Abstract
Neuropeptide FF (NPFF) is a part of a neurotransmitter system acting as a modulator of endogenous opioid functions. At this time, no non-peptide or peptide NPFF-antagonists have been discovered. Here, we demonstrate that Neuropeptide Y (NPY) ligands, in fact possess significant ability to interact with the human NPFF2 receptors. NPY Y1 antagonist BIBP3226 and mixed Y1 antagonist/Y4 agonist GR231118 are able to displace with low affinity, 50 – 100 nM, the specific binding on NPFF receptors expressed in CHO cells as well as in rat dorsal spinal cord, an affinity however superior to those determined against Y2, Y4 or Y5 receptors. Furthermore, BIBP3226 which is unable to inhibit the forskolin-stimulated cyclic AMP production mediated by NPFF2 receptors, antagonizes the effect of NPFF, revealing the first antagonist of NPFF receptors. These properties of NPY ligands on Neuropeptide FF receptors must be considered when evaluating pharmacological activities of these drugs.
Keywords: Neuropeptide FF, neuropeptide Y, G-protein coupled receptor, antagonist, agonist
Introduction
Neuropeptide FF (NPFF) is an amidated neuropeptide acting as a modulator of endogenous opioid functions (for review Roumy & Zajac, 1998). Pharmacological studies suggested the possible existence of NPFF receptor subtypes. Indeed, two different complementary DNA encoding G-protein coupled receptors (NPFF1 and NPFF2 receptors) have recently been cloned (Bonini et al., 2000; Elshourbagy et al., 2000; Hinuma et al., 2000). Interestingly, both receptors share about 30 – 35% identity with the orexin and Neuropeptide Y (NPY) receptors. Moreover, Bonini et al. (2000) reported that BIBP3226 (Y1 antagonist; (Rudolf et al., 1994)) and frog Pancreatic Polypeptide (fPP), two ligands of the NPY receptor family, were able to compete for specific binding of [125I]-1DMe, an analogue of NPFF, on recombinant NPFF receptors, and were selective for NPFF1 and NPFF2 receptors, respectively.
The possible interaction between NPFF- and NPY- preferential ligands with NPFF and NPY receptors led us to investigate the capacity of several ligands belonging to the NPY family (i) to displace the specific binding of the [125I]-EYF, an analogue of NPFF on human NPFF2 receptors expressed in CHO cells, as well as on NPFF receptors of the rat spinal cord and (ii) to inhibit adenylate cyclase activity in hNPFF2 receptor transfected cells. Our results indicate that the selective NPY Y1 receptor antagonist BIBP3226 (Rudolf et al., 1994; Schober et al., 1998), the mixed NPY Y1 receptor antagonist/ Y4 receptor agonist GR231118 (Dumont et al., 2000b; Parker et al., 1998; Schober et al., 1998) and the fPP not only display a relatively high affinity for NPFF receptors, but exhibit also antagonistic (BIBP3226) or agonistic (GR231118, fPP) activity at the hNPFF2 receptor expressed in CHO cells. These features suggest that the use of these molecules requires careful interpretation, as a potential contribution from NPFF receptors may be difficult to exclude.
Methods
Peptides of the NPFF family and frog PP were synthesized using an automated peptide synthesizer (Applied Biosystems model 433A). PYY, [Leu31,Pro34]-PYY, PYY3-36 and hPP were synthesized as previously described (Forest et al., 1990). BIBP3226, BIBP3445 and BIIE0246 were generously provided by Boehringer Ingelheim (Germany). GR231118 was a gift from GlaxoWellcome (Research Triangle Park, NC, U.S.A.) while CGP71683A and JCF109 were generously obtained from Servier (Paris, France). The rat Y1, Y2, Y4 and Y5 receptor cDNA were generously provided by Dr Herbert Herzog (Sydney, Australia).
Transfected HEK 293 cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% foetal calf serum and amphotericin B, (Gibco-BRL, Canada). All NPY binding assays were performed as previously described (Dumont et al., 2000a).
