Abstract
Neuropeptides FF (NPFF) and AF (NPAF) are involved in pain modulation and opioid tolerance. These peptides were known to act through uncharacterized G protein-coupled receptors (GPCR). We describe here, using an aequorin-based assay as screening tool, that an orphan GPCR, previously designated HLWAR77, is a functional high affinity receptor for NPFF and related peptides. This receptor is further designated as NPFFR.
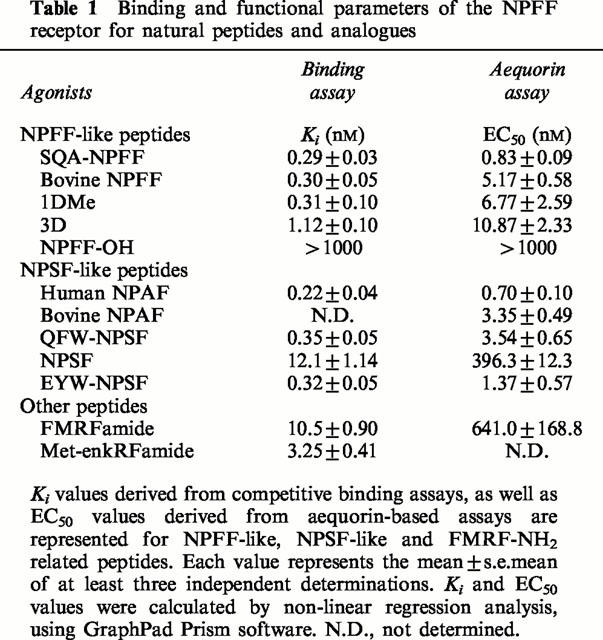
Binding experiments were performed with a new radioiodinated probe, [125I]-EYF, derived from the EFW-NPSF sequence of the rat NPFF precursor. Chinese hamster ovary (CHO) cell membranes expressing NPFFR bound [125I]-EYF with a Kd of 0.06 nM. Various NPFF analogues and related peptides inhibited [125I]-EYF specific binding with the following rank order (Ki): human NPAF (0.22 nM), SQA-NPFF (0.29 nM), NPFF (0.30 nM), 1DMe (0.31 nM), EYW-NPSF (0.32 nM), QFW-NPSF (0.35 nM), 3D (1.12 nM), Met-enk-RF-NH2 (3.25 nM), FMRF-NH2 (10.5 nM) and NPSF (12.1 nM).
The stimulatory activity of the same set of peptides was measured by a functional assay based on the co-expression of NPFFR, Gα16 and apoaequorin. The rank order of potency was consistent with the results of the binding assay.
Membranes from NPFFR expressing CHO cells bound GTPγ[35S] in the presence of SQA-NPFF. This functional response was prevented by pertussis toxin treatment, demonstrating the involvement of Gi family members.
SQA-NPFF inhibited forskolin induced cyclic AMP accumulation in recombinant CHO cells in a dose dependent manner. This response was abolished as well by pertussis toxin pre-treatment.
RT – PCR analysis of human tissues mRNA revealed that expression of NPFFR was mainly detected in placenta, thymus and at lower levels in pituitary gland, spleen and testis.
Keywords: Neuropeptide FF, neuropeptide AF, orphan receptor, G protein-coupled receptor, aequorin assay
Introduction
Neuropeptide FF (NPFF, FLFQPQRF-NH2) is a mammalian representative of a family of peptides, found throughout the animal kingdom, which possess RFamide at the C-terminal end, and which exhibit the properties of neurotransmitters.
NPFF is present in the central nervous system (Lee et al., 1993), particularly in the spinal cord, hypothalamus and pons-medulla (Kivipelto et al., 1989; Majane et al., 1989; Allard et al., 1991). It has been shown to be involved in a variety of physiological functions, including pain modulation (Yang et al., 1985; Roumy & Zajac, 1998), opioid tolerance (Gelot et al., 1998), cardiovascular regulation (Panula et al., 1996). In vivo studies demonstrated that NPFF has both pro- (Gouardères et al., 1993) and anti- (Dupouy & Zajac. 1995) opioid effects. When administered intracerebroventricularly (i.c.v.) to rats, NPFF was shown to induce analgesia or to inhibit morphine-induced analgesia, depending on the experimental paradigm (Oberling et al., 1993; Roumy & Zajac, 1998). Furthermore, NPFF potentiates the effect of opiates when administered intrathecally in rats (Gouardères et al., 1993).
NPFF and NPAF (AGEGLSSPFWSLAAPQRF-NH2) have been initially isolated from bovine brain by an antiserum directed against the molluscan cardioexcitory peptide FMRF-NH2 (Yang et al., 1985). Both peptides were found to derive from a common precursor, the gene of which was cloned from several species (Perry et al., 1997; Vilim et al., 1999). Peptides, three amino-acids longer than NPFF are predicted to be processed from the human (SQA-NPFF), bovine (SPA-NPFF), rat (NPA-NPFF) and mouse (SQA-NPFF) precursors. NPAF-like peptides are only present in the human and bovine precursors while, in murine species, shorter (eleven amino-acids long) peptides are predicted. They share with human and bovine NPAF the common sequence SLAAPQRF-NH2, called NPSF. The rat EFW-NPSF has indeed been suggested to be a physiologically active neurotransmitter (Roumy et al., 2000).
