Abstract
In a recent in vivo study, liriodenine, an aporphine alkaloid, has been identified as a prominent anti-arrhythmic agent that can prevent rats' sudden deaths, even at the dose as low as 10−7 g kg−1. The aim of this study was to determine whether liriodenine at its effective anti-arrhythmic dose of 10−7 g kg−1 had effects on the left ventricular (LV)-arterial coupling in Wistar rats.
LV pressure and ascending aortic flow signals were recorded to construct the ventricular and arterial end-systolic pressure-stroke volume relationships to calculate LV end-systolic elastance (Ees) and effective arterial volume elastance (Ea), respectively. The optimal afterload (Qload) determined by the ratio of Ea to Ees was used to measure the optimality of energy transmission from the left ventricle to the arterial system.
Liriodenine at the dose of 10−7 g kg−1 showed no significant changes in basal heart rate (HR), cardiac output (CO), LV end-systolic pressure (Pes), Ea, Ees, and Qload.
By contrast, liriodenine at the dose of 10−6 g kg−1 produced a significant fall of 2.0% in HR and a significant rise of 5.8% in CO, but no significant change in Pes. Moreover, liriodenine administration of 10−6 g kg−1 to rats significantly decreased Ees by 8.5% and Ea by 10.6%, but did not change Qload.
We conclude that liriodenine at the dose of 10−7 g kg−1 has no effects on the mechanical properties of the heart and the vasculature and the matching condition for the left ventricle coupled to its vasculature in rats. Even at 10 times the effective anti-arrhythmic dose, liriodenine shows no effects on the efficiency of energy transferred from the left ventricle to the arterial system.
Keywords: Left ventricular-arterial coupling, left ventricular end-systolic elastance, effective arterial volume elastance, optimal afterload
Introduction
As is generally recognized, myocardial ischaemia and/or reperfusion frequently contribute to malignant ventricular arrhythmia, including ventricular tachycardia and ventricular fibrillation (Penkoske et al., 1978; Janse & Kléber, 1981; Ferrier et al., 1985). Much evidence has suggested that liriodenine, an aporphine derivative isolated from the plant Fissistigma glaucescens (Lu et al., 1985), may be a prominent anti-arrhythmic agent that can enhance the force of contraction in the isolated rat cardiac muscles (Lin et al., 1994; Chang et al., 1996). It has been shown that, through the inhibition of Na+ and transient outward K+ (Ito) channels, liriodenine can suppress ventricular arrhythmia induced by myocardial ischaemia and/or reperfusion. By inhibiting the Ito channel, liriodenine has the potential to prolong the duration of action potential to enhance Ca2+ entry that can induce more free Ca2+ released from the sarcoplasmic reticulum (Chang et al., 1996). This is the possible mechanism that liriodenine can enhance the force of contraction in the isolated rat cardiac strips. These results suggest that liriodenine may be a promising drug for the treatment of cardiac arrhythmia combined with heart failure. However, cautions should be made that agents with positive inotropic action may be harmful to patients with heart failure, especially after chronic treatment.
Optimal therapy of cardiac arrhythmia requires not only knowledge of drug action on the electromechanical properties of the isolated cardiac muscle, but also information of their effects on the matching conditions for the integrative ventricular-arterial coupling. In view of the ventricular-arterial coupling, both the left ventricle and the arterial system are considered elastic chambers with left ventricular (LV) end-systolic elastance (Ees) and effective arterial volume elastance (Ea), respectively (Sunagawa et al., 1983; 1984; 1985). LV pressure and ascending aortic flow signals can be recorded to construct the ventricular and arterial end-systolic pressure-stroke volume (Pes-SV) relationships to calculate Ees and Ea, respectively. The parameter Ees can be used to describe the intrinsic contractile status of the left ventricle because of its independence of preload, afterload, and heart rate in a given constant contractile state of the ventricle (Suga et al., 1973; Sagawa 1978; 1981). In the steady state, Ea is independent of Ees and can be approximated by the ratio of physical arterial resistance to cardiac cycle length (Sunagawa et al., 1984). In this framework, stroke work represents the energy transferred from the left ventricle to the arterial system and the energy transferred reaches maximal when Ea equals Ees (Sunagawa et al., 1985).
In a recent in vivo study, it was observed that liriodenine could reduce the incidence of arrhythmia and then prevent rats' sudden deaths, even at the dose as low as 10−7 g kg−1 (Chang et al., 2000). The aim of this study was to determine whether liriodenine at its effective anti-arrhythmic dose of 10−7 g kg−1 produces effects on the matching conditions for the left ventricle coupled to the arterial system in Wistar rats. The acute effects of liriodenine on the mechanical properties of the heart and the vasculature were also evaluated at ten times the effective dose for the anti-arrhythmic activity. The ventricular-arterial coupling with special reference to the energy transmission from the left ventricle to the arterial system was studied by making use of the LV pressure-ejected volume analysis.
Methods
Animals and catheterization
Male Wistar-Kyoto rats (n=10) weighing 300 – 350 g were used to determine the acute effects of liriodenine on the matching conditions when the left ventricle is coupled to its arterial system. Animals used in this study were obtained from the colony at the Animal Center of the Medical College, National Taiwan University. All rats were maintained on Purina chow and water and were housed two to three per cage in a 12-h light-dark cycle animal room. The animal experiments were conducted according to the ‘Guide for the Care and Use of Laboratory Animals' (published by National Academy Press, Washington, D.C., U.S.A., 1996) and were approved by the ‘Laboratory Animal Care and Use Committee' of the National Taiwan University.
