Abstract
The effects of an oral daily dose (10 mg kg−1) of the flavonoid quercetin for 5 weeks in spontaneously hypertensive (SHR) and normotensive Wistar Kyoto rats (WKY) were analysed.
Quercetin induced a significant reduction in systolic (−18%), diastolic (−23%) and mean (−21%) arterial blood pressure and heart rate (−12%) in SHR but not in WKY rats.
The left ventricular weight index and the kidney weight index in vehicle-treated SHR were significantly greater than in control WKY and these parameters were significantly reduced in quercetin-treated SHR in parallel with the reduction in systolic blood pressure.
Quercetin had no effect on the vasodilator responses to sodium nitroprusside or to the vasoconstrictor responses to noradrenaline or KCl but enhanced the endothelium-dependent relaxation to acetylcholine (Emax=58±5% vs 78±5%, P<0.01) in isolated aortae.
The 24 h urinary isoprostane F2α excretion and the plasma malonyldialdehyde (MDA) levels in SHR rats were increased as compared to WKY rats. However, in quercetin-treated SHR rats both parameters were similar to those of vehicle-treated WKY.
These data demonstrate that quercetin reduces the elevated blood pressure, the cardiac and renal hypertrophy and the functional vascular changes in SHR rats without effect on WKY. These effects were associated with a reduced oxidant status due to the antioxidant properties of the drug.
Keywords: Quercetin, SHR, flavonoid, hypertension, antioxidant
Introduction
Reactive oxygen species such as superoxide anions, hydroxylradical and hydrogen peroxide have been suggested to contribute to the genesis of atherosclerosis, diabetes, ischaemic heart disease, heart failure and hypertension (Griendling & Alexander, 1997; Givertz & Colucci, 1998; Nakazono et al., 1991) and thus, prevention of oxidative stress-induced damage is an area of growing interest. Hypertensive patients show increased levels of plasma superoxide, hydrogen peroxide and lipid peroxide and reduced plasma levels of the antioxidant vitamin C (Lacy et al., 1998; Kumar & Das, 1993; Tse et al., 1994). Moreover, in vessels from spontaneously hypertensive rats (SHR) and essential hypertensives, enhanced endothelial superoxide anion production has been described (Grunfeld et al., 1995; Suzuki et al., 1995) and this effect has been related to the impairment of endothelium-dependent relaxation (Jameson et al., 1993).
Flavonoids comprise a large group of secondary metabolites occurring widely throughout the plant kingdom including food plants (Rice-Evans & Packer, 1998). The daily flavonoid intake (mainly from onions, apples, grapes, wine, tea, berries, herbs and spices) in the human diet is highly variable, estimations ranging from 23 mg day−1 (only flavonols plus flavones; Hertog et al., 1993a) to more than 500 mg day−1 (total flavonoids, Manach et al., 1996). Among dietary flavonols, quercetin is by far the most abundant. Several epidemiological studies have shown a significant inverse association between dietary flavonoids (mainly quercetin) and long term mortality from coronary heart disease (Hertog et al., 1993b; 1995; 1997; Knekt et al., 1996; Rimm et al., 1996; Yochum et al., 1999). A very wide range of biological actions of flavonoids, including antioxidant (Rice-Evans & Packer, 1998), antiaggregant (Gryglewski et al., 1987) and vasodilator effects (Duarte et al., 1993a, 1993b) support these protective effects of quercetin in cardiovascular diseases. However, there is little information about the effects of quercetin on in vivo models of cardiovascular diseases and particularly, on animal models of systemic hypertension. Therefore, in the present study we have analysed the effects of chronic administration of an oral daily dose of quercetin (10 mg kg−1) on blood pressure, vascular structure, endothelial function and oxidative status in SHR rats and normotensive Wistar Kyoto rats (WKY).
Methods
Animals and experimental protocol
Twelve-week-old, male SHR and WKY rats were obtained from Harlan Laboratories (Barcelona, Spain). All the experiments were performed in accordance with Institutional Guidelines for the ethical care of animals.
