Abstract
A study was made of the regulation of [3H]-γ-aminobutyric acid ([3H]-GABA) release from slices of rat striatum by endogenous dopamine and exogenous histamine and a histamine H3-agonist. Depolarization-induced release of [3H]-GABA was Ca2+-dependent and was increased in the presence of the dopamine D2 receptor family antagonist, sulpiride (10 μM). The sulpiride-potentiated release of [3H]-GABA was strongly inhibited by the dopamine D1 receptor family antagonist, SCH 23390 (1 μM). Neither antagonist altered basal release.
The 15 mM K+-induced release of [3H]-GABA in the presence of sulpiride was inhibited by 100 μM histamine (mean inhibition 78±3%) and by the histamine H3 receptor-selective agonist, immepip, 1 μM (mean inhibition 81±5%). The IC50 values for histamine and immepip were 1.3±0.2 μM and 16±2 nM, respectively. The inhibitory effects of histamine and immepip were reversed by the H3 receptor antagonist, thioperamide, 1 μM.
The inhibition of 15 mM K+-induced [3H]-GABA release by immepip was reversed by the H3 receptor antagonist, clobenpropit, Kd 0.11±0.04 nM. Clobenpropit alone had no effect on basal or stimulated release of [3H]-GABA.
Elevated K+ caused little release of [3H]-GABA from striatal slices from reserpinized rats, unless the D1 partial agonist, R(+)-SKF 38393, 1 μM, was also present. The stimulated release in the presence of SKF 38393 was reduced by 1 μM immepip to the level obtained in the absence of SKF 38393.
These observations demonstrate that histamine H3 receptor activation strongly inhibits the dopamine D1 receptor-dependent release of [3H]-GABA from rat striatum; primarily through an interaction at the terminals of GABA neurones.
Keywords: GABA release, rat striatum, dopamine D1 receptors, dopamine D2 receptors, histamine H3 receptors, immepip, clobenpropit, thioperamide, SKF 38393, reserpine
Introduction
We have reported previously that activation of histamine H3 receptors located on the terminals of striatonigral projection neurones in rat substantia nigra pars reticulata (SNr) selectively inhibits the component of depolarization-induced release of [3H]-γ-aminobutyric acid ([3H]-GABA) which is dependent on concomitant dopamine D1 receptor stimulation (Garcia et al., 1997). The striatonigral projection neurones have axon collaterals which remain within the striatum (Kawaguchi et al., 1990; reviewed in Gerfen & Wilson, 1996) and the release of striatal GABA is subject to the same interplay between D1 and D2 receptors as in SNr; D1 agonists and D2 antagonists both causing an increase in GABA release (Girault et al., 1986; Floran et al., 1990; Harsing & Zigmond, 1997). The striatum is also rich in histamine H3 receptors (Arrang et al., 1987a; Cumming et al., 1991b; Pollard et al., 1993; Ligneau et al., 1994; Jansen et al., 1994) and striatal quinolinic acid lesions result in a parallel decrease in the numbers of ipsilateral dopamine D1 and histamine H3 receptors, both in SNr and striatum (Ryu et al., 1994). These observations suggest that D1 and H3 receptors are colocalized on the same terminals in the striatum, as in SNr (Garcia et al., 1997), and, hence, that depolarization-induced, D1 receptor-dependent release of [3H]-GABA in striatum may be regulated by H3 receptor activation in the same way as in SNr. We report here a study of the effects of ligands acting at dopamine D1 and D2 and histamine H3 receptors on depolarization-induced release of [3H]-GABA from slices of rat striatum. A preliminary account of some of these results has been presented to the British Pharmacological Society (Arias-Montaño et al., 2000).
Methods
Measurement of [3H]-GABA release from slices of rat striatum
The striatum was dissected from vibratome-cut slices (300 μm) of rat brain (Wistar strain, males, 250 – 300 g, bred in the Centro de Investigacion), cut into smaller pieces, and incubated for 30 min at 37°C in 4.5 ml of a modified Krebs-Henseleit solution (composition in mM: NaCl 134, KCl 4.75, MgSO4 1, KH2PO4 1.25, NaHCO3 25, CaCl2 2 and D-glucose 10) gassed continuously with O2/CO2 (95 : 5, v v−1). The slices were then incubated for 30 min with 80 nM [3H]-GABA in 4.5 ml Krebs-Henseleit solution containing 10 μM aminooxyacetic acid. At the end of this period, excess radiolabel was removed by washing twice with Krebs-Henseleit solution containing 10 μM aminooxyacetic acid and 10 μM nipecotic acid, which were present in the superfusion solution for the rest of the experiment. The slices were then apportioned randomly between the chambers of a superfusion apparatus (volume of each chamber 80 μl; 20 chambers in parallel) and superfused with the medium at a rate of 0.5 ml min−1 for 30 min. The design of the superfusion chambers was essentially as described by Aceves & Cuello (1981), except that the electrodes for electrical stimulation were omitted. Basal release of [3H]-GABA was measured by collecting four or five fractions of the superfusate at 4 min intervals (each fraction 2 ml) before release was stimulated by changing to a solution containing 15 mM K+ (composition in mM: NaCl 55.6, Na2SO4 39.2; K2SO4 6.87, MgSO4 1, KH2PO4 1.25, NaHCO3 25, CaCl2 2 and D-glucose 10: Floran et al., 1988) and a further six fractions collected. In experiments in which sulpiride (10 μM) or SCH 23390 (1 μM) was present in every incubation, they were added 4 min before the first basal fraction was collected.