Each rat was anaesthetized with sodium pentobarbitone (35 mg kg−1, i.p.). The femoral vein was cannulated for the administration of supplemental pentobarbitone (30 mg kg−1 every 2 h) and for the administration of liriodenine at the dose of 10−7 or 10−6 g kg−1. Liriodenine was dissolved in dimethylsulphoxide (DMSO) and the final volume of the infusing solution did not exceed 0.1 ml kg−1 (Chang et al., 1996). Tracheotomy was performed to provide artificial ventilation with a tidal volume of 5 – 6 ml kg−1 and respiratory rate of 50 – 70 breaths min−1. The chest was opened through the right second intercostal space. An electromagnetic flow probe (model 100 series, internal circumference 8 mm, Carolina Medical Electronics, King, NC, U.S.A.) was positioned around the ascending aorta to measure the pulsatile aortic flow. A Millar catheter with a high-fidelity pressure sensor (model SPC 320, size 2F, Millar Instruments, Houston, TX, U.S.A.) was used to measure the pulsatile LV pressure. Before inserting catheter, the pressure sensor was prewarmed in 37°C saline for at least 1 h. The catheter was inserted via the isolated right carotid artery into the left ventricle. After withdrawing the catheter from each rat, the catheter was reimmersed in the bath to check for baseline drift. At the end of the experiment, the pressure reading from the sensor submerged in the saline of less than 10 mm in depth was used as the zero pressure reference (Zuckerman & Yin, 1989). The electrocardiogram (ECG) of lead II was recorded with a Gould ECG/Biotach amplifier (Cleveland, OH, U.S.A.).
After the surgical procedure, each rat was allowed to stabilize for 20 – 30 min to obtain haemodynamic data at basal state (BA). Thereafter, the animal was subjected to DMSO in a volume of 0.1 ml kg−1 intravenously. The acute effects of liriodenine on the mechanical properties of the heart and the vasculature were evaluated at the dose of 10−7 or 10−6 g kg−1 in a volume of 0.1 ml kg−1. Animals were injected with a bolus of liriodenine at the dose of 10−7 or 10−6 g kg−1, and were maintained for 5 – 10 min to obtain steady-state parameters after each injection.
The analogue waveforms were sampled at 500 Hz using a 12-bit simultaneously sampling analogue-to-digital (A/D) converter interfaced to a personal computer. Selection of signals of 5 – 10 beats at steady state was made on the basis of the following criteria: (i) recorded beats with optimal velocity profile that was characterized by a steady diastolic level, maximal systolic amplitude, and minimal late systolic negative flow; (ii) beats with a cardiac cycle length less than 5% different from the average value for all recorded beats; (iii) exclusion of ectopic and postectopic beats. The selective beats were averaged in the time domain, using the R wave (the peak wave of QRS complex) of the ECG as a fiducial point. A single-beat estimation technique was used to evaluate the ventricular-arterial coupling without altering LV loads (Takeuchi et al., 1991; Chang & Kuo, 1997; Chang, 1998).
End-systolic pressure-stroke volume relationships
The LV end-systolic elastance and the effective arterial volume elastance can be calculated from the ventricular and arterial end-systolic pressure-stroke volume relationships, respectively (Sunagawa et al., 1984; Chang & Kuo, 1997). Briefly, the pressure-ejected volume loop (dotted line in Figure 1C) can be obtained by the time integration of aortic flow (Figure 1A) and the measured LV pressure (solid line in Figure 1B). The peak isovolumic pressure of the left ventricle at the end-diastolic volume (Pisomax) is estimated by the equation described in Appendix and is shown as dashed line in Figure 1B. In Figure 1C, drawing a tangential line from the estimated peak isovolumic pressure to the right corner of the pressure-ejected volume loop yields a point referred to as the end-systolic equilibrium point (Barnea & Jaron, 1990; Kubota et al., 1992). The line connected the estimated peak isovolumic pressure to the end-systolic equilibrium point is the ventricular Pes-SV relationship, which is denoted as the solid line in Figure 1C. The slope of this solid line represents the LV end-systolic elastance, Ees, and the volume axis intercept of this line is the effective LV end-diastolic volume (Veed). Veed is the difference between the LV end-diastolic volume (Ved) and the zero-pressure volume axis intercept (V0). On the other hand, the arterial Pes-SV relationship is the line connecting the end-diastolic point to the end-systolic equilibrium point, which is denoted as the dashed line in Figure 1C. The slope of this dashed line represents the effective arterial end-systolic elastance, Ea.
Figure 1.
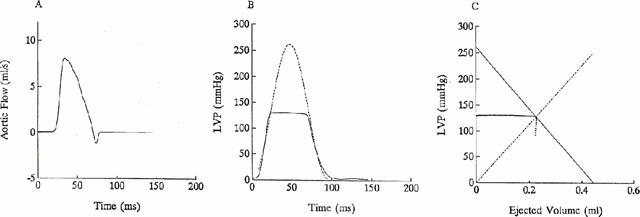
Ascending aortic flow (A), LV pressure (B), and ventricular as well as arterial end-systolic pressure-stroke volume (Pes-SV) relationships (C). (B) The dashed line represents the isovolumic pressure curve at an end-diastolic volume, which is estimated by fitting a sinusoidal function (shown in Appendix 1) to the isovolumic portions of the measured LV pressure. (C) Drawing a tangential line from Pisomax to the right corner of the pressure-ejected volume loop yields a point referred to as the end-systolic equilibrium point. The solid line connecting Pisomax to the end-systolic equilibrium point constructs the ventricular Pes-SV relationship that has the slope of Ees and the volume intercept of Veed. On the other hand, the arterial Pes-SV relationship is the dashed line connecting the end-diastolic point to the end-systolic equilibrium point, with the slope of Ea.