NPFF receptors have been localized in the central nervous system by autoradiography, using a radiolabelled NPFF analogue, [125I](1DMe)Y8Famide (Dupouy & Zajac, 1996; Gouardères et al., 1997). Abundant NPFF binding sites were detected in dorsal horn of the spinal cord, facial and trigeminal nuclei, central gray, dorsal raphe, various thalamic and hypothalamic nuclei, ventral tegmental area, cingulate, lateral septum and the head of the caudate (Zajac & Gouardères, 2000). This distribution of binding sites is consistent with the involvement of NPFF in the modulation of nociceptive pathways.
Inhibition of NPFF binding in the rat spinal cord and mouse olfactory bulb by guanine nucleotides has suggested that NPFF receptors are coupled to G proteins (Payza & Yang, 1993; Devillers et al., 1994). NPFF has also been reported to stimulate cyclic AMP generation in mouse olfactory bulb, spinal cord and cerebellum (Gherardi & Zajac, 1997).
In the present study, we identified a receptor for NPFF and related peptides in the course of functional analyses of orphan G protein-coupled receptors (GPCRs) using an aequorin-based functional assay. We performed an extensive pharmacological characterization of this receptor using a binding assay and a number of natural ligands, as well as aequorin-based, GTPγS and cyclic AMP functional assays. The same NPFF receptor has been identified independently by two other groups, and reported during the course of this study (Elshourbagy et al., 2000; Bonini et al., 2000).
Methods
Cloning and functional expression of the HLWAR77 orphan receptor
A cDNA fragment encoding a human orphan GPCR, designated HLWAR77, was amplified by polymerase chain reaction (PCR) according to a nucleotide sequence described in a patent application (Genbank/EMBL accession number: AF257210). This sequence corresponds to a short variant of the orphan receptor also reported as NPGPR (Cikos et al., 1999). Sense (5′-ATCGGAATTCACCATGAATGAGAAATGGGA CACAAAC-3′) and antisense (5′-ATCATCTAGATTAAATCTCACTGCTGTTAGTAG-3′) primers were used in a PCR experiment using cDNA from human brain (Marathon Ready cDNA, Clontech, Palo Alto, CA, U.S.A.) as a template under the following conditions: 94°C for 1 min, 55°C for 1 min, 72°C for 4 min, 3 cycles; 94°C for 1 min, 65°C for 1 min, 72°C for 4 min, 30 cycles. A 1300 bp DNA fragment was obtained as predicted, cloned between the EcoRI and XbaI sites of the pEFIN3 bicistronic vector and sequenced by both strands. The plasmid encoding HLWAR77 was transfected into CHO-K1 cells, or CHO-K1 cells expressing Gα16 and mitochondrial apoaequorin, using Fugene 6 (Roche Molecular Biochemicals, Inc., Indianapolis, IN, U.S.A.) as described previously (Blanpain et al., 1999). Selection of transfected cells was made with 400 μg ml−1 G418 (Life Technologies, Merelbeke, Belgium), and individual clones were isolated. Levels of HLWAR77 transcripts in each clone were estimated by Northern blotting, and the clones displaying the highest expression were selected for subsequent studies. A single clone co-expressing apoaequorin, and a single clone not co-expressing apoaequorin were used for generating all data presented. Similar results were obtained on the pool of transfected cells, following selection by G418, but before isolation of individual clones.
Peptides
In order to identify potential ligands of HLWAR77, we tested a library of approximately 250 peptides described to have biological activities. Peptides were dispensed at a 100 nM concentration in 96-well plates and tested in the aequorin assay. Following the identification of the receptor, the following natural peptides and analogues were used: NPFF (FLFQPQRF-NH2), SQA-NPFF (SQAFLFQPQRF-NH2), human NPAF (AGEGLNSQFWSLAAPQRF-NH2), bovine NPAF (AGEGLSSPFWSLAAPQ RF-NH2), NPSF (SLAAPQRF-NH2), QFW-NPSF (QFWSLAAPQRF-NH2), 1DMe (DYL(NMe)FQPQRF-NH2), 3D (DYDLDFQPQRF-NH2), NPFF-OH (FLFQPQRF-OH), EYW-NPSF (EYWSLAAPQRF-NH2) and FMRF-NH2.
Aequorin-based functional assay
Functional responses were analysed by recording the luminescence of aequorin following the addition of HLWAR77-expressing cells, as previously described (Detheux et al., 2000). CHO cells coexpressing HLWAR77, Gα16 and apoaequorin were collected from plates with PBS containing 5 mM EDTA, centrifuged for 2 min at 1000×g, resuspended in DMEM F-12 at a density of 5×106 cells ml−1, and incubated for 4 h in the dark in the presence of 5 μM coelenterazine H (Molecular Probes, Eugene, OR, U.S.A.). Fifty μl of cell suspension (5×105 cells ml−1) was injected on 96-well plates containing potential agonists in 50 μl DMEM f-12 in each well and luminescence was measured for 30 s in a Berthold luminometer (Perkin Elmer, Norwalk, CT, U.S.A.).
