Abstract
The pharmacological modulation of opioid actions by drugs acting on heterologous mechanisms could be useful to overcome some of the main problems associated with the use of opiate agonists. Based on previous findings on the interactions between yohimbine and opioid drugs, we have further studied the effects of yohimbine on the antinociceptive and positive-negative reinforcing effects of morphine (μ opioid receptor-preferring agonist), U-50,488 (κ agonist) and SNC80 (δ agonist).
Pretreatment with yohimbine completely blocked the antinociception provided by the three opioid agonists in the mouse tail-immersion test.
A similar blockade of SNC80 and U-50,488-induced antinociception was observed with yohimbine in the mouse hot plate test at the same doses. In this paradigm, the effect of the κ agonist was very slight and the actions of yohimbine rather variable.
In place conditioning experiments with SD (Sprague – Dawley) male rats, yohimbine alone was inactive but it limited the preference induced by morphine and SNC80 and the aversive effect of U-50,488. Impaired novelty preference was also observed with the combination of yohimbine and U-50,488.
It is concluded that yohimbine tends to limit opioid antinociception and the addictive potential of μ and δ opioid agonists. More selective drugs could help to understand the mechanisms involved in these actions.
Keywords: Opioid; morphine; SNC80; U-50,488; yohimbine; antinociception; addiction; hot plate; tail-immersion; place conditioning
Introduction
It is widely accepted that the noradrenergic function is deeply involved in opioid pharmacology. Many of the studies performed on this subject have detected bidirectional interactions between μ-opioid and α2-adrenergic mechanisms even from the development of these systems (Wang et al., 1986; Alberti et al., 1999). Some of these interactions could be of therapeutic interest: thus, it is accepted that α2-adrenergic agonists have analgesic potential and also enhance the antinociceptive effect of μ-opioids (Wilcox et al., 1987), which seems relevant for the clinical management of pain (Eisenach et al., 1994). Conversely, α2-adrenoceptor antagonists such as yohimbine or idazoxan are hyperalgesic and attenuate morphine analgesia in several pharmacological tests (Browning et al., 1982; Iglesias et al., 1992; Herrero & Solano, 1999). There is however some controversy on this last subject: some authors have found that yohimbine could provide antinociception in some experimental tests (Ageel et al., 1987) and could enhance opioid analgesia in humans (Gear et al., 1995); on the other hand, it has been proposed that the modulation of μ-opioid-induced antinociception by α2-adrenoceptor antagonists only occurs in hyperalgesic conditions but not in normal animals (Herrero & Solano, 1999). Some of these differences could be explained by the experimental test and the drug or route of application chosen by the authors.
The chronic adaptations that give rise to tolerance and dependence to μ-opioids also affect α2-adrenergic mechanisms and can be modified by ligands of these receptors. Chronic exposure to morphine generates tolerance to opioid analgesia but also downregulation of α2-adrenoceptors (Smith et al., 1989) and cross-tolerance with the antinociceptive actions of α2-agonists (Solomon & Gebhart, 1988). When opioid tolerance and dependence has developed, α2-agonists are able to control the expression of these adaptations since they inhibit many of the signs of withdrawal both in animal models and in humans (Gold et al., 1978). As expected, α2-adrenoceptor antagonists work in the opposite direction: they prevent the development of opioid dependence in several experimental paradigms when administered concomitantly with the opioid (Taylor et al., 1991; Iglesias et al., 1992; 1998; El-Kadi & Sharif, 1997) but they increase opioid withdrawal signs when acutely injected to abstinent subjects (Dwoskin et al., 1983); the effect of these drugs on opioid tolerance are however more controversial and seem to be highly dependent on the experimental paradigm used (Alguacil et al., 1987; Iglesias et al., 1992; Boronat et al., 1998). According to experimental data, Hameedi et al. (1997) have found that repeated yohimbine use partially prevents opioid withdrawal signs in methadone-maintained addicts, thus raising the possibility that this drug could be introduced for therapy of opiate abusers.
Besides physical dependence, the positive reinforcing properties of μ-opioid receptor agonists are deeply involved in their abuse. Important interactions between α2-adrenergic mechanisms and opioids have also been described concerning this subject. Thus, recent studies have shown an illicit use of the α2-agonist clonidine among opiate addicts that does not seem to be exclusively directed to alleviate withdrawal (Anderson et al., 1997; Beuger et al., 1998). This effect could be related to a possible reinforcing effect of α2-agonists such as clonidine, as shown in animal models of drug addiction (Asin & Wirtshafter, 1985; Shearman et al., 1981). Concerning α2-adrenoceptor antagonists, we have not found any reference in the literature on the possible effects of these drugs on the abuse potential of μ-opioid agonists, despite the fact that the preceding reports raise the possibility of a significant interaction.
Bearing in mind the conflicting or sparse results commented above, we have designed several experiments to further increase the knowledge of the interactions between yohimbine and the prototypic opioid morphine concerning antinociception and abuse potential. These studies have been extended with a comparative analysis of the interaction of yohimbine with δ and κ specific agonists in the same experimental conditions, which is justified by previous results suggesting an involvement of the α2-adrenergic system in the antinociceptive effect of both kind of drugs (Bianchi et al., 1989; Roerig & Fujimoto, 1989; Rady et al., 1991; Gordon et al., 1992; Harada et al., 1995; Grabow et al., 1999). Antinociception was studied with the tail immersion and the hot plate tests since they are widely used paradigms claimed to have good predictive value of spinal and supraspinal mediated-antinociception, respectively. The action of yohimbine on the abuse potential of opioid agonists was studied by means of place conditioning experiments, a widely used behavioural model to study the reinforcing properties of drugs which quantifies the incentive motivational properties of environmental stimuli that become associated with the drug through classical conditioning (Carr et al., 1989).