Recombinant CHO cells expressing the human NPFF2 receptor were grown in Ham's F12 medium supplemented with 7% foetal calf serum and G418 400 μg ml−1, (Gibco-BRL, France). For membrane preparation, cells were harvested in phosphate buffer saline, frozen at −70°C, and homogenized in 50 mM Tris-HCl, pH 7.4 in a Potter Elvehjem tissue grinder. The nuclear pellet was discarded by centrifugation at 1000×g for 15 min at 4°C, and the membrane fraction was collected upon centrifugation of the supernatant at 100,000×g for 30 min at 4°C. Binding of [125I]-EYF ([125I]-EYWSLAAPQRF-NH2), a new specific radioligand for NPFF receptors (2000 Ci mmole; Gouardères et al., 2001) on membranes (2 μg) of hNPFF2 receptor expressing CHO cells was measured by rapid filtration as described (Gouardères et al., 2001). Binding on NPFF receptors in rat (male Sprague-Dawley, 350 g; Depré, France) spinal cord sections was exactly as described in Gouardères et al. (2001).
Assay for intracellular cyclic AMP was performed essentially as described in (Mollereau et al., 1999). Briefly, 200,000 recombinant cells were incubated for 1 h at 37°C under 5% CO2 with 0.6 μCi [3H]-adenine (26 Ci/mmole Amersham) in Ham's F12 medium. Cyclic AMP production was stimulated by 2 μM Forskolin (Sigma, France) for 10 min at 37°C in 200 μl HEPES buffered Krebs-Ringer saline in the presence of 0.1 mM of the phosphodiesterase inhibitors, IBMX (Sigma, France) and Ro-20 1724 (Fisher, France). Ligands to be tested were added at the same time at the desired concentration. The reaction was stopped by addition of 20 μl HCl 2.2 N and intracellular [3H]-cyclic AMP was isolated by chromatograhic procedure on acid alumina columns (Sigma, France).
Results
Competition studies on HEK 293 cells transfected with either the rat NPY Y1, Y2, Y4 or Y5 receptor cDNA revealed that NPFF related peptides such as NPFF (FLFQPQRF-NH2), NPA-NPFF, 1DMe (D.YL(NMe)FQPQRF-NH2), NPSF (SLAAPQRF-NH2), EFW-NPSF, hNPAF (Perry et al., 1997; Vilim et al., 1999) were inactive up to 10 μM to compete against specific [125I]-GR231118, [125I]-PYY3-36, [125I]-hPP or [125I][Leu31,Pro34]PYY sites, respectively (Table 1 and data not shown). Conversely, ligands of the NPY family (Dumont et al., 2000c) including BIBP3445 (enantiomer of BIBP3226), BIIE0246 (Y2 antagonist), CGP71683A (Y5 antagonist) and JCF109 (Y5 antagonist) were not recognized (IC50>1 μM) by the human NPFF2 receptor expressed in CHO cells (not shown) except the frog pancreatic polypeptide (fPP), the Y1 antagonist/Y4 agonist GR231118 and the Y1 antagonist BIBP3226. As shown in the Figure 1A, these molecules were able to completely inhibit the specific binding of [125I]-EYF on the hNPFF2 receptor with apparent affinities (Ki) in the nM range (Table 1). Similar values were obtained against endogenously expressed NPFF receptors in the rat spinal cord (Table 1), confirming the ability of some NPY ligands to interact with native NPFF receptors. Moreover, the binding of BIBP3226 to NPFF receptors exhibited the same stereoselectivity as on NPY receptors since the enantiomer BIBP3435 was inactive. It is interesting to add that the last C-terminal nine residues of fPP were sufficient to confer affinity of fPP28-36 for NPFF receptors (Table 1).
Table 1.
Binding constants (Ki) and functional parameters (EC50) of various ligands on NPFF receptors in rat spinal cord (rNPFF column) and CHO transfected cells (hNPFF2 column), and on rat NPY receptors in HEK 293 transfected cells (rNPY Y1, Y2, Y4, Y5 columns)
Figure 1.
