Abstract
The signal transduction pathways involved in kinin B2 receptor-related vasodilation were investigated in rat isolated perfused kidneys. During prostaglandin F2α or KCl-induced constriction, the vasodilator response to a selective B2 receptor agonist, Tyr(Me)8bradykinin (Tyr(Me)8BK), was assessed.
Tyr(Me)8BK produced a concentration- and endothelium-dependent relaxation that was decreased by about 30 – 40% after inhibition of nitric oxide (NO) synthase by NG-nitro-L-arginine (L-NOARG) or of cyclo-oxygenase by indomethacin; a greater decrease (about 40 – 50%) was observed after concomitant inhibition of the two pathways.
High extracellular K+ diminished Tyr(Me)8BK-induced relaxation by about 75% suggesting a major contribution of endothelium-derived hyperpolarization. The residual response was almost completely suppressed by NO synthase and cyclo-oxygenase inhibition. The K+ channel inhibitors, tetrabutylammonium (non-specific) and charybdotoxin (specific for Ca2+-activated K+ channel), suppressed Tyr(Me)8BK-induced relaxation resistant to L-NOARG and indomethacin.
Inhibition of cytochrome P450 (clotrimazole or 7-ethoxyresorufin) decreased the NO/prostanoids-independent relaxation to Tyr(Me)8BK by more than 60%, while inhibition of the cannabinoid CB1 receptor (SR 141716A) had only a moderate effect.
Acetylcholine induced a concentration-dependent relaxation with characteristics nearly similar to the response to Tyr(Me)8BK. In contrast, the relaxation elicited by sodium nitroprusside was potentiated in the absence of NO (L-NOARG or removal of endothelium) but remained unchanged otherwise.
These results indicate that the activation of kinin B2 receptors in the rat isolated kidney elicits an endothelium-dependent vasorelaxation, mainly dependent on the activation of charybdotoxin-sensitive Ca2+-activated K+ channels. In addition, cytochrome P450 derivatives appear to be involved.
Keywords: Tyr(Me)8bradykinin, kinin B2 receptor, acetylcholine, endothelium, rat isolated kidney, nitric oxide, prostanoids, K channels, cytochrome P450, cannabinoid CB1 receptor
Introduction
After the description by Furchgott & Zawadzki (1980) of an absolute requirement of endothelium for the vasodilation elicited by acetylcholine (ACh) in rabbit aorta, endothelial cells were shown to release a variety of mediators which can act to alter the mechanical and electrical properties of the vascular smooth muscle cells (Furchgott & Vanhoutte, 1989). These factors include prostacyclin, endothelin, endothelium-derived relaxing factor and endothelium-derived hyperpolarizing factor (EDHF). Although endothelium-derived relaxing factor was rapidly recognized as nitric oxide (NO) or a compound releasing NO (Palmer et al., 1987; Ignarro et al., 1987), the identity of EDHF still remains controversial (Quilley et al., 1997; Mombouli & Vanhoutte, 1997). Endothelium-dependent vasodilation also occurs with bradykinin (BK), mainly by the activation of bradykinin B2 receptors. BK has been shown to increase the release of prostacyclin and NO from bovine aortic endothelial cells in culture (Busse et al., 1994; Blatter et al., 1995) and to produce hyperpolarization in coronary arteries with intact endothelium (Vanhoutte, 1993; Fisslthaler et al., 1999). The involvement of three major pathways, i.e. prostacyclin, NO and EDHF, in BK-induced relaxation was further documented in various blood vessels by the use of specific inhibitors of cyclo-oxygenase (COX), NO synthase (NOS) and K+ channels. These studies also revealed that, according to the vessel, each pathway did not contribute to the same degree to the vasorelaxation. In particular, NO-independent mechanisms appear to play a more important role in small resistance vessels (Garland et al., 1995).
In the rat renal vasculature, we previously reported that the main response to BK existed of a relaxation via bradykinin B2 receptors (Bagaté et al., 1999). BK-induced vasodilation was mimicked by Tyr(Me)8BK, a selective B2 receptor agonist (Regoli & Barabé, 1980), and was inhibited by icatibant, a specific B2 receptor antagonist. The question of kinin signalling pathways in renal vessels was previously addressed using BK (Fulton et al., 1992; Rapacon et al., 1996; Mieyal et al., 1998). The aim of the present study was to investigate these pathways in the isolated perfused rat kidney, with Tyr(Me)8BK. The kinin B2 receptor-mediated vasodilation was analysed before and after exclusion of transduction pathways by selective inhibitors. After having controlled requirement of intact endothelium, we evaluated the effects of NOS and/or COX inhibition. The NO/prostanoids-independent vasodilation was considered to be linked to so called ‘EDHF' although measurement of vascular smooth muscle cell membrane potential was not possible in our experimental preparation. However, suppression of the response with high extracellular K+ and by inhibitors of K+ channels supported the contribution of a hyperpolarizing factor. We finally investigated the effects of cytochrome P450 inhibition and cannabinoid CB1 receptor antagonism because epoxyeicosatrienoic acids (EETs) and endogenous cannabinoids are possible candidates for EDHF (Mombouli & Vanhoutte, 1997).
