Abstract
Inflammation may influence response to pharmacotherapy.
We investigated the effect of inflammation on response to sotalol, a β-adrenergic receptor and potassium channel antagonist. Racemic sotalol (40 mg kg−1) was administered to healthy, acutely (interferonα 2a-induced) and chronically (Mycobacterium butyricum-induced adjuvant arthritis) inflamed male Sprague-Dawley rats (n=4 – 6/group). Another group of interferon-treated rats received 3 mg kg−1 of anti-TNF antibody infliximab. Electrocardiogram (ECG) recorded and plasma sotalol concentration monitored for 6 h. The study was repeated in acutely inflamed rats following administration of stereochemically pure individual sotalol enantiomers [40 mg kg−1 S (potassium channel blocker) or 20 mg kg−1 R (β-adrenergic/potassium channel blocker)].
Chronic arthritis was readily evident. Acute arthritis was associated with elevated segmented neutrophils and increased plasma nitrite and tumour necrosis factor (TNF) concentrations. Sotalol affected ECG in all rats. In both inflamed groups, however, response to sotalol in prolongation of QT interval (potassium channel sensitivity) was reduced. The effect of PR interval (β-adrenergic activity) was also reduced following administration of the racemate and R-enantiomer. No significant differences in pharmacokinetics were observed between control and inflamed rats.
Infliximab reduced nitrite and TNF concentrations and reversed the effect of acute inflammation on both PR and QT intervals.
The reduced electrocardiographic responses to sotalol is likely due to the influence of inflammation on the action of the drug on both β-adrenergic and potassium channel receptors secondary to over-expression of pro-inflammatory cytokines and/or nitric oxide.
Our observation may have therapeutic consequences in all conditions where inflammatory mediators are increased.
Keywords: Sotalol, inflammation, stereochemistry, β-adrenergic antagonist, potassium channel blocker, infliximab, cytokines, nitric oxide
Introduction
It is known that in vitro increases in nitric oxide and/or pro-inflammatory cytokines may influence the sensitivity of β-adrenergic receptors (Rozanski & Witt, 1994) as well as the calcium (Liu et al., 1999) and potassium (Kaprielian et al., 1999) channels. The exact instigator of this down-regulation is not known since expression of any of the inflammatory mediators results in increased level of the others. Suggested mechanisms for these in vitro observations include reduced receptor density and/or affinity (Raaijmakers et al., 1989; Gulick et al., 1989; Rozanski & Witt, 1994; Liu et al., 1999). Nevertheless, the therapeutic consequence of the reduced sensitivity is not known due to the lack of in vivo data. However, it has been shown that in post-myocardial infarction patients, inflammatory status is the main determinant of therapeutic failure (Verheggen et al., 1999) or mortality (Pietila et al., 1996). In addition, as compared with the general population, a significantly higher cardiovascular-related mortality rate has been noticed in patients afflicted with systemic lupus erythematosus (Manzi et al., 1997).
A complicating factor in this context is the well-known effect of inflammation on the pharmacokinetics of drugs due to inhibition of various metabolic pathways (Schneider et al., 1981; Belpaire et al., 1982; Piquette-Miller & Jamali, 1995). Inflammation causes increased concentrations of α1-acid glycoproteins (Piafsky et al., 1978) and reduced intrinsic clearance (Belpaire et al., 1989), both of which can result in increased circulating drug concentration. This renders differentiation between the effects due to pharmacodynamics changes and those resulting from pharmacokinetic alterations difficult.
To determine the effect of inflammation on response to antagonists of β-adrenergic receptors and cardiac potassium channels, sotalol was administered to acutely and chronically inflamed as well as control rats. Sotalol is negligibly bound to plasma proteins and is mainly eliminated unchanged via the renal route (Anderson & Prystowsky, 1999). Thus, inflammation would not be expected to reduce sotalol clearance hence pharmacodynamic assessments can be carried out in the absence of altered pharmacokinetics. Single oral doses of 40 mg kg−1 of racemic sotalol which significantly prolonged both PR and QT intervals and were still in the ascending phase of the dose-response curves were chosen for the study. In addition, both racemic and R-sotalol have nonselective β-adrenergic and cardiac potassium channel blocking activities but S-sotalol is a pure potassium channel blocker. Thus, we took advantage of stereochemistry and by administering stereochemically pure enantiomers, determined the effect of inflammation on cardiac potassium channels in the absence of β-adrenergic activity. In an attempt to explore whether the effect of inflammation on the potency of sotalol was cytokine dependent, inflamed rats were treated with anti-tumour necrosis factor (TNF) antibody (infliximab) and their ECG recorded.
Methods
Animals and dosing
This investigation was performed in adherence to the principles of the Animal Ethics Committee of the University of Alberta. Adult male Sprague – Dawley rats were housed in standard rodent cages, kept on a 12 h light/dark cycle, and fed a standard diet of Purina rat chow. Racemate and enantiomers of sotalol were administered by dissolving sotalol in normal saline and giving the appropriate volume via oral gavage.