Binding assays
CHO cells coexpressing HLWAR77, Gα16 and apoaequorin were harvested in PBS buffer, frozen at −20°C for 60 min, and homogenized in 50 mM Tris-HCl, pH 7.4, in a tissue grinder. The nuclear pellet was discarded after centrifugation at 1000×g for 15 min at 4°C and the membrane fraction was collected by centrifugation of the supernatant at 100,000×g for 30 min at 4°C. Membranes (2 – 5 μg proteins) were incubated in polypropylene tubes in a final volume of 500 μl containing 50 mM Tris-HCl, pH 7.4, 60 mM NaCl, 0.1% BSA and [125I]-EYF as radioligand. Non-specific binding was determined in the presence of 1 μM EYW-NPSF. In competition binding experiments with unlabelled peptides, bestatin (25 μM) was added to the reaction mixture. After incubation for 1 h at 25°C, the samples were rapidly filtered on Whatman GF/B filters preincubated in 50 mM Tris-HCl, pH 7.4, 0.1% BSA, washed with the same ice-cold buffer, and the bound radioactivity was counted in a gamma counter (Packard, Instrument, Doners Grove, IL, U.S.A.).
GTPγ[35S] binding experiments
Membranes of CHO cells expressing HLWAR77, but not apoaequorin (about 15 μg proteins per point), were incubated in 200 μl solution containing (mM) HEPES 2, pH 7.4, NaCl 10, MgCl2 3, GDP 3, 10 μg ml−1 saponin, 0.1 nM GTPγ[35S] (1086 Ci mmol−1, New England Nuclear, Boston, MA, U.S.A.) and various concentrations of agonists at 30°C for 30 min. The membranes were collected by centrifugation at 1000×g for 10 min at 4°C, and bound GTPγ[35S] was counted.
Cyclic AMP assays
CHO cells expressing HLWAR77, but not apoaequorin (2×105 cells per well in 24-well plates), were cultured for 15 h at 37°C in Ham's F-12 medium with or without 100 ng ml−1 pertussis toxin (PTX, Sigma, St Louis, MI, U.S.A.). Cells were further incubated for 30 min at 37°C in Krebs-Ringer HEPES buffer supplemented with various concentrations of agonists and/or 10 μM forskolin. Incubations were terminated by removing the medium and adding 500 μl 0.1 M HCl. Cyclic AMP was measured by using a radioimmunoassay kit (Amersham, Buckinghamshire, U.K.) as described by Tovey et al. (1974).
Intracellular Ca2+ assays
CHO cells expressing HLWAR77, but not apoaequorin, were collected from plates in PBS supplemented with 5 mM EDTA, resuspended and incubated in Hanks' balanced salt medium (Life Technologies) containing 0.1% BSA and 2.8 μg ml−1 Fura-2 (Molecular Probes) for 45 min at 37°C in the dark. The cells were washed twice and resuspended at a density of 1×106 cells ml−1. Intracellular Ca2+ was measured following the addition of agonists using a luminescence spectrometer LS50B (Perkin Elmer, Norwalk, CT, U.S.A.).
Tissue distribution analysis
Reverse transcription-polymerase chain reaction (RT – PCR) experiments were carried out using a panel of human polyA+ RNA (Clontech). Aldolase mRNA was chosen as standard and amplified in a separate reaction. The primers were as follows: aldolase sense primer (5′-GGCAAGGGCATCCTGGCTGC-3′), aldolase antisense primer 5′-TAACGGGCCAGAACATTGGCATT-3′), HLWAR77 sense primer (5′-TTGTGCATGATGGGAAATAC-3′) and HLWAR77 antisense primer (5′-TTTTTCCTGGACACCACGTG-3′). The expected sizes of the amplified DNA bands were 443 base pairs (bp) for aldolase and 617 bp for HLWAR77. Approximately 75 ng polyA+ RNA was reverse transcribed with Superscript II (Life Technologies) and used for PCR. PCR was performed under the following conditions: denaturation at 94°C for 3 min, 30 cycles at 94°C for 1 min, 58°C for 2 min and 72°C for 2 min. Aliquots (10 μl) of the PCR reaction were analysed by 1% agarose gel electrophoresis.
Results
Identification of a receptor for NPFF and related peptides
In order to identify the ligand of the orphan receptor designated HLWAR77, approximately 250 peptides were tested using the aequorin assay. This peptide collection contained molecules described for displaying biological activities in vitro or in vivo, and acting possibly through presently unknown G protein-coupled receptors. Strong functional responses were obtained for SQA-NPFF, NPFF, human NPAF, 1DMe and 3D with CHO cells expressing HLWAR77, while no signal was observed with wild-type CHO cells.
