Abstract
Recently discovered chemically modified tetracyclines (CMTs) have shown in vitro and in vivo anti-proliferative and anti-tumour activities. Here, we evaluated in vitro the anti-proliferative and apoptotic activity of six different dedimethylamino chemically modified tetracyclines (CMT-1, CMT-3, CMT-5, CMT-6, CMT-7 and CMT-8) in sensitive and multidrug resistant myeloid leukaemia cells (HL60 and HL60R) in vitro.
Three of these compounds (CMT-5, CMT-6, CMT-7) showed low cytotoxic activity both in sensitive and in resistant cells, CMT-3 was endowed with a high anti-proliferative activity only in sensitive cells and was moderately effective as apoptosis inducing agent, with an activity similar to that shown by doxycycline. On the contrary, CMT-1 and CMT-8 were very effective as programmed cell death inducing agents.
The apoptotic pathway activated by these compounds involved the activation of caspases, especially caspase-9 and, for CMT-1, also the activation of Fas.
Interestingly CMT-8, but not CMT-1, was able to induce apoptosis in multidrug resistant HL60R and in Fas-ligand resistant HUT78B1 cell lines. These properties, together with others previously described (e.g. anti-metastatic and anti-osteolytic activities), suggest that CMT-8 may have important applications in the clinical management of cancer.
The comparative analysis of structure-activity relationship of CMT-8 and doxycycline suggests that the C-5 hydroxy moiety may play an important role in conferring activity in multidrug resistant cells. These findings appear to support the hypothesis that CMT-8 may represent an interesting lead for the development of a new class of potent apoptosis inducer agents active in multidrug resistant and Fas-ligand resistant malignancies.
Keywords: Tetracyclines, multidrug resistance, apoptosis
Introduction
Several studies demonstrated that a variety of widely used anticancer drugs kill cancer cells through induction of apoptosis (Walker et al., 1991; Vial et al., 1997). The molecular events leading to apoptosis involve a cascade of proteolytic cleavage events. The most important proteins involved are the cysteine-dependent aspartate-directed proteases (caspases) which are initially expressed as inactive zymogens and are activated by proteolytic processing (Salvesen & Dixit, 1997). Many mechanisms for caspase activation probably exist, but the best documented is that involving several members of the tumour necrosis factor (TNF) family of cytokine receptors including TNF-R1 and Fas (CD95/APO-1) (Nagata & Golstein, 1995). Although, it has been observed that antineoplastic agents can induce apoptosis through a Fas/Fas-ligand-dependent pathway (Friesen et al., 1996; Fulda et al., 1997) currently there are many controversial and diverging opinions about its implication in drug-induced apoptosis and many studies, including our previous observations, indicate that antineoplastic treatments trigger apoptosis through a Fas-independent pathway (Villunger et al., 1997; Eischen et al., 1997; Tolomeo et al., 1998). It is possible that these divergent results could depend on the different experimental models used. However, at the present, it is still not clear how chemotherapeutic drugs induce a common pathway of apoptosis in drug sensitive target cells.
Recently, it has been observed that tetracyclines, a family of well known antibiotics, also possesses non-antibiotic properties and cytotoxic activity against several tumours. Some of them are able to induce programmed cell death in target cells, but the mechanism by which they act as apoptotic agents is still not clear (Fife et al., 1997; 1998). The first non-antibiotic property discovered has been the ability of tetracyclines to inhibit various zinc-dependent enzymes of the matrix metalloproteinase (MMP) family (Hanemaaijer et al., 1998; Ramamurthy et al., 1999; Maisi et al., 1999; Brown, 1995). MMPs play an important role in the degradation of the extracellular matrix (ECM), the invasion of tumour cells, and the constitution of metastasis (Cockett et al., 1994; Himrlstein et al., 1994; Kohn & Liotta, 1995; Makela et al., 1998). Moreover, tetracyclines also showed anti-inflammatory properties, related to their ability to inhibit the PKC activity, MPPs, and mediators (oxygen radicals, arachidonic acid, and prostaglandin E2) (Patel et al., 1999; Vadas et al., 1991; Elattar et al., 1988; Milano et al., 1997; Eklund & Sorsa, 1999; D'agostino et al., 1998a; 1998b).