Coupling of the left ventricle and the arterial system
The matching condition for the left ventricle coupled to its arterial system can be described using the ratio of Ea to Ees (Burkhoff & Sagawa, 1986). In this type of analysis, the arterial system is treated as if it were an elastic chamber with a volume elastance, Ea, just as the ventricle is represented as an elastic chamber with an end-systolic volume elastance, Ees. When the ventricular elastance is connected to the arterial elastance, part of the energy stored in the ventricular elastance is transferred to the arterial elastance. Thus, the optimality of energy transmission from the left ventricle to the arterial system, i.e. the optimal afterload (Qload), can be determined from the ratio of stroke work to its theoretical maximal value and can be expressed using the ratio of Ea to Ees as follows (Burkhoff & Sagawa, 1986; Kubota et al., 1992):
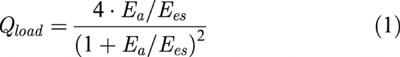 |
Note that Qload is independent of preload. Once the ratio of Ea to Ees is known, Qload can be determined from equation 1 When Ea equals Ees, Qload becomes unity and the arterial system extracts maximal energy from a given Ees and Veed.
Statistics
All data are expressed as means±s.e.m. A one way analysis of variance (ANOVA) was employed to determine the acute effects of liriodenine on the left ventricular-arterial coupling in Wistar rats. The randomized block design (repeated factor) was performed for the administration of liriodenine to experimental animals. If ANOVA for a haemodynamic variable reached the significant level, then the Tukey's honestly significant difference (HSD) method was used to determine differences among means within BA, DMSO, and effects of liriodenine at the dose of 10−7 or 10−6 g kg−1. Significant differences were assumed to be at the level of P<0.05.
Results
Before liriodenine challenge, we determined the acute effects of DMSO on the mechanical properties of the heart and the vasculature, which were shown in Figures 2, 3, 4 and 5. In comparison with basal state, BA, the administration of DMSO in a volume of 0.1 ml kg−1 to rats showed no effects on basal heart rate (HR in Figure 2A), peak LV pressure (Pmax in Figure 2B), as well as cardiac output (CO in Figure 2C). Neither LV end-systolic pressure (Pes in Figure 3A) nor stroke volume (SV in Figure 3B) was affected by DMSO challenge, which accounted for no alteration in effective arterial volume elastance Ea (Figure 3C). The DMSO intervention produced no effects on either estimated peak isovolumic pressure (Pisomax in Figure 4A) or effective LV end-diastolic volume (Veed in Figure 4B), causing no change in LV end-systolic elastance Ees (Figure 4C). Moreover, no significant alteration in the ratio of Ea to Ees was detected (Figure 5A), suggesting that the optimal afterload (Qload in Figure 5B) may not be influenced by the action of DMSO. These data demonstrated that the acute administration of DMSO in a volume of 0.1 ml kg−1 to rats had no effects on the chamber properties of the heart and the vasculature and the matching condition for the ventricular-arterial coupling.
Figure 2.
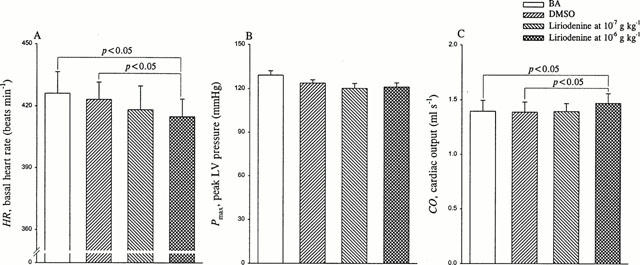
Effects of liriodenine on basal heart rate HR (A), peak LV pressure Pmax (B), and cardiac output CO (C). Liriodenine at its effective anti-arrhythmic dose of 10−7 g kg−1 showed no significant changes in HR, CO, and Pmax. By contrast, at 10 times the effective anti-arrhythmic dose 10−6 g kg−1, liriodenine produced a significant fall of 2.0% in HR and a significant rise of 5.8% in CO, but no significant change in Pmax. BA, basal state; DMSO, dimethylsulphoxide.
Figure 3.
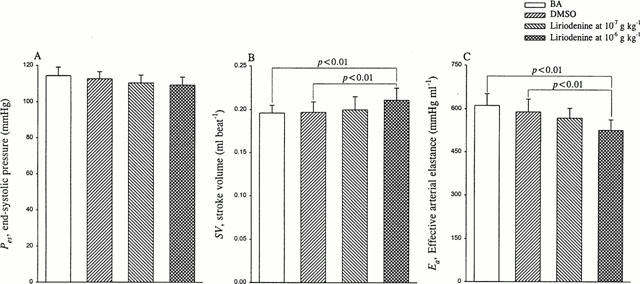
Effects of liriodenine on end-systolic pressure Pes (A), stroke volume SV (B), and effective arterial volume elastance Ea (C). Ea could be determined by the ratio of Pes to SV. Neither Pes nor SV was affected by liriodenine at the dose of 10−7 g kg−1, causing no significant change in Ea. However, liriodenine at the dose of 10−6 g kg−1 produced a rise of 7.1% in SV and a fall of 10.6% in Ea. The increased SV in the absence of any significant change in Pes accounted for the reduction in Ea. BA, basal state; DMSO, dimethylsulphoxide.