Methods
Animals
Male Wistar rats (220 – 280 g, Janvier breeding, Le Genest St Isle, France) were used. Animals were housed in a room at 20°C with a 12 h light/dark cycle (light on at 0600 h) and allowed free access to tap water and standard food (AO4 pellets, 0.2% sodium, UAR, Villemoisson/Orge, France). The rats stayed in our animal facility for at least 1 week before the start of the experiments. Experiments were performed in accordance with guidelines of the European Community and the French Government concerning the use of animals.
The isolated perfused rat kidney preparation
After sodium pentobarbitone anaesthesia (45 mg kg−1 i.p.), the right kidney was prepared with special care to avoid ischaemia and in vitro perfused via the mesenteric artery as described previously (Barthelmebs et al., 1996; Bagaté et al., 1999). Perfusion was performed without recirculation (open circuit) except for the experiment in which charybdotoxin was used (closed circuit). The perfusion medium was a prewarmed (37°C), oxygenated (95% O2 / 5% CO2), colloid free Tyrode's solution of the following composition (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1.1, NaH2PO4 0.42, NaHCO3 12, glucose 10, pH was adjusted at 7.4. Following an equilibration period of 45 min, the perfusion flow rate was adjusted at 8 ml min−1 and kept constant thereafter (Gilson Minipuls 3, Bioblock, Illkirch, France). Perfusion pressure was continuously monitored (Statham P23 Db tranducer, Statham Intruments, Hato Rey, Porto Rico) and recorded (Philips PM 8222, Philips, Bobigny, France) throughout the experiment.
Experimental protocols
Vascular tone of the isolated kidney was increased by the continuous perfusion of prostaglandin F2α at a concentration sufficient to induce a steady increase in perfusion pressure of 20 – 25 mmHg. Two concentrations of Tyr(Me)8BK (1 and 10 nM) were tested at 30 min intervals to avoid desensitization of the bradykinin B2 receptor (Bagaté et al., 1999). Acetylcholine (ACh) and sodium nitroprusside (SNP) were included as respective markers for endothelium-dependent and -independent vasodilator reactivity (Barthelmebs et al., 1996). Two concentrations of ACh (3 and 30 nM) were tested with the same time-interval as used for Tyr(Me)8BK. The highest concentrations tested (10 and 30 nM respectively) were previously shown to induce maximal renal vasodilator responses (Bagaté et al., 1999; Stephan et al., 1995). A supramaximal concentration of SNP (10 μM) was administered at the end of the experiments. All the experiments were carried out in the presence of an inhibitor of angiotensin converting enzyme (lisinopril, 1 μM) in order to prevent kinin catabolism which blunts response in our renal vascular preparation (Bagaté et al., 2000).
NG-nitro-L-arginine (L-NOARG, 100 μM) and indomethacin (30 μM) were added alone or together to the perfusate to inhibit NO synthase (NOS) and cyclo-oxygenase (COX) activity. A nonionic detergent, 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propane sulphonate (CHAPS), was used to remove endothelial cells from intrarenal blood vessels as described by Bhardwaj & Moore (1988) and used before (Stephan et al., 1995). However, the usual dose of CHAPS (4.7 mg ml−1, 30-s infusion) altered smooth muscle vascular reactivity in Tyrode-perfused kidneys as was obvious from hyporesponsiveness to prostaglandin F2α and blunting of SNP-induced vasorelaxation. Subsequent experiments were successfully carried out with a lower dose of CHAPS (4 mg ml−1, 15-s infusion).
We ascertained that EDHF contributes to vasodilation by suppression of the response in depolarizing conditions. Tyrode's solution was prepared in which KCl (25 mM) was isotonically substituded for NaCl. KCl was present from the onset of kidney perfusion and also served to restore vascular tone. L-NOARG and indomethacin were included in the KCl perfusate of some kidneys.
In further experiments aimed to investigate signal transduction pathways involved in the vasodilator response resistant to NOS and COX inhibition, L-NOARG (100 μM) and indomethacin (30 μM) were included in the perfusate from the start of the kidney perfusion. To evaluate the contribution of K+ channels, several inhibitors were used: tetrabutylammonium (TBA, 0.5 mM) as a non-selective inhibitor of K+ channels, glibenclamide (10 μM) to inhibit ATP-selective K+ channels and charybdotoxin (0.1 μM) to inhibit Ca2+-activated K+ channels. To evaluate the contribution of cytochrome P450 metabolites, vasodilator response was evaluated in the presence of clotrimazole (1 μM) or 7-ethoxyresorufin (1 μM). Finally, to investigate the possibility of CB1 receptor involvement in endothelium-derived hyperpolarization, SR 141716A (5 μM), an antagonist of these receptors (Rinaldi-Carmona et al., 1994), was also tested.
The vasodilator responses were expressed as percentage reversal of the prostaglandin F2α-induced preconstriction. In kidneys perfused with KCl, the KCl-induced vascular tone was estimated from SNP-induced relaxation (see Results).