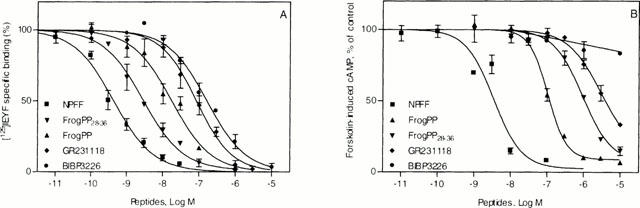
Displacement of [125I]-EYF (0.05 nM) specific binding on membranes of recombinant CHO cells (A) and accumulation of intracellular cyclic AMP (B) in CHO cells expressing the human NPFF2 receptor. Each curve represents the means±s.e.mean of at least three experiments performed in triplicate.
Functional activity of fPP, GR231118 and BIBP3226 was investigated next by measuring the decrease of forskolin-induced cyclic AMP content as we found the hNPFF2 receptor to be negatively coupled to adenylate cyclase activity in CHO cells (Figure 1B and data not shown). In recombinant hNPFF2 transfected cells, NPFF inhibited the cyclic AMP production with EC50 of 3 nM (Table 1). fPP and GR231118 were found to be full agonists (Figure 1B) but with a rather low potency (EC50=115 nM and 3000 nM respectively, Table 1). On the other hand, BIBP3226 was inactive by itself at concentration up to 10 μM (Figure 1B). However, increasing concentrations of this compound reversed the inhibitory effect of 10 nM NPFF on adenylate cyclase activity demonstrating that BIBP3226 behaved as an antagonist at hNPFF2 receptors (Figure 2).
Figure 2.

Effect of increasing concentrations of BIBP3226 on the inhibition by NPFF of forskolin-induced cyclic AMP accumulation in CHO cells expressing the human NPFF2 receptor. Intracellular cyclic AMP content induced by 2 μM forskolin (black bar) was inhibited (80 – 90%) by 10 nM NPFF (white bar), and increasing concentrations of BIBP3226 (grey bar) reversed this effect. The bars represent the means±s.e.mean of triplicate determinations of one representative experiment among three.
Discussion
In the present study, we found that among several ligands of the NPY family, frog PP, GR231118 and BIBP3226, were able to compete for the specific NPFF binding expressed in CHO cells as well as in rat dorsal spinal cord. Interestingly, the apparent affinity of the selective NPY Y1/Y4 ligand GR231118 for NPFF2 receptors (Ki=50 – 70 nM) is comparable to its well established affinities for the NPY Y2 (Ki=63 nM) and Y5 (Ki=100 nM) receptors (Parker et al., 1998 and Table 1). Moreover, the apparent affinity (Ki about 100 nM) of the selective NPY Y1 receptor antagonist BIBP3226 for NPFF2 receptors is much greater than those reported on the NPY Y2, Y4 and Y5 receptors, (Schober et al., 1998; Dumont et al., 2000c and Table 1). Furthermore, we observed relatively high affinities (Ki=1.5 – 7 nM) for fPP and its truncated analogue fPP28-36 for NPFF receptors. This is likely explained by the presence of Arg-Phe-amide residues at the C-terminus of the peptides instead of the usual Arg-Tyr-amide residues present in all mammalian pancreatic polypeptides as well as in NPY and PYY. To our knowledge, no data on the affinity of fPP for NPY receptor subtypes is available in the literature. Interestingly, we observed that the affinities of BIBP3226 and fPP for the hNPFF2 receptors expressed in CHO cells and for the rat spinal cord receptors (suspected to be of the NPFF2 receptor subtype; Bonini et al., 2000), were 10 fold better than those reported on human and rat NPFF2 receptors expressed in HEK 293 cells (Bonini et al., 2000). This apparent discrepancy remains to be explained.