Histamine, immepip, clobenpropit and thioperamide were present from 12 min before the change to the medium containing 15 mM K+. The superfusate fractions were mixed with 10 ml scintillator (4 g 2,5-diphenyloxazole+0.2 g, 1,4-bis-2-(5-phenyloxazolyl)-benzene in 1 l toluene/Triton X-100, 2 : 1, v v−1) and the tritium content determined by scintillation counting. It has been shown that >90% of the tritium released by a depolarizing stimulus from rat striatum is [3H]-GABA (Kuriyama et al., 1984; Harsing & Zigmond, 1997). To determine the total amount of tritium remaining in the tissue, the contents of each chamber were collected, treated with 1 ml 1 M HCl and allowed to stand for 1 h before addition of scintillator.
Pretreatment of animals with reserpine
Rats were pretreated with reserpine (5 mg kg−1, i.p.) 24 h before preparation of striatal slices. Control animals were treated with the same volume (1 ml kg−1) of vehicle (7% w v−1 lactic acid). The reserpine treatment has previously been shown to reduce striatal dopamine levels by 95% (Garcia et al., 1997).
Analysis of data
[3H]-GABA release was expressed initially as a fraction of the total amount of tritium remaining in the tissue. Basal fractional release per 2 ml superfusate fraction varied quite widely between chambers, but was normally in the range 0.003 – 0.020 (released tritium usually 1000 – 7000 d.p.m.), although occasional values were outside this range. The within-treatments variability in an experiment was greatly reduced by expressing the amount of tritium in each fraction as a percentage of the amount of tritium present in the fraction collected immediately before the change to the medium containing 15 mM K+ (i.e. the release in fraction 4 or 5 was set to 100%). In most experiments, 5 – 6 replicate determinations were made at each drug concentration or drug combination tested.
The effect of drugs on the basal release of [3H]-GABA was assessed by comparing the fractional release in fraction 2 or 3 (immediately before exposure of the tissue to drug, e.g. sulpiride) and fraction 4 or 5 (immediately prior to exposure to 15 mM K+), using the paired t-test.
A measure of the degree of inhibition of the release of [3H]-GABA was obtained by comparing the areas under the appropriate release curves between the first and last fractions collected after the change to high K+, making the assumption that the basal release of [3H]-GABA would have remained unchanged at the level measured in the fraction immediately preceding K+ stimulation (set to unity in the normalization procedure above). In three experiments in which this was tested the basal release in fraction 10 was 90±4% of that in fraction 5.
To obtain an unbiased estimate of IC50 values, concentration-response data for the inhibition of [3H]-GABA release by histamine and immepip, and for the reversal by clobenpropit of the inhibitory action of immepip, were fitted by non-linear regression to an hyperbola. The actual equation fitted was:
where Respmax is the maximum response (maximum per cent inhibition or per cent of control [3H]-GABA release), C is the concentration of histamine, immepip or clobenpropit and IC50 is the concentration giving the half maximal response (inhibition or reversal of inhibition). The Kd for clobenpropit in reversing the inhibition by immepip was calculated from the curves for immepip and clobenpropit using the method of Lazareno & Roberts (1987); Dickenson & Hill (1993).
To test for statistical differences between treatments, the area under the release curve in the presence of elevated K+ was calculated for each individual chamber and the data then analysed as described previously (Garcia et al., 1997).
Chemicals
[2,3-3H]-γ-Aminobutyric acid ([3H]-GABA), specific activity 82 Ci.mmol−1, was obtained from Amersham Pharmacia Biotech. Aminooxyacetic acid hemihydrochloride, 2,5-diphenyloxazole and (±)-nipecotic acid were purchased from Sigma; histamine dihydrochloride, R(+)-SCH 23390 hydrochloride (R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride), R(+)-SKF 38393 hydrochloride ((±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride), (±)-sulpiride and thioperamide maleate from Research Biochemicals International; and 1,4-bis-2-(5-phenyloxazolyl)-benzene from Packard. Clobenpropit dihydrobromide and immepip dihydrobromide were kind gifts from Prof H. Timmerman, Vrije Universiteit, Amsterdam.
Stock solutions of sulpiride (1 mM) were made in 100 μM ascorbic acid.
Results
Effect of D2 and D1 dopamine receptor blockade and of omission of Ca2+ from the medium on [3H]-GABA release
In the absence of the dopamine D2 receptor antagonist, sulpiride, increasing the K+ concentration in the superfusion medium from 6 – 15 mM caused only a small increase in the release of [3H]-GABA from striatal slices (Figure 1). However, in the presence of 10 μM sulpiride the release was markedly enhanced (mean stimulation 2.9±0.2 fold of basal, n=3; Figure 1) and was well maintained over the period for which samples were collected. In contrast, sulpiride had no significant effect on basal [3H]-GABA release (Figure 1). Sulpiride (10 μM) was therefore included in all subsequent experiments, except where specifically indicated.