Figure 4.
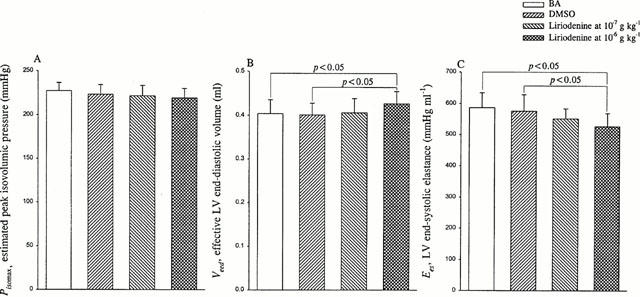
Effects of liriodenine on estimated peak isovolumic pressure Pisomax (A), effective LV end-diastolic volume Veed (B), and LV end-systolic elastance Ees (C). Ees could be determined by the ratio of Pisomax to Veed. Liriodenine at the dose of 10−7 g kg−1 showed no effects on either Pisomax or Veed, causing no significant change in Ees that is indicative of the intrinsic contractility of the left ventricle. By contrast, liriodenine administration of 10−6 g kg−1 to rats decreased Ees by 8.5%. In the absence of any significant change in Pisomax, liriodenine at the dose of 10−6 g kg−1 contributed to a decline in Ees via its positive effect on Veed. BA, basal state; DMSO, dimethylsulphoxide.
Figure 5.
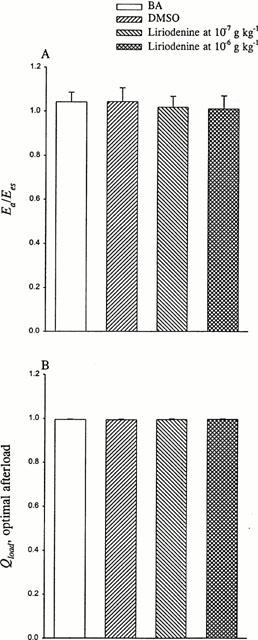
Effects of liriodenine on the ratio of Ea to Ees (A) and optimal afterload Qload (B). The optimal afterload can be determined by the ratio of Ea to Ees. The ratio of Ea to Ees was unaffected by the acute administration of liriodenine to rats, at either 10−7 or 10−6 g kg−1. No significant change in Ea/Ees suggests that even at 10 times the effective anti-arrhythmic dose, liriodenine shows no effect on the efficiency of energy transferred from the left ventricle to the arterial system. BA, basal state; DMSO, dimethylsulphoxide.
Compared with either BA or DMSO, the acute administration of liriodenine at the dose of 10−7 g kg−1 showed no significant changes in HR (Figure 2A) as well as CO (Figure 2C). However, rats treated with liriodenine at the dose of 10−6 g kg−1 had lower HR (414.9±8.6 beats min−1) than did rats at BA (426.2±10.4 beats min−1) and rats treated with DMSO (423.2±8.4 beats min−1). The ECG intervals (cardiac cycle length) for BA, DMSO, liriodenine at 10−7, and 10−6 g kg−1 were 141.8±3.0, 142.1±2.9, 143.5±3.6, and 145.0±3.0 ms, respectively. In contrast, CO was enhanced from 1.395±0.100 ml s−1 in rats at BA and 1.387±0.093 ml s−1 in rats treated with DMSO to 1.470±0.089 ml s−1 in rats treated with liriodenine at the dose of 10−6 g kg−1. Pmax was unaffected by the action of liriodenine at the dose of either 10−7 or 10−6 g kg−1 (Figure 2B).
Neither Pes (Figure 3A) nor SV (Figure 3B) was affected by the action of liriodenine at the dose of 10−7 g kg−1, suggesting that liriodenine at this effective anti-arrhythmic dose may not modify the chamber property of the arterial system (Ea in Figure 3C). However, rats treated with liriodenine at the dose of 10−6 g kg−1 had smaller Ea (525.8±36.8 mmHg ml−1) than did rats at BA (611.5±40.8 mmHg ml−1) and rats treated with DMSO (589.6±44.5 mmHg ml−1). As for the chamber properties of the left ventricle, liriodenine at the dose of 10−7 g kg−1 exerted no effects on either Pisomax (Figure 4A), or Veed (Figure 4B), producing no alteration in the physical property of the heart (Ees in Figure 4C). By contrast, the mechanical property of the left ventricle was depressed by the action of liriodenine at the dose of 10−6 g kg−1, as evidenced by the reduction in Ees. The quantity in Ees was decreased from 586.9±47.7 mmHg ml−1 in rats at BA and 575.8±53.2 mmHg ml−1 in rats treated with DMSO to 526.9±42.2 mmHg ml−1 in rats treated with liriodenine at the dose of 10−6 g kg−1. The increased Veed in the absence of any significant change in Pisomax accounted for the diminished Ees, when liriodenine at 10 times its effective anti-arrhythmic dose was administered to rats.