The i.p. and s.c. routes of administration have been chosen for the experiments since these routes are closer to the potential clinical application of these drugs than other methods for drug delivery frequently used in similar studies; it must be taken into account that previous discrepancies on the effects of α2-adrenoceptor antagonists on opioid analgesia have been attributed to the use of more specialized methods for drug administration (Herrero & Solano, 1999). This approach to the clinical scene has also been determinant to choose the widely used analgesic morphine instead of other, more specific μ-opioid receptor agonists; this could also permit comparisons with previous experiments performed in our laboratory on morphine-yohimbine interactions. Preliminary results of these experiments have been reported (Morales et al., 1998; 1999; 2000).
Methods
Animals and drugs
Male OF1 mice (25 – 30 g; IFFA – CREDO, France) and male SD rats (300 – 350 g, San Pablo CEU University breeding) were used. Animals had free access to water and standard diet and were maintained in a controlled environment (20 – 22°C, 12 – 12 h dark-light cycle) throughout the experiments. The drugs used were: U-50,488 (trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzene-acetamide methane sulphonate) (κ agonist; RBI, U.S.A.); SNC80 ((+)-4-[(α-R*)-α-((2S*,5R*)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide) (δ agonists; RBI, U.S.A.) morphine sulphate (nonselective, μ-receptor preferring agonist) and yohimbine hydrochloride (Sigma, Spain); they were dissolved in either physiological saline or 20% DMSO (dimethyl sulfoxide) for administration. The doses of the different opioid receptor agonists used had previously shown antinociceptive effects (Iglesias et al., 1992; Hayes et al., 1987; Bilsky et al., 1995) and were reported to produce either preference or aversion in place conditioning experiments (Randall et al., 1998; Bals-Kubik et al., 1989; Longoni et al., 1998). The doses of yohimbine were able to block morphine analgesia and physical dependence in previous studies performed by our team (Iglesias et al., 1992).
Effects of yohimbine on opioid-induced antinociception
Spinal analgesia (tail-immersion test)
Antinociception was assessed using the 55°C warm-water tail-flick assay. In this test, the latency to the first sign of a rapid tail-flick was taken as the behavioural end point (Heyman et al., 1986). Each mouse was first tested for baseline latency by immersing its tail in the water and recording the time to response. Mice not responding within 5 s were excluded from further testing. Just following baseline determination, animals were treated with yohimbine (2 mg kg−1, i.p.) or an equivalent volume of saline (10 ml kg−1) and 5 min later were injected with morphine (5 mg kg−1, i.p.), U-50,488 (6 mg kg−1, s.c.), SNC80 (57 mgkg−1, i.p.), saline or 20% DMSO once more. Reaction times to noxious stimulus were obtained at 15, 30, 60 and 120 min postinjection. Cut-off time was 30 s.
Supraspinal analgesia (hot plate test)
In the hot plate test (Woolfe & MacDonald, 1944) mice were placed on a heated surface (55°C) and either licking of the paws or jumping was taken as the end point. Baseline was obtained 15 min before drug administration, and only those animals with latency less than 15 s were further used. Animals were treated with yohimbine (2 mg kg−1, i.p.) or an equivalent volume of saline (10 ml kg−1) and 5 min later were injected with U-50,488 (6 mg kg−1, s.c.), SNC80 (57 mg kg−1, i.p.), saline or 20% DMSO again. The time of latency was determined at 15, 30, 60 and 120 min postinjection, with a cut-off time of 30 s.
Effects of yohimbine on opioid-induced place conditioning
Apparatus
The apparatus consisted of three square Plexiglas compartments of the same size (40×35×35 cm), situated at 120° angles from each other and separated by a triangular passage area; each compartment was communicated with the passage area by a guillotine door and had different floor and wall drawings. The first compartment had cork floor and black walls, the second had a black Plexiglas floor and walls with white circles (7.5 cm diameter) and the third had corncob bedding and black walls painted with white strips.
Experiments were based on the method of Rodríguez de Fonseca et al. (1995) adapted to our experimental conditions (Pérez-García et al., 1999). The procedure consisted of a 5-day schedule with three phases: preconditioning, conditioning and testing.
Preconditioning
Animals were placed in the middle of the apparatus and were allowed to freely explore the whole set for 30 min. Animal behaviour was monitored by means of a videotracking system (San Diego, CA, U.S.A.), which enabled the calculation of the time spent in each compartment. Rats that exhibited strong unconditioned aversion (less than 10% of the session) and/or preference (more than 60% of the session) for any of the compartments were discarded from the study.
Conditioning
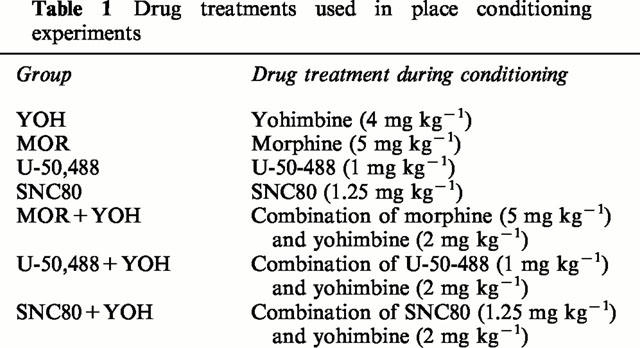
This phase consisted of a 3-day schedule of double conditioning sessions. The first one involved a morning session in which animals received a single injection of saline (10 ml kg−1, i.p.) and were immediately confined to a randomly paired compartment for 30 min. In the evening session the animals received a drug treatment (10 ml kg−1, i.p.) and were confined to another randomly paired compartment for 30 min. There was a delay of at least 3 h between the morning and the evening sessions. On the following 2 days, the procedure used was the same, but the order of the treatments (morning – evening) was changed to avoid the influence of circadian variability. The drug treatment for any given animal consisted in single injections of: (a) an opioid agonist, (b) yohimbine, or (c) a combination of both; these experimental groups are fully explained in Table 1.
Table 1.
Drug treatments used in place conditioning experiments
Testing
On the 5th day of the schedule the animals moved freely throughout the apparatus, exactly as in the preconditioning phase. The permanence in the saline-paired compartment, the drug-paired compartment and the neutral compartment (the one which was not used for conditioning) was automatically registered and was expressed as the percentage of the total time spent in any of the three compartments of the apparatus (the time spent in the passage area was not significantly modified by any drug treatment and was not considered for calculations).
