Abstract
The aim of this study was to investigate the structure-activity relationship of S-alkyl-L-isothiocitrulline-containing dipeptides towards three partially purified recombinant human nitric oxide synthase (NOS) isozymes, as well as the effects of these compounds on cytokine-induced NO production by human DLD-1 cells.
In an in vitro assay, S-methyl-L-isothiocitrulline (L-MIT) was slightly selective for human neuronal NOS (nNOS) over the inducible (iNOS) or endothelial (eNOS) isozyme, but the combination of a hydrophobic L-amino acid (L-Phe, L-Leu or L-Trp) with L-MIT dramatically altered the inhibition pattern to give selective iNOS inhibitors. Introduction of a hydroxy, nitro, amino or methoxy group at the para position of the aromatic ring of L-MIT-L-Phe (MILF) decreased the selectivity and inhibitory potency. A longer or larger S-alkyl group also decreased the selectivity and potency. Dixon analysis showed that all of the dipeptides were competitive inhibitors of the three isoforms of human NOS. The enzymatic time course curves indicated that MILF was a slow binding inhibitor of human iNOS.
These results suggest that the human NOS isozymes have different-sized cavities in the binding site near the position to which the C-terminal of L-arginine binds, and the cavity of iNOS is hydrophobic. Interestingly, L-MIT-D-Phe (MIDF) showed little inhibitory activity or selectivity, suggesting that the cavity of human iNOS is located in a well-defined direction from the α carbon atom.
NO production in cytokine-stimulated human DLD-1 cells was measured with a fluorescent indicator, DAF-FM. MILF, L-MIT-L-Trp(-CHO) (MILW) and L-MIT-L-Tyr (MILY) showed more potent activity than L-MIT in this whole-cell assay.
Thus, S-alkyl-L-isothiocitrulline-containing dipeptides are selective inhibitors of human iNOS, and work efficiently in cell-based assay.
Keywords: Nitric oxide, human nitric oxide synthase, isozyme-selective inhibition, structure-activity relationship study, hydrophobic L-amino acids, depeptide, human DLD-1 cells
Introduction
Nitric oxide (NO) is involved in the regulation of diverse physiological processes, including actions of immune cells (Schmitt & Walter, 1994). NO and L-citrulline are produced in the two-step oxidation of L-arginine by the neuronal, inducible and endothelial isozymes of nitric oxide synthase (nNOS, iNOS and eNOS), all of which use NADPH, FAD, FMN, haeme, and tetrahydrobiopterin as cofactors (Nathan & Xie, 1994).
iNOS activity is readily induced by bacterial endotoxins and cytokines and NO plays an important role in host defence against parasites (Liew et al., 1990). However, some human tissues, such as human articular cartilage, produce NO copiously in response to cytokine stimulation (Rediske et al., 1994). There is also evidence that iNOS is present in diseased tissues – iNOS mRNA and protein are present in joint tissues from patients with rheumatoid arthritis (Sakurai et al., 1995) and calcium-independent NOS activity has been detected in homogenates of colonic tissue from patients with ulcerative colitis (Boughton-smith et al., 1993). The production of NO in inflamed tissues is likely to contribute to disease pathology by increasing blood flow, thereby potentiating plasma leakage from inflamed microvessels (Laszlo et al., 1994). In addition, NO may promote tissue injury by reacting with superoxide anion to produce peroxynitrite in vivo (Haddad et al., 1994). The finding that NO production is enhanced in human inflamed tissues through the induction of iNOS suggests that inhibitors of iNOS would modulate smooth muscle contractility, platelet reactivity, and central and peripheral neurotransmission, and have therapeutic potential, particularly those that do not affect the protective and physiological roles of eNOS.
The generation of NO by NOS can be inhibited by analogues of the substrate, L-arginine. But, the most commonly used inhibitors of NOS, namely NG-monomethyl-L-arginine (L-NMMA), NG-nitro-L-arginine (L-NA) and its methyl ester (L-NAME), inhibits eNOS at least as strongly as they inhibit iNOS (Gross et al., 1991). Non-amino acid analogues of L-arginine such as aminoguanidine (Misako et al., 1993), alkylguanidines (Hansan et al., 1993) and S-alkylisothiourea (Garvey et al., 1994) have also been reported to inhibit NOS. These compounds show some selectivity for iNOS. However, the need for isoform selectivity is critical, and safe inhibitors that show high selectivity and good cell penetration are also needed to delineate the role of the isoforms of NOS in disease models.
Recently, we reported that L-arginine analogue-containing dipeptides inhibit mouse iNOS without inhibiting rat nNOS activity (Kobayashi et al., 1999). Accordingly, we proposed that L-arginine analogue-containing dipeptides might provide a good starting point for development of novel, clinically useful analgesics. However, detailed structure-activity relationship studies on the C-terminal of the L-arginine derivatives had not been conducted, and we had used animal NOS and neglected the effect of these agents on eNOS.