Chronic inflammation
Eight rats (232±6 g) were divided to two groups of four. One group (adjuvant arthritis, AA) received heat-killed freeze dried Mycobacterium butyricum (Difco Laboratories, Detroit, MI, U.S.A.) via an intralymphatic tail base inoculation (35 mg in 0.05 ml squalene). The control group received saline instead of the adjuvant. Pharmacokinetic-pharmacodynamic experiments started 14 days after inoculation when control and AA rats weighed 298±21 and 278±31, respectively. Before sotalol dosing, severity of the arthritis was determined (Whitehouse, 1988). Briefly, an arthritic index score was assigned by quantifying hind paw and fore paw swelling and number of joints affected. Each hind paw was visually graded using a score from zero to four with zero representing no swelling or joint involvement, while four indicating severe swelling of several joints. Each fore paw was graded from zero to three with zero representing no swelling while a score of three indicates excessive swelling of the wrist and joints. The highest score attainable was 14 indicating severe inflammation with extensive joint involvement.
Acute inflammation
Twelve rats (310±17 g) were divided to two groups of six. Inflammation was induced in the test group by s.c. injections of two doses of 5.0×104 units of interferonα 2a (Roche Pharmaceuticals, Mississauga, Ontario, Canada) 12 and 3 h prior to sotalol administration. The control group received saline instead of interferon. To determine affliction with inflammation, before commencement of the experiment, a differential blood stain was performed on each rat. A total of 100 white cells were counted to determine the percentage of lymphocytes, neutrophils and segmented (mature) neutrophils. The number of segmented neutrophils (mature neutrophils) were counted since activation of the inflammatory response is thought to accelerate the maturation process (Davies et al., 1999). In addition, administration of interferonα 2a is reported to enhance neutrophil respiratory burst, a step in which oxidative metabolism of neutrophils increase before phagocytosis, which occurs with bacterial and viral infection (Little et al., 1994). Thus, greater amounts of segmented neutrophils in the interferonα 2a-treated rat indicated acute inflammatory disease.
Plasma nitrite assay
Nitrite (NO2−, a stable breakdown product of NO) was measured in plasma of all rats using a method reported by Archer et al. (1995) and Grisham et al. (1996). Briefly, 100 μl of plasma was incubated with Asperigillus nitrate reductase to reduce all nitrate (NO3−) to nitrite (NO2−). This was then treated with the Griess reagent and absorbance measured at 540 nm. Calibration was performed using standard solutions of NaNO2 and NaNO3. A comparison of NaNO2 and NaNO3 calibration curves was used to test the enzyme efficiency which was 98.8±1.2% for the experiment. The assay was linear from 5 – 200 μM with a coefficient of variation <10%.
Plasma TNF assay
TNFα was measured using an ELISA kit (Endogen, Woburn, MA, U.S.A.) with the minimum quantifiable concentration of 10 pg ml−1 based on 50 μl of plasma. The inter- and intra-coefficient of variation were <10%.
Electrocardiograph (ECG) recording
Prior to sotalol administration, under a light general anaesthesia using methoxyflurane, three stainless steel Teflon coated electrodes (Cooner wire, Chatsworth, CA, U.S.A.) were attached to the rats for Lead I monitoring. Two electrodes near the right and left axilla regions, and the third at the xiphoid cartilage.
To avoid interference of anaesthesia with the effect of sotalol, rats were allowed to recover for 2 h and were studied while conscious (Mayo & Jamali, 1999).
ECG measurements, i.e., PR, and QT intervals as well as the heart rate, were continually monitored using an Electronics for Medicine Honeywell V1207A Electrocardiograph Amplifier and recorded using Acknowledge 3.0 Data Acquisition software (Biopac Systems, Inc., Goleta, CA, U.S.A.). The PR interval represents the time required for an impulse to conduct through the tissues located above the ventricles (i.e., atria, AV node and His bundle). Since β-adrenergic antagonists act by sympathetic blockage, decreased SA node automaticity and AV node conduction, PR interval prolongation is a measure of β1-adrenergic antagonism (Opie, 1998). The QT interval, conduction through Purkinjie fibres and ventricular muscle represents drug effect on ventricular depolarization and repolarization, is used as a measure of cardiac potassium channel blocking activity (Opie, 1998). The PR interval is measured as the distance from the crest of the P wave to the crest of the R wave. The heart rate is measured as the distance from the crest of one R wave to another. In the rat, the ST segment of the electrocardiogram cycle forms a plateau that is not seen in a human electrocardiogram. Therefore, in order to quantify the QT interval the distance from the Q dip to the bottom of the ST segment is measured. Prolongation of PR and QT intervals were not corrected for changes in heart rate since a consistent relationship between the duration of both PR and QT intervals and heart rate in the rat has not been shown (Detweiler, 1981).
The interval measurements were conducted before blood sample collection. For each interval at every sampling time five consecutive cycles were averaged.
Dose-response study
A dose-response study was conducted by administering single oral doses of racemic sotalol to healthy rats (10, 20, 40, 80, 100 and 120 mg kg−1; n=6 per group) and recording ECG. Single oral doses of 40 mg kg−1 racemic sotalol were chosen for pharmacokinetic-pharmacodynamic studies.
Pharmacokinetic-pharmacodynamic study
For simultaneous ECG recording and blood sample collection, in addition to the electrodes, the right jugular vein was catheterized with silastic tubing (0.025 in. i.d.×0.037 o.d.; Dow Corning, Midland, MI, U.S.A.) under general anaesthesia using methoxyflurane (Jansen Pharmaceuticals, North York, Ontario, Canada). The animals were allowed to recover overnight before the experiment. Rats were allowed access to water but were fasted over the night before the experiment.