All rats were maintained five per cage at a constant temperature (24±1°C), with a 12-h dark/light cycle and on standard rat chow. An adaptation period of 2 weeks for vehicle administration and blood pressure measurements was allowed before the initiation of the experimental protocols. Fourteen WKY and 20 SHR were randomly assigned to a control group (vehicle, 1 ml of 1% methylcellulosa) or a quercetin group (10 mg kg−1, mixed in 1 ml of 1% methylcellulosa). Rats were treated orally by gavage for 5 weeks. During the experimental periods rats had free access to tap water and chow. Body weight was measured every week. The last day of the experimental period the animals were placed on metabolic cages to collect urine. The quercetin treatment was stopped 2 days before the end of the experiment in order to study the long-term effects of quercetin without the involvement of the effects of acute administration. Four control and four quercetin-treated SHR rats were used at the end of the study for direct blood pressure measurements and the remaining six SHR in each group were used for the analysis of vascular function and structure.
Blood pressure measurements
Systolic blood pressure (SBP) was measured weekly 18 – 20 h after administration of the drugs in conscious, prewarmed, restrained rats by tail-cuff plethysmography (Tamargo et al., 1995). At least seven determinations were made in every session and the mean of the lowest three values within 5 mmHg was taken as the systolic blood pressure level. At the end of the fifth week, direct blood pressure was measured in conscious SHR rats. For this purpose, the rats were anaesthetized with ethyl ether and the right femoral artery was cannulated to obtain direct measurements of arterial blood pressure. The catheter was tunnelled subcutaneously, exteriorized through the skin on the dorsal side of the neck and protected with a silver spring. Rats were allowed to recover for 24 h and, after connecting the catheter to a transducer and a two-channel recorder (TRA-021 and Letigraph 2000, respectively; Letica SA, Barcelona, Spain), blood pressure and heart rate were continuously recorded for 60 min.
Cardiac and vascular morphology
Immediately after exsanguination, the superior mesenteric artery was cannulated and perfused for 30 min at the mean blood pressure of the rat with formol (10%) containing phosphate-buffered saline. A sample of the mesenteric vessels corresponding to the second branch (approximately 140 – 200 μm of average diameter) was obtained from each rat by dissection, immersed in 10% formol and then dehydrated in graded ethanol solutions and embedded in paraffin. In each artery a series of four 5 μm cross sections were made on a precision microtome and stained with hematoxylin-eosin. Arterial wall thickness (WT), media thickness (MT), lumen diameter (LD), media cross-sectional area (MCSA) and media-lumen ratio (M/L) were measured using a computer equipped with a Leica Q500MC image analyser connected to a video camera of a Leica Leitz DMRB microscope. The heart and kidneys were excised, cleaned and weighed. The atria and the right ventricle were then removed and the remaining tissue (left ventricle plus septum) weighed. The heart weight index (HW/BW), the left ventricular weight index (LVW/BW) and the kidney weight index (KW/BW) were calculated by dividing the heart weight (HW), the left ventricular weight (LVW) and the kidney weight (KW) by the body weight (BW).
Vascular function ex vivo
Descending thoracic aortic rings (3 mm) were dissected and mounted in organ chambers filled with Krebs solution (composition in mM): NaCl 118, KCl 4.75, NaHCO3 25, MgSO4 1.2, CaCl2 2, KH2PO4 1.2 and glucose 11) at 37°C and gassed with 95% O2 and 5% CO2. Rings were stretched to 2 g of resting tension by means of two L-shaped stainless-steel wires inserted into the lumen and attached to the chamber and to an isometric force-displacement transducer (Letigraph, model 2000, Letica S.A., Barcelona, Spain), respectively, as previously described (Duarte et al., 1993a, 1993b). Concentration-response curves to noradrenaline (10−9 – 10−6 M) or KCl (8 – 80 mM) were constructed by cumulative addition of the drugs. The concentration-relaxation response curves to acetylcholine (10−8 – 10−5 M) were performed in rings pre-contracted by 10−7 M phenylephrine. The concentration-relaxation response curves to nitroprusside (10−10 – 10−5 M) were performed in the dark in rings pre-contracted by 10−6 M noradrenaline. Endothelium-dependent contractions to acetylcholine were tested in rings which were initially stimulated with 80 mM KCl. After washing in Krebs solution and incubation for 30 min with L-NAME (10−4 M), acetylcholine was added in a cumulative fashion (10−8 – 10−4 M). In these experiments, the contractile responses to acetylcholine were expressed as a percentage of the response to KCl.