Figure 1.
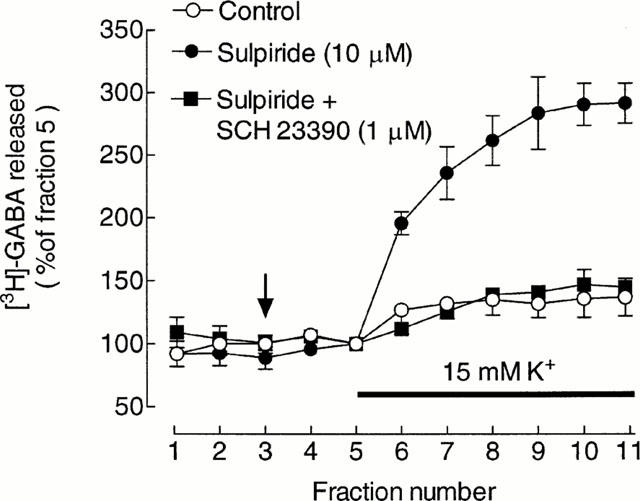
Modulation of depolarization-induced [3H]-GABA release by dopamine receptor antagonists. Values are expressed as a percentage of the fractional release of [3H]-GABA in fraction 5 and represent the means±s.e.mean from three experiments. Drugs were added at the vertical arrow and the K+ in the medium increased for the period indicated by the horizontal bar.
The depolarization-induced release of [3H]-GABA in the presence of sulpiride was highly dependent on concomitant D1 receptor activation, since it was strongly inhibited by the dopamine D1 receptor antagonist, SCH 23390 (1 μM) (Figure 1); mean inhibition 84±6%, n=3. SCH 23390 had no significant effect on basal [3H]-GABA release (Figure 1).
The depolarization-induced release of [3H]-GABA was strongly Ca2+-dependent, since omission of Ca2+ from the superfusion medium and increasing Mg2+ from 1 – 3 mM reduced depolarization-induced [3H]-GABA release to a low level; mean inhibition 84±6%, n=3 (data not shown).
Effect of histamine and immepip on [3H]-GABA release
Depolarization-induced [3H]-GABA release was strongly inhibited by 100 μM histamine (Figure 2A) and by the selective histamine H3 receptor agonist, immepip (1 μM) (Figure 2B). The mean inhibitions in this series of experiments were 78±3% and 81±5% for histamine and immepip, respectively, both n=3. Neither 100 μM histamine nor 1 μM immepip had any significant effect on basal [3H]-GABA release.
Figure 2.
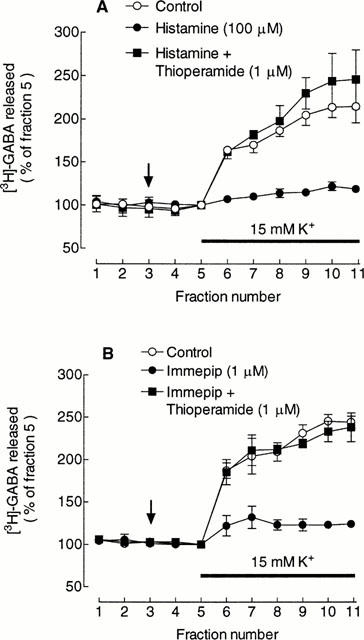
Inhibition of depolarisation-induced [3H]-GABA release by histamine and immepip and reversal by thioperamide. (A) Effect of 100 μM histamine in the absence and presence of 1 μM thioperamide. (B) Effect of 1 μM immepip in the absence and presence of 1 μM thioperamide. In both panels values are expressed as a percentage of the fractional release of [3H]-GABA in fraction 5 and represent the means±s.e.mean from three experiments. Drugs were added at the vertical arrow and the K+ in the medium increased for the period indicated by the horizontal bar. Sulpiride (10 μM) was present throughout.
The inhibitory effect of the agonists on depolarization-induced [3H]-GABA release was blocked by the H3 receptor antagonist, thioperamide (1 μM) (Figure 2A,B). In the presence of histamine/immepip+thioperamide, the extent of the release was not significantly different from control.
The inhibitory actions of histamine and immepip on depolarisation-induced [3H]-GABA release were concentration-dependent (Figure 3). The value for 300 μM histamine is from a single experiment, since in two other experiments this concentration of histamine caused a statistically significant release of [3H]-GABA in normal K+ medium. The best-fit values of log IC50±estimated s.e.mean for histamine and immepip were 3.10±0.12 and 1.19±0.15, respectively, (concentrations expressed as nM) (IC50 1.3±0.2 μM for histamine and 15.5±2.3 nM for immepip).
Figure 3.