Here we describe the structure-activity relationship of S-alkyl-L-isothiocitrulline-containing dipeptides towards three recombinant human NOS isozymes and the effects of these compounds on NO production by a human tumor cell line (DLD-1).
Methods
Expression and purification of human NOS
Human NOS isozymes were expressed in Sf-9 (a Spodoptera frugiperda insect cell line) as described in detail previously (Nakane et al., 1995). Baculovirus transfer vectors for human nNOS (EcoI – EcoRI)/pVL1393, human iNOS (NotI – DraI)/pVL1392 and human eNOS (NotI – XbaI)/pVL1392 were provided by Dr Masaki Nakane (Abbott Labs, IL, U.S.A.). Calmodulin (CaM) cDNA was obtained from Dr Hiroyuki Hori. An EcoI to BamHI fragment containing the coding region of CaM was isolated and subcloned into a pBACgus-2cp transfer plasmid (Novagen; baculovirus transfer vector). The vectors were employed to prepare recombinant baculovirus using a BaculoGold kit from Pharmingen. Monolayer cultures of Sf-9 cells were infected with each human NOS recombinant baculovirus and incubated for 72 h at 27°C in Ex-Cell 420 insect serum-free medium supplemented with 1.5 μg ml−1 hemin, 1 μM riboflavin, 5 μM nicotinic acid and 10 μM sepiapterin. For iNOS, Sf-9 cells were co-infected with the CaM baculovirus. The cells were harvested by centrifugation and homogenized in 5 volumes of ice-cold buffer ((mM) Tris-HCl 50, pH 7.5 containing EGTA 1, DTT 1, PMSF 1), pepstatin 1 μg ml−1, leupeptin 1 μg ml−1, chymostatin 20 μM, FAD 5 μM, FMN 5 μM, BH4 10 μM: for eNOS, 10 mM CHAPS was included, and centrifuged at 40,000×g for 30 min. The supernatant fractions were applied to a 2-ml 2′, 5′-ADP Sepharose affinity column (Pharmacia). The column was washed with 0.5 M NaCl and eluted with 10 mM NADPH. The eluted fraction was concentrated to 0.2 mg ml−1 with a Centricon 30 (Amicon). For eNOS, 10 mM CHAPS was included in the buffer to maintain solubility of the enzyme.
Enzyme assay
Recombinant human NOS activity was measured by monitoring the conversion of [14C]-L-arginine to [14C]-L-citrulline as described previously (Hori et al., 1997). Enzyme solution (5 μl) was added to 25 μl of buffer containing HEPES 50 mM (pH 7.4), [14C]-L-arginine 4.2 – 16.7 μM, CaCl2 1.8 mM, DTT 150 μM, CaM 10 μg ml−1, BH4 15 μM, EGTA 1 mM, FAD 15 μM, FMN 15 μM, BSA 0.9 mg ml−1, NADPH 1.5 mM and the test compound in the concentration range from 0.1 μM – 1 mM. After incubation for 10 min at 37°C, the reaction was terminated by adding 20 μl of 5 mM cold L-arginine and 5 mM L-citrulline and boiling the mixture at 90 – 95°C for 3 min. An aliquot (5 μl) was spotted onto a cellulose plate and separated by thin layer chromatography using methanol-pyridine-water (20 : 1 : 5, v:v:v). Radioactivity was measured by autoradiography with a Fuji BAS1500 Bioimaging analyzer.
Measurement of NO production by DLD-1
DLD-1 cells (JCRB, Tokyo, Japan; human colorectal adenocarcinoma cell line) were grown in RPMI (Sigma) with L-glutamine, penicillin-streptomycin and 10% heat-inactivated foetal calf serum. Prior to assay, the cells were seeded in 96-well plates (2×105 cells per well) and allowed to attach overnight. NO production induced by exposure of the cells to 200 u ml−1 human interferon-γ (hIFN-γ), 10 ng human tumour necrosis factor-α (hTNF-α) and 2 ng human interleukin-1β (hIL-1β) for 24 h at 37°C (Sherman et al., 1993) was measured with the recently described fluorescent indicator DAF-FM (Kojima et al., 1999; Nakatsubo et al., 1998). A dimethyl sulphoxide (DMSO; final concentration, 0.02%) solution of DAF-FM (1 μM) and the test compound at a concentration of 0.3 μM – 1 mM were added at the same time as cytokines. L-Arginine (1.15 mM) was included in the medium. After the incubation, 180 μl of the supernatant was transferred to a black microplate well and fluorescence was measured in a fluorescence microplate reader (Titertek Fluorescence II, Flow Laboratories) calibrated for excitation at 485 nm and emission at 538 nm.
Data analysis
The IC50 values of the inhibitors were calculated by using linear regression between data points just above and below 50% activity. The kinetic data were obtained by Dixon analysis (Dixon, 1953). Calculations were performed using Excel software.