Analytical procedures
For total 8-iso-prostaglandin F2α determination, urine samples were hydrolysed by incubation at 40°C for 90 min with 10 N NaOH. The samples were allowed to cool and neutralized with 2 N HCl. After centrifugation at 600×g for 15 min the supernatant was collected for assay. The total 8-iso-prostaglandin F2α concentration was measured by a competitive enzyme immunoassay (R&D Systems, Inc., Minneapolis, MN, U.S.A.).
Plasma malonyldialdehyde (MDA) content was evaluated as described by Esterbauer & Cheeseman (1990). Hundred μl of plasma reacted with a chromogenic reagent, 1-methyl-2-phenylindole (10.3 mmol l−1) in acetonitrile and 37% aqueous HCl (10.4 mol l−1). After incubation of the reaction mixture for 40 min in a 45°C water bath, the absorbance was measured at 586 nm in a GBC 920 spectrophotometer.
All drugs and chemicals were purchased from Sigma (Alcobendas, Madrid, Spain).
Statistical analysis
Results are expressed as means±s.e.means of measurements. The evolution of tail SBP and with time was compared using the nested design, with groups treatment and days as fixed factors and the rat as random factor. When the overall difference was significant comparisons were made using Bonferroni's method with an appropriate error. Analysis of the nested design was also carried out with groups and doses to compare the dose response curves to ACh. The rest of variables were compared using a two-way factor design, where group and treatment were fixed effect factors with unequal sample sizes in the different groups. When interaction was significant Bonferroni's method was used for pairwise comparisons. P<0.05 was considered statistically significant. Concentration-response curves were fitted to the logistic equation: 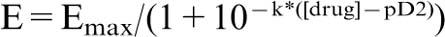 , where Emax is the maximal effect, k is a factor which represents the slope of the curve, and pD2 is the drug concentration exhibiting 50% of the Emax expressed as negative log molar.
, where Emax is the maximal effect, k is a factor which represents the slope of the curve, and pD2 is the drug concentration exhibiting 50% of the Emax expressed as negative log molar.
Results
Blood pressure and heart rate
Long-term quercetin administration induced a progressive reduction in SBP in SHR and this effect reached statistical significance after the first week of treatment while no changes were observed in WKY rats (Figure 1A). At the end of the 5 weeks of treatment, direct measurements of blood pressure in conscious rats showed that quercetin induced a significant reduction in systolic (−18%), diastolic (−23%) and mean (−21%) arterial blood pressure in SHR (Figure 1B). Heart rate was also significantly reduced by quercetin in SHR (−12%) (Figure 1B).
Figure 1.
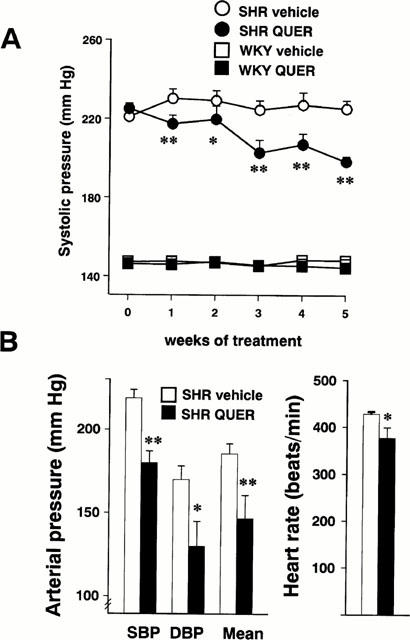
Effects of quercetin on arterial blood pressure and heart rate. (A) Represents the time course of the systolic arterial pressure as measured by tail-cuff plethysmography in the SHR vehicle (n=10), SHR quercetin (QUER, n=10), WKY vehicle (n=7) and WKY quercetin (n=7) groups. (B) Shows the direct measurements of systolic (SBP), diastolic (DBP) and mean arterial pressure and heart rate in the SHR vehicle (solid bars, n=4) and SHR quercetin (open bars, n= 4) groups. Values are expressed as means±s.e.mean. *P<0.05, **P<0.01 vs the SHR vehicle group (Bonferroni's test).
Morphological variables
Treatment with quercetin did not modify the body weight in either SHR or WKY rats. The heart weight index (HW/BW), left ventricular weight index (LVW/BW) and kidney weight index (KW/BW) in SHR were significantly greater than in WKY. These parameters were significantly reduced in quercetin-treated SHR as compared to vehicle treated SHR (Table 1). Moreover, the reduction in LVW and KW indices in SHR treated with quercetin correlated with the observed reduction in systolic blood pressure (Figure 2A,B).