NPFF and the various analogues available were further tested in dose-response experiments using the same assay (Figure 1). The natural human peptides were characterized by high potencies, with EC50 in the low nanomolar range (Table 1). Human NPAF (EC50: 0.70±0.10 nM) was the most potent agonist, followed by SQA-NPFF (0.83±0.09 nM), QFW-NPSF (3.54±0.65 nM), NPFF (5.17±0.58 nM), and NPSF (396.3±12.3 nM). Bovine NPAF (EC50: 3.35±0.49 nM) was also a potent agonist at the human receptor, as well as the synthetic analogues EYW-NPSF (EC50: 1.37±0.57 nM), 1DMe (6.77±2.59 nM), and 3D (10.87±2.33 nM). The unrelated RFamide peptide FMRF amide (EC50: 641.0±168.80 nM) was active, although much less potent, and NPFF-OH, the non-amidated analogue of NPFF, was totally inactive. This demonstrates that the RFamide moiety is essential for biological activity. The potency rank order observed for the tested natural ligands and analogues (NPAF=SQA-NPFF=EYW-NPSF>bovine NPAF=QFW-NPSF=NPFF=1DMe=3D>NPSF>FMRFamide) was consistent with the previous pharmacological characterization of the NPFF receptor in mouse and rat preparations (Gherardi & Zajac, 1997; Roumy et al., 2000). These results indicated that HLWAR77 was the human receptor for NPFF and related peptides, and it was tentatively designated as NPFFR.
Figure 1.
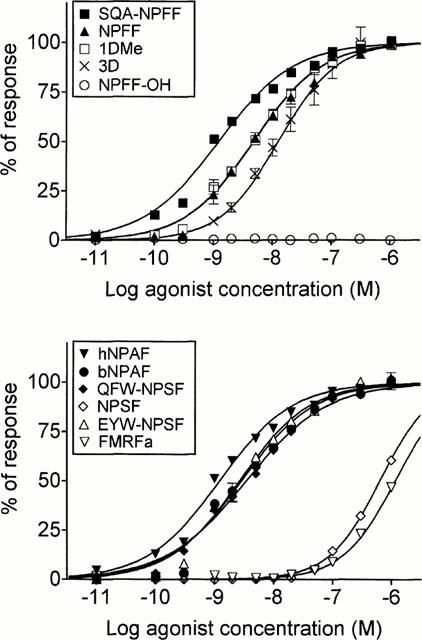
Functional response of the NPFF receptor (HLWAR77) to various peptides. A CHO-K1 cell line co-expressing HLWAR77, Gα16 and apoaequorin was tested in an aequorin assay with neuropeptide FF and related peptides. Data points represent the mean±s.e.mean for triplicates, and were normalized relative to the luminescent signal obtained following stimulation by 100 μM ATP. The data shown are representative of at least three independent experiments that generated similar results.
Table 1.
Binding and functional parameters of the NPFF receptor for natural peptides and analogues
Binding characteristics of the NPFF receptor
Characterization of the binding profile of the NPFFR stably expressed in CHO-K1 cells, co-expressing apoaequorin and Gα16, was determined by using a high affinity radioligand, [125I]-EYF, described recently (Gouardères et al., 2001). In membrane preparations from the recombinant cell line, [125I]-EYF bound specifically (Figure 2) to a homogeneous population of sites (Bmax=497±74 fmoles mg−1 protein, n=3) with high affinity (Kd=0.06±0.01 nM, n=3). No binding was detected in non-transfected cells. Several peptides of the NPFF and NPSF series derived from NPFF precursors, the synthetic peptides 1DMe, 3D, NPFF-OH, EYW-NPSF and unrelated RFamide ligands (FMRF-NH2, Met-enkephalin RF-NH2), were assayed for their ability to inhibit the specific binding of [125I]-EYF. As shown in Figure 3, all these peptides competed with [125I]-EYF with high affinities, with the exception of NPSF, FMRFamide and Met-enkephalin RFamide that exhibited lower affinities. In contrast to these ligands, unamidated NPFF-OH did not compete for [125I]-EYF binding.
Figure 2.
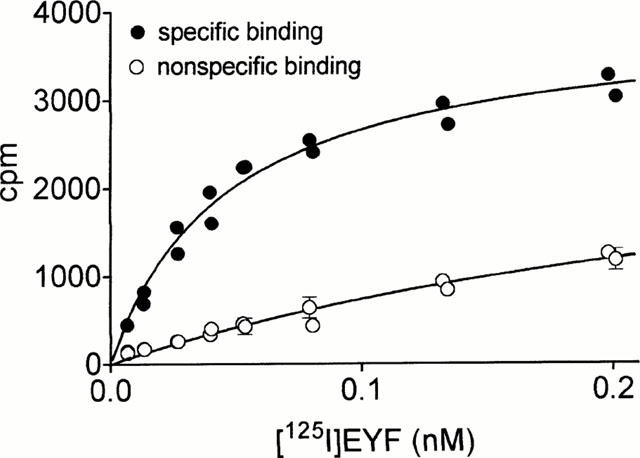
Saturation binding assay performed with membranes of CHO cells expressing the human NPFF receptor, using [125I]-EYF as tracer. Specific (•) and nonspecific (○) binding are represented. Nonspecific binding was determined in the presence of 1 μM unlabelled EYW-NPSF. Each point represents the mean±s.e.mean of triplicate determinations. The data are representative of three independent experiments.
Figure 3.