Synthesis and purification of S-alkyl-L-isothiocitrulline-containing dipeptides
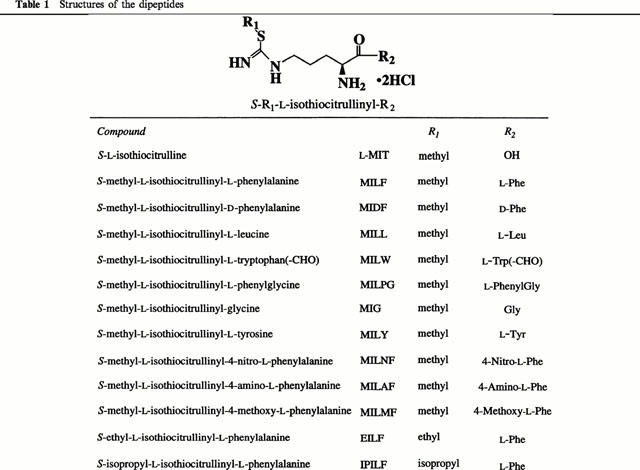
S-Alkyl-L-isothiocitrulline-containing dipeptides used in this study, the structures of which are given in Table 1, were obtained as follows: Nε-R-Nα-Boc-L-ornithine was condensed with various amino acid O-t-butyl esters. The R group (Z- or Fmoc-) of Nε-R-Nα-Boc-L-ornithinyl-amino acid O-t-butyl ester was cleaved with Pd-C and H2 or diethylamine, respectively. Nα-Boc-L-ornithinyl-amino acid O-t-butyl ester was converted to Nα-Boc-L-thiocitrullinyl-amino acid O-t-butyl ester with thiophosgene and ammonia gas. After S-methylation, S-methyl-Nα-Boc-L-isothiocitrullinyl-amino acid O-t-butyl ester was deprotected with trifluoroacetic acid. Finally, the TFA salt was changed to the hydrochloride salt with a (−) ion exchange column. In the case of synthesis of S-methyl-L-isothiocitrullinyl-4-amino-L-phenylalanine, the 4-nitro group was converted to a 4-amino group with Pt-C and H2, to which the Fmoc group is stable. The 4-amino group was protected with a Boc group to prevent methylation at the time of S-methylation. When S-methyl-L-isothiocitrullinyl-4-methoxy-L-phenylalanine was synthesized, the hydroxy group of the Nε-Z-Nα-Boc-L-Orn-L-Tyr O-t-butyl ester was converted to a methoxy group with methyl iodide. In the case of synthesis of S-ethyl-L-isothiocitrullinyl-L-phenylalanine and S-isopropyl-L-isothiocitrullinyl-L-phenylalanine, L-thiocitrullinyl-L-phenylalanine was S-alkylated with ethyl iodide or isopropyl iodide. All dipeptides were confirmed to be pure by 1H-NMR spectral examination.
Table 1.
Structures of the dipeptides
Materials
S-Alkyl-L-isothiocitrulline-containing dipeptides were synthesized in our own laboratories (see Methods). hIFN-γ, hTNF-α and hIL-1β were purchased from Boehringer Mannheim (Tokyo, Japan). DAF-FM was synthesized according to the reported method in our own laboratories (Kojima et al., 1999). [14C]-L-Arginine was purchased from NEN Life Science Products (Boston, MA, U.S.A.). S-Methylisothiocitrulline (L-MIT) was purchased from Wako (Osaka, Japan). Cellulose plates were from Funakoshi (Tokyo, Japan). All other chemicals were of the highest grade available.