Liriodenine at the dose of 10−7 g kg−1 showed no significant change in the ratio of Ea to Ees (Figure 5A), causing no effects on the matching condition for the left ventricular-arterial coupling in rats (Qload in Figure 5B). Although contributing to a fall in Ea and Ees, liriodenine at the dose of 10−6 g kg−1 produced no significant change in Ea/Ees. This suggests that even at 10 times the effective anti-arrhythmic dose, liriodenine may have little influence on the optimality of energy transferred from the left ventricle to the arterial system.
Discussion
The major findings of this study are that liriodenine at the dose of 10−7 g kg−1 produces no effects on the mechanical properties of the heart and the vasculature and the matching condition for the ventricular-arterial coupling in rats. However, at 10 times its effective anti-arrhythmic dose, liriodenine may alter the chamber properties of the heart and the vasculature, but not the optimality of energy transferred from the left ventricle to the arterial system.
Acute effects of liriodenine on the systolic mechanical property of the left ventricle
Our data did not show any significant changes in myocardial contractility in rats after the acute administration of liriodenine at the dose of 10−7 g kg−1. However, liriodenine at the dose of 10−6 g kg−1 contributed to a decline in the contractile state of the left ventricle, as evidenced by the reduction in Ees. These results could not meet the conclusion that liriodenine possessed the positive inotropic effects on the rat isolated ventricular myocyte (Lin et al., 1994; Chang et al., 1996). With an electrophysiological study, the anti-arrhythmic action of liriodenine, at a concentration of 1 – 10 μM, may be mediated through blockade mainly of the Na+ and Ito channels (Chang et al., 1996). Through the Ito inhibition, liriodenine could prolong the action potential duration (APD) of the cardiomyocyte to diminish its spontaneous rate. The subsequent prolongation of APD may be responsible for the increased sarcoplasmic reticulum Ca2+ loading and release, accounting for the force enhancement of this anti-arrhythmic agent. Force itself, however, may not necessarily be the best index of a change in contractile state, even at a given muscle length. In this study, we used the system parameter Ees to describe the intrinsic contractility of the left ventricle because of its independence of preload, afterload, and heart rate in a given constant contractile state (Sunagawa et al., 1983; 1984; 1985). In addition, the dose of 10−6 g kg−1 for liriodenine approximates the concentration of 0.03 μM in the intact rat; the concentration used in this study is far smaller than those used in the in vitro studies. Being an aporphine alkaloid, liriodenine at this low dose is supposed to exert another mechanism of the action to reduce the intrinsic contractile state of the left ventricle in the intact animal. Further cellular studies are needed to delineate the negative inotropic effect of liriodenine at the dose of 10−6 g kg−1 on the intact heart.
Acute effects of liriodenine on the chamber property of the arterial system
No significant change in effective arterial volume elastance was observed in rats after the acute administration of liriodenine at the dose of 10−7 g kg−1. However, liriodenine at the dose of 10−6 g kg−1 caused a fall in arterial chamber stiffness, as evidenced by the reduction in Ea. If a small difference between LV end-systolic pressure and mean aortic pressure is ignored, then LV end-systolic pressure can approximate mean aortic pressure (Sunagawa et al., 1984). Total peripheral resistance of the systemic circulation (Rp) can be calculated as LV end-systolic pressure divided by mean aortic flow. In this study, Rp was unaffected by the action of liriodenine at the dose of 10−7 g kg−1. However, rats treated with liriodenine at the dose of 10−6 g kg−1 had lower Rp (1.265±0.082 mmHg s ml−1) than did rats at BA (1.376±0.108 mmHg s ml−1) and rats treated with DMSO (1.393±0.103 mmHg s ml−1). These data suggest that the diminished tension of the vascular smooth muscle cells may occur in the resistance arterioles by the action of liriodenine at the dose of 10−6 g kg−1 but not at the dose of 10−7 g kg−1. It has been shown that Ea can be reasonably approximated by the ratio of physical arterial resistance to cardiac cycle length (Sunagawa et al., 1984). Thus, the decreased total peripheral resistance and basal heart rate by the action of liriodenine at the dose of 10−6 g kg−1 may account for the reduction in effective arterial volume elastance.
Acute effects of liriodenine on the matching condition for the ventricular-arterial coupling
Equilibrium stroke volume can be determined as the interaction between the ventricular and arterial end-systolic pressure-stroke volume relationships (Figure 1C). It has been shown that the equilibrium stroke volume is directly proportional to the effective LV end-diastolic volume Veed and is inversely related to the ratio of Ea to Ees (Burkhoff & Sagawa, 1986). Our data did not show any significant changes in Ea/Ees in rats after the acute administration of liriodenine at the dose of either 10−7 or 10−6 g kg−1. As mentioned earlier, liriodenine at the dose of 10−6 g kg−1 gave rise to a decline in either Ea or Ees. Unchanged Ea/Ees indicated that liriodenine at the dose of 10−6 g kg−1 altered the chamber properties of the left ventricle and the arterial system to the same extent. Thus, liriodenine at the dose of 10−6 g kg−1 had increased equilibrium stroke volume due to a rise in Veed in the absence of any significant change in Ea/Ees. Despite lower heart rate and depressed cardiac contractility, rats treated with liriodenine at the dose of 10−6 g kg−1 are able to enhance stroke volume, causing an increase in blood flow and maintaining LV end-systolic pressure as seen in rats before liriodenine administration. This is due to changes in the peripheral circulation by the action of liriodenine at the dose of 10−6 g kg−1 and reserves in Frank-Starling operation of the rat heart.