Drugs
The following drugs were used: ACh hydrochloride, CHAPS, charybdotoxin, clotrimazole, 7-ethoxyresorufin, indomethacin, L-NOARG, SNP, tetrabutylammonium (all from Sigma, St Quentin Fallavier, France); prostaglandin F2α tromethamine salt (Dinolytic®, Upjohn Laboratories, Paris, France); sodium pentobarbitone (Nembutal®, Sanofi Santé, Libourne, France); sodium heparinate (Léo, St Quentin Yvelines, France); SR 141716A (Sanofi-Synthélabo, Montpellier, France); Tyr(Me)8BK (Dr Regoli, Sherbrooke, Canada). All other chemicals were of pro-analysis quality from Merck (Darmstadt, Germany).
Clotrimazole was dissolved in absolute ethanol. Indomethacin was prepared as a N-methyl-D-glucamine salt. Glibenclamide and SR 141716A were dissolved in DMSO. L-NOARG was dissolved in HCl and pH adjusted to 7 with NaOH. Peptides were prepared as stock solutions (1 mg ml−1 in distilled water), stored in aliquots at −20°C and diluted to the desired final concentration with 0.9% saline just prior to use. To avoid adsorption of peptides, infusion material was coated with a 1% silicone solution (Aquasil, Interchim, Monluçon, France). Solutions of other drugs were freshly prepared in distilled water before each experiment.
Statistical analysis
Results are expressed as means±s.e.mean. Differences were tested for statistical significance by one-way or two-way variance analysis (ANOVA) on repeated measurements when appropriate. Multiple comparisons between groups were performed by Student – Newman – Keuls test. A P value less than 0.05 was considered significant. Square root transformation was used when necessary to normalize data or equalize variance between groups. All statistics were run with GraphPad Prism (GraphPad, San Diego, CA, U.S.A.) or SigmaStat (SPSS Inc., Chicago, IL, U.S.A.).
Results
Characteristics of the isolated perfused rat kidneys
After pentobarbitone anaesthesia and just before starting perfusion of the kidneys, an overall mean arterial blood pressure of 89.3±1.7 mmHg (n=80) was measured. Kidneys were perfused at a fixed flow rate of 8 ml min−1, allowing a physiological perfusion pressure in the range of 70 – 100 mmHg. Calculated basal renal vascular resistance of control kidneys amounted 9.0±0.22 mmHg min ml−1 (Table 1). Vascular resistance was increased in kidneys perfused with L-NOARG+indomethacin without any effect of other co-treatments (data were therefore pooled). Similar increase in vascular resistance was observed in kidneys lacking intact endothelium. Renal vascular tone was increased by 23.2±0.5 mmHg at a mean prostaglandin F2α concentration of 1.2±0.1 μM (n=71) without change between groups. SNP-induced relaxation was enhanced in the groups treated with L-NOARG or pretreated with CHAPS (Table 1). Since pilot studies showed that high extracellular K+ did not affect SNP-induced relaxation, we subsequently used the vasodilator response to SNP for evaluating the increase in renal vascular tone in KCl-perfused kidneys. Response to SNP (10 μM) was considered, respectively in KCl controls and KCl+L-NOARG+indomethacin experiments, as 95% and 110% reversal of KCl-elevated renal vascular tone, according to mean values observed in the other groups (Table 1).
Table 1.
Characteristics of the isolated perfused rat kidneys
Endothelium-dependent renal vasodilation elicited by Tyr(Me)8BK and ACh
Tyr(Me)8BK and ACh elicited concentration-dependent vasodilation of endothelium-intact perfused rat kidney precontracted with prostaglandin F2α (Figure 1). The maximum response to ACh (30 nM) was about one-third higher than that to Tyr(Me)8BK (10 nM, Figure 1) but remained less than that to SNP (10 μM, Table 1).
Figure 1.
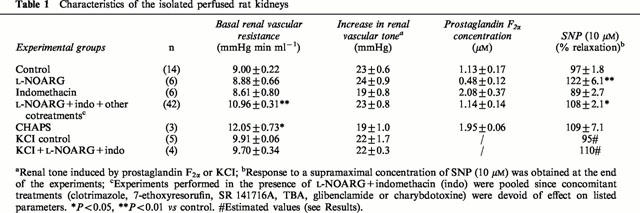
Effect of L-NOARG (100 μM), indomethacin (30 μM), L-NOARG+indomethacin, or CHAPS (4 mg ml−1 during 15 s) on the vasodilator responses to Tyr(Me)8BK (1 and 10 nM) and ACh (3 and 30 nM) in the isolated perfused rat kidney. Results are given as means±s.e.mean with the number of individual experiments in brackets. aP<0.05, bP<0.01, cP<0.001 vs control; *P<0.05, ***P<0.001 for other comparisons as indicated.