Statistics
Latencies in the antinociceptive studies and percentages of time spent in the different compartments in place conditioning experiments were all analysed by ANOVA followed by multiple comparison post hoc tests (least significant differences). Significance was considered at the 0.05 level.
Results
Figures 1, 2 and 3 show that the antinociceptive effect of the three opioid agonists in the tail-immersion test was completely prevented by yohimbine pretreatment; this reversal could not be attributed to yohimbine hyperalgesia since this drug did not modify animal response when compared with saline, although the very low basal latencies could have prevented further reductions after yohimbine injection.
Figure 1.
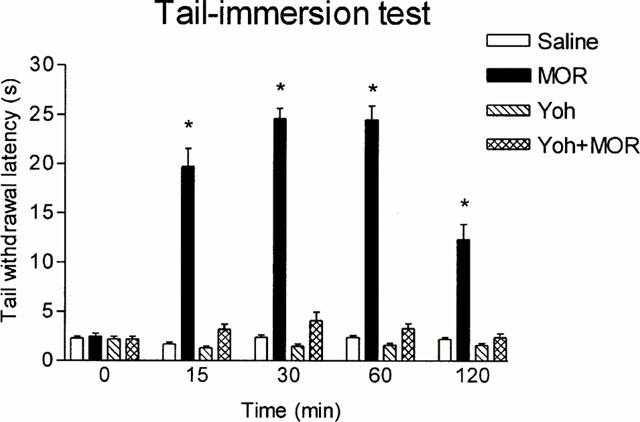
Latencies in the tail-immersion test in animals pretreated i.p. with saline or yohimbine (Yoh) (2 mg kg−1) and treated 5 min later i.p. with saline or morphine (MOR) (5 mg kg−1). Animals per group were n=10. *P<0.05 vs saline. Data are presented as mean±s.e.mean.
Figure 2.
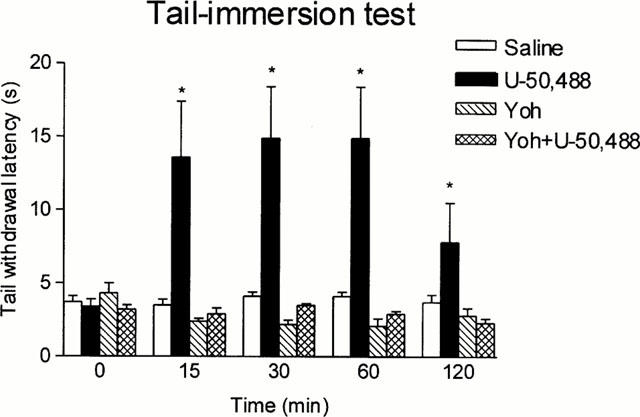
Latencies in the tail-immersion test in animals pretreated i.p. with saline or yohimbine (Yoh) (2 mg kg−1) and treated 5 min later s.c. with saline or U-50,488 (6 mg kg−1). Animals per group were n=10. *P<0.05 vs saline. Data are presented as mean±s.e.mean.
Figure 3.
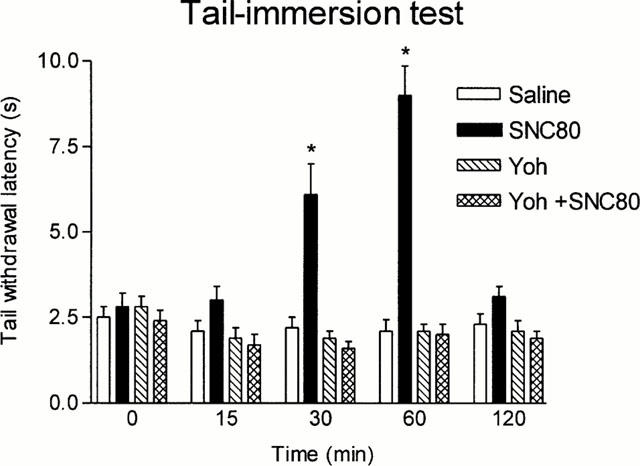
Latencies in the tail-immersion test in animals pretreated i.p. with saline or yohimbine (Yoh) (2 mg kg−1) and treated 5 min later i.p. with 20% DMSO or SNC80 (57 mg kg−1). Animals per group were n=10. *P<0.05 vs saline. Data are presented as mean±s.e.mean.
In the hot plate test, U-50,488 only increased the time of reaction 15 min after injection (Figure 4). Yohimbine also elicited a transient increase of the reaction time at 15 min, but this was followed by a more persistent hyperalgesic effect (Figure 4). The concomitant use of yohimbine and U-50,488 did not elicit analgesia 15 min postinjection and produced an effect very similar to that of yohimbine alone at longer periods of time (Figure 4). It should be noted that control animals exhibited increasing latencies upon repeated exposure to the plate in these experiments; this effect reached statistical significance 60 and 120 min postinjection (Figure 4). The antinociceptive effect of SNC80 in the hot plate test appeared before in the tail-immersion test and was also yohimbine-sensitive; in these last experiments, yohimbine alone did not produce any effect by itself and saline-treated animals exhibited a stable response along the test (Figure 5).
Figure 4.
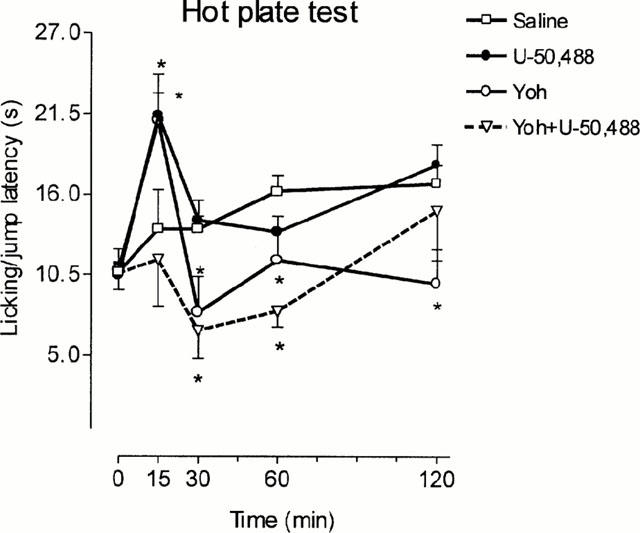
Paw-lick and jump latencies in the hot plate assay. Animals were pretreated i.p. with saline or yohimbine (Yoh) (2 mg kg−1) and treated 5 min later s.c. with saline or U-50,488 (6 mg kg−1). Animals per group were: saline n=27, U-50,488 n=29, Yoh n=7 and Yoh+U-50,488 n=7. *P<0.05 vs saline. Data are presented as mean±s.e.mean.