Modification of molecular functional groups within the upper peripheral region of tetracyclines seem to alter their bioactivity drastically. For example, the antibiotic properties are related to the presence of a dimethylamino group in C4. Conversely, the removal of the C4 functional group through synthetic modification of the parent tetracycline increases the activity of these compounds against non-antibiotic targets but abolishes antibiotic potency (Nelson, 1998). Recently, it has been observed that doxycycline, a semi-synthetic tetracycline characterized by the presence of a dimethylamino group in C4 and a hydroxyl group in C5, was able to inhibit MMP activity and cell proliferation in different cancer cell lines (Fife et al., 1997; Fife & Sledge, 1995; 1998). The antiproliferative effect of doxycycline seems to have a direct effect on the regulation of cell proliferation, which may be distinct from its ability to inhibit MMP activity (Fife & Sledge, 1995; 1998).
In this work we have studied the cytotoxic activity of six chemically modified non-antimicrobial tetracyclines (CMTs), characterized by the absence of the dimethylamino group in C4, against sensitive and multidrug resistant (MDR) leukaemia cell lines. Three of these compounds (CMT-1, CMT-3, and CMT-8) were more active than doxycycline and one of them (CMT-8) was able to induce programmed cell death (PCD), or apoptosis, also in multidrug resistant and apoptosis resistant cell lines. Interestingly, the most active compounds tested here, CMT-1 and CMT-8, induced apoptosis through a different pathway, Fas dependent for CMT-1 and Fas independent but caspases dependent for CMT-8, indicating that a little modification of the chemical structure of the same compound can result in molecules that induce apoptosis through Fas activation or independently by Fas.
Methods
Cell culture
Cells were grown in RPMI-1640 (Gibco, Grand Island, NY, U.S.A.) containing 10% FCS (Gibco), 100 U ml−1 penicillin (Gibco), 100 μg ml−1 streptomycin (Gibco), and 2 mM L-glutamine (Sigma Chemical Co, St Louis, MO, U.S.A.) in a 5% CO2 atmosphere at 37°C. The concentrations of penicillin and streptomycin in the culture medium did not modify the cell growth of cell lines used in this work.
Chemicals
CMTs were obtained from CollaGenex Pharmaceuticals (Newtown, PA, U.S.A.). The purity of these compounds was more than 96%. The caspase inhibitors Ac-DEVD-CHO (acetyl-Asp-Glu-Val-Asp-aldehyde), Ac-ZLEHD-fmk (Z-Leu-Glu(Ome)-His-Asp(Ome)-fmk), Ac-YVAD-CHO (Ac-Tyr-Val-Ala-Asp-CHO) and Ac-ZVAD-fmk (acetyl-Tyr-Val-Ala-Asp-chloromethyl ketone) were purchased from Alexis Biochemicals (Laufelfingen, Switzerland). The Fas agonistic antibody CH11 and the anti-Fas Ab ZB4 were purchased from Upstate Biotechnology (Lake Placid, NY, U.S.A.) All other reagents were analytical grade.
Drug preparation
Each CMT (CollaGenex, Newtown, PA, U.S.A.) derivative was dissolved in dimethylsulfosside (DMSO, Sigma). They were diluted in appropriate experimental concentrations in tissue medium and protected from light. In each experiment DMSO never exceeded 0.5% and this percentage did not interfere with cell growth and did not induce differentiation in HL60 cells at 48 and 72 h (Simoni et al., 1999).
Cytotoxicity assays
To determine the cytotoxic activity of the drugs tested, 2×105 cells were plated into 25 mm wells (Costar, Cambridge, U.K.) in 1 ml of complete medium and treated with different concentrations of each drug. After 48 h of incubation, the number of viable and dead cells was determined by light microscopy after staining with Trypan blue and expressed as a per cent of control proliferation.
Flow cytometry analysis of cell cycle and apoptosis
Cells were washed once in ice-cold PBS and resuspended at 1×106 ml−1 in a hypotonic fluorochrome solution containing propidium iodide (Sigma) 50 μg ml−1 in 0.1% sodium citrate plus 0.03% (v v−1) nonidet P-40 (Sigma). After 30 min of incubation, the samples were filtered through nylon cloth, and their fluorescence was analysed as single-parameter frequency histograms using a FACScan flow cytometer. Apoptosis was determined by evaluating the percentage of hypoploid nuclei accumulated in the sub-G0Gi peak after labelling with propidium iodide (Darziynkiewick et al., 1992).