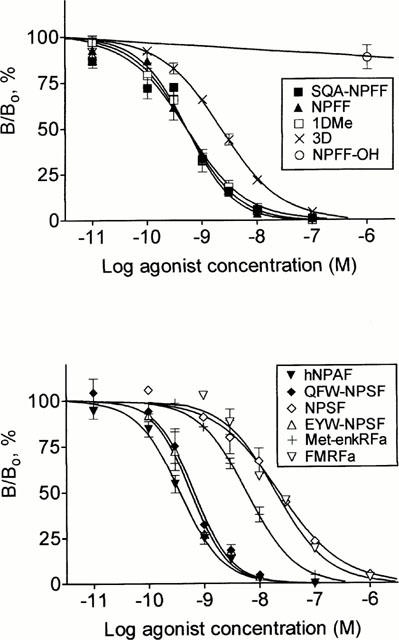
Competition binding assay. Competition binding using [125I]-EYF (0.05 nM) as tracer, and NPFF-like, NPSF-like and RF-NH2-like peptides as competitors. Bo and B refer to specifically bound radioligand in the absence and presence of inhibitor, respectively. Each point represents the mean±s.e.mean of 2 – 4 experiments performed in triplicate.
Functional coupling of the NPFF receptor
GTPγ[35S] binding assays, cyclic AMP measurements, and Ca2+ mobilization assays were performed on a CHO-K1 cell line expressing NPFFR, but not apoaequorin or Gα16. As shown in Figure 4, SQA-NPFF enhanced binding of GTPγ[35S] to the membranes in a dose-dependent manner, with a 7 fold increase over basal level at the highest concentrations. Following PTX treatment, SQA-NPFF did not increase GTPγ[35S] binding to membranes. SQA-NPFF inhibited forskolin-induced cyclic AMP accumulation in these cells (Figure 5A) by up to 93%, as compared to the level obtained following forskolin stimulation alone. This functional response was completely abolished by PTX pretreatment. SQA-NPFF was unable to stimulate cyclic AMP accumulation over basal levels up to micromolar concentrations. A dose-response curve of inhibition of cyclic AMP accumulation by SQA-NPFF is illustrated in Figure 5B (EC50: 0.66 nM). We also investigated whether NPFFR agonists could mobilize intracellular calcium in the CHO cell line. No calcium release could be detected following incubation of the cells with up to 1 μM SQA-NPFF (data not shown).
Figure 4.
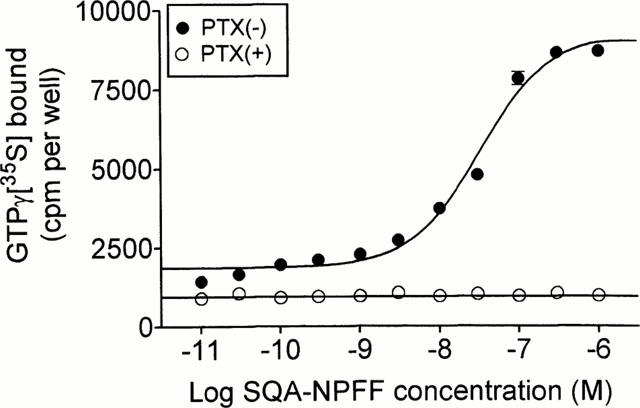
GTPγ[35S] assay. Binding of GTPγ[35S] to membranes of CHO-K1 cells expressing NPFFR was measured following stimulation by SQA-neuropeptide FF, with (○) or without (•) pretreatment with PTX. Each point represents the mean±s.e.mean of triplicate determinations. The data are representative of three experiments performed independently.
Figure 5.
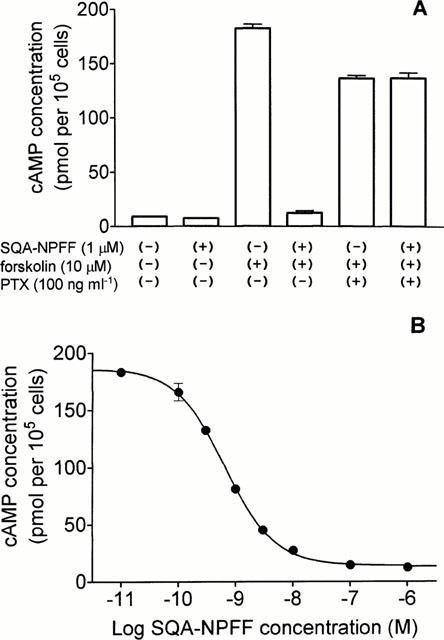
Cyclic AMP accumulation in CHO cells expressing NPFFR. (A) Cells pretreated or not with PTX were incubated with vehicle or 1 μM SQA-neuropeptide FF in the presence or absence of 10 μM forskolin as described in the Methods section. Each value represents the mean±s.e.mean for triplicate determinations. The data are representative of three independent experiments. (B) Cells were incubated in the presence of the various concentrations of SQA-neuropeptide FF and 10 μM forskolin as described. Each point represents the mean±s.e.mean of triplicate determinations.
Distribution of the NPFF receptor
NPFFR mRNA was assayed by RT – PCR in 16 human tissues (Figure 6). A strong band of expected size was detected in thymus and placenta, and at lower levels in pituitary gland, spleen, testis and brain. Faint bands were detected in spinal cord, pancreas, small intestine, uterus, stomach, lung, heart and skeletal muscle, while not in liver or kidney.
Figure 6.