Results
Inhibitory activity of S-alkyl-L-isothiocitrulline-containing dipeptides on partially purified recombinant human NOS isozymes
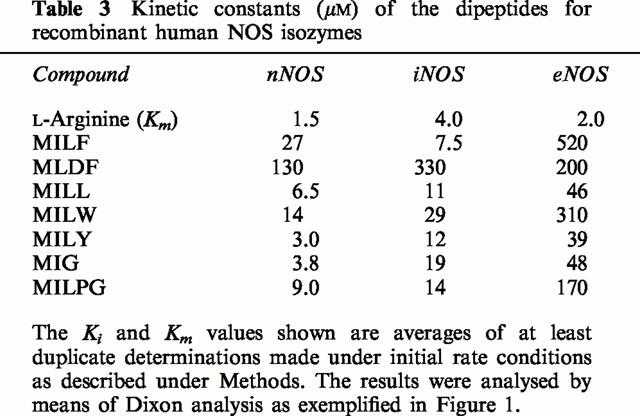
The IC50 values of the dipeptides were obtained from data on the concentration dependence of inhibitory activity. As shown in Table 2, L-MIT was slightly selective for nNOS, but S-methyl-L-isothiocitrullinyl-L-phenylalanine (MILF), S-methyl-L-isothiocitrullinyl-L-leucine (MILL), S-methyl-L-isothiocitrullinyl-L-tryptophan(-CHO) (MILW), in which hydrophobic L-amino acids are combined with L-MIT, showed a dramatically altered inhibition pattern, exhibiting selectivity to iNOS. Interestingly, S-methyl-L-isothiocitrullinyl-D-phenylalanine (MIDF) showed only very weak inhibition with no selectivity. S-Methyl-L-isothiocitrullinyl-L-phenylglycine (MILPG) inhibited both nNOS and iNOS, but its iNOS inhibitory activity was less potent than that of MILF. S-Methyl-L-isothiocitrullinyl-glycine (MIG) showed similar selectivity to L-MIT, but with weaker inhibitor potency for all the NOS isozymes. S-Methyl-L-isothiocitrullinyl-L-tyrosine (MILY), bearing a hydroxy group at the para position of the aromatic ring of MILF, inhibited nNOS as well as iNOS, and its iNOS inhibitory activity was weaker than that of MILF. Introduction of a nitro (S-methyl-L-isothiocitrullinyl-4-nitro-L-phenylalanine; MILNF), amino (S-methyl-L-isothiocitrullinyl-4-amino-L-phenylalanine; MILAF), or methoxy (S-methyl-L-isothiocitrullinyl-4-methoxy-L-phenylalanine; MILMF) group at the para position of the aromatic ring of MILF resulted in a reduction of selectivity and inhibitory potency. Furthermore, a longer or larger S-alkyl group, as in (S-ethyl-L-isothiocitrullinyl-L-phenylalanine (EILF) and S-isopropyl-L-isothiocitrullinyl-L-phenylalanine (IPILF)), also decreased selectivity and inhibitory potency. The dipeptides generally had little or no inhibitory effect on human eNOS activity. The greatest selectivity was observed with MILF, which was at least 256-fold more potent against human iNOS than human eNOS.
Table 2.
IC50 values (μM) for inhibition of human NOS isozymes by L-MIT and the dipeptides

Dixon plots for inhibition by MILF are shown in Figure 1. All of the dipeptides were competitive inhibitors of the three isoforms of human NOS (Table 3). These results suggest that the dipeptides bind at the L-arginine-binding site.
Figure 1.
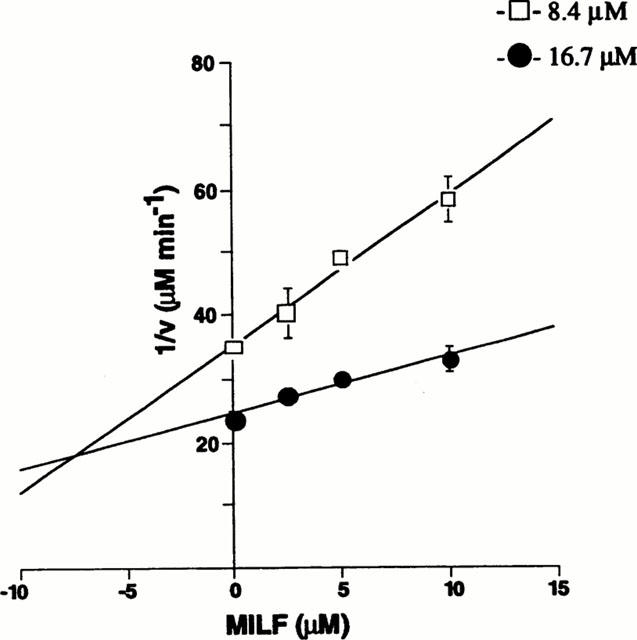
Dixon analysis of inhibition of human iNOS by MILF. Measurement of human iNOS activity under initial velocity conditions is described under Methods. [14C]-L-Arginine concentration was 8.4 or 16.7 μM. The data represent the mean±s.e.mean of three individual experiments.
Table 3.
Kinetic constants (μM) of the dipeptides for recombinant human NOS isozymes
Inhibition of human iNOS by MILF was time-dependent. The first-order rate constant for the onset of inhibition of iNOS by 10 μM MILF at 16.7 μM L-arginine was 0.0026 s−1 (Figure 2).
Figure 2.
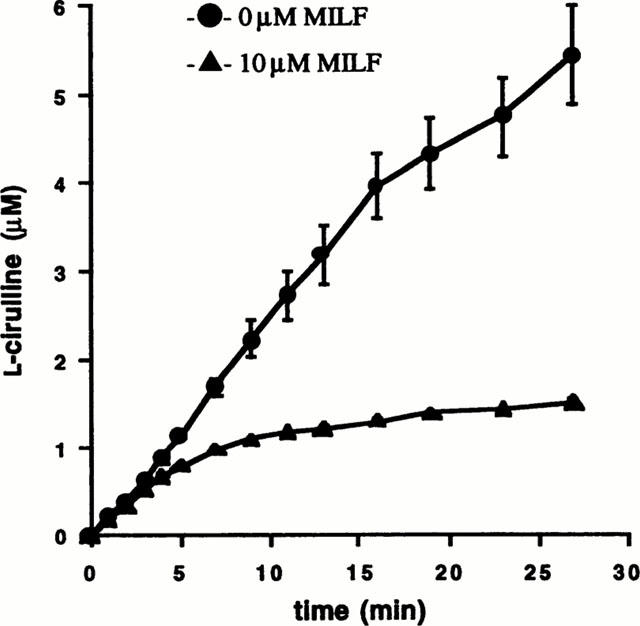
Time dependence of human iNOS inhibition by MILF. Progress curves for L-citrulline formation with 16.7 μM [14C]-L-arginine in assay media (Methods) and 0 or 10 μM MILF. The data represent the mean±s.e.mean of three individual experiments.