Morphological evaluation of apoptosis and necrosis
Drug induced apoptosis and necrosis were determined morphologically after labelling with acridine orange and ethidium bromide (Duke & Cohen, 1992). Cells (2×105) were centrifuged (300×g) and the pellet was resuspended in 25 μl of the dye mixture. Ten μl of the mixture was examined in oil immersion with a 100×objective using a fluorescence microscope. At least 300 cells were counted. Live cells were determined by the uptake of acridine orange (green fluorescence) and exclusion of ethidium bromide (red fluorescence) stain. Live and dead apoptotic cells were identified by perinuclear condensation of chromatin stained by acridine orange or ethidium bromide, respectively, and by the formation of apoptotic bodies. Necrotic cells were identified by uniform labelling of the cells with ethidium bromide.
Photometric enzyme-immunoassay for the quantitative determination of apoptosis
Internucleosomal degradation of genomic DNA occurring during apoptosis was evaluated by ELISAPLUS kit (Boehringer Mannheim, Germany) which is based on a quantitative sandwich enzyme immunoassay principle using mouse monoclonal antibodies directed against histone-associated-DNA fragments. Cells (1×104), after the incubation, were treated with lysis buffer. Twenty μl of lysate (cytoplasmic fraction) were transferred into the streptavidin coated microtiter plate. Subsequently, a mixture of anti-histone-biotin anti-DNA-peroxidase conjugated were added. After the removal of unbound antibodies by a washing step, the amount of nucleosomes was quantified by the peroxidase retained in the immunocomplex. Samples were red in a spectrophotometer at 405 nm. Fold increase in the levels of mono- and oligonucleosomes in the cytoplasmic fraction was determined by comparing these results with the levels of the uninduced controls.
Results
Effects of CMTs on cell growth and on apoptosis of sensitive and multidrug resistant cell lines
Figure 1 shows the structure of six different CMTs characterized by the absence of the dimethylamino group in C4. Only three of these compounds (CMT-1, CMT-3 and CMT-8) shows a cell growth inhibitory activity in the myeloblastic leukaemia HL60 cell line similar or greater than doxycycline (Table 1). Interestingly, the compounds CMT-8 was also active in MDR-P-glycoprotein (Pgp) expressing HL60R cells, a cell line derived from sensitive parental HL60 cells by gradually increasing concentrations of daunorubicin (Table 1).
Figure 1.
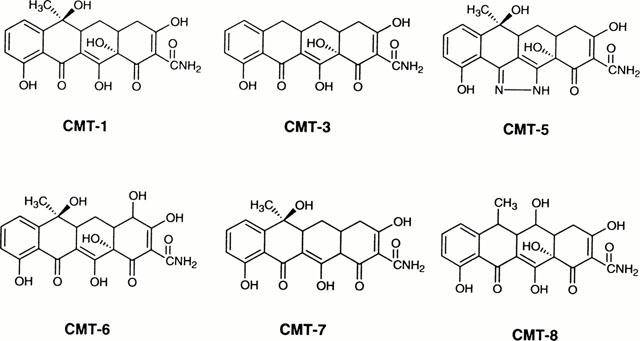
Structure of chemically modified tetracyclines (CMT) studied in this work.
Table 1.
IC50 and AC50 (concentration able to induce 50% apoptosis) expressed as μg ml−1 of doxycycline and CMTs in sensitive HL60 and MDR HL60R cell lines
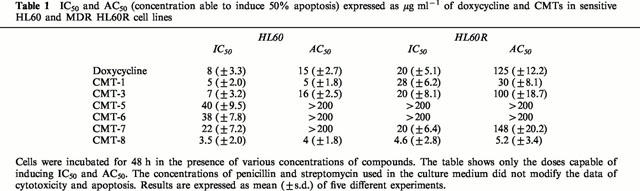
Morphological analysis demonstrated that CMT-1 and CMT-8 were able to induce programmed cell death or apoptosis when used at concentrations of 5 μg ml−1 in the myeloblastic leukaemia cell line HL60 (Figure 2a,c). On the contrary, these compounds did not induce necrosis also when used at concentrations of 20 μg ml−1 (Figure 2b,d). The IC50 of these two compounds corresponds approximately to the concentration able to induce 50% apoptosis (AC50), while, for doxycycline and the others CMTs, apoptosis was induced at concentrations higher than IC50 (Table 1). As shown in Figure 2a, the apoptosis-induced activity of CMT-1 in MDR-Pgp expressing HL60R cells was lower than that shown in parental HL60 cells. On the contrary, the apoptosis-inducing activity of CMT-8 was similar in both HL60 and HL60R cell lines (Figure 2b). All data were confirmed using DNA fragmentation ELISA assay (Figure 3a).