To the chronic arthritic rats and their control (n=4 per group), 40 mg kg−1 racemic sotalol was administered and serial blood samples (0.2 ml per sample) were taken at 0, 0.25, 0.50, 0.75, 1.0, 1.5, 2.0, 3.0, 4.0 and 6.0 h via the right jugular vein.
The rats with acute inflammation were divided to three groups of six. Each group was tested along with a control group (n=6 per group). One group received 40 mg kg−1 racemic sotalol. Since sotalol enantiomers are equipotent in potassium channel antagonism but only R-sotalol possesses β-adrenergic blocking activity, single oral doses of 40 mg kg−1 S- or 20 mg kg−1 R-sotalol were administered to rats with acute inflammation and their respective controls. We expected equipotent potassium channel and β-adrenergic antagonism following administration of racemate versus S and R enantiomers, respectively. Blood samples were taken at 0, 0.25, 0.75, 1.0, 1.5, 4.0 and 6.0 h via the right jugular vein.
In another experiment we monitored ECG in four healthy and four acutely-inflamed rats in the absence of jugular catheterization and concluded that the catheterization had no effect on the baseline indices during the baseline and after a 40 mg kg−1 dose of sotalol.
Effect of anti-TNF
Rats (n=6 per group) were divided to four groups: (1) Control; (2) interferon-treated; (3) interferon/anti-TNF-treated; and (4) Control-anti-TNF-treated under the same conditions described under Acute inflammation. Single i.v. doses of 3 mg kg−1 infliximab (Remicade, Schering Canada, Inc.) were administered 8 h after the second dose of interferon treatment. Ten hours after this point, single oral doses of 40 mg kg−1 racemic sotalol was administered. ECG was recorded throughout the experiment. Plasma nitrite and TNF concentrations were measured at the time of sotalol administration.
Sotalol assay
Sotalol was quantified using a validated stereospecific normal-phase HPLC assay (Carr et al., 1991). This method involves liquid extraction from 100 μl plasma and formation of diastereomers of both racemic sotalol and (±)-atenolol (internal standard). The S-(+)-1-(1-napthyl)ethyl isocyanate diastereomers were separated using a silica column and fluorescence detection.
Data elaboration
The area under the plasma concentration-time (AUC) and that of effect-time (AUEC) curves from 0 to 6 h were calculated using the trapezoidal rule. Detail sotalol pharmacokinetics analysis was made only on the data collected from the rats with chronic inflammation since sufficient number of blood samples were collected only from this group. The AUC to infinity is determined from CLast/β where CLast is the last point on the concentration-time curve. The terminal elimination rate constant (β) was calculated using log-linear regression of at least three points in the log-linear terminal phase of the plasma concentration-time curve. Oral clearance (CL/F) was calculated using Dose/AUC0-∞ where F is the oral bioavailability. The volume of distribution (Vd/F) after an oral dose was estimated from Dose/AUC0-∞*β. To determine the correlation between dose of sotalol and ECG interval prolongation for the dose-response study, the effect of escalating doses of sotalol racemate on PR and QT interval prolongation were fitted to a Hill equation using SigmaPlot for Windows version 4.0 (SPSS Inc., Chicago, IL, U.S.A.).
The statistical significance of the observed differences were tested using the two tailed Student t-test or ANOVA followed by the Duncan's New Multiple Range test at α=0.05 for two and more means, respectively. The results are reported as mean±standard deviation.
Results
Inflammatory conditions
In the chronic arthritis group, 10 – 14 days after inoculation with Mycobacterium butyricum, skin nodules on the ears and tail, as well as fore and hind paw swelling were noted. The arthritic index scores ranged from 10 – 12 indicating moderate adjuvant arthritis. The rats with acute inflammation demonstrated significantly greater counts of segmented neutrophils as compared with control rats (Table 1). Baseline plasma nitrite was significantly elevated from 13.3±4.4 μM in controls to 37.3±7.6 μM in the rats with acute inflammation and from 12.8±6.2 to 41.8±13.2 μM in chronic arthritis.
Table 1.
Mean±s.d. of hematological parameters after administration of interferonα2a (acute inflammation)
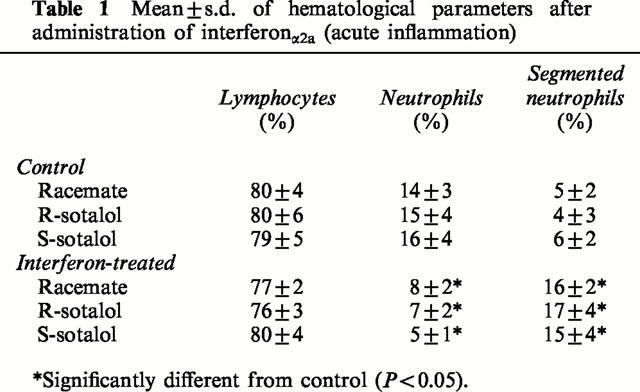
Sotalol dose-response
All doses of sotalol affected ECG significantly (Figure 1). Plots of effect versus sotalol concentration exhibited high degrees of variability. Nevertheless, PR and QT interval-concentration relationships tend to exhibit sigmoidal shape. On the other hand, heart rate-concentration curves were better described as a counter-clockwise hysteresis. Attempts to collapse the latter failed due, perhaps, to excessive variability. No further interpretation of pharmacokinetic-pharmacodynamic data was made due to the observed variability. From plots of racemic sotalol doses versus prolongation of PR and QT intervals in healthy rats, the 40 mg kg−1 dose was chosen for pharmacokinetic-pharmacodynamic studies since it produced effects in the ascending phase of the dose-response curves (Figure 1). The effect of a single 40 mg kg−1 dose on both PR and QT intervals was significantly greater than that of a 20 mg kg−1 and smaller than that of an 80 mg kg−1.