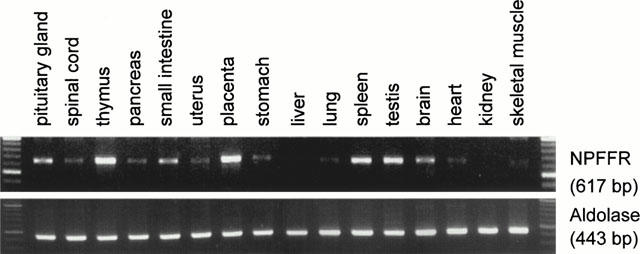
RT – PCR distribution of NPFFR in human tissues. PolyA+ RNA from various human tissues were reverse transcribed and PCR was performed using primers specific for NPFFR and aldolase as control. The expected sizes of the bands were respectively 617 and 443 bp.
Discussion
In the present study, we have identified a G protein-coupled receptor interacting specifically with NPFF and related peptides using an aequorin based functional assay, through the systematic testing of potential ligands collected in a peptide library. We have characterized further the pharmacology of this NPFF receptor by binding, GTPγ[35S] and cyclic AMP assays, and have studied its tissue distribution by RT – PCR.
In competition binding assays, using the new tracer [125I]-EYF (Gouardères et al., 2001), NPFF-like peptides and NPSF-like peptides appeared as equally potent, indicating that the human NPFF receptor is not able to discriminate between the two families of bioactive peptides derived from the NPFF precursor (Table 1). NPSF itself was, however, 40 fold less active than NPFF. The non-amidated analogue NPFF-OH was unable to bind the receptor, as might be expected from earlier structure-activity studies (Payza et al., 1993). Other RFamides peptides unrelated to the NPFF family, such as the amidated Met-enkephalin analogue and FMRFamide, bound to the NPFF receptor, but with much lower affinities (Table 1). It appears therefore that the RFamide moiety of the peptides plays an important role in the binding to the receptor, but that other residues contribute to high affinity binding. These results are in good agreement with the pharmacological data reported in the literature, deduced from studies on brain tissue preparations (Payza et al., 1993; Devillers et al., 1994; Gherardi & Zajac, 1997; Gouardères et al., 1997; Roumy et al., 2000).
The functional pharmacological profile of the NPFF receptor was determined by using a cell line coexpressing the receptor, Gα16 and apoaequorin. This situation does not necessarily represent the natural coupling of the receptor, as Gα16 is known to couple most GPCRs to intracellular Ca2+ release. The potencies found for the various peptides in this assay were essentially consistent with the affinities obtained from binding assays. SQA-NPFF and human NPAF appeared, however, slightly more potent in the functional assay than NPFF and QFW-NPSF, although the four ligands had very similar Ki values in the binding assay. These results are consistent with the prevailing hypothesis that, in vivo, SQA-NPFF and human NPAF are the main peptides generated from the human precursor.
In CHO cells expressing only NPFFR, we demonstrated that the NPFF receptor is negatively coupled to adenylyl cyclase, through the Gi class of G proteins. Indeed, NPFF analogues did not induce calcium release in cells lacking Gα16, nor did they stimulate the accumulation of cyclic AMP, but NPFFR agonists inhibited very efficiently the forskolin-induced accumulation of cyclic AMP. This effect was prevented by PTX pretreatment, as well as the stimulation of GTPγ[35S] binding to membranes. It has previously been suggested that NPFF stimulates cyclic AMP accumulation in the mouse olfactory bulb, spinal cord and cerebellum (Gherardi & Zajac, 1997), although at much higher concentrations than those used here on the recombinant receptor. During the course of the present study, Elshourbagy et al. (2000) and Bonini et al. (2000) have reported the functional characterization of an NPFF receptor identical to ours and its coupling to inhibition of adenylyl cyclase by cyclic AMP responsive element-directed luciferase reporter assay in HEK 293 cells (Elshourbagy et al., 2000) or Ca2+ mobilization in COS-7 cells expressing chimeric Gq proteins (Bonini et al., 2000).
Tissue distribution by RT – PCR revealed that NPFFR transcripts were present in human central nervous system and a wide variety of peripheral organs, which is consistent with previous reports (Bonini et al., 2000; Elshourbagy et al., 2000). Of particular interest in this study is the presence of abundant NPFFR transcripts in human thymus, suggesting that NPFFR could be involved in the control of lymphocyte proliferation by NPFF as reported by Lecron et al. (1992).
In conclusion, we have identified a human receptor for NPFF and related peptides. According to Bonini et al. (2000) and Hinuma et al. (2000), who have identified another G protein coupled receptor for NPFF, the one described in the present study is assumed to be the NPFFR 2 subtype. The availability of the cloned receptor will lead to a better understanding of the physiological and pathophysiological roles of NPFF and related peptides in the central nervous system.
Acknowledgments
We thank Sophie Lamoral, Marie-Eve Decobecq and Pierre Libert for their expert technical assistance. This work was supported by the Belgian program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, the Fondation Médicale Reine Elisabeth, the BIOTECH program of the European Community (grant BIO4-CT96-0699) and the Fonds de la Recherche Scientifique Médicale of Belgium.