The functional properties of fPP, GR231118 and BIBP3226 were investigated next on the basis of cyclic AMP accumulation assays in hNPFF2 receptors transfected cells. Interestingly, fPP and GR231118 exhibit agonistic activity. Although the potency of GR231118 is 500 – 1000 fold lower than that observed for NPY Y4 receptors (Parker et al., 1998; Schober et al., 1998), it is in the same range order than those described for Y2 and Y5 NPY receptors (Parker et al., 1998). On the other hand, the Y1 antagonist BIBP3226 which is inactive by itself at up to 10 μM, is able to antagonize in a concentration-dependent manner the inhibition of forskolin-stimulated cyclic AMP production induced by NPFF (10 nM). Hence, BIBP3226 is the first antagonist to be reported for NPFF2 receptors and could therefore be considered as a lead compound in an effort to develop more potent antagonists for the NPFF2 receptor subtype.
Taken together our data suggest that NPFF receptors are related to NPY (most particularly Y1 and Y4) receptors not only on sequence homology but also on binding affinity and functional properties. Both families may have conserved an ancestral binding pocket that has evolved towards the Arg-Phe-amide or Arg-Tyr-amide interactions. This hypothesis should be explored in detailed mutagenesis and structure-activity studies.
NPY agonists are known to stimulate appetite (Dumont et al., 2000c). In contrast, the only report on the effect of NPFF on ingestive behaviour described reduction of food intake in rats (Murase et al., 1996). Similarly, GR231118 although acting as a NPY Y4 agonist, has been found to decrease food intake in rats (Schober et al., 1998). Whether this effect is due to a possible interaction with a NPFF receptor subtype should be investigated in future studies.
In conclusion, our results describe the first NPFF receptors antagonist (BIBP3226) and suggest cross-reaction between BIBP3226 and GR231118 with NPFF receptors when using these compounds to investigate the NPY receptors.
Acknowledgments
We thank H. Mazarguil for the synthesis of peptides. This study was supported by CNRS and MIDLT/INSERM/CNRS and grants from the Canadian Institute of Health Research (CIHR) to R. Quirion. R. Quirion is a ‘chercheur-boursier' of the ‘Fonds de la Recherche en Santé du Québec'.
Abbreviations
- BIBP3226
R-N2-(Diphenylacetyl)-N-(4-hydrophenyl)-methyl argininamide
- GR231118
homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2
References
- BONINI J.A., JONES K.A., ADHAM N., FORRAY C., ARTYMYSHYN R., DURKIN M.M., SMITH K.E., TAMM J.A., BOTEJU L.W., LAKHLANI P.P., RADDATZ R., YAO W.J., OGOZALEK K.L., BOYLE N., KOURANOVA E.V., QUAN Y., VAYSSE P.J., WETZEL J.M., BRANCHEK T.A., GERALD C., BOROWSKY B. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000;275:39324–39331. doi: 10.1074/jbc.M004385200. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., FOURNIER A., QUIRION R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIB03304 (Y1) and CGP71683A (Y5) Can. J. Physiol. Pharmacol. 2000a;78:116–125. [PubMed] [Google Scholar]
- DUMONT Y., JACQUES D., ST PIERRE J.A., TONG Y., PARKER R., HERZOG H., QUIRION R.Neuropeptide Y, peptide YY and pancreatic polypeptide receptors proteins and mRNAs in mammalian brains Handbook of chemical Neuroanatomy 2000c16Peptides receptors Part I 375-475. Eds R. Quirion, A. BJörklund, T. Hökfelt [Google Scholar]