Effect of S-alkyl-L-isothiocitrulline-containing dipeptides on NO production by human colorectal adenocarcinoma cell line DLD-1
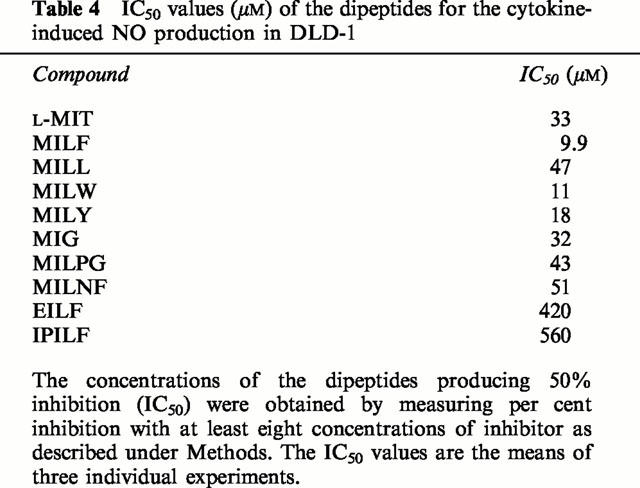
We investigated their effect on cytokine-induced NO production by human DLD-1 cells. NO production was measured with the fluorescent indicator DAF-FM. As shown in Table 4, NO production in cytokine-stimulated DLD-1 was highly sensitive to inhibition by the dipeptides. Although the dipeptides showed lower inhibitory activity than L-MIT in the assay of recombinant human iNOS (IC50=0.3 μM for L-MIT, IC50=3.9 μM for MILF, IC50=15 μM for MILW and IC50=6.7 μM for MILY), MILF, MILW and MILY showed more potent inhibitory activity than L-MIT in the whole-cell assay. MILW most efficiently entered DLD-1 cells. However, EILF did not efficiently inhibit cellular NO production (IC50=13 μM for recombinant human iNOS).
Table 4.
IC50 values (μM) of the dipeptides for the cytokine-induced NO production in DLD-1
Discussion
A series of S-alkyl-L-isothiocitrulline-containing dipeptides was synthesized and screened for biological activity in vitro against the three isoforms of human NOS. L-MIT was slightly selective for human nNOS, but the combination of a hydrophobic L-amino acid (L-Phe, L-Leu or L-Trp) with L-MIT dramatically altered the inhibition pattern, affording selective iNOS inhibitors. Introduction of a hydroxy, nitro, amino or methoxy group at the para position of the aromatic ring of MILF resulted in a reduction of selectivity and inhibitory activity. Dixon analysis showed that all of the dipeptides were competitive inhibitors of the three isoforms of human NOS. Further, MILF showed higher affinity for human iNOS than MIG, which contains glycine without a functional group (Ki=7.5 μM for MILF and Ki=19 μM for MIG). These results suggest that the NOS isozymes have different-sized cavities in the binding site near the position at which the C-terminal of L-arginine binds, and the cavity of human iNOS is hydrophobic. The cavity of human iNOS is well fitted by a benzyl group, since the dipeptide containing L-phenylalanine was more potent than that containing L-leucine or L-tryptophan(-CHO) (IC50=3.9 μM for MILF, IC50=9.7 μM for MILL and IC50=15 μM for MILW). Further, the distance of the cavity from the C-terminal is optimal for L-phenylalanine, because the iNOS-inhibitory activity of MILPG, containing L-phenylglycine, is reduced in contrast with that of MILF (IC50=6.7 μM for MILPG). Interestingly, MIDF showed little inhibitory activity or selectivity. These results indicate that the hydrophobic cavity of human iNOS has affinity for L-amino acid, but not D-amino acid. This may reflect its location in a specific direction from the α carbon atom. Furthermore, a longer or larger S-alkyl group decreased selectivity and inhibition. The X-ray structure of the complex of human iNOS and S-ethylisothiourea has been reported (Fischmann et al., 1999). Unlike the substrate side chain, the ethyl group of S-ethylisothiourea is packed near the haeme and Phe 369 side chain. In the structure-activity relationship data of S-ethylisothioureas (Garvey et al., 1994), ethyl and isopropyl moieties appear optimal for the NOS binding cavity (Ki=0.019 μM for S-ethylisothiourea and 0.0098 μM for S-isopropylisothiourea), and the cavity is relatively small and narrow (Ki=0.24 μM for S-n-propylisothiourea and 11 μM for S-n-butylisothiourea). Grounded on that finding, we suggest that introduction of an S-alkyl group into an L-arginine analogue impairs binding of the hydrophobic amino acid and the S-alkyl group to their respective binding sites.