Figure 5.
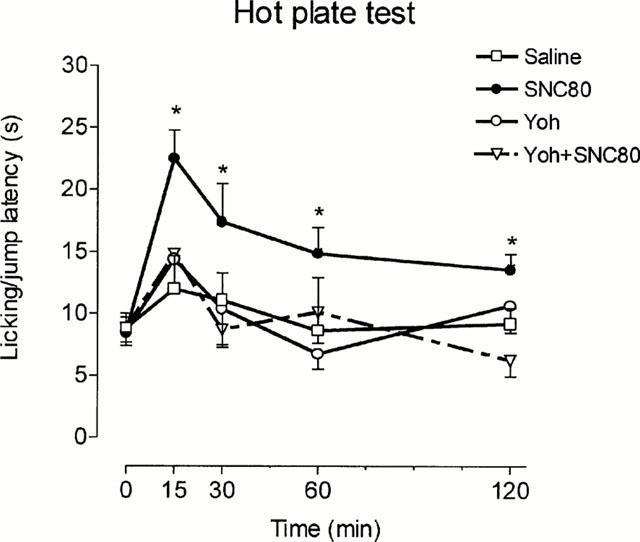
Paw-lick and jump latencies in the hot plate assay. Animals were pretreated i.p. with saline or yohimbine (Yoh) (2 mg kg−1) and treated 5 min later i.p. with 20% DMSO or SNC80 (57 mg kg−1). Animals per group were: saline n=10, SNC80 n=10, Yoh n=9 and Yoh+SNC80 n=10. *P<0.05 vs saline. Data are presented as mean±s.e.mean.
In place conditioning experiments, yohimbine per se did not elicit neither preference nor aversion (Figure 6). Figure 7 shows that morphine conditioning elicited a significant preference for the opioid-paired chamber when compared with neutral and saline-paired compartments; concomitantly, the rats spent more time in the neutral compartment compared with the saline-paired one. Morphine preference was significantly reduced when the opioid was administered together with yohimbine, whilst the tendency to prefer the neutral compartment remained preserved.
Figure 6.
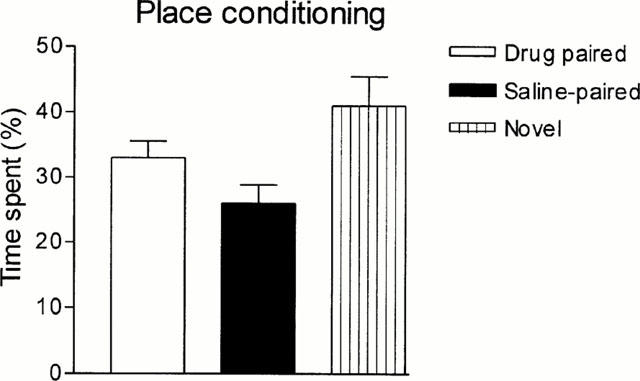
Effect of yohimbine (4 mg kg−1, i.p.) on place preference conditioning test. Bars represent mean±s.e.mean. (n=10).
Figure 7.
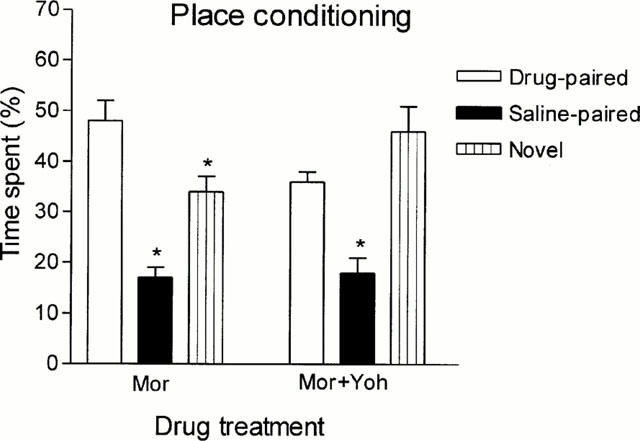
Effect of yohimbine (Yoh) on morphine-induced conditioned place preference. Rats were treated i.p. with morphine (5 mg kg−1, n=20) or with a mixture of morphine (5 mg kg−1) and yohimbine (2 mg kg−1, n=9) Bars represent mean±s.e.mean. *P<0.05 with respect to the drug-paired compartment.
U-50,488 conditioning produced a significant place aversion for the opioid-paired compartment which was prevented by yohimbine co-treatment; the combination of yohimbine with U-50,488 also abolished preference for the neutral compartment (Figure 8).
Figure 8.
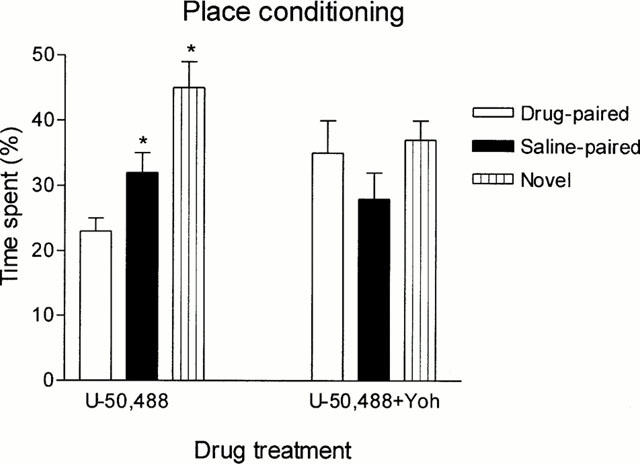
Effect of yohimbine (Yoh) on U-50,488-induced conditioned place aversion. Rats were treated i.p. with U-50,488 (1 mg kg−1, n=20) or with a mixture of U-50,488 (1 mg kg−1) and yohimbine (2 mg kg−1, n=11) Bars represent mean±s.e.mean. *P<0.05 with respect to the drug-paired compartment.