The infusion of CHAPS (4 mg ml−1, 15-s infusion) induced an increase in basal perfusion pressure (Table 1). It abolished the vasodilator responses to Tyr(Me)8BK completely and that to ACh almost completely (Figure 1). In contrast, the endothelium-independent vasorelaxation to SNP tended to increase and the vascular smooth muscle cell reactivity to prostaglandin F2α remained unchanged (Table 1), showing selective alteration in endothelium-dependent responses.
Effects of L-NOARG and indomethacin on renal vasodilation to Tyr(Me)8BK and ACh
The inhibition of NO and prostanoids synthesis by L-NOARG (100 μM) and indomethacin (30 μM) respectively, decreased the vasodilator responses to Tyr(Me)8BK and ACh (Figure 1). The decrease was about 30 – 40% for each inhibitor, whatever the concentration of agonists. The concomitant administration of L-NOARG+indomethacin further reduced the vasodilator responses to Tyr(Me)8BK and ACh (Figure 1). The vasodilation which was resistant to the simultaneous NOS and COX inhibition, represented about 40 – 50% of the responses elicited by Tyr(Me)8BK and ACh in control kidneys.
Effects of high extracellular K+ on renal vasodilation to Tyr(Me)8BK and ACh
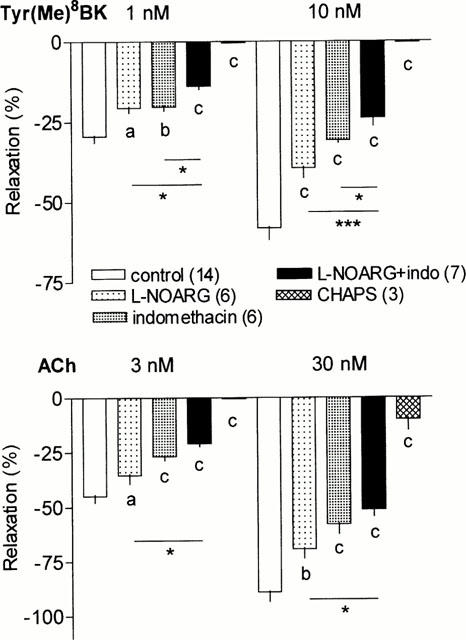
Raising the extracellular concentration of K+ to 25 mM decreased the renal vasorelaxation elicited by Tyr(Me)8BK and ACh (Figure 2). The decrease approached 75% of the response of Tyr(Me)8BK and ACh in control kidneys. This decrease was significantly higher than that observed with the combined inhibition of NOS and COX activity. In the presence of high K+, the subsequent addition of L-NOARG (100 μM) and indomethacin (30 μM) completely abolished the vasodilator response to the low concentrations of Tyr(Me)8BK and ACh (Figure 2). Although further diminished by this combination, some vasodilation however persisted at the high concentrations of the agonists (Figure 2).
Figure 2.
Effect of KCl alone (25 mM) or together with L-NOARG+indomethacin (100 and 30 μM respectively) on the vasodilator responses to Tyr(Me)8BK (1 and 10 nM) and ACh (3 and 30 nM) in the isolated perfused rat kidney. Results are given as means±s.e.mean with the number of individual experiments in brackets. cP<0.001 vs control; *P<0.05, **P<0.01, ***P<0.001 for other comparisons as indicated.
Effect of K+ channel blockers on renal L-NOARG/indomethacin insensitive vasodilation to Tyr(Me)8BK and ACh
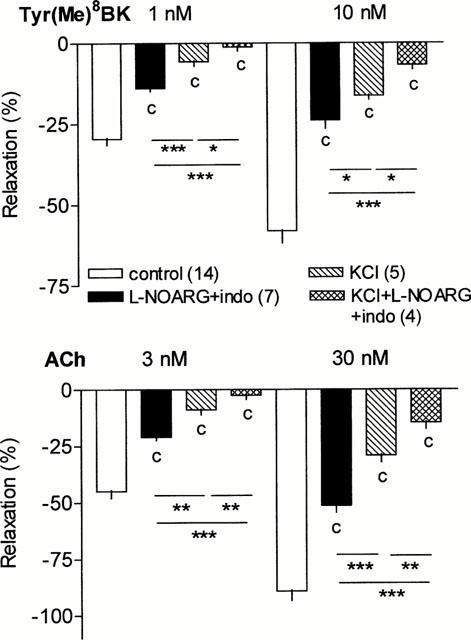
Different K+ channel blockers were used to determine the type of K+ channel involved in the renal vasodilator responses to Tyr(Me)8BK and ACh which occurred independent from NO/prostanoids. The non-selective inhibitor of K+ channels, TBA (0.5 mM), completely abolished the vasodilator responses to Tyr(Me)8BK and ACh, whatever the concentrations (Figure 3). The selective inhibitor of Ca2+-sensitive K+ channel, charybdotoxin (0.1 μM), also abolished the vasodilator responses elicited by the agonists. In contrast, a selective inhibitor of ATP-sensitive K+ channel, glibenclamide (10 μM), did not modify the vasodilator responses to Tyr(Me)8BK and ACh (Figure 3). The vascular responses to Tyr(Me)8BK and ACh were identical in open circuit and closed circuit perfused kidneys (Figure 3). Inhibitors of K+ channels did not change SNP-elicited vasorelaxation (not shown).