Figure 2.
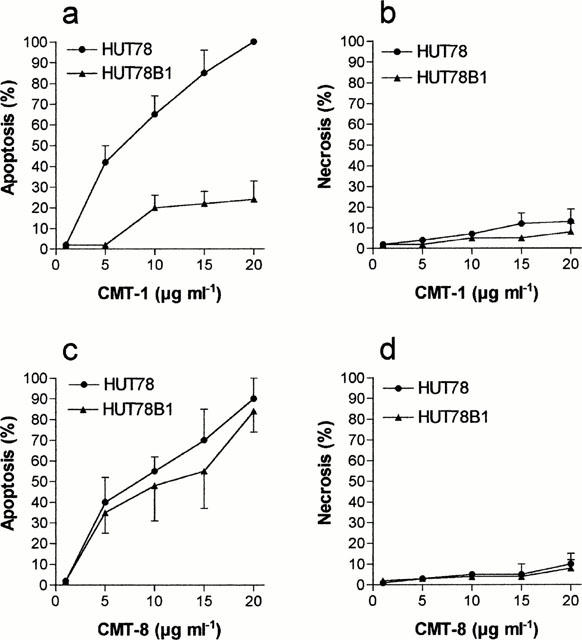
Percentage of apoptotic (a and c) and necrotic cells (b and d) induced by different concentrations of CMT-1 and CMT-8 in HL60 and HL60R cell lines. Evaluation by morphologic assay after 48 h of treatment. Results are means±s.d. of five experiments.
Figure 3.
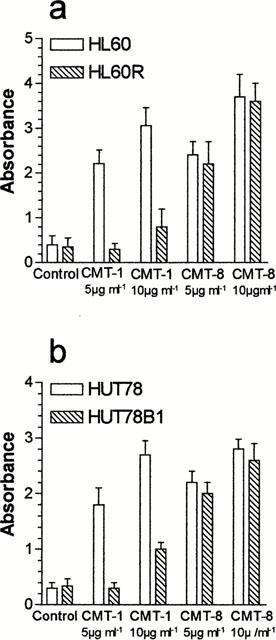
Quantitative evaluation of apoptosis in HL60 (a), HL60R (a), HUT78 (b), and HUT78B1 (b) cells by photometric enzyme-immunoassay after 24 h treatment with CMT-1 or CMT-8.
The analysis of cell cycle by flow cytometry showed an evident apoptotic sub-G0-G1 peak in HL60 treated with CMT-1 (Figure 4b) or CMT-8 (Figure 4c), without important modifications in the cell cycle distribution. These data indicate that the antitumour activity of these compounds is related principally to their ability to induce programmed cell death in target cells. Moreover flow cytometry assay showed a sub-G0-G1 apoptotic peak in HL60R cells treated with CMT-8 (Figure 4f), whereas any important modifications in cell cycle distribution were observed in HL60R cells treated with CMT-1 (Figure 4e).
Figure 4.
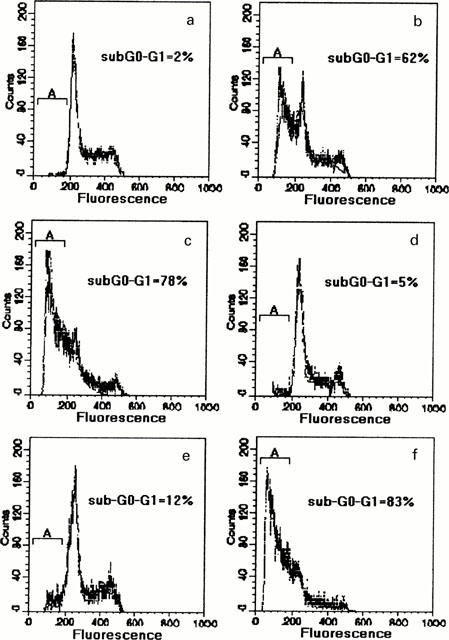
Flow cytometric assay of cell cycle distribution of HL60 and HL60R cells after 48 h exposure to 10 μg ml−1 of CMT-1 or CMT-8. (a) HL60 (control); (b) CMT-1 in HL60; (c) CMT-8 in HL60; (d) HL60R (control); (e) CMT1 in HL60R; (f) CMT-8 in HL60R. The concentrations of penicillin and streptomycin used in the culture medium did not modify the cell cycle of control and treated cells.