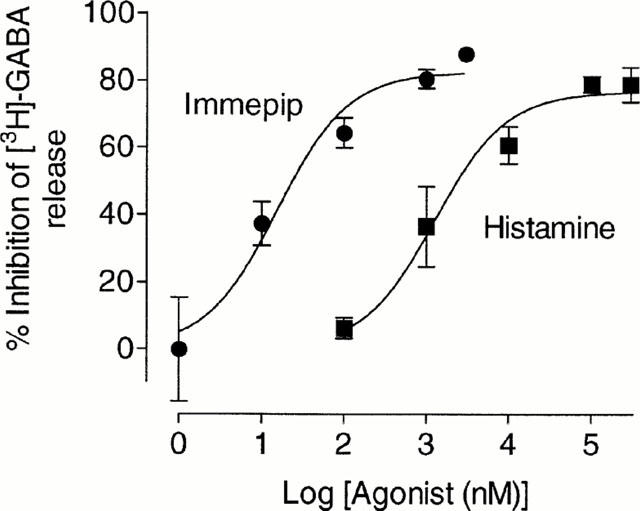
Concentration-dependence of the inhibition by histamine and immepip of depolarisation-induced [3H]-GABA release. Values are means±s.e.mean from 3 – 6 independent determinations at each concentration, except for 300 μM histamine, which is from a single experiment (see text). The curves drawn are best-fit lines to an hyperbola (see Methods).
The high degree to which the release of [3H]-GABA is inhibited by SCH 23390 makes it difficult to test whether the inhibitory action of H3 agonists is selective for the D1 receptor-dependent component of release. In three experiments in which comparison was made, the extent of the inhibition of depolarization-induced [3H]-GABA release in the presence of 1 μM SCH 23390, 84±6%, was not significantly different from that in the presence of 1 μM SCH 23390+1 μM immepip, 88±9% inhibition.
Reversal of the inhibitory action of immepip by clobenpropit
The potent effect of immepip and its reversal by thioperamide strongly suggests that the inhibition is mediated by histamine H3 receptors. To gain more quantitative evidence, we have investigated the concentration-dependence of the effect of the selective H3 receptor antagonist, clobenpropit. Acting alone, 1 μM clobenpropit had no significant effect on either basal or depolarization-induced release of [3H]-GABA (Figure 4A). However, clobenpropit reversed in a concentration-dependent manner the inhibition of depolarization-induced [3H]-GABA release by 1 μM immepip (Figure 4B). There was a marked variability in some of the experiments in this series, which is reflected in the large estimated error associated with the best-fit value of the IC50, 7.3±2.1 nM. The calculated Kd, for clobenpropit was 0.11±0.04 nM.
Figure 4.
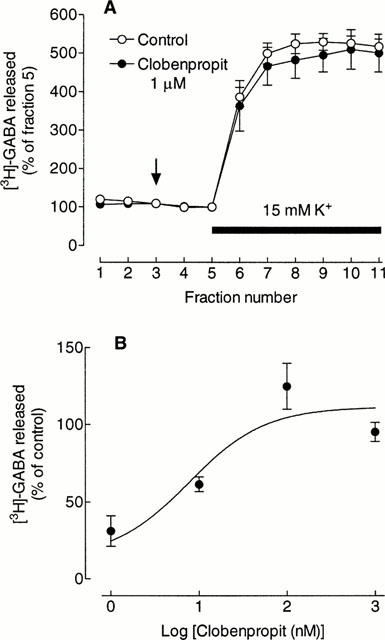
Effect of clobenpropit on [3H]-GABA release. (A) Action of clobenpropit alone. Values are expressed as a percentage of the fractional release of [3H]-GABA in fraction 5 and represent the means±s.e.mean from three experiments. Clobenpropit was added at the vertical arrow and the K+ in the medium increased for the period indicated by the horizontal bar. (B) Concentration-dependence of the reversal by clobenpropit of the inhibition of [3H]-GABA release by immepip. Immepip (1 μM) was present in all incubations with clobenpropit. Values are the per cent of control release of [3H]-GABA, calculated from the relative areas under the curves (see Methods) and are the means±s.e.mean from 3 – 5 determinations. The curve drawn is the best-fit line to an hyperbola (see Methods). The foot of the curves has been fixed at 13% (mean inhibition by 1 μM immepip in this series of experiments 87.0±2.5%). Sulpiride (10 μM) was present throughout in (A) and (B).
Effect of SKF 38393 and immepip on depolarization-induced [3H]-GABA release in striatum from reserpinized rats
The evidence indicates that H3 receptor activation inhibits dopamine-dependent release of [3H]-GABA in rat striatum, but does not indicate whether the action is at the level of dopamine release or is a direct effect at GABA terminals. To establish whether there is an interaction at GABA terminals, measurements were made on depolarization-induced [3H]-GABA released from striatal slices from animals treated with reserpine 24 h previously, which reduces striatal dopamine to very low levels (Garcia et al., 1997). Sulpiride was also omitted from the superfusion medium in these experiments.