Figure 3.
Effect of K+ channel inhibitors, TBA (0.5 mM), glibenclamide (10 μM) or charybdotoxin (0.1 μM), on the NOS and COX inhibition resistant vasodilator responses to Tyr(Me)8BK (1 and 10 nM) and ACh (3 and 30 nM) in the isolated perfused rat kidney. Since charybdotoxin was tested in a closed circuit perfused kidney, corresponding controls were also performed in the presence of L-NOARG+indomethacin. Results are given as means±s.e.mean with the number of individual experiments in brackets. bP<0.01, cP<0.001 vs corresponding L-NOARG+indomethacin group.
Role of cytochrome P450 and cannabinoid CB1 receptor in renal L-NOARG/indomethacin insensitive vasodilation to Tyr(Me)8BK and ACh
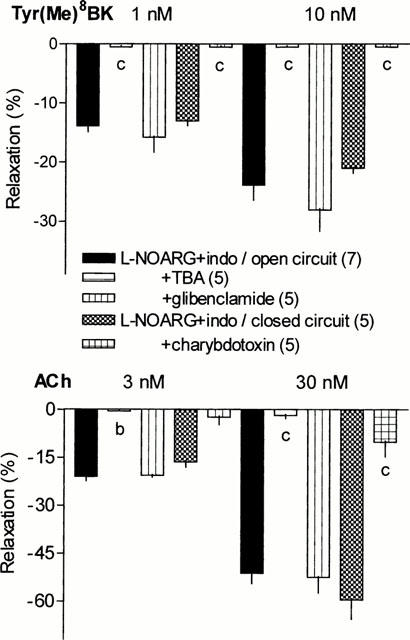
During perfusion of indomethacin (30 μM) and L-NOARG (100 μM), the cytochrome P450 inhibitors, clotrimazole (1 μM) or 7-ethoxyresorufin (1 μM), markedly reduced the vasodilator responses to Tyr(Me)8BK and ACh (Figure 4). The inhibitory effect of 7-ethoxyresorufin on Tyr(Me)8BK-induced vasorelaxation tended to be higher than that of clotrimazole which was not used at higher concentration due to possible non-specific effects. The inhibition approached 80% at the highest concentration of Tyr(Me)8BK, although it only was of about 60% for ACh-induced vasorelaxation.
Figure 4.
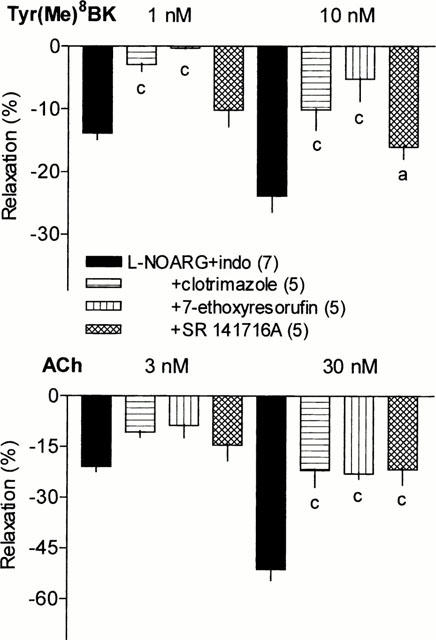
Effect of clotrimazole (1 μM), 7-ethoxyresorufin (1 μM) or SR 141716A (5 μM) on the NOS and COX inhibition resistant vasodilator responses to Tyr(Me)8BK (1 and 10 nM) and ACh (3 and 30 nM) in the isolated perfused rat kidney. Results are given as means±s.e.mean with the number of individual experiments in brackets. aP<0.05, cP<0.001 vs L-NOARG+indomethacin group.
The antagonist of cannabinoid CB1 receptor, SR 141716A (5 μM), only moderately decreased the renal vasodilator response to Tyr(Me)8BK (two-way ANOVA, treatment factor P=0.07), although the effect became significant at the highest concentration of the kinin. SR 141716A elicited greater reduction in the vasodilation induced by ACh, particularly at the highest concentration of the agonist (Figure 4).
Discussion
The results of this study demonstrate that kinin B2 receptor-dependent vasodilation in the rat isolated perfused kidney strictly depends on the presence of an intact endothelium as shown by its complete suppression with CHAPS, in conditions preserving vascular smooth muscle reactivity. Three signalling pathways contribute to Tyr(Me)8BK-induced relaxation: NO release, prostanoids production and a K+-sensitive mechanism which might have been due to hyperpolarizing factors. The NO- and prostaglandin-independent response occurred via a Ca2+-activated K+ channel and appeared to utilize cytochrome P450 derivatives.