The tissue perfusion of vital organs, such as brain, heart, and kidney, depends not only on adequacy of cardiac output or stroke volume, but also on a sufficient level of arterial blood pressure. As a result, the stroke work should measure the performance of the left ventricle, accounting for both the blood flow and the arterial pressure (Sunagawa et al., 1985). The optimality of the afterload, Qload, has been defined as the ratio of stroke work to its maximal value and used as a measure for the matching condition, when the left ventricle is coupled to the arterial system. Since no significant changes in Ea/Ees were observed in rats after the acute administration of liriodenine at the dose of either 10−7 or 10−6 g kg−1, liriodenine produced little influence on the matching condition for the left ventricular-arterial coupling. Unaltered optimal afterload suggests that even at 10 times the effective dose for the anti-arrhythmic activity, liriodenine may maintain the optimality of energy transferred from the left ventricle to the arterial system as seen in rats before liriodenine challenge.
Differences in cardiovascular dynamics between liriodenine and quinidine
Quinidine, an optical isomer of quinine, has been considered the prototypical anti-arrhythmic drug for the patients with atrial fibrillation. Herein, we also studied the effects of quinidine on the left ventricular-arterial coupling in another seven rats. Animals with NaCl infusion served as controls. As mentioned earlier, liriodenine at its effective anti-arrhythmic dose of 10−7 g kg−1 produces no effects on the mechanical properties of the heart and the vasculature and the matching condition for the ventricular-arterial coupling. On the contrary, after infusion of quinidine at its effective anti-arrhythmic dose of 3 mg kg−1, we observed a significant reduction (P<0.01) in HR from 417.0±12.9 to 385.1±14.8 beats min−1, and in Pes from 117.4±5.4 to 73.7±3.7 mmHg. Although there was a trend toward decreasing CO after quinidine, the difference was not significant (1.485±0.113 vs 1.298±0.133 ml s−1, P>0.05). These data were in accordance with other reports in the literature (Nwangwu et al., 1982; Hoffmeister et al., 1988; 1989; Mariano et al., 1992). It is evident that hypotension due to quinidine is caused by vasodilatation via α-adrenergic blocking effect of the drug. Quinidine reduces cardiac rate by increasing threshold via blocking fast Na+ channels (Lee et al., 1981) and increasing action potential duration via blocking Ikr or Ito channels (Wang et al., 1990; 1995). The vasodilatation and the bradycardia caused by quinidine may account for the decrease (P<0.01) in Ea from 561.3±32.1 to 309.3±23.6 mmHg ml−1. By contrast, the vasodilatation caused by quinidine may be associated with baroreflex-mediated increases in sympathetic nerve activity to maintain contractile state of the myocardium (Ees, at 638.3±55.6 of quinidine vs 665.3±43.8 mmHg ml−1 of controls, P>0.05). Thus, the discrepancy between Ea and Ees was augmented by quinidine to worsen the matching condition for the left ventricle coupled to the arterial system, leading to a reduction (P<0.01) in Qload from 0.990±0.003 to 0.872±0.004. This suggests that the energy transferred from the left ventricle to the vasculature may be deteriorated in rats after infusion of quinidine. In this study, we suggest that liriodenine but not quinidine at its effective anti-arrhythmic dose may be useful in the therapy of cardiac arrhythmia.
Limitations
There is a concern regarding the estimation of the isovolumic pressure from an ejection contraction. The duration of the isovolumic contraction by occluding the ascending aorta in diastole is significantly longer than that of the ejecting contraction, and the cardiac cycle length of the estimated isovolumic pressure is therefore shorter than that of the measured isovolumic pressure. Despite this observation, the predicted peak isovolumic pressure has good correlation with that obtained by actual aortic occlusion at the end of diastole (Sunagawa et al., 1980). The advantage of this single-beat estimation technique is that the specific drug effects on the integrative nature of the left ventricular-arterial coupling can be measured without altering ventricular loads.
Another concern is the cumulative effect of DMSO on the mechanical properties of the heart and the vasculature. In this study, the four measurements (i.e., basal, vehicle, liriodenine at 10−7 and 10−6 g kg−1) have been taken in temporal sequence. Since the body weight of the rat used was between 300 and 350 g, the cumulative dose of DMSO given three times did not exceed 0.1 ml. Although we did not show data on a series of control animals in which DMSO has been administered at the same dose, there is evidence that DMSO has little influence on arterial blood pressure and blood gasses, even at the higher dose of 1.0 ml (Shimizu et al., 1997).
In summary, we determined the acute effects of liriodenine on the matching condition for the left ventricular-arterial coupling in Wistar rats. Our data suggest that liriodenine at its effective anti-arrhythmic dose of 10−7 g kg−1 may produce no effects on the mechanical properties of the left ventricle and the arterial system and the optimality of afterload. By contrast, liriodenine at the dose of 10−6 g kg−1 diminishes the LV end-systolic elastance Ees and the effective arterial elastance Ea, but not the ratio of Ea to Ees. Unaltered Ea/Ees and then the optimal afterload suggest that even at 10 times the effective anti-arrhythmic dose, liriodenine may maintain the optimality of energy transferred from the left ventricle to the arterial system as seen in rats before liriodenine challenge. Further studies are needed to delineate the negative inotropic and chronotropic effects of liriodenine at the dose of 10−6 g kg−1 on animals.