Table 1.
Body weight and cardiac and renal indices
Figure 2.
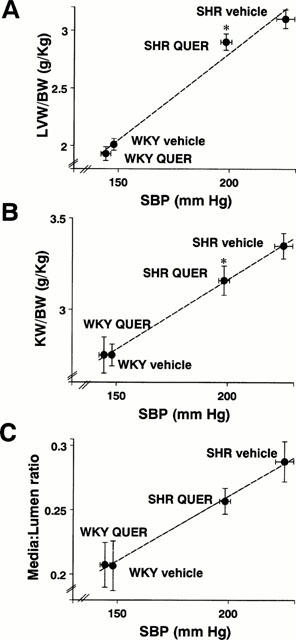
(A) Left ventricular weight index (LVW/BW), (B) Kidney weight index (KW/BW) and (C) Media to lumen ratio in resistance mesenteric arteries plotted against systolic blood pressure (SBP) in the SHR vehicle, SHR quercetin (QUER), WKY vehicle and WKY quercetin groups. Data, taken from Figure 1A and Tables 1 and 2, are expressed as means±s.e.mean. (n=7 and 10 for WKY and SHR groups, respectively, except for media : lumen ratio data in SHR where n=6). *P<0.05 vs the SHR vehicle group (Bonferroni's test).
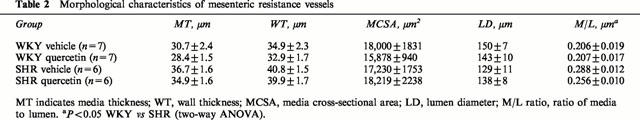
SHR rats showed vascular structural alterations characterized by a non significant increase in MT (19%), a decrease in LD (14%) and a significant increase (43%) in the M/L as compared to WKY (Table 2). The MCSA was similar in both groups. Treatment with quercetin had no effect on the morphological parameters of mesenteric vessels in WKY. In SHR, quercetin tended to increase the LD and to reduce the MT. Although the reduction in M/L ratio (12%) did not achieve statistical significance (Table 2) it correlated with the reduction in systolic blood pressure (Figure 2C).
Table 2.
Morphological characteristics of mesenteric resistance vessels
Ex vivo vascular reactivity
In aortae from WKY and SHR rats treated with quercetin, no differences were observed in the endothelium-independent vasodilator responses to the NO donor sodium nitroprusside in vessels pre-contracted with noradrenaline or to the vasoconstrictor responses induced by noradrenaline or KCl as compared with their respective control WKY and SHR groups (Table 3). Aortae from vehicle-treated SHR rats showed significant reduced endothelium-dependent vasodilator responses to acetylcholine in arteries stimulated by phenylephrine as compared to aortae from control WKY (Emax=58.0±4.7% vs 81.6±3.4%). In addition, in the presence of L-NAME (10−4 M), aortae from SHR also showed increased endothelium-dependent vasoconstrictor responses to acetylcholine than their normotensive WKY counterparts (40.6±10.0% vs 3.8±0.4% of the response to 80 mM KCl, respectively). Quercetin produced a weak significant increase in the relaxation induced by 10−7 M and 10−6 M acetylcholine in WKY while in SHR rats, it increased the vasodilation induced by acetylcholine at all concentrations tested (Emax=78.2±5.2%, P<0.01) without changes in the endothelium-dependent vasoconstriction (Figure 3).
Table 3.
Parameters of the concentration-response curves to endothelium-independent vasoactive factors

Figure 3.
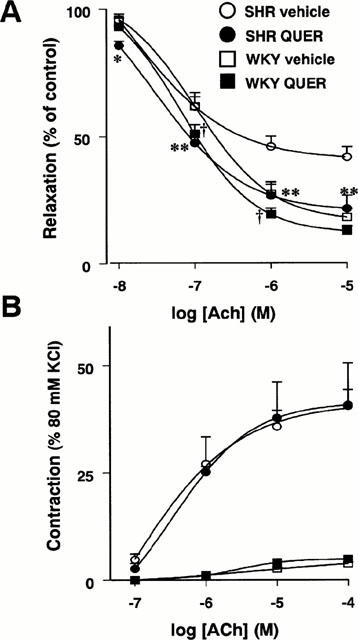
Endothelium-dependent relaxation (A) and endothelium-dependent contractions (B) induced by ACh in aortae from the SHR vehicle (n=6), SHR quercetin (n=6), WKY vehicle (n=7) and WKY quercetin (n=7) groups. ACh-induced relaxations were induced in arteries contracted by 10−7 M phenylephrine. ACh-induced contractions were evoked in arteries treated with L-NAME (10−4 M) and expressed as a per cent of the response to 80 mM KCl. Values are expressed as means±s.e.mean. *P<0.05 and **P<0.01 SHR QUER vs the SHR vehicle group and †P<0.05 WKY QUER vs WKY vehicle (Bonferroni's test).