The inhibition of NO synthesis by L-NOARG only partially blocked the B2 vasodilator response as has been reported previously by Fulton et al. (1992) in the rat isolated kidney. The inhibition of COX by indomethacin similarly decreased the kinin B2 receptor-mediated relaxation in our study although indomethacin has been reported to be inactive on BK-induced renal vasodilation (Fulton et al., 1992). We used a high concentration of indomethacin (30 instead 2.8 μM) since the inhibition of prostaglandin synthesis in the kidney was not complete even at 15 μM (Cooper & Malik, 1986). Indomethacin did not affect SNP-induced vasorelaxation. The inhibition of NOS can be considered as complete at 100 μM L-NOARG. As expected, SNP-elicited relaxation was increased as reported for other nitrovasodilators (Katusic & Vanhoutte, 1989).
A major part of the vasodilator response to Tyr(Me)8BK was suppressed with depolarizing perfusate. Moreover, the subsequent addition of NOS and COX inhibitors, or the addition of K+ channels inhibitors to preceding NOS and COX inhibition, almost completely suppressed the residual renal vasorelaxation to Tyr(Me)8BK. Taken together, the present results suggest that the NO/prostanoids-independent renal vasodilation to the kinin may be due to hyperpolarizing factors. BK has been reported to elicit endothelium-dependent hyperpolarization of vascular smooth muscle of various species including humans (Mombouli et al., 1992; Mombouli & Vanhoutte, 1995; Fisslthaler et al., 1999). Similar results were obtained with ACh in our study. It is of interest to note that the hyperpolarizing component represented up to 75% of the renal vasodilator response to the agonists, as shown in the experiments with raised extracellular K+. The 50 – 60% decrease in vasorelaxation observed after concomitant inhibition of NOS and COX might overestimate the contribution of NO/cyclic GMP and prostanoids/cyclic AMP pathways since, in some vessels, part of the response to NO and prostanoids was also linked to activation of K+ channels (Mombouli & Vanhoutte, 1997). The role of hyperpolarization in the renal vasodilator response to the agonists is in line with considering the kidney as a resistance vasculature. Relaxation by EDHF seems more prominent in arteries with small diameter, unlike NO that plays a bigger role in large vessels (Hwa et al., 1994; Garland et al., 1995). A limited contribution of NO in vasodilation has previously been reported in the kidney for BK (Fulton et al., 1992) and for ACh (Stephan et al., 1995). The apparent predominance of EDHF in endothelium-dependent vasodilation in small resistance arteries is therefore not specific for an agonist. It exists with kinins which are synthesized in vessels and act in an autocrine/paracrine way to exert effects.
The use of K+ channels inhibitors further confirmed the contribution of a K+-mediated mechanism in the residual response to the kinin after inhibition of NO/prostanoids synthesis. The potent inhibitory effect of TBA and charybdotoxin is in favour of the involvement of Ca2+-activated K+ channels, while the inactivity of glibenclamide excluded the contribution of ATP-sensitive K+ channels. Similar conclusions can be made for ACh. Non-specific effects of the inhibitors seem unlikely since SNP-induced relaxation was unchanged. Present results are in accordance with the mechanisms previously proposed for BK and/or ACh regarding the renal vasculature but also for perfused coronary arteries and various other vessels (Rapacon et al., 1996; Mombouli & Vanhoutte, 1997; Quilley et al., 1997; Mieyal et al., 1998). Moreover, since specific inhibitors respectively for large and small conductance Ca2+-activated K+ channels, iberiotoxin and leiurotoxin, have been reported to be inactive on BK NO-independent renal vasodilation and since charybdotoxin inhibits both large and intermediate conductance Ca2+-activated K+ channels, present results favour a prominent contribution of intermediate conductance channels (Rapacon et al., 1996). K+ channels are present in both endothelial and vascular smooth muscle cells (Himmel et al., 1993; Nelson & Quayle, 1995) and hyperpolarization can be induced by BK in endothelial cells (Bény & Chabaud, 1996). It therefore remains unclear if smooth muscle cell hyperpolarization is related to a diffusible endothelial factor or to transfer of an electrical or chemical signal through myoendothelial gap junctions.