We have previously reported that L-arginine analogue-containing dipeptides show isozyme-selective inhibition of mouse iNOS over rat nNOS (Kobayashi et al., 1999). The IC50 values of MILF, MIDF and MILL for rat nNOS were 39, 50 and 20 μM, respectively, and those for mouse iNOS were 0.59, 47 and 1 μM, respectively. There seems to be no marked difference between rat nNOS and human nNOS, but the dipeptides were stronger inhibitors of mouse iNOS than human iNOS. The dipeptide binding sites of human and mouse iNOS may be structurally distinct. Indeed, rat and human nNOS have very similar amino acid sequences (93% identical; Nakane et al., 1993), whereas iNOS exhibits only 80% amino acid identity between mouse and human (Geller et al., 1993).
The dipeptides were next assayed in cultured cells (DLD-1; human colorectal adenocarcinoma cell line). MILF (IC50=9.9 μM), MILW (IC50=11 μM) and MILY (IC50=18 μM) exhibited more potent iNOS-inhibitory activity than L-MIT (IC50=33 μM) in this whole-cell assay. When these results are compared with those using recombinant human NOS isozymes (IC50=0.3 μM for L-MIT, IC50=3.9 μM for MILF, IC50=15 μM for MILW, IC50=6.7 μM for MILY), it is clear that the inhibitory activity towards cellular NO production increased with increasing hydrophobicity of the L-amino acid at the C-terminal. At present, the mechanism involved is not clear. We hypothesize that the introduction of a hydrophobic L-amino acid enhanced cell permeability by allowing the dipeptides to be taken up by system L, a transporter of large neutral amino acids such as Phe and Tyr. Addition of L-phenylalanine to an enkephalin analogue, DPDPE, significantly increased permeation across bovine endothelial cell monolayers (Greene et al., 1996), but this compound had a short life in both serum and brain homogenate, being cleaved by aminopeptidase. S-Alkyl-L-isothiocitrulline-containing dipeptides may also be enzymatically cleaved in blood. However, it is conceivable that this may be overcome by isosteric replacement of the peptidic bond, e.g. conversion to sulfonamide, N-methylation or replacement of the amidic bond with an ester (Fauchère, 1986).
EILF did not inhibit well the cellular NO production (IC50=13 μM for recombinant human iNOS, IC50=420 μM for NO production in DLD-1 cells). L-MIT and S-ethylisothiocitrulline (L-EIT) have been identified as inhibitors of nNOS (Furfine et al., 1994), with L-EIT showing similar inhibitory activity to L-MIT in rat brain cytosol. However, L-EIT did not inhibit nNOS as potently as did L-MIT in rat brain slices. Although detailed analysis of the inhibition in rat brain slices was not done, the data suggest that L-EIT does not enter well into neuronal cells. This is consistent with the idea that EILF enters DLD-1 cells somewhat more inefficiently than MILF.
Recently, dipeptides containing NG-nitro-L-arginine were reported (Huang et al., 1999) to be selective inhibitors of bovine nNOS. The most potent compound was NG-nitro-L-arginine-L-2, 4-diaminobutyramide, which also exhibited the highest selectivity over bovine eNOS (>1500 fold), together with a 192-fold selectivity over mouse iNOS. These authors suggested an electrostatic or hydrogen bonding interaction between the dipeptide side chain and nNOS, but not eNOS or iNOS. However, it is important to inhibit iNOS, since there is increasing evidence that iNOS-like activity is present in inflamed human tissues. Moreover, iNOS plays a role in the pathophysiology of a variety of other diseases, including diabetes and meningitis (Corbett et al., 1994; Buster et al., 1995). But, non-isoform-selective inhibition of NO formation may lead to side effects by inhibiting the physiological and protective roles of eNOS (Billiar et al., 1990; Tracey et al., 1995).
S-Substituted isothioureas have been identified as highly potent inhibitors of NOS, S-Methylisothiourea (SMT) shows some selectivity for iNOS over eNOS and had beneficial effects in rodent models of septic shock (Szabó et al., 1994). Detailed structure-activity relationship studies in the isothiourea series identified S-(2-aminoethyl)isothiourea (aminoethyl-TU) as a relatively selective iNOS inhibitor (Southan et al., 1995). However, strict isoform selectivity is still desirable, and good cell penetration is also required.
Our findings here confirm that S-alkyl-L-isothiocitrulline-containing dipeptides are selective inhibitors of human iNOS and are effective in cell-based assay. They may be useful as lead compounds for developing new iNOS inhibitors to treat human disease, such as arthritis, inflammation, diabetes and meningitis.