Interestingly, the CMT-1 apoptosis-resistance observed in HL60R cells was only partially reversed by common MDR reversing agents such as verapamil or cyclosporin A (data not shown). This data suggest that mechanisms, other than Pgp may be implicated in the low response of HL60R cells to CMT-1-inducing apoptosis.
Effect of CMTs on Fas and caspase dependent apoptosis
We previously observed that, in contrast to HL60, HL60R cells do not express Fas/Apo1 (CD95) and that they are resistant to the Fas-agonistic monoclonal antibody (MoAb) CH11 (Tolomeo et al., 1999). To verify the implication of the lack of Fas in CMT-1-resistance we studied the effects of CMT-1 and CMT-8 in HUT78B1 cells, a CH11 resistant human T-lymphoma cell line characterized by a deletion of Fas involving death domain. As shown in Figure 5a, CMT-1 was inactive in HUT78B1 cells, while it showed an apoptotic activity in parental CH11-sensitive HUT78 similar to that shown in HL60 cell line. Conversely, CMT-8 was a potent inducer of apoptosis both in HUT78B1 and in the parental HUT78 cell line (Figure 5c). Both drugs did not induce necrosis (Figure 5b,d). The studies on apoptosis using DNA-fragmentation ELISA assay confirmed the data obtained with morphological evaluation of apoptosis.
Figure 5.
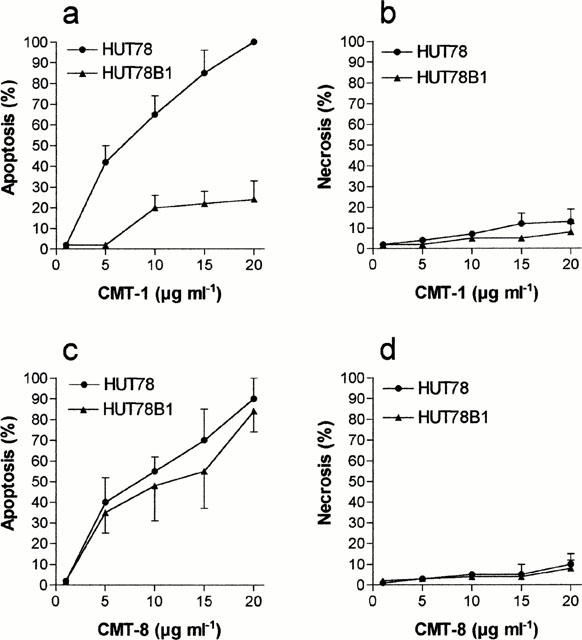
Percentage of apoptotic (a and c) and necrotic cells (b and d) cells induced by different concentrations of CMT-1 and CMT-8 in HUT78 and HUT78B1 cell lines. Evaluation by morphologic assay after 48 h of treatment. Results are means±s.d. of five experiments.
To confirm the implication of the Fas/Fas-ligand system in apoptosis induced by CMT-1, we evaluated its activity in HUT78 cell exposed to the Fas-blocking monoclonal antibody DX2.
