.: Academic Press; 425–456.ed. Sies, H. pp [DOI] [PubMed] [Google Scholar]
- ESTERBAUER H., STRIEGL G., PUHL H., ROTHENEDER M. Continuous monitoring of in vivo oxidation of human low density lipoprotein. Free Radic. Res. Commun. 1989;6:67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., KELM M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem. Biophys. Res. Commun. 1991;180:286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., OSTROWSKI J., NOACK E. On the mechanism of NO-release from sydnonimines. J. Cardiovasc. Pharmacol. 1989;14 suppl. 11:S13–S22. [PubMed] [Google Scholar]
- FIELD L., DILTS R.V., RAVICHANDRAN R., LENHERT G., CARNAHAN G.E. An unusual stable thionitrite from N-acetyl-D,L-penicillamine; x-ray crystal and molecular structure of 2-(acetylamino)-2-carboxy-1,1-dimethyl thionitrite. JCS Chem. Commun. 1978;1157:249–250. [Google Scholar]
- FINK B., BASSENGE E. Unexpected, tolerance-devoid vasomotor and platelet actions of pentaerythrityl tetranitrate. J. Cardiovasc. Pharmacol. 1997;30:831–836. doi: 10.1097/00005344-199712000-00020. [DOI] [PubMed] [Google Scholar]
- FREIMAN P.C., MITCHELL G.G., HEISTAD D.D., ARMSTRONG M.L., HARRISON D.G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ. Res. 1986;58:783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- GOLINO P., PISCIONE F., WILLERSON J.T., CAPELLI-BIGAZZI M., FOCACCIO A., VILLARI B., INDOLFI C., RUSSOLILLO E., CONDORELLI M., CHIARIELLO M. Divergent effects of serotonin on coronary-artery dimensions and blood flow in patients with coronary atherosclerosis and control patients. N. Engl. J. Med. 1991;324:641–648. doi: 10.1056/NEJM199103073241001. [DOI] [PubMed] [Google Scholar]
- GUO J., MILHOAN K.A., TUAN R.S., LEFER A.M. Beneficial effect of SPM-5185, a cysteine-containing nitric oxide donor, in rat carotid artery intimal injury. Circ. Res. 1994;75:77–84. doi: 10.1161/01.res.75.1.77. [DOI] [PubMed] [Google Scholar]
- HAFNER D., HEINEN E., NOACK E. Mathematical analysis of concentration-response-relationships. Arzneim. Forsch. 1977;27:1871–1873. [PubMed] [Google Scholar]
- HARRISON D.G. Cellular and Molecular Mechanisms of Endothelial Cell Dysfunction. J. Clin. Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAYAKODY T.L., SENARATNE M.P.J., THOMPSON A.B.R., KAPPAGODA C.T. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can. J. Physiol. Pharmacol. 1985;63:1206–1209. doi: 10.1139/y85-199. [DOI] [PubMed] [Google Scholar]
- KOJDA G., HARRISON D.G. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- KOJDA G., HACKER A., NOACK E. Effects of non-intermittent treatment of rabbits with pentaerythritol tetranitrate on vascular reactivity and vascular superoxide production. Eur. J. Pharmacol. 1998a;355:23–31. doi: 10.1016/s0014-2999(98)00460-9. [DOI] [PubMed] [Google Scholar]
- KOJDA G., HÜSGEN B., HACKER A., SCHNAITH E.M., KOTTENBERG E., NOACK E. Impairment of endothelium dependent vasorelaxation in experimental atherosclerosis is dependent on gender. Cardiovasc. Res. 1998b;37:738–747. doi: 10.1016/s0008-6363(97)00268-x. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KLAUS W., WERNER G., FRICKE U. The influence of 3-ester side chain variation on the cardiovascular profile of nitrendipine in porcine isolated trabeculae and coronary arteries. Naunyn Schmiedeberg's Arch. Pharmacol. 1991;344:488–494. doi: 10.1007/BF00172590. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KOTTENBERG K., HACKER A., NOACK E. Alterations of the vascular and the myocardial guanylate cyclase/cGMP-system induced by long-term hypertension in rats. Pharm. Acta Helv. 1998c;73:27–35. doi: 10.1016/s0031-6865(97)00044-7. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KOTTENBERG K., NIX P., SCHLÜTER K.D., PIPER H.M., NOACK E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ. Res. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- KOJDA G., STEIN D., KOTTENBERG E., SCHNAITH E.M., NOACK E. In vivo effects of pentaerythrityl-tetranitrate and isosorbide-5-mononitrate on the development of atherosclerosis and endothelial dysfunction in cholesterol-fed rabbits. J. Cardiovasc. Pharmacol. 1995;25:763–773. doi: 10.1097/00005344-199505000-00012. [DOI] [PubMed] [Google Scholar]
- LUDMER P.L., SELWYN A.P., SHOOK T.L., WAYNE R.R., MUDGE G.H., ALEXANDER R.W., GANZ P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- MILES A.M., BOHLE D.S., GLASSBRENNER P.A., HANSERT B., WINK D.A., GRISHAM M.B. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J. Biol. Chem. 1996;271:40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- MOROI M., ZHANG L., YASUDA T., VIRMANI R., GOLD H.K., FISHMAN M.C., HUANG P.L. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J. Clin. Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜLLENHEIM J., FRÄßDORF J., THÄMER V., KOJDA G.Negligible Afterload reduction induced by pentaerythritol tetranitrate Naunyn Schmiedebergs Arch. Pharmacol. 2000361Suppl(Abstract) [Google Scholar]
- MÜNZEL T., HARRISON D.G. Evidence for a role of oxygen-derived free radicals and protein kinase C in nitrate tolerance. J. Mol. Med. 1997;75:891–900. doi: 10.1007/s001090050181. [DOI] [PubMed] [Google Scholar]
- NÄGELE U., HÄGELE E.O., SAUER G., WIEDEMANN E., LEHMANN P., WAHLEFELD A.W., GRUBER W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J. Clin. Chem. Clin. Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- PARKER J.C., DICARLO F.J., DAVIDSON I.W. Comparative vasodilator effects of nitroglycerin, pentaerythritol trinitrate and biometabolites, and other organic nitrates. Eur. J. Pharmacol. 1975;31:29–37. doi: 10.1016/0014-2999(75)90075-8. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MOLLACE V., PISTELLI A., ANGGARD E., VANE J. Metabolism of glyceryl trinitrate to nitric oxide by endothelial cells and smooth muscle cells and its induction by Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 1992;89:982–986. doi: 10.1073/pnas.89.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG D. Clinical trials of antioxidants in atherosclerosis: Are we doing the right thing. Lancet. 1995;346:36–38. doi: 10.1016/s0140-6736(95)92657-7. [DOI] [PubMed] [Google Scholar]
- WEBER W., MICHAELIS K., LUCKOW V., KUNTZE U., STALLEICKEN D. Pharmacokinetics and bioavailability of pentaerythrityl tetranitrate and two of its metabolites. Arzneim. Forsch. 1995;45:781–784. [PubMed] [Google Scholar]
- WISSLER R.W. Theories and new horizons in the pathogenesis of atherosclerosis and the mechanisms of clinical effects. Arch. Pathol. Lab. Med. 1992;116:1281–1291. [PubMed] [Google Scholar]
- ZEIHER A.M., DREXLER H., SAURBIER B., JUST H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J. Clin. Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]