Similarly to morphine, SNC80 conditioning resulted in a robust preference for the opioid-paired compartment; when yohimbine was added to the opioid during conditioning, preference for the drug-paired compartment disappeared whilst the tendency of the animals to the neutral compartment persisted (Figure 9).
Figure 9.
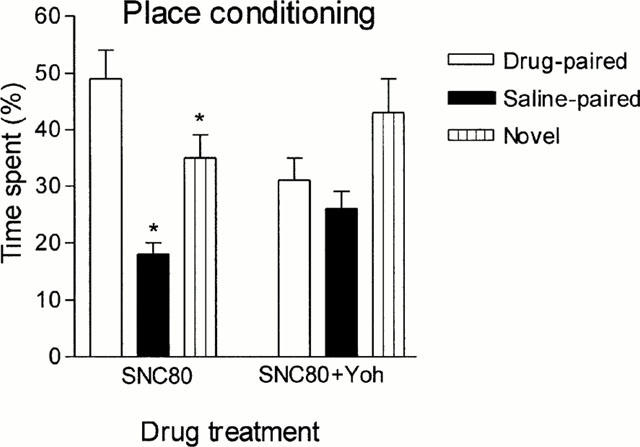
Effect of yohimbine (Yoh) on SNC80-induced conditioned place preference. Rats were treated i.p. either with SNC80 (1.25 mg kg−1, n=10) or with a mixture of SNC80 (1.25 mg kg−1) and yohimbine (2 mg kg−1, n=9). Bars represent mean±s.e.mean. *P<0.05 with respect to the drug-paired compartment.
Discussion
The results obtained in the tail-immersion test clearly show the ability of systemic yohimbine to block the spinal antinociception mediated by the three main opioid receptor types. Yohimbine reversal of the spinal component of morphine antinociception has been repeatedly described in the literature and was previously observed in our laboratory by using the rat tail-flick test (Iglesias et al., 1992). Furthermore, the blockade of α2 adrenoceptors has been also claimed to reduce spinal analgesia mediated by both δ and κ opioid receptors (Roerig et al., 1992; Roerig & Fujimoto, 1989). These results together with many other available in the literature tend to show that a combined systemic use of yohimbine and any other opioid agonist is expected to reduce the acute spinal analgesic effect of the opioid.
A similar conclusion could be achieved concerning supraspinal analgesia as indicated by our interaction studies in the hot plate test, since yohimbine reduced the analgesic effect of U-50,488 and SNC80 as it was previously reported in the case of morphine (Iglesias et al., 1991). However, the actions of yohimbine per se in this test seem to be irregular and highly dependent on slight experimental variations. Thus, we have found that yohimbine provokes a transient increase of hot plate latencies followed by hyperalgesia in a first set of experiments, but not in a second one. This is not surprising: analgesia, hyperalgesia, a biphasic effect or no effect at all have been observed with yohimbine in the hot plate test, which seems dependent on the dose, the plate temperature and the basal response of the animals (Ageel et al., 1987; Iglesias et al., 1991). Moreover, it is possible that the experimental conditions could have varied from one experiment to the other, since the response of control animals was not exactly the same: thus, basal latencies were slightly different and increasing times of reaction, probably related to stress-induced antinociception, were only seen in the saline-treated animals from the first experiment. In our experience, the hot plate test is very sensitive to subtle changes of environmental factors (temperature, humidity, light, animal condition. . .) which are frequently undetected but can influence quantitatively the results of the experiments. Despite these considerations, the main conclusion that can be achieved from these experiments together with others previously conducted in our laboratory (Iglesias et al., 1991) is that yohimbine consistently reduces the antinociceptive effect of the agonists of the three opioid receptors in the hot plate test. In the case of morphine, this is in agreement with the results obtained in other models of supraspinal analgesia (Izenwasser & Kornetsky, 1990). It is interesting to note that the antinociceptive effect of U-50,488 was potent and sustained in the tail immersion test, but it was slight and short-lasting in the hot plate test. This last finding is consistent with previous reports that showed significant analgesia after U-50,488 treatment in the hot plate test only at doses close to the threshold for motor impairment (Hayes et al., 1987). These results suggest a reduced activity of U-50,488 at supraspinal sites, at least when administered by general routes; in fact, κ-opioid agonists were once claimed to provide analgesia only by spinal mechanisms, although the intracerebroventricular administration of κ-receptor ligands and more recent experiments with κ-opioid receptor knockout mice clearly show that κ receptors are also involved in supraspinal analgesia (see reviews by Millan, 1990 and Kieffer, 1999). A high intensity of the noxious stimulus or a rapid drug redistribution have been previously suggested to explain the slight antinociceptive actions of κ-agonists (Leighton et al., 1988). Concerning the antinociceptive effects of SNC80, our experiments suggest that the supraspinal effect of this drug is more rapidly achieved than the spinal action after systemic administration; this comparison could not be established in previous studies on the antinociceptive effect of SNC80 in the hot plate and the tail-flick tests since appropriate time response curves were not reported (Bilsky et al., 1995). The fast activity of SNC80 in the hot plate test that has been obtained in our study has a good correspondence with the brain levels of the radiolabelled form of this drug (termed SNC121) that peaked at 20 min after peripheral injection in previous studies (Schetz et al., 1996); on the other hand, the causes leading to the slower onset of the antinociceptive effect in the tail immersion test remain to be determined.