Figure 1.
Maximum effect observed following administration of various doses of racemic sotalol to healthy rats. Error bars represent standard deviation of the mean (n=6 per group), lines through the data points represent the best fit using hyperbola function.
Effect of inflammation on ECG
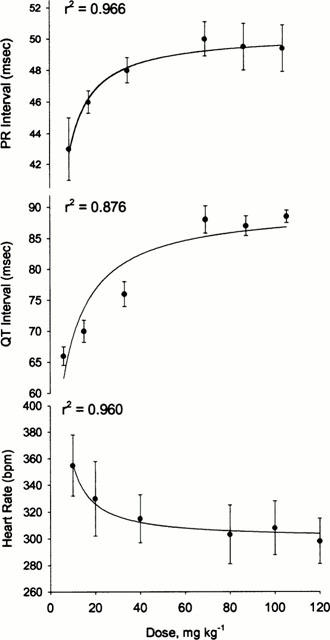
To determine the effect of inflammation on ECG response to sotalol, both individual data points (Figures 2 and 3) and AUEC0-6h (Table 2) were assessed. No significant differences in baseline PR and QT intervals were found between the control and inflamed rats (Figures 2 and 3). These baseline values were close to those previously reported (Detweiler et al., 1981). Similarly, the baseline heart rate was unaffected by acute inflammation but was significantly elevated in response to chronic arthritis (Figure 2).
Figure 2.
Time courses of sotalol effect following administration of single 40 mg kg−1 oral doses of racemic sotalol to healthy and chronically arthritic rats. Error bars represent standard deviation of the mean (n=4 per group); *Denotes significantly different (P<0.05).
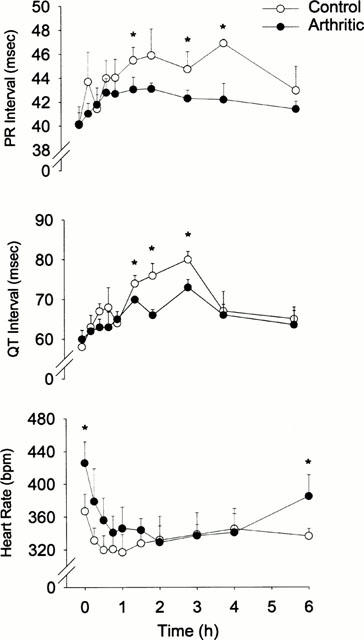
Figure 3.
Time courses of sotalol effect following administration of single oral doses of 40 mg kg−1 sotalol racemate, S-sotalol (40 mg kg−1) and R-sotalol (20 mg kg−1) to control and interferonα 2a (IFN)α2a treated rats. Error bars represent standard deviation of the mean (n=6 per group); *Denotes significantly different (P<0.05).
Table 2.
Area under the effect-time curves from 0 to 6 h following oral administration of sotalol to control and rats with chronic (adjuvant arthritic) and acute (interferon-treated) inflammation
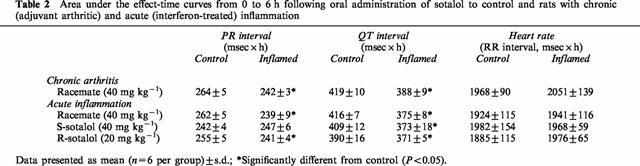
Figures 2 and 3, and Table 2 depict the observed pharmacodynamic data following a single 40 mg kg−1 dose. All ECG indices were affected by 40 mg kg−1 doses of sotalol in all rats. As expected, while both racemic and R-sotalol prolonged PR and QT intervals, S-sotalol lengthened only QT interval. However, the effect of the treatments on PR and QT intervals was significantly reduced by both types of inflammation. On the other hand, inflammation did not significantly influence the effect of sotalol on heart rate.
Effect of anti-TNF antibody
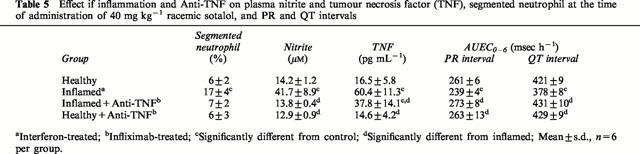
Administration of anti-TNF antibody to rats with acute inflammation resulted in normalization of nitrite concentration and all pharmacodynamic responses to sotalol as well as reduced plasma TNF concentration (Table 5).
Table 5.