Acknowledgments
We thank Dr Masaki Nakane of Abbott Laboratory for providing human NOS isozyme plasmids. We are also grateful to Dr Tsutomu Ogura of the National Cancer Center Research Institute and Dr Hidetaka Miyoshi of Tanabe Seiyaku Co. Ltd for valuable discussions. This work was supported in part by the Ministry of Education, Science, Sports and Culture of Japan (grants numbers; 11794026, 12470475, 12557217 to T Nagano and 11470494, 12793009, 12020217 to T Higuchi), as well as the Mitsubishi Foundation and the Research Foundation for Opt-Science and Technology.
Abbreviations
- CaM
calmodulin
- e
endothelial
- EILF
S-ethyl-L-isothiocitrullinyl-L-phenylalanine
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- i
inducible
- IPILF
S-isopropyl-L-isothiocitrullinyl-L-phenylalanine
- MIDF
S-methyl-L-isothiocitrullinyl-D-phenylalanine
- MIG
S-methyl-L-isothiocitrullinyl-glycine
- MILAF
S-methyl-L-isothiocitrullinyl-4-amino-L-phenylalanine
- MILF
S-methyl-L-isothiocitrullinyl-L-phenylalanine
- MILL
S-methyl-L-isothiocitrullinyl-L-leucine
- MILMF
S-methyl-L-isothiocitrullinyl-4-methoxy-L-phenylalanine
- MILNF
S-methyl-L-isothiocitrullinyl-4-nitro-L-phenylalanine
- MILPG
S-methyl-L-isothiocitrullinyl-L-phenylglycine
- MILW
S-methyl-L-isothiocitrullinyl-L-tryptophan(-CHO)
- MILY
S-methyl-L-isothiocitrullinyl-L-tyrosine
- L-MIT
S-methyl-L-isothiocitrulline
- n
neuronal
- NADPH
nicotinamide adenine dinucleotide phosphate
- L-NAME
NG-nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
- L-NMMA
NG-monomethyl-L-arginine
- TFA
trifluoroacetic acid
References
- BILLIAR T.R., CURRAN R.D., HARBRECHT B.G., STUEHR D.J., DEMETRIS A.J., SIMMONS R.L. Modulation of nitrogen oxide synthesis: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrite/nitrate biosynthesis while promoting hepatic damage. J. Leukocyte Biol. 1990;48:565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., HAWKEY C.J., COLE A.T., BALSITIS M., WHITTLE B.J.R., MONCADA S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- BUSTER B.L., WEINTROB A.C., TOWNSEND G.C., SCHELD W.M. Potential role of nitric oxide in the pathophysiology of experimental bacterial meningitis in rats. Infect. Immun. 1995;63:3835–3839. doi: 10.1128/iai.63.10.3835-3839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBETT J.A., KWON G., MISKO T.P., RODI C.P., MCDANIEL M.L. Tyrosine kinase involvement in IL-1 beta-induced expression of iNOS by beta-cells purified from islets of Langerhans. Am. J. Physiol. 1994;267:C48–C54. doi: 10.1152/ajpcell.1994.267.1.C48. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem. J. 1953;55:170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUCHÈRE J.-L. Elements for the rational design of peptide drugs. Advances in Drug Research. 1986;15:29–69. [Google Scholar]
- FISCHMANN T.O., HRUZA A., NIU X.D., FOSSETTA J.D., LUNN C.A., DOLPHIN E., PRONGAY A.J., REICHERT P., LUNDELL D.J., NARULA S.K., WEBER P.C. Structure characterization of nitric oxide synthase isoforms reveals striking active-site conversion. Nature Structural Biology. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- FURFINE E.S., HARMON M.F., PAITH J.E., KNOWLES R.G., SALTERS M., KIFF R.J., DUFFY C., HAZELWOOD R., OPLINGER J.A., GARVEY E.P. Potent and selective inhibition of human nitric oxide synthase. J. Biol. Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.P., TANOUR Y, , SHERMAN P.A., FOWLER M., MARSHALL S., HARMON M.F., PAITH J.E., FURFINE E.S. Potent and selective inhibition of human nitric oxide synthases. J. Biol. Chem. 1994;94:2407–2413. [PubMed] [Google Scholar]
- GELLER D.A., LOWENSTEIN C.J., SHAPIRO R.A., NUSSLER A.K., SILVIO M.D., WANG S.C., NAKAYAMA D.K., SIMMONS R.L., SNYDER S.H., BILLIAR T.R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE D.L., HAU V.S., ABBRUSCATO T.J., BARTOSZ H., MISICKA A., LIPKOWSKI A.W., HOM S., GILLESPIE T.J., HRUBY V.J., DAVIS T.P. Enkephalin analog prodrugs: Assessment of in vitro conversion, enzyme cleavage characterization and blood-brain barrier permeability. J. Pharm. Exp. Ther. 1996;277:1366–1375. [PubMed] [Google Scholar]