Gear et al. (1995) have reported that systemic yohimbine increases morphine analgesia in a study of dental postoperatory pain in humans, which seems to be contradictory with the above conclusions. However, they reported potentiation of opioid analgesia after a 3 day treatment with three daily doses of 5.4 mg yohimbine. The time of exposure to yohimbine can explain this effect, since rodent studies have shown that repeated yohimbine did not affect morphine analgesia (Taylor et al., 1991) and even could potentiate it by delaying the development of tolerance (Kihara & Kaneto, 1986). It seems therefore that yohimbine could interfere with opioid analgesics in acute conditions, but repeated exposure could have rather different effects since it could counteract some of the neuroadaptations induced by long-term opioid use. In agreement with this idea, yohimbine has been shown to prevent the development of opioid physical dependence in rodents (Taylor et al., 1991; Iglesias et al., 1992; 1998; Ambrosio et al., 1997; El-Kadi & Sharif, 1997) and to reduce the severity of methadone abstinence in humans after repeated use (Hameedi et al., 1997).
The potential beneficial effects of yohimbine could be even more interesting if we take into account that this drug seemed to reduce the positive reinforcing effects of morphine and SNC80 in the conditioned place preference paradigm. Interestingly, yohimbine reduced the place preference induced by these drugs but did not alter preference for the neutral environment, which is partially novel for the animals since it was not used during conditioning. Novelty preference in this paradigm has been reported by other authors (Carr et al., 1989; Parker, 1992) and served us to verify that the cognitive function seems preserved in conditioned place preference studies (Pérez-García et al., 1999); consequently, we think that yohimbine could reduce the motivational properties of both μ and δ opioid agonists. By contrast, we cannot be so confident on the specificity of yohimbine reversal of U-50,488 place aversion, since the animals neither showed novelty preference and therefore the results could reflect an impairment of learning during conditioning.
It is important to remark that the effects of yohimbine on the pharmacological properties of opioid drugs could be related to mechanisms other than α2-adrenoceptor blockade. Although yohimbine is still considered a useful tool to study α2-adrenergic function and it is perhaps the best known α2-adrenoceptor blocker, it should be also considered a serotoninergic drug especially active on 5-HT1A receptors (Winter & Rabin, 1992). Furthermore, some authors have reported significant modifications of dopaminergic transmission by yohimbine (Van Oene et al., 1984; Scatton et al., 1980). Either the serotoninergic or the dopaminergic components of yohimbine could account for the effects observed on pain transmission and motivation and therefore further experiments with other reference drugs must be performed to clarify the exact mechanism of yohimbine actions on these variables.
Acknowledgments
This work was supported by grants from DGICYT (PM 97-0027), Comunidad de Madrid (08.8/4/97) and San Pablo CEU University (2/99).
Abbreviations
- MOR
morphine
- SD
Sprague – Dawley
- YOH
yohimbine
References
- AGEEL A.M., GINAWI O.T., ZAKI M.A. Modification of morphine analgesia by yohimbine in mice. Res. Commun. Subs. Abuse. 1987;8:85–92. [Google Scholar]
- ALBERTI I., FERNÁNDEZ B., ALGUACIL L.F., AGUILAR A., CAAMAÑO M., ROMERO E.M., VIVEROS M.P. Preweanling naltrindole administration differentially affects clonidine induced antinociception and plasma adrenaline levels in male and female neonatal rats. Br. J. Pharmacol. 1999;128:953–960. doi: 10.1038/sj.bjp.0702852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALGUACIL L.F., ALAMO C., SANTOS C., CUENCA E. Yohimbine reduces morphine tolerance in guinea-pig ileum. Life Sci. 1987;40:155–160. doi: 10.1016/0024-3205(87)90354-7. [DOI] [PubMed] [Google Scholar]
- AMBROSIO E., IGLESIAS V., GARCÍA-LECUMBERRI C., ORENSANZ L., ALGUACIL L.F. Effect of yohimbine on the development of morphine dependence in the rat: lack on involvement of cortical b-adrenoceptor modifications. Pharmacol. Biochem. Behav. 1997;3:487–491. doi: 10.1016/s0091-3057(96)00243-2. [DOI] [PubMed] [Google Scholar]
- ANDERSON F., PALUZZI P., LEE J., HUGGINS G., SVIKIS D. Illicit use of clonidine in opiate-abusing pregnant women. Obstet. Gynecol. 1997;90:790–794. doi: 10.1016/S0029-7844(97)00413-4. [DOI] [PubMed] [Google Scholar]
- ASIN K.E., WHIRTSHAFTER D. Clonidine produces a conditioned place preference in rats. Psychopharmacol. 1985;85:383–385. doi: 10.1007/BF00428206. [DOI] [PubMed] [Google Scholar]
- BALS-KUBIK R., HERZ A., SHIPPENBERG T.S. Evidence that aversive effects of opioid antagonists and κ-agonists are centrally mediated. Psychopharmacol. 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- BEUGER M., TOMMASELLO A., SCHWARTZ R., CLINTON M. Clonidine use and abuse among methadone program applicants and patients. J. Subst. Abuse Treatment. 1998;15:589–593. doi: 10.1016/s0740-5472(97)00309-7. [DOI] [PubMed] [Google Scholar]
- BIANCHI M., BRINI A., ROVATI L., DE GIULI-MORGHEN C., PANERAI A.E. Possible involvement of dynorohin in clonidine induced analgesia. Med. Sci. Res. 1989;17:351–352. [Google Scholar]