Acknowledgments
This study was supported by grants from the National Science Council, Taiwan (NSC 87-2314-B-002-274 and NSC 89-2320-B-002-115).
Abbreviations
- APD
action potential duration
- BA
basal state
- CO
cardiac output (ml s−1)
- DMSO
dimethylsulphoxide
- Ea
effective arterial volume elastance (mmHg ml−1)
- Ees
left ventricular end-systolic elastance (mmHg ml−1)
- HR
basal heart rate (beats min−1)
- Pes
end-systolic pressure of the left ventricle (mmHg)
- Pisomax
peak isovolumic pressure of the left ventricle (mmHg)
- Pmax
peak pressure of the left ventricle (mmHg)
- Qload
optimal afterload
- Rp
total peripheral resistance (mmHg s ml−1)
- SV
stroke volume (ml beat−1)
- Ved
end-diastolic volume of the left ventricle (ml)
- Veed
effective end-diastolic volume of the left ventricle (ml)
- V0
the zero-pressure volume (ml)
Appendix
Estimation of the isovolumic pressure from an ejecting contraction
To estimate the isovolumic pressure curve Piso(t) from an ejecting beat, a nonlinear least-squares approximation technique is used (Sunagawa et al., 1980):
where Pidmax is an estimated peak isovolumic developed pressure, ω is an angular frequency, c is a phase shift angle of the sinusoidal curve, and Pd is a LV end-diastolic pressure. The parameter Pisomax is the estimated peak isovolumic pressure that is the sum of Pidmax and Pd. Piso(t) is obtained by fitting the measured LV pressure curve segments from the end-diastolic pressure point to the peak positive dP/dT and from the pressure point of the peak negative dP/dT to the same level as the end-diastolic pressure of the preceding beat (Takeuchi et al., 1991). The peak R wave of the ECG is used to identify the LV end-diastolic point. Figure 1B schematically represents the relationship between the ejection contraction and the estimated isovolumic contraction in the pressure-time diagram.
References
- BARNEA O, JARON D. A new method for the estimation of the left ventricular pressure-volume area. IEEE Trans.Biomed. Eng. 1990;37:109–111. doi: 10.1109/10.43623. [DOI] [PubMed] [Google Scholar]
- BURKHOFF D, SAGAWA K. Ventricular efficiency predicted by an analytical model. Am. J. Physiol. 1986;250:R1021–R1027. doi: 10.1152/ajpregu.1986.250.6.R1021. [DOI] [PubMed] [Google Scholar]
- CHANG G.J., WU M.H., WU Y.C, SU M.J. Electrophysiological mechanisms for antiarrhythmic efficacy and positive inotropy of liriodenine, a natural aporphine alkaloid from Fissistigma glaucenscens. Br. J. Pharmacol. 1996;118:1571–1583. doi: 10.1111/j.1476-5381.1996.tb15577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG K.C. Theoretical maximal flow of the left ventricle is sensitive to change in ventricular afterload. J. Theor. Biol. 1998;194:409–417. doi: 10.1006/jtbi.1998.0773. [DOI] [PubMed] [Google Scholar]
- CHANG K.C, KUO T.S. Single-beat estimation of the ventricular pumping mechanics in terms of the sytolic elastance and resistance. J. Theor. Biol. 1997;189:89–95. doi: 10.1006/jtbi.1997.0498. [DOI] [PubMed] [Google Scholar]
- CHANG W.L., WU Y.C, SU M.J.Ischemia and/or reperfusion-induced arrhythmia in anesthetized rats: prevention by liriodenine 3rd International Congress on CORONARY ARTERY DISEASE from Prevention to Intervention 2000. Abstract No 23
- FERRIER G.R., MOFFAT M.P, LUKAS A. Possible mechanisms of ventricular arrhythmias elicited by ischemia followed by reperfusion: studies on isolated canine ventricular tissue. Circ. Res. 1985;56:184–194. doi: 10.1161/01.res.56.2.184. [DOI] [PubMed] [Google Scholar]
- HOFFMEISTER H.M., HEPP A, SEIPEL L. Experimental studies of the hemodynamics of disopyramide in comparison with quinidine. Zeitschrift fur Kardiologie. 1988;77:48–52. [PubMed] [Google Scholar]
- HOFFMEISTER H.M., PFLUG A., KRAMER B, SEIPEL L. Circulatory and myocardial effects of different sodium antagonistic drugs in comparison to calcium antagonist verapamil. Arzneimittel-Forschung. 1989;39:1425–1429. [PubMed] [Google Scholar]
- JANSE M.J, KLÉBER A.G. Electrophysiological changes and ventricular arrhythmias in the early phases of regional myocardial ischemia. Circ. Res. 1981;49:1069–1081. doi: 10.1161/01.res.49.5.1069. [DOI] [PubMed] [Google Scholar]