Abbreviations
- BSA
bovine serum albumin
- cyclic AMP
cyclic adenosine monophosphate
- CHO
Chinese hamster ovary
- GPCR
G protein-coupled receptor
- NPAF
neuropeptide AF
- NPFF
neuropeptide FF
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RT
reverse transcription
References
- ALLARD M., THEODOSIS D.T., ROUSSELOT P., LOMBARD M.C, SIMONNET G. Characterization and localization of a putative morphine-modulating peptide, FLFQPQRFamide, in the rat spinal cord: biochemical and immunocytochemical studies. Neuroscience. 1991;40:81–92. doi: 10.1016/0306-4522(91)90176-o. [DOI] [PubMed] [Google Scholar]
- BLANPAIN C., DORANZ B.J., VAKILI J., RUCKER J., GOVAERTS C., BAIK S.S.W., LORTHIOIR O., MIGEOTTE I., LIBERT F., BALEUX F., VASSART G., DOMS R.W, PARMENTIER M. Multiple charged and aromatic residues in CCR5 amino-terminal domain are involved in high affinity binding of both chemokines and HIV-1 env protein. J. Biol. Chem. 1999;274:34719–34727. doi: 10.1074/jbc.274.49.34719. [DOI] [PubMed] [Google Scholar]
- BONINI J.A., JONES K.A., ADHAM N., FORRAY C., ARTYMYSHYN R., DURKIN M.M., SMITH K.E., TAMM J.A., BOTEJU L.W., LAKHLANI P.P., RADDATZ R., YAO W.J., OGOZALEK K., BOYLE N., KOURANOVA E.V., QUAN Y., VAYSSE P.J., WETZEL J.M., BRANCHEK T.A., GERALD C, BOROWSKY B. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000;275:39324–39331. doi: 10.1074/jbc.M004385200. [DOI] [PubMed] [Google Scholar]
- CIKOS S., GREGOR P, KOPPEL J. Sequence and tissue distribution of a novel G protein-coupled receptor expressed prominently in human placenta. Biochem. Biophys. Res. Commun. 1999;256:352–356. doi: 10.1006/bbrc.1999.0332. [DOI] [PubMed] [Google Scholar]
- DETHEUX M., STANDKER L., VAKILI J., MUNCH J., FORSSMANN U., ADERMANN K., POHLMANN S., VASSART G., KIRCHHOFF F., PARMENTIER M, FORSSMANN W.G. Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J. Exp. Med. 2000;192:1501–1508. doi: 10.1084/jem.192.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVILLERS J.-P., MAZARGUIL H., ALLARD M., DICKENSON A.H., ZAJAC J.-M, SIMONNET G. Characterization of a potent agonist for NPFF receptors: binding study in rat spinal cord membranes. Neuropharmacology. 1994;33:661–669. doi: 10.1016/0028-3908(94)90172-4. [DOI] [PubMed] [Google Scholar]
- DUPOUY V, ZAJAC J.-M. Effects of neuropeptide FF analogs on morphine analgesia in the nucleus raphe dorsalis. Regul. Pept. 1995;59:349–356. doi: 10.1016/0167-0115(95)00091-o. [DOI] [PubMed] [Google Scholar]
- DUPOUY V, ZAJAC J.-M. Neuropeptide FF receptors in rat brain: a quantitative light-microscopic autoradiographic study using [125I][D.Tyr1, (NMe)Phe3]NPFF. Synapse. 1996;24:282–296. doi: 10.1002/(SICI)1098-2396(199611)24:3<282::AID-SYN11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., AMES R.S., FITZGERALD L.R., FOLEY J.J., CHAMBERS J.K., SZEKERES P.G., EVANS N.A., SCHMIDT D.B., BUCKLEY P.T., DYTKO G.M., MURDOCK P.R., MILLIGAN G., GROARKE D.A., TAN K.B., SHABON U., NUTHULAGANTI P., WANG D.Y., WILSON S., BERGSMA D.J, SARAU H.M. Receptor for the pain modulatory neuropeptide FF and AF is an orphan G protein-coupled receptor. J. Biol. Chem. 2000;275:25965–25971. doi: 10.1074/jbc.M004515200. [DOI] [PubMed] [Google Scholar]
- GELOT A., FRANCES B., GICQUEL S, ZAJAC J.-M. Antisense oligonucleotides to human SQA-neuropeptide FF decrease morphine tolerance and dependence in mice. Eur. J. Pharmacol. 1998;358:203–206. doi: 10.1016/s0014-2999(98)00625-6. [DOI] [PubMed] [Google Scholar]
- GHERARDI N, ZAJAC J.-M. Neuropeptide FF receptors of mouse olfactory bulb: binding properties and stimulation of adenylate cyclase activity. Peptides. 1997;18:577–583. doi: 10.1016/s0196-9781(97)00071-5. [DOI] [PubMed] [Google Scholar]
- GOUARDÈRES C., MOLLEREAU C., TAFANI J.A.M., MAZARGUIL H, ZAJAC J-M.[125I]EYF: a new high affinity radioligand to Neuropeptide FF receptors Peptides 2001. in press [DOI] [PubMed]
- GOUARDÈRES C., SUTAK M., ZAJAC J.-M, JHAMANDAS K. Antinociceptive effects of intrathecally administered F8Famide and FMRFamide in the rat. Eur. J. Pharmacol. 1993;237:73–81. doi: 10.1016/0014-2999(93)90095-y. [DOI] [PubMed] [Google Scholar]
