Abstract
The regional haemodynamic effects of rat or human urotensin II (U-II) 3, 30, 300 and 3000 pmol kg−1, i.v.) were assessed in separate groups of conscious, unrestrained, male, Sprague-Dawley rats (n=8 in each). Rat and human U-II had similar effects. At a dose of 3 pmol kg−1, neither peptide had any significant action, while at a dose of 30 pmol kg−1, there was a transient mesenteric vasodilatation (significant only for rat U-II). At doses of 300 and 3000 pmol kg−1, there were dose-dependent tachycardias, and mesenteric and hindquarters hyperaemic vasodilatations. Thus, in conscious rats, the predominant cardiovascular action of rat and human U-II is vasodilatation. This is in contrast to recent findings with human U-II in non-human primates, but is consistent with effects on human isolated resistance vessels.
Keywords: Human urotensin-II, rat urotensin-II, vasodilatation, haemodynamics
Introduction
The cloning of the cDNA encoding human urotensin II (U-II) (Coulouarn et al., 1998), and the identification of this peptide as the endogenous ligand for the hitherto orphan receptor, GPR 14 (Ames et al., 1999; Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999), has stimulated interest in the potential physiological and pathophysiological role(s) of human U-II. The report of Ames et al. (1999), showing a predominance of GPR 14 expression in cardiovascular tissues (vascular smooth muscle, cardiac tissue and endothelial cells), together with a powerful in vitro vasoconstrictor action in non-human primate vasculature, and intense vasoconstriction leading to fatal cardiovascular collapse following systemic administration of human U-II to anaesthetized non-human primates, concluded with speculation about the possible involvement of the peptide in cardiovascular homeostasis and pathology (Ames et al., 1999).
Ames et al. (1999) described potent vasoconstrictor activity of human U-II in arterial, but not venous, preparations from a wide range of vascular territories in cynomolgus monkeys, whereas the vasoconstrictor activity, in preparations from rats, was restricted to the thoracic aorta. Marked species differences, and regional variations in the vasoconstrictor effects of human U-II, have since been shown by others (Bottrill et al., 2000; Douglas et al., 2000; Maclean et al., 2000; Maguire et al., 2000). Vascular contractions induced by human U-II are described as being slow in onset, sustained and resistant to washout, and the potency of human U-II to cause constriction is generally found to be either greater than, or equal to, that of endothelin. Human U-II has, therefore, been described as ‘the most potent mammalian vasoconstrictor identified to date' (Douglas et al., 2000).
In addition to a vasoconstrictor effect of human U-II, there is evidence for it having a vasodilator action in some vascular beds. For example, rat small mesenteric arteries showed no constrictor response to human U-II, but dose-dependent vasodilatation when pre-constricted with methoxamine (Bottrill et al., 2000). Furthermore, rat coronary vasculature (isolated vessels (Bottrill et al., 2000) and perfused heart (Katano et al., 2000)), showed endothelium-dependent dilatation in response to human U-II, in addition to a variable degree of constriction. Moreover, human pulmonary artery preparations showed variable constrictor responses to human U-II (3 out of 10 preparations tested), and only apparent in the presence of nitric oxide synthase inhibition (Maclean et al., 2000), indicating a possible dual vasoconstrictor and vasodilator action of the peptide. More recently, it was shown that human small pulmonary and abdominal adipose tissue arteries, with an intact endothelium, showed relaxations in response to human U-II when pre-contracted with endothelin, but whether or not the relaxations were endothelium-dependent was not determined (Stirrat et al., 2000). Thus, in contrast to the general view that human U-II may be an important vasoconstrictor (see above), Stirrat et al. (2000) concluded with the statement that ‘human U-II was a potent vasodilator of human resistance arteries'.
To date, the only published in vivo study with human U-II, to our knowledge, is that of Ames et al. (1999) in anaesthetized, cynomolgus monkeys, demonstrating a profound vasoconstrictor action in that species. Douglas et al. (2000) alluded to unpublished observations showing the lack of systemic pressor responses to human U-II in anaesthetized or pithed rats, but clearly, as stated by Bottrill et al. (2000), the marked regional variations in the vascular responses to U-II make it difficult to interpret data restricted to measurements of blood pressure. Therefore, the aim of the present study was to establish the regional haemodynamic effects of human U-II in conscious, freely-moving rats. For comparison, studies were also performed using rat U-II, which differs from human U-II in the number (14 vs 11) and identity of amino acid residues, but shares the cyclohexapeptide sequence, which is highly conserved across species (Coulouarn et al., 1998; 1999) and is, therefore, likely to be important in ligand binding.