Effect if inflammation and Anti-TNF on plasma nitrite and tumour necrosis factor (TNF), segmented neutrophil at the time of administration of 40 mg kg−1 racemic sotalol, and PR and QT intervals
Effect of inflammation on pharmacokinetics of sotalol
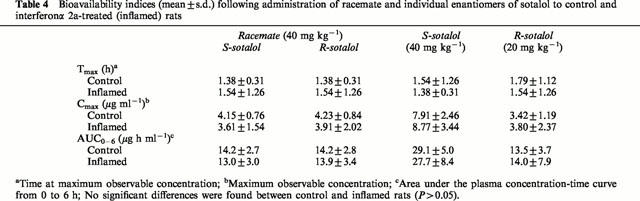
As anticipated chronic and acute inflammation did not influence pharmacokinetics of sotalol enantiomers after administration of racemate and stereoisomers (Figure 4, Tables 3 and 4). No stereoselectivity in pharmacokinetics of sotalol was observed
Figure 4.
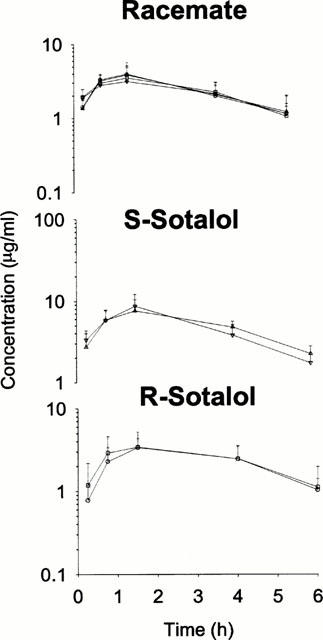
Plasma sotalol enantiomer concentration-time plots in control and interferonα 2a treated rats following racemate (40 mg kg−1), S-sotalol (40 mg kg−1) and R-sotalol (20 mg kg−1) oral doses. Control R (○) and S (□sim;), interferon treated R (□amp;) and S (▿), error bars represent standard deviation of the mean no significant differences between control and inflamed rats are observed (P>0.05).
Table 3.
Mean±s.d. of pharmacokinetic indices following administration of 40 mg kg−1 racemic sotalol to healthy and chronic arthritic rats
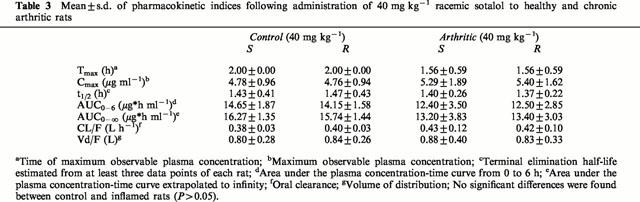
Table 4.
Bioavailability indices (mean±s.d.) following administration of racemate and individual enantiomers of sotalol to control and interferonα 2a-treated (inflamed) rats
Discussion
The present data demonstrate, for the first time, a reduced response to sotalol in prolonging PR and QT intervals in rats with both acute and chronic inflammation. This may be due to an altered pharmacokinetics or a reduced β-adrenergic and potassium channel receptors responsiveness. The former explanation can be ruled out since the pharmacokinetics of sotalol remained unaffected by inflammation (Figure 4, Tables 3 and 4). The similar disposition of sotalol enantiomers in the healthy and inflamed rats is due, perhaps, to negligible binding to plasma proteins and its complete dependency on the renal route of elimination (Anderson & Prystowsky, 1999). Hence, the two major inflammation-induced changes, i.e., increased serum α1-acid glycoproteins and decreased intrinsic hepatic clearance (Piafsky et al., 1978; Belpaire et al., 1989) do not play any significant role in clearance of sotalol.
A series of published in vitro observations point to the possibility of a reduced β-adrenergic responsiveness in the presence of pro-inflammatory mediators such as interleukin (IL)-1β (Liu et al., 1999; Gulick et al., 1989); TNF (Gulick et al., 1989) and nitric oxide (Rozanski and Witt, 1994; Muller-Werdan et al., 1998). Therefore, the observed reduced potency of sotalol to prolong PR interval in inflamed rats is due, perhaps, to an altered cardiovascular receptor function causing an alteration in β-adrenergic receptor configuration, density and/or chemical messenger activity. This alteration is likely secondary to an over-expression of nitric oxide and/or pro-inflammatory cytokines. Indeed, we observed approximately 3 fold greater plasma concentration of nitrite in both acute and chronic inflammation. In addition, in a separate experiment, we found significant elevations of plasma TNF and nitrite concentrations in acutely inflamed rats at the time of sotalol administration (Table 5). Both these inflammatory mediators were significantly reduced in response to a single dose of infliximab. The anti-TNF antibody resulted in the reversal of the diminishing effect of inflammation (Table 5). Infliximab is a novel drug for the treatment of inflammatory diseases. It is a chimeric monoclonal antibody to TNFα which binds to both soluble and transmembrane forms of TNFα. Through inhibition of TNFα, infliximab reduces concentrations of TNF and other inflammatory mediators and decreases lymphocyte migration into the joints of patients with rheumatoid arthritis (Markham & Lamb, 2000). Our observation confirms, for the first time, a significant in vivo link between pro-inflammatory mediators and reduced responsiveness of β-adrenergic and potassium channel receptors.
β-adrenergic antagonists have been found to be less efficacious (Brodde et al., 1995; Messerli et al., 1998; Tenero et al., 1990) or less beneficial (MRC Working Party, 1992) in treating hypertension in the elderly as compared with younger individuals. Decreased sensitivity of calcium channels to verapamil in rheumatoid arthritis (Mayo et al., 2000), in older individuals (Abernethy et al., 1993) and in obese hypertensive patients (Abernethy & Schwartz, 1988) have also been reported. Increased expression of pro-inflammatory cytokines in these subjects (Bruunsgaard et al., 1999; Liao et al., 1993; Visser et al., 1999; Mayo et al., 2000) may explain these reduced sensitivities.