Oxidant status
The 24 h urinary isoprostane F2α excretion and the plasma MDA levels in SHR rats were significantly increased as compared to normotensive WKY rats (Figure 4A,B, respectively). However, in quercetin-treated SHR rats, isoprostane F2α excretion and plasma MDA were significantly reduced as compared to vehicle-treated SHR rats, showing similar values to those of vehicle-treated WKY.
Figure 4.

Oxidant status. Urinary iso-PGF2α excretion (A) and total plasma malondialdehyde (MDA) content (B) in the vehicle- (open bars) and quercetin-treated rats (solid bars). Data are means±s.e.mean (n=7 – 10). *P<0.05, **P<0.01, n.s. not significant (Bonferroni's test).
Discussion
In the present study, a single oral daily dose of the bioflavonoid quercetin reduced blood pressure and heart rate, the cardiac and renal hypertrophy, the endothelial dysfunction and the oxidant status in SHR, a rat model of hypertension, but had no effect on normotensive WKY. To our knowledge this is the first report showing the chronic antihypertensive effect of a flavonoid.
Quercetin and related bioflavonoids show vasodilator effects in isolated aortae stimulated with noradrenaline, KCl or phorbol esters and these effects are independent of the presence of endothelium (Duarte et al., 1993a, 1993b). Thus, this direct vasodilator effect might contribute to its antihypertensive effects observed in the present study. However, the direct blood pressure measurements were carried out 42 – 48 h after the last administration of quercetin, when plasma quercetin and its metabolites fall below 25% of the peak plasma levels, suggesting that the direct vasodilator effect is not essential for the maintenance of low blood pressure (Hollman et al., 1997). Therefore, although the direct vasodilator effect of quercetin may play a role, quercetin does not appear to be merely an acute vasodilator.
The SHR model is characterized by an increased oxidative stress (Suzuki et al., 1995). Urinary levels of isoprostane F2α, a prostaglandin-like compound produced in a non enzymatic reaction of arachidonic acid and superoxide, as well as plasma levels of MDA have been proposed to be reliable markers of lipid peroxidation and oxidative stress (Morrow & Roberts, 1996; Kitts et al., 1998) and in the present study, these parameters were enhanced in SHR as compared to normotensive WKY rats. Increased superoxide anion production is thought to contribute to hypertension in SHR rats (Grunfeld et al., 1995; Suzuki et al., 1995). In fact, reduction of superoxide anions with alloxanthine, an inhibitor of xantine oxidase (Miyamoto et al., 1996), or CuZn superoxide dismutase (SOD, Nakazono et al., 1991) acutely decreased mean arterial pressure in the SHR. Additionally, administration of tempol, a membrane-permeable SOD mimetic, for 7 days also reduced blood pressure in SHR (Schnackenberg et al., 1998). One possible explanation for the hypertensive effects of superoxide may be that this free radical can inactivate NO and, therefore, impair the NO-dependent vasodilation (Vanhoutte & Lüscher, 1987). In addition, some products of oxidative stress, such as 8-iso-prostaglandin F2α, have been shown to be potent vasoconstrictors and to raise blood pressure (Morrow et al., 1990; Morrow & Roberts, 1996; Takahashi et al., 1992). Furthermore, excess active oxygen species may also stimulate cellular proliferation by direct mitogenic effects on vascular smooth muscle cells (Rao & Berk, 1992; Gong et al., 1996) and by decreasing the effective concentrations of NO and prostacyclin (Garg & Hassid, 1989).