Methods
Experiments were performed in male, Sprague Dawley rats (Charles River) weighing 380 – 450 g. Under anaesthesia (fentanyl and meditomidine (300 μg kg−1 of each, i.p.) reversed with nalbuphine and atipamezole (1 mg kg−1 of each, s.c.)), miniaturized pulsed Doppler flow probes and intravascular catheters were implanted in a two-stage procedure, separated by at least 10 days (see Gardiner et al., 1994). Experiments were performed at least 24 h after the last surgical procedure (catheterization), with the animals fully conscious and freely moving in their home cages.
After a period of baseline recordings, rat or human U-II were administered as i.v. bolus doses in ascending order (3, 30, 300 and 3000 pmol kg−1), to two separate groups of rats (n=8 in each), with 30 min between the first and second dose, 30 min between the second and third dose, and 60 min between the third and fourth dose. Injections were given in a volume of 0.1 ml over a period of 5 s. U-II (rat or human) was obtained from the Peptide Institute. Stock solutions (120 nmol ml−1) were prepared in sterile distilled water and subsequently diluted with sterile saline.
Data analysis
Within-group comparisons were made with Friedman's test, and between-group comparisons were made using the Kruskal-Wallis test. A P value <0.05 was taken as significant.
Results
At the two lower doses, neither rat U-II nor human U-II had any cardiovascular effects, with the exception of a small and very transient (1 min duration) increase in mesenteric vascular conductance following the 30 pmol kg−1 dose. This effect was significant for rat U-II (+6±3% change) but not for human U-II (+5±2% change).
At doses of 300 and 3000 pmol kg−1, rat and human U-II caused dose-dependent tachycardia, and, whilst mean arterial blood pressure was not affected by the 300 pmol kg−1 dose, it fell substantially following the 3000 pmol kg−1 dose (Figure 1). There was a fall in renal blood flow following administration of the highest dose of U-II (Figure 2), but no significant effects on renal vascular conductance were observed at any of the doses tested (Figure 3). In contrast, U-II caused dose-dependent hyperaemic vasodilatations in the mesenteric and hindquarters vascular beds (Figures 2 and 3). The increases in mesenteric flow and vascular conductance occurred sooner, and were more transient, than the increases in hindquarters flow and vascular conductance (Figures 2 and 3). Indeed, even 2 h after administration of the highest dose, heart rate and hindquarters vascular conductances were still elevated (data not shown). There were no significant differences between the effects of rat U-II and human U-II (Figures 1, 2 and 3).
Figure 1.
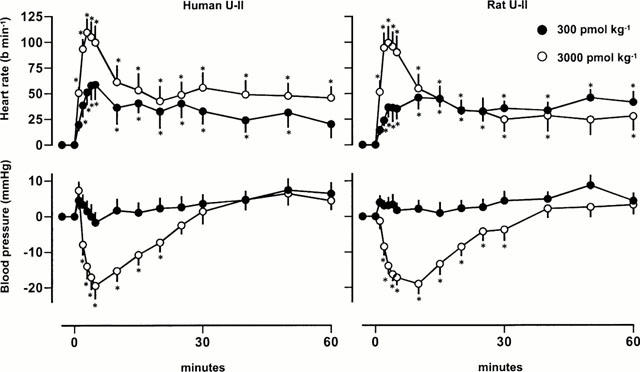
Changes in heart rate and mean arterial blood pressure in conscious Sprague Dawley rats following administration of human or rat U-II (n=8 in each group). Values are mean and vertical bars show s.e.mean; *P<0.05 for a change from baseline (Friedman's test).
Figure 2.
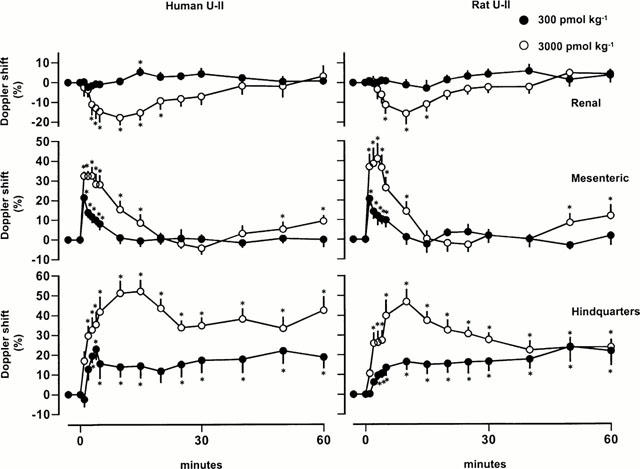
Changes in Doppler shift (blood flow) in conscious Sprague Dawley rats following administration of human or rat U-II (n=8 in each group). Values are mean and vertical bars show s.e.mean; *P<0.05 for a change from baseline (Friedman's test).
Figure 3.