The proposal that EDHF may be cytochrome P450 derivatives arised from a body of observations which, at least in coronary arteries, support this possibility: endothelial cells are rich in cytochrome P450, release EETs which hyperpolarize and relax coronary arteries via a charybdotoxin-sensitive mechanism as described for BK and coronary relaxation to BK is reduced by cytochrome P450 inhibitors of different structures (Fulton et al., 1995; Campbell et al., 1996; Quilley et al., 1997; Fisslthaler et al., 1999). The renal vasculature, at least in the rat, ressembles the coronary circulation. In the isolated rat kidney, arachidonic acid is metabolized by endothelial cytochrome P450 to vasodilator metabolites (Oyekan et al., 1991) and 5,6-EET induces vasodilation (Fulton et al., 1996). We observed a marked reduction by clotrimazole and 7-ethoxyresorufin of the Tyr(Me)8BK-elicited renal NO/prostanoids-independent vasodilation. Incomplete inhibition of renal BK-induced relaxation was also described by Fulton et al. (1992). Although the selectivity of clotrimazole for cytochrome P450 has been a matter of discussion (Alvarez et al., 1992), we observed similar or more pronounced inhibition with 7-ethoxyresorufin, a structurally different and more specific cytochrome P450 inhibitor (Adeagbo, 1997), thus confirming the contribution of cytochrome P450 derivatives in kinin-elicited renal vasorelaxation
In the last few years, the contribution of cannabinoids to EDHF action has also been considered. Anandamide, the putative endogenous ligand for CB1 receptors, was shown to induce endothelium-independent vasodilation and hyperpolarization in the rat mesenteric artery. SR 141716A, a CB1 receptor antagonist, inhibited anandamide as well as carbachol-elicited relaxation (Randall et al., 1996; Plane et al., 1997). Other investigations documented that CB1 receptors also exists on endothelial cells, namely in the kidney which is rich in the enzyme that degrades anandamide and where anandamide produces CB1 receptor-mediated, NO-dependent endothelium-dependent relaxation of juxtamedullary afferent arterioles (Deutsch et al., 1997). In our experiments, SR 141716A was used at a concentration (5 μM) virtually devoid of non-specific effects on Ca2+ and K+ channels (White & Hiley, 1998). SR 141716A however reduced only moderately the renal NO/prostanoids-independent vasodilation to Tyr(Me)8BK, although a 5 fold lower concentration abolished anandamide-induced relaxation in renal afferent arterioles perfused in vitro (Deutsch et al., 1997). Our result therefore does not support a role for endogenous cannabinoids in kinin-induced release of EDHF in the rat renal vasculature. A similar result was recently reported for BK in the rat coronary artery (Fulton & Quilley, 1998). SR 141716A however seems to reduce the renal NO/prostanoids-independent relaxation to ACh more extensively. This point warrants further investigations to confirm relation with endocannabinoids.
Taken together, the present results demonstrate that activation of bradykinin B2 receptors by a specific agonist, Tyr(Me)8BK, elicited an endothelium-dependent vasorelaxation in the rat isolated kidney whose vascular tone had previously been restored by prostaglandin F2α. This response, like that of ACh, mainly depended on the activation of charybdotoxin-sensitive Ca2+-activated K+ channels. In addition, cytochrome P450 derivatives appeared to be involved.
Acknowledgments
The authors are indebted to Prof D. Regoli and Dr F. Gobeil (Medical School, Sherbrooke, Canada) for the gift of Tyr(Me)8BK, to Dr A. Roccon (Sanofi Synthelabo, Montpellier, France) for the gift of SR 141716A and to Mr Arfi (Upjohn Laboratories, Paris, France) for the gift of Dinolytic®. The authors are grateful to Antoine Jund for skilful technical assistance. Mariette Barthelmebs is Research Director in C.N.R.S.
Abbreviations
- ACh
acetylcholine
- BK
bradykinin
- CHAPS
3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propane sulphonate
- COX
cyclo-oxygenase
- EDHF
endothelium-derived hyperpolarizing factor
- EETs
epoxyeicosatrienoic acids
- L-NOARG
NG-nitro-L-arginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- SNP
sodium nitroprusside
- SR 141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-diclorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- Tyr(Me)8BK
Tyr(Me)8bradykinin
References
- ADEAGBO A.S. Endothelium-derived hyperpolarizing factor: characterization as a cytochrome P450 1A-linked metabolite of arachidonic acid in perfused rat mesenteric prearteriolar bed. Am. J. Hypertens. 1997;10:763–771. doi: 10.1016/s0895-7061(97)00057-5. [DOI] [PubMed] [Google Scholar]
- ALVAREZ J., MONTERO M., GARCIA-SANCHO J. High affinity inhibition of Ca++-dependent K+ channels by cytochrome P-450 inhibitors. J. Biol. Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- BAGATÉ K., DEVELIOGLU L., IMBS J.L., MICHEL B., HELWIG J.J., BARTHELMEBS M. Vascular kinin B1 and B2 receptor-mediated effects in the rat isolated perfused kidney–differential regulations. Br. J. Pharmacol. 1999;128:1643–1650. doi: 10.1038/sj.bjp.0702961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAGATÉ K., DEVELIOGLU L., GRIMA M., DE JONG W., SIMMONS W.H., IMBS J.L., BARTHELMEBS M. Vascular catabolism of bradykinin in the rat isolated perfused kidney. Eur. J. Pharmacol. 2000;407:317–325. doi: 10.1016/s0014-2999(00)00744-5. [DOI] [PubMed] [Google Scholar]