Flavonoids are considered as an important part of the food antioxidants. The flavonoid intake exceeds that of other dietary antioxidants such as β-carotene (2 – 3 mg day−1) and vitamin E (7 – 10 mg day−1) and is about one-third that of vitamin C (70 – 100 mg day−1) (Rice-Evans & Packer, 1998). In vitro, quercetin inhibits hypoxanthine-xanthine oxidase activity and scavenges superoxide, hydroxyl radical, and peroxynitrite (Rice-Evans & Packer, 1998) and after oral administration quercetin metabolites retain the antioxidant properties of the parent compound (Manach et al., 1998). Furthermore, treatment with intravenous quercetin scavenged superoxide anions released in the brain cortex during reperfusion (Shutenko et al., 1999). In the present study, long-term treatment with quercetin reduced the 24 h urinary excretion of isoprostane F2α and the plasma MDA in SHR rats to levels similar to those of WKY, indicating that chronic oral treatment quercetin reduced the oxidative stress in the SHR. Furthermore, quercetin restored the endothelium-dependent vasodilatation to ACh observed in SHR but not in WKY rats while it had no effect on the relaxant effects of SNP. This endothelium-dependent effect might result from the superoxide scavenging properties of quercetin, preventing the superoxide-induced NO degradation and thus prolonging its half-life. Therefore, the restoration of NO-induced vasodilation together with the reduced production of the vasoconstrictor 8-iso-prostaglandin F2α may explain, at least partially, the antihypertensive effects of quercetin.
Sustained high blood pressure is one of the most powerful determinants of the development of cardiac and renal hypertrophy (Frochlich et al., 1993). Additionally, resistance vessels from essential hypertensive patients and SHR show inward eutrophic remodelling, i.e. increased M/L ratio without changes in the amount of material (Heagerty et al., 1993; Mulvany et al., 1996). Some studies in SHR have also found medial hypertrophy, particularly in aged animals (Intengan et al., 1999). In our study, SHR showed increased left ventricular and renal weight indices and M/L ratio as compared to normotensive WKY rats. However, we did not observe medial hypertrophy. The reduction in the left ventricular and renal weight indices induced by quercetin in SHR rats run in parallel with its antihypertensive effects. Quercetin also tended to reduce the M/L ratio in SHR following the trend of blood pressure reduction. However, this effect did not achieve statistical significance which might be attributed to the lower accuracy of the method employed as compared to measurements in pressurized vessels mounted in a myograph. Thus, the beneficial effect of quercetin on cardiovascular structure seems to be related to its blood pressure lowering effect. However, other effects beyond the antihypertensive properties, such as the decrease in heart rate, the antioxidant effects and the protection from NO breakdown, might also play a role in the prevention of morphological changes observed in SHR rats treated with quercetin.
The equivalent human dose of quercetin used in the present study in a weight to weight basis is 10 – 30 times higher than the average human daily intake. However, this dose can be achieved in the human diet in subjects consuming selected meals rich in vegetables, fruit and wine. The supplementation with an oral daily dose of 1 g quercetin for 28 days to healthy normotensive subjects has been recently compared to placebo, showing no differences in selected cardiovascular risk factors including blood pressure (Conquer et al., 1998). However, a diet rich in fruits and vegetables (and presumably rich in flavonoids) lowered blood pressure in hypertensive but not in normotensive subjects (Appel et al., 1997; Moore et al., 1999). Accordingly, we also found no changes on blood pressure in normotensive WKY while a significant reduction was observed in SHR rats treated with quercetin. Prospective clinical studies addressing the antihypertensive effect of flavonoids in essential hypertensive patients are needed.
In conclusion, these data demonstrate that quercetin reduces the elevated blood pressure, the cardiac and renal hypertrophy and the functional vascular changes in SHR rats. These effects were associated with a reduced oxidant status due to the antioxidant properties of the drug.
Acknowledgments
This work was supported by a CICYT (SAF 98-0160, 98-0157, 99-0069) Grants. F. Pérez-Vizcaíno is supported by a grant from Comunidad Autónoma de Madrid. We wish to thank Juan de Dios Luna for the statistical analysis (Dept of Biostatistics, University of Granada).