- GOUARDÈRES C., TAFANI J.A., MAZARGUIL H, ZAJAC J.-M. Autoradiographic characterization of rat spinal neuropeptide FF receptors by using [125I][D.Tyr1, (NMe)Phe3]NPFF. Brain Res. Bull. 1997;42:231–238. doi: 10.1016/s0361-9230(96)00261-4. [DOI] [PubMed] [Google Scholar]
- HINUMA S., SHINTANI Y., FUKUSUMI S., IIJIMA N., MATSUMOTO Y., HOSOYA M., FUJII R., WATANABE T., KIKUCHI K., TERAO Y., YANO T., YAMAMOTO T., KAWAMATA Y., HABATA Y., ASADA M., KITADA C., KUROKAWA T., ONDA H., NISHIMURA O., TANAKA M., IBATA Y, FUJINO M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat. Cell. Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- KIVIPELTO L., MAJANE E.A., YANG H.-Y.T, PANULA P. Immunohistochemical distribution and partial characterization of FLFQPQRFamide-like peptides in the central nervous system of rats. J. Comp. Neurol. 1989;286:269–287. doi: 10.1002/cne.902860211. [DOI] [PubMed] [Google Scholar]
- LECRON J.C., MINAULT M., ALLARD M., GOUBE DE LAFOREST P., GOMBERT J, SIMONNET G. Modulation of human lymphocyte proliferation by FLFQPQRFamide, a FMRFamide-like peptide with anti-opiate properties. J. Neuroimmunol. 1992;38:1–8. doi: 10.1016/0165-5728(92)90084-x. [DOI] [PubMed] [Google Scholar]
- LEE C.-H., WASOWICZ D.H., BROWN R., MAJANE E.A., YANG H.-Y.T, PANULA P. Distribution and characterization of neuropeptide FF-like immunoreactivity in the rat nervous system with a monoclonal antibody. Eur. J. Neurosci. 1993;5:1339–1348. doi: 10.1111/j.1460-9568.1993.tb00920.x. [DOI] [PubMed] [Google Scholar]
- MAJANE E.A., PANULA P, YANG H.-Y.T. Rat brain distribution and spinal cord neuronal pathway of FLFQPQRF-NH2, a mammalian FMRF-NH2-like peptide. Brain Res. 1989;494:1–12. doi: 10.1016/0006-8993(89)90137-6. [DOI] [PubMed] [Google Scholar]
- OBERLING P., STINUS L., LE MOAL M, SIMONNET G. Biphasic effect on nociceptin and antiopiate activity of neuropeptide FF (FLFQPQRFamide) in the rat. Peptides. 1993;14:919–924. doi: 10.1016/0196-9781(93)90067-q. [DOI] [PubMed] [Google Scholar]
- PANULA P., AARNISALO A.A, WASOWICZ K. Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog. Neurobiol. 1996;48:461–487. doi: 10.1016/0301-0082(96)00001-9. [DOI] [PubMed] [Google Scholar]
- PAYZA K., AKAR C.A, YANG H.Y. Neuropeptide FF receptors: structure-activity relationship and effect of morphine. J. Pharmacol. Exp. Ther. 1993;267:88–94. [PubMed] [Google Scholar]
- PAYZA K, YANG H.-Y.T. Modulating of neuropeptide FF receptors by guanine nucleotides and cations in membranes of rat brain and spinal cord. J. Neurochem. 1993;60:1894–1899. doi: 10.1111/j.1471-4159.1993.tb13417.x. [DOI] [PubMed] [Google Scholar]
- PERRY S.J., YI-KUNG HUANG E., CRONK D., BAGUST J., SHARMA R., WALKER R.J., WILSON S, BURKE J.F. A human gene encoding morphine modulating peptides related to NPFF and FMRFamide. FEBS Lett. 1997;409:426–430. doi: 10.1016/s0014-5793(97)00557-7. [DOI] [PubMed] [Google Scholar]
- ROUMY M., GOUARDERES C., MAZARGUIL H, ZAJAC J.-M. Are neuropeptides FF and SF neurotransmitters in the rat. Biochem. Biophys. Res. Commun. 2000;275:821–824. doi: 10.1006/bbrc.2000.3394. [DOI] [PubMed] [Google Scholar]
- ROUMY M, ZAJAC J.-M. Neuropeptide FF, pain and analgesia. Eur. J. Pharmacol. 1998;345:1–11. doi: 10.1016/s0014-2999(97)01604-x. [DOI] [PubMed] [Google Scholar]
- TOVEY K.C., OLDHAM K.G, WHELAN J.A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin. Chim. Acta. 1974;56:221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- VILIM F.S., AARNISALO A.A., NIEMINEN M.L., LINTUNEN M., KARLSTEDT K., KONTINEN V.K., KALSO E., STATES B., PANULA P, ZIFF E. Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli. Mol. Pharmacol. 1999;55:804–811. [PubMed] [Google Scholar]
- YANG H.-Y.T., FRATTA W., MAJANE E.A, COSTA E. Isolation, sequencing, synthesis, and pharmacological characterization of the two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC J.-M, GOUARDÈRES C.Neuropeptide FF receptors Handbook of Chemical Neuroanatomy Peptide Receptors Part I 200016Amsterdam: Elsevier; 163–193.eds. Quirion, R., Bjökland, A., Hökfelt, T. pp [Google Scholar]