- BARTHELMEBS M., KRIEGER J.P., GRIMA M., NISATO D., IMBS J.L. Vascular effects of [Arg8]vasopressin in the isolated perfused rat kidney. Eur. J. Pharmacol. 1996;314:325–332. doi: 10.1016/s0014-2999(96)00584-5. [DOI] [PubMed] [Google Scholar]
- BÉNY J.L., CHABAUD F.Kinins and endothelium-dependent hyperpolarization in porcine coronary arteries Endothelium-Derived Hyperpolarizing Factor 1996Harwood Academic Publishers: Amsterdam; 41–49.ed. Vanhoutte P.M. pp [Google Scholar]
- BHARDWAJ R., MOORE P.K. Endothelium-derived relaxing factor and the effects of acetylcholine and histamine on resistance blood vessels. Br. J. Pharmacol. 1988;95:835–843. doi: 10.1111/j.1476-5381.1988.tb11712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLATTER L.A., TAHA Z., MESAROS S., SHACKLOCK P.S., WIER W.G., MALINSKI T. Simultaneous measurements of Ca++ and nitric oxide in bradykinin-stimulated vascular endothelial cells. Circ. Res. 1995;76:922–924. doi: 10.1161/01.res.76.5.922. [DOI] [PubMed] [Google Scholar]
- BUSSE R., HECKER M., FLEMING I. Control of nitric oxide and prostacyclin synthesis in endothelial cells. Arneimittelforschung/Drug Res. 1994;44:392–396. [PubMed] [Google Scholar]
- CAMPBELL W.R., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- COOPER C.L., MALIK K.U. Contribution of Ca++ and calmodulin to the action of norepinephrine on renal prostaglandin synthesis and vascular tone. J. Pharmacol. Exp. Ther. 1986;236:424–431. [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.O., DAS S.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–496. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FULTON D., QUILLEY J. Evidence against anandamide as the hyperpolarizing factor mediating the nitric oxide-independent coronary vasodilator effect of bradykinin in the rat. J. Pharmacol. Exp. Ther. 1998;286:1146–1151. [PubMed] [Google Scholar]
- FULTON D., BALAZY M., MCGIFF J.C., QUILLEY J. Possible contribution of platelet cyclooxygenase to the renal vascular action of 5,6-epoxyeicosatrienoic acid. J. Pharmacol. Exp. Ther. 1996;277:1195–1199. [PubMed] [Google Scholar]
- FULTON D., MAHBOUBI K., MCGIFF J.C., QUILLEY J. Cytochrome P450-dependent effects of bradykinin in the rat heart. Br. J. Pharmacol. 1995;114:99–102. doi: 10.1111/j.1476-5381.1995.tb14911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON D., MCGIFF J.C., QUILLEY J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br. J. Pharmacol. 1992;107:722–725. doi: 10.1111/j.1476-5381.1992.tb14513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., VANHOUTTE P.M. Endothelium-derived relaxing and constricting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F., KEMP B.K., COCKS T.M. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. TIPS. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- HIMMEL H.M., WHORTON A.R., STRAUSS H.C. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHATTERJEE M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WOOD K.S., BYRNS R.E. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATUSIC Z.S., VANHOUTTE P.M. Effects of SIN-1 on isolated canine basilar arteries. J. Cardiovasc. Pharmacol. 1989;14 suppl 11:S72–S75. [PubMed] [Google Scholar]
- MIEYAL P., FULTON D., MCGIFF J.C., QUILLET J. NO-independent vasodilation to acetylcholine in the rat isolated kidney utilizes a charybdotoxin-sensitive, intermediate-conductance Ca++-activated K+ channel. J. Pharmacol. Exp. Ther. 1998;285:659–664. [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor(s) and the potentiation of kinins by converting enzyme inhibitors. Am. J. Hypertension. 1995;8:19S–27S. doi: 10.1016/0895-7061(95)00029-o. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor(s): updating the unknown. TIPS. 1997;18:252–256. [PubMed] [Google Scholar]
- MOMBOULI J.V., ILIANO S., NAGAO T., SCOTT-BURDEN T., VANHOUTTE P.M. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ. Res. 1992;71:137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- OYEKAN A.O., MCGIFF J.C., QUILLEY J. Cytochrome P450-dependent vasodilator responses to arachidonic acid in the isolated, perfused kidney of the rat. Circ. Res. 1991;68:958–965. doi: 10.1161/01.res.68.4.958. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PLANE F., HOLLAND M., WALDRON G.J., GARLAND C.J., BOYLE J.P. Evidence that anandamide and EDHF act via different mechanisms in rat mesenteric arteries. Br. J. Pharmacol. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUILLEY J., FULTON D., MCGIFF J.C. Hyperpolarizing factors. Biochem. Pharmacol. 1997;54:1059–1070. doi: 10.1016/s0006-2952(97)00039-7. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Comm. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RAPACON M., MIEYAL P., MCGIFF J.C., FULTON D., QUILLEY J. Contribution of calcium-activated potassium channels to the vasodilator effect of bradykinin in the isolated, perfused kidney of the rat. Br. J. Pharmacol. 1996;118:1504–1508. doi: 10.1111/j.1476-5381.1996.tb15566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- STEPHAN D., BILLING A., KRIEGER J.P., GRIMA M., FABRE M., HOFNER M., IMBS J.L., BARTHELMEBS M. Endothelium-dependent relaxation in the isolated rat kidney: impairment by cyclosporine A. J. Cardiovasc. Pharmacol. 1995;26:859–868. doi: 10.1097/00005344-199512000-00003. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Other endothelium-derived vasoactive factors. Circulation. 1993;87:V-9–V-17. [Google Scholar]
- WHITE R., HILEY C.R. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]