Abbreviations
- BW
body weight
- HW
heart weight
- KW
kidney weight
- LD
lumen diameter
- LVW
left ventricular weight
- MCSA
media cross-sectional area
- MDA
total plasma malondialdehyde
- M/L
media – lumen ratio
- MT
media thickness
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rat
- SOD
superoxide dismutase
- WKY
Wistar Kyoto rat
- WT
arterial wall thickness
References
- APPEL L.J., MOORE T.J., OBARZANEK E., VOLLMER W.M., SVETKEY L.P., SACKS F.M., BRAY G.A., VOGT T.M., CUTLER J.A., WINDHAUSER M.M., LIN P.-H., KARANJA N. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997;33:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- CONQUER J.A., MAIANI G., AZZINI E., RAGUZZINI A., HOLUB B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- DUARTE J., PÉREZ-VIZCAÍNO F., UTRILLA M.P., JIMÉNEZ J., TAMARGO J., ZARZUELO A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen. Pharmacol. 1993a;24:857–864. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- DUARTE J., PÉREZ-VIZCAÍNO F., ZARZUELO A., JIMÉNEZ J., TAMARGO J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1993b;239:1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- ESTERBAUER H., CHEESEMAN K.H. Determination of aldehydic lipid peroxidation product: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- FROCHLICH E., APSTEIN C., CHOBANIAN A., DEVEREUX R., DUSTAN H., DZAU V., FAUAD-TARAZI F., HORAN M., MARCUS M., MASSIE B., PFEFER M., RE R., ROCCELLA E., SAVAGE D., SHUB C. The heart in hypertension. N. Engl. J. Med. 1993;327:998–1008. doi: 10.1056/NEJM199210013271406. [DOI] [PubMed] [Google Scholar]
- GARG U.C., HASSID A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscular cells. J. Clin. Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIVERTZ M.M., COLUCCI W.S. New targets for heart-failure therapy: endothelin, inflammatory cytokines, and oxidative stress. Lancet. 1998;52 Suppl 1:SI34–SI38. doi: 10.1016/s0140-6736(98)90017-4. [DOI] [PubMed] [Google Scholar]
- GONG K.-W., ZHU G.-Y., WANG L.-H., TANG C.S. Effects of active oxygen species on intimal proliferation in rat aorta after arterial injury. J. Vasc. Res. 1996;33:42–46. doi: 10.1159/000159130. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., ALEXANDER R.W. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- GRUNFELD S., HAMILTON C.A., MESAROS S., MCCLAIN S.W., DOMINICZAK A.F., BOHR D.F., MALINSKI T. Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension. 1995;26:854–857. doi: 10.1161/01.hyp.26.6.854. [DOI] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., KORBUT R., ROBAK J., SWIES J. On the mechanism of antithrombotic action of flavonoids. Biochem. Pharmacol. 1987;36:317–322. doi: 10.1016/0006-2952(87)90288-7. [DOI] [PubMed] [Google Scholar]
- HEAGERTY A.M., AALKJAER C., BUND S.J., KORSGAARD N., MULVANY M.J. Small artery structure in hypertension: dual processes of remodeling and growth. Hypertension. 1993;21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G.L., FESKENS E.J.M., HOLLMAN P.C.H., KATAN M.B., KROMHOUT D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993b;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G.L., FESKENS E.J.M., KROMHOUT D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G.L., HOLLMAN P.C.H., KATAN M.B., KROMHOUT D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer. 1993a;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G., KROMHOUT D., ARAVANIS C., BLACKBURN H., BUZINA R., FIDANZA F., GIAMPAOLI S., JANSEN A., MENOTTI A., NEDELJKOVIC S., PEKKARINEN M., SIMIC B.S., TOSHIMA H., FESKENS E.J.M., HOLLMAN P.C.H., KATAN M.B. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. [PubMed] [Google Scholar]
- HOLLMAN P.C.H., VAN TRIJP J.M.P., BUYSMAN M.N.C.P., VAN DER GAAG M.S., MENGELERS M.J.B., DE VRIES J.H.M., KATAN M.B. Relative bioavalability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- INTENGAN H.D., THIBAULT G., LI J.-S., SCHIFFRIN E.L. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats. Circulation. 1999;100:2267–2275. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- JAMESON M., DAI F.-X., LÜSCHER T., SKOPEC J., DIEDERICH A., DIEDERICH D. Endothelium-derived contracting factors in resistence arteries of young spontaneously hypertensive rats before development of overt hypertension. Hypertension. 1993;21:280–288. doi: 10.1161/01.hyp.21.3.280. [DOI] [PubMed] [Google Scholar]