- KUBOTA T., ALEXANDER J.J.R., ITAYA R., TODAKA K., SUGIMACHI M., SUNAGAWA K., NOSE Y, TAKESHITA A. Dynamic effects of carotid sinus baroreflex on ventriculoarterial coupling studied in anesthetized dogs. Circ. Res. 1992;70:1044–1053. doi: 10.1161/01.res.70.5.1044. [DOI] [PubMed] [Google Scholar]
- LEE K.S., HUME J.R., GILES W, BROWN A.M. Sodium current depression by lidocaine and quinidine in isolated ventricular cells. Nature (London) 1981;291:325–327. doi: 10.1038/291325a0. [DOI] [PubMed] [Google Scholar]
- LIN C.H., CHANG G.J., SU M.J., WU Y.C., TENG C.M, KO F.N. Pharmacological characteristics of liriodenine, isolated from Fissistigma glaucescens, a novel muscarinic receptor antagonist in guinea-pigs. Br. J. Pharmacol. 1994;113:275–281. doi: 10.1111/j.1476-5381.1994.tb16205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU S.T., WU Y.C, LEOU S.P. Alkaloids of Formosan Fissistigma and Goniothalamas species. Phytochemistry. 1985;24:1829–1834. [Google Scholar]
- MARIANO D.J., SCHOMER S.J, REA R.F. Effects of quinidine on vascular resistance and sympathetic nerve activity in humans. J. Am. Coll. Cardiol. 1992;20:1411–1416. doi: 10.1016/0735-1097(92)90256-m. [DOI] [PubMed] [Google Scholar]
- NWANGWU P.U., HOLCSLAW T.L., STOHS S.J., ROSENBERG H., SMALL L.D, MODRAK J.B. Hemodynamic properties of a new quinidine analog, cupreidine (6′-hydroxycinchonine) J. Cardiovas. Pharmacol. 1982;4:124–128. doi: 10.1097/00005344-198201000-00020. [DOI] [PubMed] [Google Scholar]
- PENKOSKE P.A., SOBEL B.E, CORR P.B. Disparate electrophysiological alterations accompanying dysrhythmia due to coronary occlusion and reperfusion in the cat. Circulation. 1978;58:1023–1035. doi: 10.1161/01.cir.58.6.1023. [DOI] [PubMed] [Google Scholar]
- SAGAWA K. The ventricular pressure-volume diagram revisited. Circ. Res. 1978;43:677–687. doi: 10.1161/01.res.43.5.677. [DOI] [PubMed] [Google Scholar]
- SAGAWA K. The end-systolic pressure-volume relation of the ventricle: definition, modifications, and clinical use. Circulation. 1981;63:1223–1227. doi: 10.1161/01.cir.63.6.1223. [DOI] [PubMed] [Google Scholar]
- SHIMIZU S., SIMON R.P, GRAHAM S.H. Dimethylsulfoxide (DMSO) treatment reduces infarction volume after permanent focal cerebral ischemia in rats. Neurosci. Lett. 1997;239:125–127. doi: 10.1016/s0304-3940(97)00915-4. [DOI] [PubMed] [Google Scholar]
- SUGA H., SAGAWA K, SHOUKAS A.A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 1973;32:314–322. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- SUNAGAWA K., MAUGHAN W.L., BURKHOFF D, SAGAWA K.Left ventricular interaction with arterial load studied in isolated canine ventricle Am. J. Physiol. 1983245H773–H780.(Heart Circ Physiol) [DOI] [PubMed] [Google Scholar]
- SUNAGAWA K., MAUGHAN W.L, SAGAWA K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ. Res. 1985;56:586–595. doi: 10.1161/01.res.56.4.586. [DOI] [PubMed] [Google Scholar]
- SUNAGAWA K., SAGAWA K, MAUGHAN W.L. Ventricular interaction with the loading system. Ann. Biomed. Eng. 1984;12:163–189. doi: 10.1007/BF02584229. [DOI] [PubMed] [Google Scholar]
- SUNAGAWA K., YAMADA A., SENDA Y., KIKUCHI Y., NAKAMURA M., SHIBAHARA T, NOSE Y. Estimation of the hydromotive source pressure from ejection beats of the left ventricle. IEEE Trans. Biomed. Eng. 1980;27:299–305. doi: 10.1109/TBME.1980.326737. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI M., IGARASHI Y., TOMIMOTO S., ODAKE M., HAYASHI T., TSUKAMOTO T., HATA K., TAKAOKA H, FUKUZAKI H. Single-beat estimation of the slope of the end-systolic pressure-volume relation in the human left ventricle. Circulation. 1991;83:202–212. doi: 10.1161/01.cir.83.1.202. [DOI] [PubMed] [Google Scholar]
- WANG Z.G., FERMINI B, NATTEL S. Effects of flexaimide, quinidine, and 4-aminopyridine on transient outward rectifier currents in human atrial myocytes. J. Pharmacol. Exp. Ther. 1995;272:184–196. [PubMed] [Google Scholar]
- WANG Z.G., PELLETIER L.C., TALAJIC M, NATTEL S. Effects of flecaimide and quinidine on human atrial action potentials. Role of rate-dependence and comparison with guinea pig, rabbit, and dog tissues. Circulation. 1990;82:274–283. doi: 10.1161/01.cir.82.1.274. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN B.D, YIN F.C.P. Aortic impedance and compliance in hypertensive rats. Am. J. Physiol. 1989;257:H553–H562. doi: 10.1152/ajpheart.1989.257.2.H553. [DOI] [PubMed] [Google Scholar]