Our data also suggest reduced response to potassium channel receptors antagonists in addition to that of β-adrenergic antagonists in both chronic and acute inflammations. Racemic sotalol antagonizes both these receptors (Anderson & Prystowsky, 1999). Hence, both PR and QT intervals are prolonged and inflammation seems to reduce the effect of sotalol on both of these ECG intervals. The effect of S-sotalol, the specific potassium channel blocker enantiomer (Anderson & Prystowsky, 1999), on QT intervals was also reduced by inflammation. It should be mentioned that both racemic and R-sotalol also affected QT interval but, as expected, this was accompanied by a similar effect on PR interval due to their lack of specificity. Previously reported in vitro data support our finding (Nishio et al., 1999; Kaprielian et al., 1999; Neumann et al., 1995). Similarly, over-expression of pro-inflammatory cytokines and potassium channel down-regulation is observed in patients with congestive heart failure (Kapadia et al., 1998; Nabauer & Kaab, 1998). Hence, the decreased QT interval prolongation in sotalol-treated rats with acute and chronic arthritis is also due, perhaps, to increased expression of pro-inflammatory cytokines.
The decreased β-adrenergic receptor and cardiac potassium channel function was observed in both acute and chronic inflammations despite the inherent differences between the two types of inflammations (Kuby, 1997). However, our data suggest an increased baseline heart rate in adjuvant but not in the interferon-treated rats. We cannot offer an explanation for this observation except the chronic nature of adjuvant arthritis which is accompanied by various pathophysiological changes. Acute inflammation is characterized by a sudden onset as well as a short duration and is accompanied by production of acute phase proteins by the liver. On the other hand, chronic inflammatory diseases are associated with a persistent antigen that resists phagocytosis and produces greater inflammatory responses and tissue damage.
The reduced response to sotalol in rats inoculated with interferonα 2a may also be considered a drug interaction since interferon and other cytokines are used to treat various diseases. The clinical significance of our observation remains to be explored in patients with conditions such as chronic active hepatitis B and renal cell carcinoma who are recipients of interferonα 2a therapy. Interestingly, in these disorders, development of arthritis as a complication of interferon treatment has been reported (Nesher & Ruchlemer, 1998).
The altered response to sotalol in the rat with both acute and chronic inflammation emphasizes the importance of disease-drug interactions. With most drugs there are discrete sub-populations (e.g., disease, age, concurrent therapy) for whom concentration-effect relationships differ from what is commonly seen and understood (Levy, 1998). Interestingly, inflammatory status has been shown to determine the clinical course of post-myocardial infarction patients who have unstable angina and receive standard drug therapy (Verheggen et al., 1999). In addition, mortality in post-myocardial infarction was greater in individuals with higher concentrations of inflammatory marker, C-reactive protein, independent of drug treatment (Pietila et al., 1996). It is also probable that the higher rate of cardiovascular complications observed in patients afflicted with inflammatory diseases to be due to the presence of high concentrations of pro-inflammatory mediators and/or the diminishing effect of drugs used in the treatment of cardiovascular complications.
Our observation in the rat and that recently reported in humans (Mayo et al., 2000) may shed light into the variability in response to agents used in the treatment of cardiovascular diseases in patients with elevated pro-inflammatory cytokine concentrations due to a variety of pathophysiological changes (Kulmatycki & Jamali, 2001).
Acknowledgments
This work was supported by the Canadian Institute of Health Research grant 98-3587. Presented in part at the American Association of Pharmaceutical Scientists annual meeting, November 16, 1999, New Orleans, LA, U.S.A.
Abbreviations
- AUC
area under the plasma concentration-time curve
- AUEC
area under the effect-time curve
- CL/F
oral clearance
- ECG
electrocardiograph
- HPLC
high performance liquid chromatography
- IL
interleukin
- TNF
tumour necrosis factor
- NO
nitric oxide
- Vd/F
volume of distribution after oral doses
References
- ABERNETHY D.R., WAINER I.W., LONGSTRETH J.A., ANDRAWIS N.S. Stereoselective verapamil disposition and dynamics in aging during racemic verapamil administration. J. Pharmacol. Exp. Ther. 1993;266:904–911. [PubMed] [Google Scholar]