In the absence of added drugs, depolarization with raised K+ had a minimal effect on the release of [3H]-GABA (Figure 5), consistent with the absence of significant dopamine release from the reserpinized slices. However, in the presence of the dopamine D1 receptor agonist, R(+)-SKF 38393, 1 μM, the depolarization-induced release was markedly stimulated (Figure 5). Addition of 1 μM immepip reduced the depolarization-induced release in the presence of SKF 38393 to control levels (Figure 5), indicating that histamine H3 and dopamine D1 receptors are probably colocalized on the GABA terminals. Immepip alone was without effect on release (Figure 5).
Figure 5.
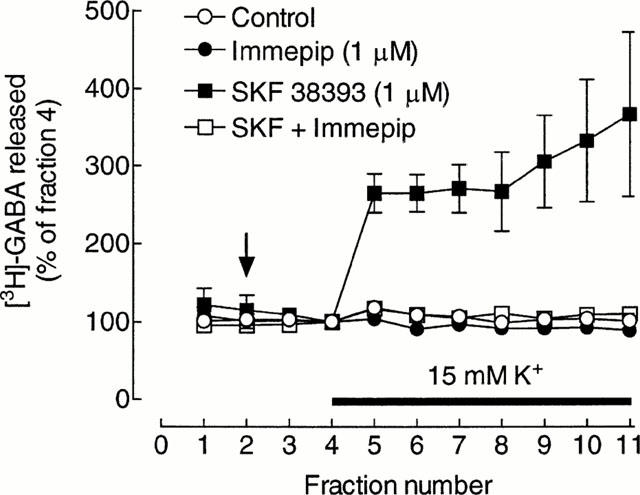
Effect of immepip on depolarization-induced, dopamine D1 receptor-dependent, release of [3H]-GABA from striatal slices from reserpinised rats. Values are the means±s.e.mean from four (control and immepip) or five replicate determinations within a single experiment. Drugs were added at the vertical arrow and and K+ in the medium increased for the period indicated by the horizontal bar. Similar results were obtained in two further experiments, except that the magnitudes of the release in the presence of SKF 38393 differed (max. 1.9 and 7.3 fold of basal release).
Discussion
It is clear that histamine inhibits dopamine-dependent [3H]-GABA release from rat striatal slices in a manner similar to that reported using slices of rat SNr (Garcia et al., 1997). However, the larger amount of striatal tissue has made it possible to provide much stronger evidence that the inhibition is mediated by histamine H3 receptors. The estimated Kd of 0.11±0.04 nM for the H3 receptor antagonist clobenpropit is in accord with literature values of 0.13 nM (Leurs et al., 1995a), 0.03 nM (Harper et al., 1999) and 0.08 nM (Valentine et al., 1999) and the potency of immepip as an inhibitor of dopamine-dependent [3H]-GABA release (IC50 16±2 nM) is similar to that reported for inhibition of electrically-evoked twitches of guinea-pig jejunum (IC50 10 nM; Leurs et al., 1995a). In addition, the effect of histamine and immepip is fully reversed by 1 μM thioperamide, although thioperamide may be a less selective H3 antagonist than clobenpropit (Leurs et al., 1995b). There is no evidence for the involvement of either histamine H1 or H2 receptors in the inhibition of [3H]-GABA release, since the inhibition is completely reversed by clobenpropit and thioperamide and the extent of the inhibition by histamine is the same, within error, as that produced by immepip.
Rat striatum has a low to moderate density of histaminergic fibres (Inagaki et al., 1988; Panula et al., 1989), but there is no indication of any release of endogenous histamine in our slices, since clobenpropit, in the absence of immepip, had no significant effect on either basal or depolarization-stimulated release of [3H]-GABA. The lack of effect on basal release is consistent with the estimate of 50 nM for the lower limit of the extracellular concentration of histamine in rat striatum (Cumming et al., 1991a), which is well below the IC50 for histamine for inhibition of [3H]-GABA release, 1.3±0.2 μM. Histamine is released from histaminergic fibres on increasing extracellular K+ (Arrang et al., 1983), but apparently not in sufficient amounts from striatal fibres to produce any inhibition of [3H]-GABA release, as indicated by the lack of effect of clobenpropit. This is consistent with a report that no change was detected in the release of endogenous histamine from rat striatum, measured by microdialysis, when the K+ concentration in the probe was increased to 156 mM (Russell et al., 1990).
The IC50 for histamine for inhibition of [3H]-GABA release is in close agreement with the recently reported IC50 for histamine inhibition of corticostriatal transmission, 1.6 μM (Doreulee et al., 2001), but is much higher than that reported for histamine-induced inhibition of depolarization-induced release of [3H]-histamine from rat brain slices, 40 nM (Arrang et al., 1983; Leurs et al., 1995a). However, the IC50 for inhibition of [3H]-GABA release is nearer to the IC50 values for histamine-induced inhibition of depolarization-induced histamine synthesis in rat cerebral cortex, 0.34 μM (Arrang et al., 1987b) and for inhibition by histamine of the electrically-evoked release of [3H]-noradrenaline (Schlicker et al., 1992) and [3H]-serotonin (Smits & Mulder, 1991), circa 0.1 μM, from brain slices. The IC50 for histamine-induced inhibition of [3H]-dopamine release in mouse striatum is not well defined (Schlicker et al., 1993), but appears to be of the same order as that we observe for inhibition of [3H]-GABA release. The variation in IC50 values between tissues could reflect differences in receptor reserve or a difference in G protein coupling, possibly involving subtypes of the H3 receptor (reviewed in Hill et al., 1997).