- GROSS S.S., JAFFE E.A., LEVI R., KILBOURN R.G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogues with a rank-order of potency characteristic of activated macrophages. Biochem. Biophy. Res. Commun. 1991;187:823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- HADDAD I.Y., PATAKI G., HU P., GALLIANI C., BECKMAN J.S., MATALONM S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J. Clin. Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSAN K., HEESEN B.J., CORBETT J.A., MCDANIEL M.L., CHANG K., ALLISON W., WOLFENBUTTEL B.H.R., WILLIAMSON J.R., TILTON R.G. Inhibition of nitric oxide formation by guanidines. Eur. J. Pharmacol. 1993;249:101–106. doi: 10.1016/0014-2999(93)90667-7. [DOI] [PubMed] [Google Scholar]
- HORI H., IWASAKI T., KURAHASHI Y., NISHINO T. Calcium-dependent inactivation of neuronal nitric oxide synthase: evidence for the existence of stabilization/activation factor. Biochem. Biophys. Res. Commun. 1997;234:476–480. doi: 10.1006/bbrc.1997.6664. [DOI] [PubMed] [Google Scholar]
- HUANG H., MARTASEK P., ROMAN L.J., MASTERS B.S.S., SILVERMAN R.B. Nω-Nitroarginine-containing dipeptide amides. Potent and highly selective inhibitors of neuronal nitric oxide synthase. J. Med. Chem. 1999;42:3147–3153. doi: 10.1021/jm990111c. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI N., HIGUCHI T., URANO Y., KIKUCHI K., HIROBE M., NAGANO T. Dipeptides containing L-arginine analogues: new isozyme-selective inhibitors of nitric oxide synthase. Biol. & Pharm. Bull. 1999;22:936–940. doi: 10.1248/bpb.22.936. [DOI] [PubMed] [Google Scholar]
- KOJIMA H., URANO Y., KIKUCHI K., HIGUCHI T., NAGANO T. Fluorescent indicators for imaging nitric oxide production. Angew. Chem. Int. Ed. 1999;38:3209–3212. doi: 10.1002/(sici)1521-3773(19991102)38:21<3209::aid-anie3209>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- LASZLO F., WHITTLE B.J.R., MONCADA S. Time-dependent enhancement or inhibition of endotoxin-induced vascular injury in rat intestine by nitric oxide synthase inhibitors. Br. J. Pharmacol. 1994;111:1309–1315. doi: 10.1111/j.1476-5381.1994.tb14887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEW F.Y., MILLOTT S., PARKINSON C., PALMER R.M.J., MONCADA S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J. Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- MISAKO T.P., MOORE W.M., KASTEN T.P., NICKOLS D.A., CORBETT J.A., TILTON R.G., MCDANIEL M.L., WILLIAMSON J.R., CURRIE M.G. Selective inhibition of the inducible NO synthase by aminoguanidine. Eur. J. Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- NAKANE M., POLLOCK J.S., KLINGHOFER V., BASHA F., MARSDEN P.A., HOKARI A., OGURA T., ESUMI H., CARTER G.W. Functional expression of three isoforms of human nitric oxide synthase in baculovirus-infected insect cells. Biochem. Biophy. Res. Commun. 1995;206:511–517. doi: 10.1006/bbrc.1995.1073. [DOI] [PubMed] [Google Scholar]
- NAKANE M., SCHMIDT H.H.H.W., POLLOCK J.S., FÖRSTERMANN U., MURAD F. Cloned human brain nitric oxide is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- NAKATSUBO N., KOJIMA H., KIKUCHI K., NAGOSHI H., HIRATA Y., MAEDA D., IMAI Y., IRIMURA T., NAGANO T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- NATHAN C., XIE Q. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- REDISKE J.J., KOEHNE C.F., ZHANG B., LOTZ M. The inducible production of nitric oxide by articular cell types. Osteoarthritis Cartilage. 1994;2:199–206. doi: 10.1016/s1063-4584(05)80069-x. [DOI] [PubMed] [Google Scholar]
- SAKURAI H., KOSAKA H., LIU M.-F., HIGASHIYAMA H., HIRATA Y., SAITO I, MIYASAKA N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J. Clin. Invest. 1995;96:2357–2363. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMITT H.H.H.W., WALTER U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- SHERMAN P.A., LAUBACH V.E., REEP B.R., WOOD E.R. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993;32:11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABÓ C., THIEMERMANN C. Isothiourea: potent inhibitors of nitric oxide synthase with variable isoform selectivity. Br. J. Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., SOUTHAN G.J., THIEMERMANN C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRACEY W.R., NAKANE M., BASHA F., CARTER G. In vivo pharmacological evaluation of two novel type II (inducible) nitric oxide synthase inhibitors. Can. J. Physiol. Pharmacol. 1995;73:665–669. doi: 10.1139/y95-085. [DOI] [PubMed] [Google Scholar]