- ABERNETHY D.R., SCHWARTZ J.B. Verapamil pharmacodynamics and disposition in obese hypertensive patients. J. Cardiovasc. Pharmacol. 1988;11:209–215. [PubMed] [Google Scholar]
- ANDERSON J.L., PRYSTOWSKY E.N. Sotalol: an important new antiarrhythmic. Am. Heart J. 1999;137:388–409. doi: 10.1016/s0002-8703(99)70484-9. [DOI] [PubMed] [Google Scholar]
- ARCHER S., SHULTZ P., WARREN J., HAMPL V., DEMASTER E. Preparation of standards and measurement of nitric oxide, nitroxyl and related oxidation products. Methods Enzymolog. 1995;7:21–24. [Google Scholar]
- BELPAIRE F.M., DE SMET F., CHINDAVIJAK B., FRAEYMAN N., BOGAERT M.G. Effect of turpentine-induced inflammation on the disposition kinetics of propranolol, metoprolol, and antipyrine in the rat. Fundam. Clin. Pharmacol. 1989;3:79–88. doi: 10.1111/j.1472-8206.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- BELPAIRE F.M., BOGAERT M.G., ROSSENEU M. Binding of β-adrenoceptor blocking drugs to human serum albumin, to α1-acid glycoprotein and to human serum. Eur. J. Clin. Pharmacol. 1982;22:253–256. doi: 10.1007/BF00545224. [DOI] [PubMed] [Google Scholar]
- BRODDE O.E., ZERKOWSKI H.R., SCHRANZ D., BROEDE-SITZ A., MICHEL-REHER M., SCHAFER-BEISENBUSCH E., PIOTROWSKI J.A., OELTERT H. Age-dependent changes in the β-adrenergic-G-protein(s)-adenylyl cyclase system in human right atrium. J. Cardiovasc. Pharmacol. 1995;26:20–26. doi: 10.1097/00005344-199507000-00004. [DOI] [PubMed] [Google Scholar]
- BRUUNSGAARD H., ANDERSON-RANBERG K., JUENE B., PEDERSEN A.N., SKINHOJ P., PEDERSEN B.K. A high plasma concentration of TNF-α is associated with dementia in centenarians. J. Gerentol. A Biol. Sci. Med. Sci. 1999;54A:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- CARR R.A., FOSTER R.T., BHANJI N.H. Stereospecific high-performance liquid chromatographic assay of sotalol in plasma. Pharm. Res. 1991;8:1195–1198. doi: 10.1023/a:1015870805757. [DOI] [PubMed] [Google Scholar]
- DAVIES D.H., HALABLAB M.A., CLARKE J., COX F.E.G., YOUNG T.W.K. Infection and Immunity. Pennsylvania, U.S.A.: Taylor and Francis; 1999. The immune system; pp. 1–31. [Google Scholar]
- DETWEILER D.K.The use of electrocardiograph in toxicology studies with rats The Rat Electrocardiogram in Pharmacology and Toxicology 1981New York: Pergamon Press; 83–115.eds. Budden R., Detweiler, D.K. & Zbinden, G. pp [Google Scholar]
- GRISHAM M.B., JOHNSON G.G., LANCASTER J.R. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- GULICK T., CHUNG M.K., PIEPER S.J., LANGE L.G., SCHREINER G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte β-adrenergic responsiveness. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPADIA S., DIBBS Z., KURRELMEYER K., KALRA D., SETA Y., WANG F., BOZKURT B., ORAL H., SIVASUBRAMANIAN N., MANN D.L. The role of cytokines in the failing human heart. Cardiol. Clin. 1998;16:645–656. doi: 10.1016/s0733-8651(05)70041-2. [DOI] [PubMed] [Google Scholar]
- KAPRIELIAN R., WICKENDEN A.D., KASSIRI Z., PARKER T.G., LIU P.P., BACKX P.H. Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. J. Physiol. 1999;517:229–245. doi: 10.1111/j.1469-7793.1999.0229z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBY J. Immunology. New York: WH Freeman and Company; 1997. Leukocyte migration and inflammation; pp. 357–378. [Google Scholar]
- KULMATYCKI K., JAMALI F.Therapeutic relevance of altered cytokine expression Cytokyne 2001. in press [DOI] [PubMed]
- LEVY G. Predicting effective drug concentrations for individual patients determinants of pharmacodynamic variability. Clin. Pharmacokinet. 1998;34:323–333. doi: 10.2165/00003088-199834040-00005. [DOI] [PubMed] [Google Scholar]
- LIAO Z., TU J.H., SMALL C.B., SCHNIPPER S.M., ROSENSTREICH D.L. Increased urine interleukin-1 levels in aging. Gerontology. 1993;39:19–27. doi: 10.1159/000213510. [DOI] [PubMed] [Google Scholar]
- LITTLE R., WHITE M.R., HARTSHORN K.L. Interferon-α enhances neutrophil respiratory burst responses to stimulation with influenza A virus and FMLP. JID. 1994;170:802–810. doi: 10.1093/infdis/170.4.802. [DOI] [PubMed] [Google Scholar]
- LIU S.J., ZHOU W., KENNEDY R.H. Suppression of β-adrenergic responsiveness of L-type Ca2+ current by IL-1β in rat ventricular myocytes. Am. J. Physiol. 1999;276:H141–H148. doi: 10.1152/ajpheart.1999.276.1.H141. [DOI] [PubMed] [Google Scholar]
- MANZI S., MEILAHN E.N., RAIRIE J.E., CONTE C.G., MEDSGER T.A., JR, JANSEN-MCWILLIAMS L., D'AGOSTINO R.B., KULLER L.H. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- MARKHAM A., LAMB H.M. Infliximab: a review of its use in the management of rheumatoid arthritis. Drugs. 2000;59:1341–1359. doi: 10.2165/00003495-200059060-00010. [DOI] [PubMed] [Google Scholar]