It should be noted that the overall inhibitory action of H3-agonists on [3H]-GABA release in striatum could involve some inhibition of striatal dopamine release, since in mouse striatum histamine and the H3-agonist R(-)-α-methylhistamine are reported to cause circa 30% inhibition of the electrically-stimulated release of [3H]-dopamine (Schlicker et al., 1993). H3 receptors also appear to be present on dopaminergic terminals in rat striatum, since immepip produces a marked inhibition of depolarization-induced dopamine synthesis (Molina-Hernandez et al., 2000). However, the almost complete inhibition of dopamine-dependent [3H]-GABA release in the reserpinized animals indicates that the major site of action of H3 agonists is almost certainly on the terminals of GABA neurones. This is consistent with the reports that striatal quinolinic acid lesions result in a parallel decrease in the numbers of ipsilateral D1 and H3 receptors in striatum, as in SNr, (Ryu et al., 1994) and that H3 receptor expression in the striatum is regulated, at least in part, by dopamine D1 receptors (Ryu et al., 1996). The collaterals of the projection neurones would thus seem to be the most likely site of the D1/H3 interaction. The effects of H3 agonists and dopamine on acetylcholine release in the ventral striatum (Prast et al., 1999) can also be explained by an interaction on GABA collaterals.
The GABA projection neurones make up over 90% of all the neurones in the striatum (Kawaguchi et al., 1995). However, the striatum also possesses at least two classes of GABA interneurone, at least one of which possesses D1 receptors (Kawaguchi et al., 1995), and the possibility must be considered that some of the [3H]-GABA release measured might be from these interneurones. It may be noted that the pattern of depolarization-induced [3H]-GABA release from the striatal slices (sustained or increasing with time) differs from the pattern observed in SNr (initial peak, then declining release) (Garcia et al., 1997). There is a report that [3H]-GABA microinjected into the striatum of anaesthetized rats is taken up preferentially by one type of interneurone (Bolam et al., 1983), presumably reflecting a highly active GABA uptake system. This interneurone constitutes only 3 – 5% of striatal neurones, but has a dense arborization of local axon collaterals, stains strongly for GABA and glutamic acid decarboxylase, and has different electrophysiological properties from those of the projection neurones (Kawaguchi, 1993; Kawaguchi et al., 1995). However, the labelling conditions used in the present study, in which slices were exposed to an excess of [3H]-GABA over an extended time, differ considerably from those employing a single microinjection of [3H]-GABA. It should also be noted that there is currently no evidence that any of the classes of GABA interneurones express both D1 and H3 receptors and, consequently, that they might be a locus for the H3/D1 receptor interaction.
There is at present only limited evidence for the involvement of H3 receptors in locomotor activity (Clapham & Kilpatrick, 1994), whereas the importance of the permissive role of D1 receptors in the so-called ‘direct' pathway through the basal ganglia is well documented (Gerfen & Wilson, 1996). However, the extent to which the D1 receptor-dependent release of [3H]-GABA in striatum and SNr is sensitive to inhibition by H3 agonists is striking and could be important in circumstances in which there is a high local release of histamine, as may occur secondary to ischaemia (Adachi et al., 1991).
Acknowledgments
This project was supported by Grant 28276N from CONACyT (Mexico). Part of the work was carried out during the tenure by J.M. Young of an Exchange Fellowship between the Royal Society and the Mexican Academy of Sciences.
Abbreviations
- [3H]-GABA
[3H]-γ-aminobutyric acid
- SNr
substantia nigra pars reticulata
References
- ACEVES J., CUELLO A.C. Dopamine release induced by electrical stimulation of microdissected caudate-putamen and substantia nigra. Neuroscience. 1981;6:2069–2075. doi: 10.1016/0306-4522(81)90045-2. [DOI] [PubMed] [Google Scholar]
- ADACHI N., OISHI R., SAEKI K. Changes in the metabolism of histamine and monoamines after occlusion of the middle cerebral artery in rats. J. Neurochem. 1991;57:61–66. doi: 10.1111/j.1471-4159.1991.tb02099.x. [DOI] [PubMed] [Google Scholar]