- BILSKY E.J., CALDERON S.N., WANG T., BERNSTEIN R.N., DAVIS P., HRUBY V.J., MCNUTT R.W., ROTHMAN R.B., RICE K.C., PORRECA F. SNC 80, a selective, nonpeptidic and systemicall active opioid delta agonist. J. Pharmacol. Exp. Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- BORONAT M.A., OLMOS G., GARCÍA-SEVILLA J.A. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br. J. Pharmacol. 1998;125:175–185. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWNING D.L., LIVINGSTON A., MORRIS B. Interactions of drugs active at opiate receptors and drugs active at α2 receptors on various test systems. Br. J. Pharmacol. 1982;77:487–491. doi: 10.1111/j.1476-5381.1982.tb09322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR G.D., FIBIGER H.C., PHILLIPS A.G.Conditioned place preference as a measure of drug reward The Neuropharmacological Basis of Reward 1989Oxford: Clarendon Press; 264–319.ed. Liebman, J.M. & Cooper, S.J. pp [Google Scholar]
- DWOSKIN L.P., NEAL B.S., SPARBER S.B. Yohimbine exacerbates and clonidine attenuates acute morphine withdrawal in rats. Eur. J. Pharmacol. 1983;90:269–273. doi: 10.1016/0014-2999(83)90248-0. [DOI] [PubMed] [Google Scholar]
- EISENACH J.C., D'ANGELO R., TAYLOR C., HOOD D. An isobolographic study of epidural clonidine and fentanyl after cesarean section. Anesth. Analg. 1994;79:285–290. doi: 10.1213/00000539-199408000-00014. [DOI] [PubMed] [Google Scholar]
- EL-KADI A.O.S., SHARIF S.I. The influence of chronic treatment with clonidine, yohimbine and idazoxan on morphine withdrawal. Psychopharmacol. 1997;132:67–73. doi: 10.1007/s002130050321. [DOI] [PubMed] [Google Scholar]
- GEAR R.W., GORDON N.C., HELLER P.H., LEVINE J.D. Enhancement of morphine analgesia by the α2-adrenergic antagonist yohimbine. Neuroscience. 1995;66:5–8. doi: 10.1016/0306-4522(95)00053-l. [DOI] [PubMed] [Google Scholar]
- GOLD M.S., REDMOND D.E., KLEBER H.D. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978;2:599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- GORDON N.C., HELLER P.H., LEVINE J.D. Enhancement of pentazocine analgesia by clonidine. Pain. 1992;48:167–169. doi: 10.1016/0304-3959(92)90054-F. [DOI] [PubMed] [Google Scholar]
- GRABOW T.S., HURLEY R.W., BANFOR P.N., HAMMOND D.L. Supraspinal and spinal delta-2 opioid receptor-mediated antinociceptive synergy is mediated by spinal alpha-2 adrenoceptors. Pain. 1999;83:47–55. doi: 10.1016/s0304-3959(99)00084-6. [DOI] [PubMed] [Google Scholar]
- HAMEEDI F.A., WOODS S.W., ROSEN M.I., PEARSALL H.R., KOSTEN T.R. Dose dependent effects of yohimbine on methadone maintained patients. Am. J. Drug Alcohol Abuse. 1997;23:327–333. doi: 10.3109/00952999709040950. [DOI] [PubMed] [Google Scholar]
- HARADA Y., NIHIOKA K., KITAHATA L.M., COLLINS J.G. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50488H. Anesthesiology. 1995;83:344–352. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- HAYES A.G., SHEEHAN M.J., TYERS M.B. Differential sensitivity of antinociception in the rat, mouse and guinea-pig to μ- and κ-opioid receptor agonists. Br. J. Pharmacol. 1987;91:823–832. doi: 10.1111/j.1476-5381.1987.tb11281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERO J.F., SOLANO R.E. The antinociceptive effect of the μ-opioid fentanyl is reduced in presence of the α2-adrenergic antagonist idazoxan in inflammation. Brain Res. 1999;840:106–114. doi: 10.1016/s0006-8993(99)01780-1. [DOI] [PubMed] [Google Scholar]
- HEYMAN J.S., KOSLO R.J., MOSBERG H.I., TALLARIDA R.J., PORRECA F. Estimation of the affinity of naloxone at supraespinal and spinal opioid receptors in vivo: Studies with receptor selective agonist. Life Sci. 1986;39:1795–1803. doi: 10.1016/0024-3205(86)90099-8. [DOI] [PubMed] [Google Scholar]
- IGLESIAS V., ALAMO C., CUENCA E., MORALES L., PÉREZ-GARCIA C., ALGUACIL L.F. Effect of oral yohimbine on withdrawal jumping behaviour of morphine-dependent mice. Addiction Biol. 1998;3:459–463. doi: 10.1080/13556219872001. [DOI] [PubMed] [Google Scholar]
- IGLESIAS V., ALGUACIL L.F., ALAMO C., ARIAS A., CUENCA E.Efectos de yohimbina sobre la analgesia y el desarrollo de dependencia morfínica en el ratón Abstracts 3rd Congress Sociedad Española Toxicomanías 1991. Madrid
- IGLESIAS V., ALGUACIL L.F., ALAMO C., CUENCA E. Effects of yohimbine on morphine analgesia and physical dependence in the rat. Eur. J. Pharmacol. 1992;211:35–38. doi: 10.1016/0014-2999(92)90258-6. [DOI] [PubMed] [Google Scholar]
- IZENWASSER S., KORNETSKY C. Effects of clonidine and yohimbine, alone and in combination with morphine, on supraspinal analgesia. Neuropharmacol. 1990;29:25–29. doi: 10.1016/0028-3908(90)90079-7. [DOI] [PubMed] [Google Scholar]
- KIEFFER B.L. Opioids: first lessons from knockout mice. Trends Pharmacol. Sci. 1999;20:19–25. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- KIHARA T., KANETO H. Important role of adrenergic function in the development of analgesic tolerance to morphine in mice. Japan J. Pharmacol. 1986;42:419–423. doi: 10.1254/jjp.42.419. [DOI] [PubMed] [Google Scholar]