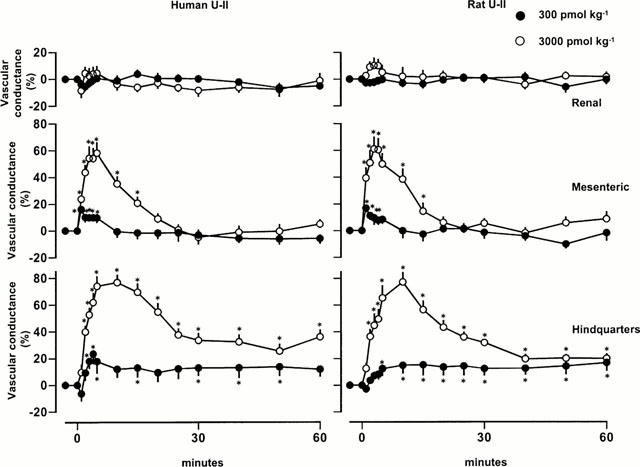
Changes in regional vascular conductances in conscious Sprague Dawley rats following administration of human or rat U-II (n=8 in each group). Values are mean and vertical bars show s.e.mean; *P<0.05 for a change from baseline (Friedman's test).
Discussion
The present study is the first to describe the cardiovascular effects of systemic administration of rat or human U-II in conscious animals, and shows that, in rats, there were marked, dose-dependent tachycardias and hyperaemic vasodilatations in the mesenteric and hindquarters vascular beds, associated with hypotension at the highest dose tested. These findings are in stark contrast to the findings of Ames et al. (1999), who showed pronounced vasoconstriction, leading to cardiovascular collapse, following systemic administrations of similar doses of human U-II to anaesthetized cynomolgus monkeys (although they did allude to some vasodilator effects of human U-II at low doses (<30 pmol kg−1)). However, the lack of pressor and vasoconstrictor effects of human U-II in our study is perhaps not surprising, in view of the marked species differences in the reported in vitro actions of this peptide (see Introduction). Thus, in rats, consistent vasoconstrictor action has only been reported in the thoracic aorta (i.e., a conduit vessel, probably not involved directly in the control of systemic arterial blood pressure), whereas in cynomolgus monkeys, vasoconstriction to human U-II was found in all arterial vessels tested (Ames et al., 1999).
Urotensin was initially isolated and sequenced from the fish neurosecretory system; fish (goby) and human isoforms differ in length by only one amino acid (Coulouarn et al., 1999). Earlier studies (Gibson 1987) showed complex vasodilator (endothelium-dependent) and vasoconstrictor effects of goby U-II in rat aortic strip preparations. The hypotensive effects we observed are consistent with earlier reports in anaesthetized and pithed rats in which it was shown that systemic administration of goby U-II caused a fall in blood pressure (Gibson et al., 1986; Hasegawa et al., 1992), although the regional vascular effects of U-II were not described. The mesenteric vasodilatation we observed is consistent with the observations of Bottrill et al. (2000) showing only vasodilator responses to human U-II in isolated rat mesenteric vasculature.
The particular points of note from the present study are as follows:
There were no differences between the rat and human isoforms of the peptide, indicating that the conserved cyclohexapeptide sequence is likely involved in initiating the effects.
The onset and duration of vasodilator action differed between the mesenteric and hindquarters vascular beds, possibly indicating different mechanisms of action (direct and/or indirect). The vasoconstrictor effect of human U-II has been described by many as being remarkably slow in onset (e.g., 20 min (Opgaard et al., 2000)). Here, the onset of the vasodilatation, particularly in the mesenteric vascular bed, was relatively rapid (time to peak=3.9±4 min and 3.3±3 min for human and rat U-II, respectively). The vasoconstrictor action depends on U-II binding to receptors on the vascular smooth muscle cells, and involves phospholipase C-dependent increases in inositol phosphates (Opgaard et al., 2000). Whether or not the vasodilator effects observed here involved endothelium-dependent mechanisms, and the possibility that, in their absence, an underlying vasoconstrictor effect would be revealed, remains to be determined.
The renal vascular conductance was apparently unaffected by U-II. The haemodynamic profile seen here with U-II, namely, early onset mesenteric vasodilatation, and delayed hindquarters vasodilatation, with little or no effect on renal vascular conductance, closely resembles the regional vascular profile following administration of CRF (Gardiner et al., 1988). There are, however, no structural similarities between CRF and U-II. Intriguingly, it is the other form of urotensin isolated from the fish neurosecretory system (U-I), or the mammalian equivalent (i.e., urocortin), which shows structural homology with CRF and binds to CRF receptors (Conlon, 2000). So, whether or not there is any involvement of endogenous CRF in the responses to exogenous U-II is worthy of further investigation.