- ARIAS-MONTAÑO J.A., FLORAN B., GARCIA M., ACEVES J., YOUNG J.M. Histamine inhibits depolarisation-induced dopamine-dependent release of GABA in rat striatum via an action on H3-receptors. Br. J. Pharmacol. 2000;129:66P. doi: 10.1038/sj.bjp.0704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRANG J.M., GARBARG M., LANCELOT J.-C., LECOMTE J.-M., POLLARD H., ROBBA M., SCHUNACK W., SCHWARTZ J.-C. Highly potent selective and potent ligands for histamine H3 receptors. Nature. 1987a;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- ARRANG J.M., GARBARG M., SCHWARTZ J.-C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- ARRANG J.M., GARBARG M., SCHWARTZ J.C. Autoinhibition of histamine synthesis mediated by presynaptic H3-receptors. Neuroscience. 1987b;23:149–157. doi: 10.1016/0306-4522(87)90279-x. [DOI] [PubMed] [Google Scholar]
- BOLAM J.P., CLARKE D.J., SMITH A.D., SOMOGYI P. A type of aspiny neuron in the rat neostriatum accumulates [3H]γ-aminobutyric acid: combination of golgi-staining, autoradiography, and electron microscopy. J. Comp. Neurol. 1983;213:121–134. doi: 10.1002/cne.902130202. [DOI] [PubMed] [Google Scholar]
- CLAPHAM J., KILPATRICK G.J. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur. J. Pharmacol. 1994;259:107–114. doi: 10.1016/0014-2999(94)90498-7. [DOI] [PubMed] [Google Scholar]
- CUMMING P., DAMSMA G., FIBIGER H.C., VINCENT S.R. Characterization of extracellular histamine in the striatum and bed nucleus of the stria terminalis of the rat: an in vivo microdialysis study. J. Neurochem. 1991a;56:1797–1803. doi: 10.1111/j.1471-4159.1991.tb02083.x. [DOI] [PubMed] [Google Scholar]
- CUMMING P., SHAW C., VINCENT S.R. High affinity histamine binding site is the H3 receptor: characterization and autoradiographic localization in rat brain. Synapse. 1991b;8:144–151. doi: 10.1002/syn.890080208. [DOI] [PubMed] [Google Scholar]
- DICKENSON J.M., HILL S.J. Adenosine A1-receptor stimulated increases in intracellular calcium in the smooth muscle cell line, DDT1MF-2. Br. J. Pharmacol. 1993;108:85–92. doi: 10.1111/j.1476-5381.1993.tb13444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOREULEE N., YANOVSKY Y., FLAGMEYER I., STEVENS D.R., HAAS H.L., BROWN R.E. Histamine H3 receptors depress synaptic transmission in the corticostriatal pathway. Neuropharmacol. 2001;40:106–113. doi: 10.1016/s0028-3908(00)00101-5. [DOI] [PubMed] [Google Scholar]
- FLORAN B., ACEVES J., SIERRA A., MARTINEZ-FONG D. Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci. Lett. 1990;116:136–140. doi: 10.1016/0304-3940(90)90399-t. [DOI] [PubMed] [Google Scholar]
- FLORAN B., SILVA I., ACEVES J. Presynaptic modulation of the release of GABA by GABAA receptors in pars compacta and by GABAB receptors in pars reticulata of the rat substantia nigra. Eur. J. Pharmacol. 1988;150:277–286. doi: 10.1016/0014-2999(88)90008-8. [DOI] [PubMed] [Google Scholar]
- GARCIA M., FLORAN B., ARIAS-MONTAÑO J.A., YOUNG J.M., ACEVES J. Histamine H3 receptor activation selectively inhibits dopamine D1 receptor-dependent [3H]-γ-aminobutyric acid release from depolarisation-stimulated slices of rat substantia nigra pars reticulata. Neuroscience. 1997;80:241–249. doi: 10.1016/s0306-4522(97)00100-0. [DOI] [PubMed] [Google Scholar]
- GERFEN C.R., WILSON C.J.The basal ganglia Integrated Systems of the CNS, Part III (Handbook of Chemical Neuroanatomy 199612Amsterdam: Elsevier; 369–466.ed. Björklund, A., Hökfelt, T. & Swanson, L. pp [Google Scholar]
- GIRAULT J.A., SPAMPINATO U., GLOWINSKI J., BESSON M.J. In vivo release of [3H]γ-aminobutyric acid in the rat neostriatum – II. Opposing effects of D1 and D2 dopamine receptor stimulation in the dorsal caudate putamen. Neuroscience. 1986;19:1109–1117. doi: 10.1016/0306-4522(86)90127-2. [DOI] [PubMed] [Google Scholar]
- HARPER E.A., SHANKLEY N.P., BLACK J.W. Characterization of the binding of [3H]-clobenpropit to histamine H3-receptors in guinea-pig cerebral cortex membranes. Br. J. Pharmacol. 1999;128:881–890. doi: 10.1038/sj.bjp.0702860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARSING L.G., JR, ZIGMOND M.J. Influence of dopamine on GABA release in striatum: evidence for D1-D2 interactions and non-synaptic influences. Neuroscience. 1997;77:419–429. doi: 10.1016/s0306-4522(96)00475-7. [DOI] [PubMed] [Google Scholar]
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- INAGAKI N., YAMATODANI A., ANDO-YAMAMOTO M., TOHYAMA M., WATANABE T., WADA H. Organization of histaminergic fibres in the rat brain. J. Comp. Neurol. 1988;273:283–300. doi: 10.1002/cne.902730302. [DOI] [PubMed] [Google Scholar]