- DUMONT Y., QUIRION R. [(125)I]- GR231118: a high affinity radioligand to investigate neuropeptide Y Y(1) and Y(4) receptors. Br. J. Pharmacol. 2000b;129:37–46. doi: 10.1038/sj.bjp.0702983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., AMES R.S., FITZGERALD L.R., FOLEY J.J., CHAMBERS J.K., SZEKERES P.G., EVANS N.A., SCHMIDT D.B., BUCKLEY P.T., DYTKO G.M., MURDOCK P.R., MILLIGAN G., GROARKE D.A., TAN K.B., SHABON U., NUTHULAGANTI P., WANG D.Y., WILSON S., BERGSMA D.J., SARAU H.M. Receptor for the pain modulatory neuropeptides FF and AF is an orphan G protein-coupled receptor. J. Biol. Chem. 2000;275:25965–25971. doi: 10.1074/jbc.M004515200. [DOI] [PubMed] [Google Scholar]
- FOREST M., MARTEL J.C., ST-PIERRE S., QUIRION R., FOURNIER A. Structural study of the N-terminal segment of neuropeptide tyrosine. J. Med. Chem. 1990;33:1615–1619. doi: 10.1021/jm00168a014. [DOI] [PubMed] [Google Scholar]
- GOUARDERES CH., MOLLEREAU C., TAFANI J.A.M., MAZARGUIL H., ZAJAC J-M.[125I]EYF: a new high affinity radioligand to Neuropeptide FF receptors Peptides 2001(in press) [DOI] [PubMed]
- HINUMA S., SHINTANI Y., FUKUSUMI S., IIJIMA N., MATSUMOTO Y., HOSOYA M., FUJII R., WATANABE T., KIKUCHI K., TERAO Y., YANO T., YAMAMOTO T., KAWAMATA Y., HABATA Y., ASADA M., KITADA C., KUROKAWA T., ONDA H., NISHIMURA O., TANAKA M., IBATA Y., FUJINO M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat. Cell. Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., MOULEDOUS L., LAPALU S., CAMBOIS G., MOISAND C., BUTOUR J.L., MEUNIER J.C. Distinct mechanisms for activation of the opioid receptor-like 1 and kappa-opioid receptors by nociceptin and dynorphin A. Mol. Pharmacol. 1999;55:324–331. doi: 10.1124/mol.55.2.324. [DOI] [PubMed] [Google Scholar]
- MURASE T., ARIMA H., KONDO K., OISO Y. Neuropeptide FF reduces food intake in rats. Peptides. 1996;17:353–354. doi: 10.1016/0196-9781(95)02137-x. [DOI] [PubMed] [Google Scholar]
- PARKER E.M., BABIJ C.K., BALASUBRAMANIAM A., BURRIER R.E., GUZZI M., HAMUD F., MUKHOPADHYAY G., RUDINSKI M.S., TAO Z., TICE M., XIA L., MULLINS D.E., SALISBURY B.G. GR231118 (1229U91) and other analogues of the C-terminus of neuropeptide Y are potent neuropeptide Y Y1 receptor antagonists and neuropeptide Y Y4 receptor agonists. Eur. J. Pharmacol. 1998;349:97–105. doi: 10.1016/s0014-2999(98)00171-x. [DOI] [PubMed] [Google Scholar]
- PERRY S.J., YI-KUNG HUANG E., CRONK D., BAGUST J., SHARMA R., WALKER R.J., WILSON S., BURKE J.F. A human gene encoding morphine modulating peptides related to NPFF and FMRFamide. FEBS Lett. 1997;409:426–430. doi: 10.1016/s0014-5793(97)00557-7. [DOI] [PubMed] [Google Scholar]
- ROUMY M., ZAJAC J.M. Neuropeptide FF, pain and analgesia. Eur. J. Pharmacol. 1998;345:1–11. doi: 10.1016/s0014-2999(97)01604-x. [DOI] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A., WILLIM K.D., ENTZEROTH M., WIENEN W., BECK-SICKINGER A.G., DOODS H.N. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur. J. Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- SCHOBER D.A., VAN ABBEMA A.M., SMILEY D.L., BRUNS R.F., GEHLERT D.R. The neuropeptide Y Y1 antagonist, 1229U91, a potent agonist for the human pancreatic polypeptide-preferring (NPY Y4) receptor. Peptides. 1998;19:537–542. doi: 10.1016/s0196-9781(97)00455-5. [DOI] [PubMed] [Google Scholar]
- VILIM F.S., AARNISALO A.A., NIEMINEN M.L., LINTUNEN M., KARLSTEDT K., KONTINEN V.K., KALSO E., STATES B., PANULA P., ZIFF E. Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli. Mol. Pharmacol. 1999;55:804–811. [PubMed] [Google Scholar]