The tachycardia was not straightforwardly associated with the fall in blood pressure and was, therefore, likely to be attributable to an action of U-II in addition to activation of the baroreceptor reflex. The time courses of effect of U-II on heart rate and hindquarters vascular conductance were similar. In conscious rats, tachycardia and hindquarters vasodilatation are characteristic of β-adrenoceptor activation (Gardiner et al., 1991), raising the possibility that, as in the case of some other peptides (e.g., Gardiner et al., 1994), some of the effects of U-II are secondary to, for example, adrenomedullary adrenaline release.
In summary, the present findings show a predominant vasodilator effect of human U-II in conscious rats, in contrast to the vasoconstrictor action reported in non-human primates (Ames et al., 1999). In the light of recent findings, showing a potent vasodilator action of human U-II on human isolated systemic resistance arteries (Stirrat et al., 2000), it is clearly important to determine whether vasoconstriction or vasodilatation is the predominant vascular action of the peptide in humans in vivo.
Abbreviations
- CRF
corticotrophin-releasing factor
- U-II
urotensin-II
References
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.-S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BOTTRILL F.E., DOUGLAS S.A., HILEY C.R., WHITE R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br. J. Pharmacol. 2000;130:1865–1870. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONLON J.M. Singular contributions of fish neuroendocrinology to mammalian regulatory peptide research. Regul. Pept. 2000;93:3–12. doi: 10.1016/s0167-0115(00)00172-5. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., JÉGOU S., TOSTIVINT H., VAUDRY H., LIHRMANN I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Letts. 1999;457:28–32. doi: 10.1016/s0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., LIHRMANN I., JEGOU S., ANOUAR Y., TOSTIVINT H., BEAUVILLAIN J.C., CONLON J.M., BERN H.A., VAUDRY H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T. Regional haemodynamic effects of depressor neuropeptides in conscious, unrestrained, Long Evans and Brattleboro rats. Br. J. Pharmacol. 1988;95:197–208. doi: 10.1111/j.1476-5381.1988.tb16565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5′-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br. J. Pharmacol. 1991;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., RAKHIT T., KEMP P.A., MARCH J.E., BENNETT T. Regional haemodynamic responses to pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide in conscious rats. Br. J. Pharmacol. 1994;111:589–597. doi: 10.1111/j.1476-5381.1994.tb14778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A. Complex effects of Gillichthys urotensin II on rat aortic strips. Br. J. Pharmacol. 1987;91:205–212. doi: 10.1111/j.1476-5381.1987.tb09000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., WALLACE P., BERN H.A. Cardiovascular effects of urotensin II in anesthetized and pithed rats. Gen. Comp. Endocrinol. 1986;64:435–439. doi: 10.1016/0016-6480(86)90080-8. [DOI] [PubMed] [Google Scholar]
- HASEGAWA K., KOBAYASHI Y., KOYAYASHI H. Vasodepressor effects of urotensin II in rats. Neuroendocrinol. Lett. 1992;14:357–363. [Google Scholar]
- KATANO Y., ISHIHATA A., AITA T., OGAKI T., HORIE T. Vasodilator effect of urotensin II, one of the most potent vasoconstricting factors, on rat coronary arteries. Eur. J. Pharmacol. 2000;402:209–211. doi: 10.1016/s0014-2999(00)00506-9. [DOI] [PubMed] [Google Scholar]
- LIU Q., PONG S.-S., ZENG Z., ZHANG Q., HOWARD A.D., WILLIAMS D.L., DAVIDOFF M., WANG R., AUSTIN C.P., MCDONALD T.P., BAI C., GEORGE S.R., EVANS J.F., CASKEY C.T. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., ALEXANDER D., STIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., MORECROFT I., POLLAND K. Contractile responses to human urotensin-II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br. J. Pharmacol. 2000;130:201–204. doi: 10.1038/sj.bjp.0703314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORI M., SUGO T., ABE M., SHIMOMURA Y., KURIHARA M., KITADA C., KIKUCHI K., SHINTANI Y., KUROKAWA T., ONDA H., NISHIMURA O., FUJINO M. Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor. SENR (GPR14) Biochem. Biophys. Res. Commun. 1999;265:123–129. doi: 10.1006/bbrc.1999.1640. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.-P., WANG Z., MCNEILL A.M., SAITO Y., MERTEN S., O'DOWD B., DUCKLES S.P., CIVELLI O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nature Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- OPGAARD O.S., NOTHACKER H.-P., EHLERT F.J., KRAUSE D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- STIRRAT A., DOUGLAS S.A., KIRK A., BERRY C., RICHARDSON M., MACLEAN M.R. Vasodilator effect of human urotensin-II on human pulmonary and resistance arteries. Br. J. Pharmacol. 2000;131 Suppl.:88P. [Google Scholar]