- JANSEN F.P., WU T.S., VOSS H.-P., STEINBUSCH H.W.M., VOLLINGA R.C., RADEMAKER B., BAST A., TIMMERMAN H. Characterization of the binding of the first selective radiolabelled histamine H3-receptor antagonist, [3H]-iodophenpropit, to rat brain. Br. J. Pharmacol. 1994;113:355–362. doi: 10.1111/j.1476-5381.1994.tb16995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI Y. Physiological, morphological, and histochemical characterization of three classes of interneurone in rat neostriatum. J. Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI Y., WILSON C.J., EMSON P.C. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J. Neurosci. 1990;10:3421–3428. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI Y., WILSON C.J., AUGOOD S.J., EMSON P.C. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- KURIYAMA K., KANMORI K., TAGUCHI J.-I., YONEDA Y. Stress-induced enhancement of suppression of [3H]GABA release from striatal slices by presynaptic autoreceptor. J. Neurochem. 1984;42:943–950. doi: 10.1111/j.1471-4159.1984.tb12695.x. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., ROBERTS F.F. Measuring muscarinic antagonist potency using stimulated phosphoinositide breakdown in rat cortex slices. Br. J. Pharmacol. 1987;92:677P. [Google Scholar]
- LEURS R., SMIT M.J., TIMMERMAN H. Molecular pharmacological aspects of histamine receptors. Pharmacol. Ther. 1995a;66:413–463. doi: 10.1016/0163-7258(95)00006-3. [DOI] [PubMed] [Google Scholar]
- LEURS R., TULP M.T.M., MENGE W.M.B.P., ADOLFS M.J.P., ZUIDERVELD O.P., TIMMERMAN H. Evaluation of the receptor selectivity of the H3 receptor antagonists, iodophenpropit and thioperamide: an interaction with the 5-HT3 receptor revealed. Br. J. Pharmacol. 1995b;116:2315–2321. doi: 10.1111/j.1476-5381.1995.tb15071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGNEAU X., GARBARG M., VIZUETE M.L., DÍAZ J., PURAND K., STARK H., SCHUNACK W., SCHWARTZ J.-C. [125I]Iodoproxyfan, a new antagonist to label and visualize cerebral histamine H3 receptors. J. Pharmacol Exp. Ther. 1994;271:452–459. [PubMed] [Google Scholar]
- MOLINA-HERNANDEZ A., NUÑEZ A., ARIAS-MONTAÑO J.-A. Histamine H3-receptor activation inhibits dopamine synthesis in rat striatum. NeuroReport. 2000;11:163–166. doi: 10.1097/00001756-200001170-00032. [DOI] [PubMed] [Google Scholar]
- PANULA P., PIRVOLA U., AUVINEN S., AIRAKSINEN M.S. Histamine-immunoreactive nerve fibres in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- POLLARD H., MOREAU J., ARRANG J.M., SCHWARTZ J.-C. A Detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience. 1993;52:169–189. doi: 10.1016/0306-4522(93)90191-h. [DOI] [PubMed] [Google Scholar]
- PRAST H., TRAN M.H., FISCHER H., KRAUS M., LAMBERTI C., GRASS K., PHILIPPU A. Histaminergic neurones modulate acetylcholine release in the ventral striatum: role of H3 histamine receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:558–564. doi: 10.1007/s002109900097. [DOI] [PubMed] [Google Scholar]
- RUSSELL W.L., HENRY D.P., PHEBUS L.A., CLEMENS J.A. Release of histamine in rat hypothalamus and corpus striatum in vivo. Brain Res. 1990;512:95–101. doi: 10.1016/0006-8993(90)91175-g. [DOI] [PubMed] [Google Scholar]
- RYU J.H., YANAI K., IWATA R., IDO T., WATANABE T. Heterogenous distribution of histamine H3, dopamine D1 and D2 receptors in rat brain. NeuroReport. 1994;5:621–624. doi: 10.1097/00001756-199401000-00022. [DOI] [PubMed] [Google Scholar]
- RYU J.H., YANAI K., ZHAO X.-L., WATANABE T. The effect of dopamine D1 receptor stimulation on the up-regulation of histamine H3-receptors following destruction of the ascending dopaminergic neurones. Br. J. Pharmacol. 1996;118:585–592. doi: 10.1111/j.1476-5381.1996.tb15441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLICKER E., BEHLING A., LÜMMEN G., GÖTHERT M. Histamine H3 receptor-mediated inhibition of noradrenaline release in the mouse brain cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;345:489–493. doi: 10.1007/BF00176630. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., FINK K., DETZNER M., GÖTHERT M. Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J. Neural. Transm. 1993;93:1–10. doi: 10.1007/BF01244933. [DOI] [PubMed] [Google Scholar]
- SMITS R.P.J.M., MULDER A.H. Inhibitory effects of histamine on the release of serotonin and noradrenaline from rat brain slices. Neurochem. Int. 1991;18:215–220. doi: 10.1016/0197-0186(91)90188-j. [DOI] [PubMed] [Google Scholar]
- VALENTINE A.F., RIZZO C.A., RIVELLI M.A., HEY J.A. Pharmacological characterization of histamine H3 receptors in human saphenous vein and guinea pig ileum. Eur. J. Pharmacol. 1999;366:73–78. doi: 10.1016/s0014-2999(98)00904-2. [DOI] [PubMed] [Google Scholar]