- KITTS D.D., YUAN Y.V., GODIN D.V. Plasma and lipoprotein lipid composition and hepatic antioxidant status in spontaneously hypertensive (SHR) and normotensive (WKY) rats. Can. J. Physiol. Pharmacol. 1998;76:202–209. [PubMed] [Google Scholar]
- KNEKT P., JÄRVINEN R., REUNANEN A., MAATELA J. Flavonoid intake and coronary mortality in Finland: a cohort study. Br. Med. J. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR K.V., DAS U.N. Are free radicals involved in the pathobiology of human essential hypertension. Free Radic. Res. Commun. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- LACY F., O'CONNOR D.T., SCHMID-SCHONBEIN G.W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998;16:291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- MANACH C., MORAND C., CRESPY V., DEMIGNE C., TEXIER O., REGERAT F., REMESY C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- MANACH C., REGERAT F., TEXIER O., AGULLO G., DEMIGNE C., REMESY C. Bioavaliability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr. Res. 1996;16:517–544. [Google Scholar]
- MIYAMOTO Y., AKAIKE T., YOSHIDA M., GOTO S., HORIE H., MAEDA H. Potentiation of nitric oxide-mediated vasorelaxation by xantine oxidase inhibitors. Proc. Soc. Exp. Biol. Med. 1996;211:366–373. doi: 10.3181/00379727-211-43982. [DOI] [PubMed] [Google Scholar]
- MOORE T.J., VOLLMER W.M., APPEL L.J., SACKS F.M., SVETKEY L.P., VOGT T.M., CONLIN P.R., SIMONS-MORTON D.G., CARTER-EDWARDS L., HARSHA D.W. Effect of dietary patterns on ambulatory blood pressure. Results from the dietary approaches to stop hypertension (DASH) Trial. Hypertension. 1999;34:472–477. doi: 10.1161/01.hyp.34.3.472. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., HILL K.E., BURK R.F., NAMMOUR T.M., BADR K.F., ROBERTS L.J. A series of prostaglandin F2 like compounds are produced in vivo by humans by a non–cyclooxygenase, free radical catalysed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J.D., ROBERTS L.J. The isoprostanes. Current knowledge and directions for future research. Biochem. Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., BAUMBACH G.L., AALKJAER C., HEAGERTY A., KORSGAARD N., SCHIFFRIN E.L., HEISTAD D.D. Vascular remodeling. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- NAKAZONO K., WATANABE N., MATSUNO K., SASAKI J., SATO T., INOUE M. Does superoxide underlie the pathogenesis of hypertension. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO G.N., BERK B.C. Does oxygen species stimulate vascular smooth-muscle cell growth and proto-oncogen expression. Circ. Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- RICE-EVANS A., PACKER L. Flavonoids in health and disease. New York: Marcel Dekker Inc; 1998. [Google Scholar]
- RIMM E.R., KATAN M.B., ASCHERIO A., STAMPFER M., WILLET W. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann. Intern. Med. 1996;125:384–389. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- SCHNACKENBERG C.G., WELCH W.J., WILCOX C.S. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic. Role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- SHUTENKO Z., HENRY Y., PINARD E., SEYLAZ J., POTIER P., BERTHET F., GIRARD P., SERCOMBE R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1999;57:199–208. doi: 10.1016/s0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., SWEI A., ZWEIFACH B.W., SCHMID-SCHÖNBEIN G.W. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats: hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., NAMMOUR T.M., FUKUNAGA M., EBERT J., MORROW J.D., ROBERTS I.L.J., HOOVER R.L., BADR K.F. Glomerular action of a free radical generated novel prostaglandin, 8-epi-prostaglandin F2α in the rat. J. Clin. Invest. 1992;90:136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMARGO J., PÉREZ O., DELPÓN E., GARCÍA-RAFANELL J. Cardiovascular effects of UR8225, a novel potassium channel opener: Comparison with levcromakalim. J. Cardiovasc. Pharmacol. 1995;26:295–305. doi: 10.1097/00005344-199508000-00016. [DOI] [PubMed] [Google Scholar]
- TSE W.Y., MAXWELL S.R., THOMASON H., BLANN A., THORPE G.H., WAITE M., HOLDER R. Antioxidant status in controlled and uncontrolled hypertension and its relationship to endothelial damage. J. Hum. Hypertens. 1994;8:843–849. [PubMed] [Google Scholar]
- VANHOUTTE P.M., LÜSCHER T.F. Vascular endothelium and hypertension. J. Cardiovasc. Pharmacol. 1987;10:S19–S24. [Google Scholar]
- YOCHUM L., KUSHI L.H., MEYER K., FOLSOM A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]