- LEIGHTON G.E., RODRIGUEZ R.E., HILL R.O., HUGHES J. Kappa-opioid agonist produce antinociception after i.v. and i.c.v. but not intrathecal administration in rat. Br. J. Pharmacol. 1988;93:523–530. doi: 10.1111/j.1476-5381.1988.tb10310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGONI R., CADONI C., MULAS A., DI CHIARA G., SPINA L. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 2. Place-preference and brain microdialysis studies in rats. Behav. Pharmacol. 1998;9:9–14. [PubMed] [Google Scholar]
- MILLAN M.J. κ-opioid receptors and analgesia. Trends Pharmacol. Sci. 1990;11:70–76. doi: 10.1016/0165-6147(90)90321-x. [DOI] [PubMed] [Google Scholar]
- MORALES L., PÉREZ-GARCÍA C., HERRADÓN G., AMBROSIO E., ALGUACIL L.F. Abstracts III European Opioid Conference. Guildford, United Kingdom; 2000. Yohimbine prevents δ opioid receptor-mediated analgesia and conditioned place preference in rodents. [Google Scholar]
- MORALES L., PÉREZ-GARCÍA C., SANCHO I., AMBROSIO E., ALGUACIL L.F. Effect of yohimbine on morphine place preference in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358 Suppl 1:R177. [Google Scholar]
- MORALES L., PÉREZ-GARCÍA C., SANCHO I., CANO M.V., SÁNCHEZ C., AMBROSIO E., ALGUACIL L.F. Effects of the α2-adrenoceptor antagonist yohimbine on U-50,488-induced analgesia and conditioned place aversion. Dolor. 1999;14 Suppl 1:43. [Google Scholar]
- PARKER L.A. Place conditioning in a three- or four-choice apparatus: Role of stimulus novelty in drug-induced place conditioning. Behav. Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- PÉREZ-GARCÍA G., MORALES L., CANO M.V., SANCHO I., ALGUACIL L.F. Effect of thioperamide on the conditioned place preference and the physical dependence induced by morphine in the rat. Pharmacol. Rev. Commun. 1999;10:291–298. [Google Scholar]
- RADY J.J., ROERIG S.C., FUJIMOTO J.M. Heroin acts on different opioid receptors than morphine in Swiss Webster and ICR mice to produce antinociception. J. Pharmacol. Exp. Ther. 1991;262:365–374. [PubMed] [Google Scholar]
- RANDALL C.K., KRAEMER P.J., BARDO M.T. Morphine-induced conditioned place preference in preweanling and adult rats. Pharmacol. Biochem. Behav. 1998;60:217–222. doi: 10.1016/s0091-3057(97)00585-6. [DOI] [PubMed] [Google Scholar]
- RODRÍGUEZ DE FONSECA F., RUBIO P., MARTÍN-CALDERÓN J.L., CAINE S.B., KOOB G.F., NAVARRO M. The dopamine receptor agonist 7-OH-DPAT modulates the acquisition and expression of morphine-induced place preference. Eur. J. Pharmacol. 1995;274:47–55. doi: 10.1016/0014-2999(94)00708-f. [DOI] [PubMed] [Google Scholar]
- ROERIG S.C., FUJIMOTO J.M. Multiplicative interaction between intrathecally and intracerebro ventriculary administered μ opioid agonists but limited interactions between δ and κ agonists for antinociception in mice. J. Pharmacol. Exp. Ther. 1989;249:762–768. [PubMed] [Google Scholar]
- ROERIG S.C., LEI S., KITTO K., HYLDEN J.L.K., WILCOX G.L. Spinal interactions between opioid and noradrenergic agonists in mice: multiplicativity involves delta and alpha-2 receptors. J. Pharmacol. Exp. Ther. 1992;262:365–374. [PubMed] [Google Scholar]
- SCATTON B., ZIVKOVIC B., DEDEK J. Antidopaminergic properties of yohimbine. J. Pharmacol. Exp. Ther. 1980;215:494–499. [PubMed] [Google Scholar]
- SCHETZ J.A., CALDERON S.N., BERTHA C.M., RICE K., PORRECA F. Rapid in vivo metabolism of a methylether derivative of (±)-BW373U86: the metabolic fate of [3H]SNC121 in rats. J. Pharmacol. Exp. Ther. 1996;279:1069–1076. [PubMed] [Google Scholar]
- SHEARMAN G.T., HYNES M., LAL H.Self-administration of clonidine by the rat Psychopharmacology of Clonidine 1981New York: Liss; ed. H. Lal & S. Fielding [PubMed] [Google Scholar]
- SMITH C.B., MOISES H.C., SPENGLER R.N., HOLLINGSWORTH P.J. Changes in α2-adrenoceptor number and function in brains of morphine-dependent rats. Eur. J. Pharmacol. 1989;161:111–119. doi: 10.1016/0014-2999(89)90833-9. [DOI] [PubMed] [Google Scholar]
- SOLOMON R.E., GEBHART G.F. Intrathecal morphine and clonidine: antinociceptive tolerance and cross-tolerance and effects on blood pressure. J. Pharmacol. Exp. Ther. 1988;245:444–454. [PubMed] [Google Scholar]
- TAYLOR J.R., LEWIS V.O., ELSWORTH J.D., PIVIROTTO P., ROTH R.H., REDMOND D.E., JR Yohimbine co-treatment during chronic morphine administration attenuates naloxone precipitated withdrawal without diminishing tail-flick analgesia in rats. Psychopharmacol. 1991;103:407–414. doi: 10.1007/BF02244297. [DOI] [PubMed] [Google Scholar]
- VAN OENE J.C., DE VIRES J.B., HORNA A.S. The effectiveness of yohimbine in blocking rat central dopamine autoreceptors in vivo. Naunyn-Schmeidebergs Arch. Pharmacol. 1984;327:304–311. doi: 10.1007/BF00506241. [DOI] [PubMed] [Google Scholar]
- WANG C., PAULSKA P., PERRY B., PIZZI W.J., SCHNOLL S.H. Effect of perinatal exposure to methadone on brain opioid and α2-adrenergic receptors. Neurobehav. Toxicol. Teratol. 1986;8:399–402. [PubMed] [Google Scholar]
- WILCOX G.L., CARLSSON K.H., JOCHIM A., JURNA I. Mutual potentiation of antinociceptive effects of morphine and clonidine on motor and sensory responses in rat spinal cord. Brain Res. 1987;405:84–93. doi: 10.1016/0006-8993(87)90992-9. [DOI] [PubMed] [Google Scholar]
- WINTER J.C., RABIN R.A. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J. Pharmacol. Exp. Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- WOOLFE G., MACDONALD A.D. The evaluation of the analgesic action of pethidine hydrochloride (Demerol) J. Pharmacol. Exp. Ther. 1944;80:300–307. [Google Scholar]