- MAYO P.R., JAMALI F. Methoxyflurane anesthesia augments the chronotropic and dromotropic effects of verapamil. J. Pharm. Pharmaceut. Sci. 1999;2:30–35. [PubMed] [Google Scholar]
- MAYO P.R., SKEITH K., RUSSELL A.S., JAMALI F. Decreased dromotropic response to verapamil despite pronounced increased drug concentration in rheumatoid arthritis. Br. J. Clin. Pharmacol. 2000;50:605–613. doi: 10.1046/j.1365-2125.2000.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESSERLI F.H., GROSSMAN E., GOLDBOURT U. Are β-blockers efficacious as first-line therapy for hypertension in the elderly. JAMA. 1998;279:1903–1907. doi: 10.1001/jama.279.23.1903. [DOI] [PubMed] [Google Scholar]
- MRC WORKING PARTY Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ. 1992;304:405–412. doi: 10.1136/bmj.304.6824.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER-WERDAN U., SCHUMANN H., LOPPNOW H., FUCHS R., DARMER D., STADLER J., HOLTZ J., WERDAN K. Endotoxin and tumor necrosis factor α exert a similar proinflammatory effect on neonatal rat cardiomyocytes, but have different cardiodepressant profiles. J. Mol. Cell. Cardiol. 1998;30:1027–1036. doi: 10.1006/jmcc.1998.0667. [DOI] [PubMed] [Google Scholar]
- NABAUER M., KAAB S. Potassium channel down-regulation in heart failure. Cardiovasc. Res. 1998;37:324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- NESHER G., RUCHLEMER R. α-interferon-induced arthritis: clinical presentation, treatment, and prevention. Semin. Arthritis Rheum. 1998;27:360–365. doi: 10.1016/s0049-0172(98)80015-2. [DOI] [PubMed] [Google Scholar]
- NEUMANN F.-J., OTT I., GAWAZ M., RICHARDT G., HOLZAPFEL H., JOCHUM M., SCHOMIG A. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction. Circulation. 1995;92:748–775. doi: 10.1161/01.cir.92.4.748. [DOI] [PubMed] [Google Scholar]
- NISHIO M., HABUCHI Y., TANAKA H., MORIKAWA J., OKANOUE T., KASHIMA K. Tyrosine kinase-dependent modulation by interferon-alpha of the ATP-sensitive K+ current in rabbit ventricular myocytes. FEBS Lett. 1999;445:87–91. doi: 10.1016/s0014-5793(99)00083-6. [DOI] [PubMed] [Google Scholar]
- OPIE L.H. The Heart Physiology, from Cell to Circulation. Pennsylvania, USA: Lippincott-Raven Publishers; 1998. Channels, pumps, and exchangers; pp. 71–114. [Google Scholar]
- PIAFSKY K.M., BORGA O., ODAR-CEDERLOF I., JOHANSSON C., SJOQVIST F. Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma α1-acid glycoprotein. N. Engl. J. Med. 1978;299:1435–1439. doi: 10.1056/NEJM197812282992604. [DOI] [PubMed] [Google Scholar]
- PIETILA K.O., HARMOINEN A.P., JOKINITTY J., PASTERNACK A.I. Serum C-reactive protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur. Heart J. 1996;17:1345–1349. doi: 10.1093/oxfordjournals.eurheartj.a015068. [DOI] [PubMed] [Google Scholar]
- PIQUETTE-MILLER M., JAMALI F. Influence of severity of inflammation on the disposition kinetics of propranolol enantiomers in ketoprofen-treated and untreated adjuvant rats. Drug Metab. Dispos. 1995;23:240–245. [PubMed] [Google Scholar]
- RAAIJMAKERS J.A., BENEKER C., VAN GEFFEN E.C., MEISTERS T.M., POVER P. Inflammatory mediators and beta-adrenoceptor function. Agents Actions. 1989;26:45–47. doi: 10.1007/BF02126558. [DOI] [PubMed] [Google Scholar]
- ROZANSKI G.J., WITT R.C. IL-1 inhibits β-adrenergic control of cardiac calcium current: role of L-arginine/nitric oxide pathway. Am. J. Physiol. 1994;267:H1753–H1758. doi: 10.1152/ajpheart.1994.267.5.H1753. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER R.E., BISHOP H., KENDALL M.J., QUATERMAN C.P. Effect of inflammatory disease on plasma concentrations of three β-adrenoceptor blocking agents. Int. J. Clin. Pharmacol. Ther. Toxicol. 1981;19:158–162. [PubMed] [Google Scholar]
- TENERO D.M., BOTTORFF M.B., BURLEW B.S., WILLIAMS J.B., LALONDE R.L. Altered β-adrenergic sensitivity and protein binding to 1-propranolol in the elderly. J. Cardiovasc. Pharmacol. 1990;16:702–707. doi: 10.1097/00005344-199011000-00003. [DOI] [PubMed] [Google Scholar]
- VERHEGGEN P.W.H.M., DE MAAT M.P.M., MANGER CATS V., HAVERKATE F., ZWINDERMAN A.H., KLUFT C., BRUSCHKE A.V.G. Inflammatory status as a main determinant of outcome in patients with unstable angina, independent of coagulation activation and endothelial cell function. Eur. Heart J. 1999;20:567–574. doi: 10.1053/euhj.1998.1312. [DOI] [PubMed] [Google Scholar]
- VISSER M., BOUTER L.M., MCQUILLAN G.M., WENER M.H., HARRIS T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M.W.Adjuvant-induced polyarthritis in rats CRC Handbook of Animal Models for the Rheumatic Diseases Volume 1 1988Florida: CRC Press; 3–16.eds. Greenwald, R.A. & Diamond, H.S. pp [Google Scholar]