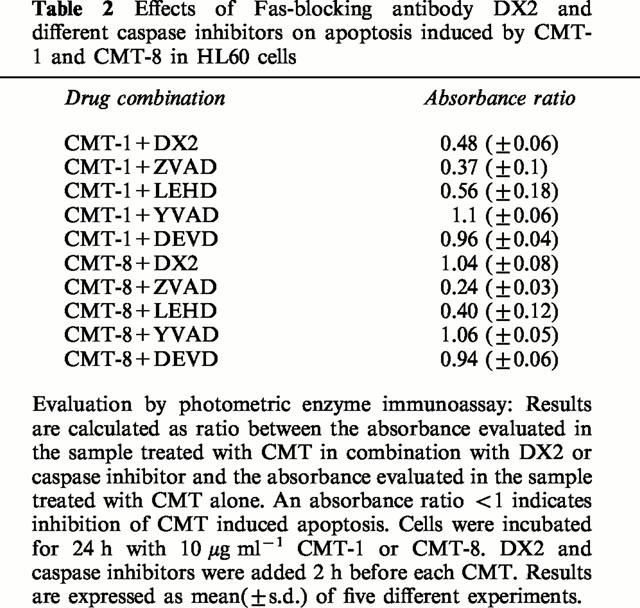
As shown in Figure 6 and in Table 2, the block of the Fas receptor by DX2 decreased, at least in part, the sensitivity of HUT78 cells to CMT-1, whereas it did not modify the apoptosis-inducing activity of CMT-8, suggesting a possible role of Fas in apoptosis induced by CMT-1 but not in apoptosis induced by CMT-8. However, the pan-caspase inhibitor ZVAD-fmk and caspase-9 inhibitor ZLEHD-fmk inhibited apoptosis by CMT-1 and CMT-8 indicating that the activation of caspases is a downstream event common for programmed cell death induced by both these chemically modified tetracyclines.
Figure 6.
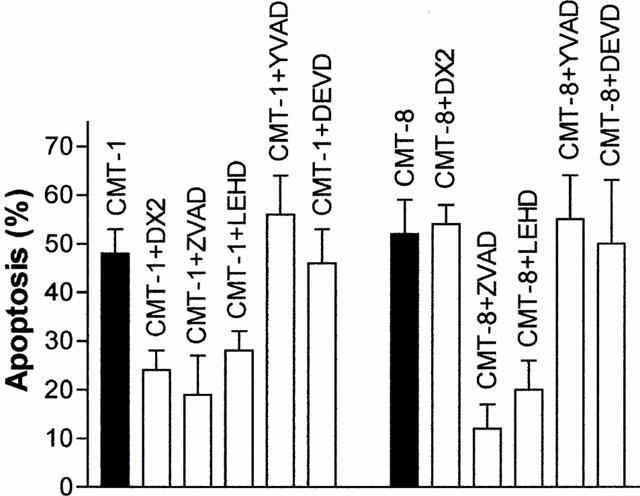
Effects of Fas-blocking antibody DX2, pan-caspase inhibitor ZVAD-fmk (50 μM), caspase-9 inhibitor ZLEHD-fmk (50 μM), caspase-1 inhibitor YVAD-CHO (200 μM), caspase-3 inhibitor DEVD-CHO (200 μM) in HL60 cells treated 24 h with 10 μg ml−1 CMT-1 or CMT-8. DX2 and caspase inhibitors was added 2 h before each CMT. Results are means±s.d. of five experiments.
Table 2.
Effects of Fas-blocking antibody DX2 and different caspase inhibitors on apoptosis induced by CMT-1 and CMT-8 in HL60 cells
Discussion
Data here demonstrate that newly designed chemically modified tetracyclines are significantly cytotoxic in leukaemia cell lines, and that, some of them act as potent programmed cell death-inducing agents (Cillari et al., 1998). Several studies demonstrated that a variety of widely used anticancer drugs kill cancer cells through induction of apoptosis (Walker et al., 1991; Vial et al., 1997), and some authors observed that antineoplastic agents can induce apoptosis through a Fas/Fas-ligand-dependent pathway (Friesen et al., 1996; Fulda et al., 1997).
Previously, Kroon et al. (1984) demonstrated that tetracyclines inhibit mitochondrial protein synthesis causing ‘cytostasis', a condition where cell proliferation is inhibited but cells are not killed. More recently, Fife et al. (1997; 1998) showed that doxycycline was able to kill neoplastic cells through the induction of apoptosis. We have observed that the appearance of cytostasis or apoptosis is correlated to the concentration of doxycycline or CMT employed. Doxycycline and most of the CMTs, induce apoptosis when used at concentrations greater than IC50. Conversely, the IC50 of CMT-1 and CMT-8 correspond to their AC50, indicating that these CMTs exert their cytotoxic activity prevalently by inducing programmed cell death. Interestingly, doxycycline and almost all CMTs tested here are inactive or show a low activity in the MDR HL60R cell line. CMT1, one of the most active compounds on the HL60 cells, is 6 fold less cytotoxic in the HL60R cell line. This is only partially related to the presence of the Pgp, as shown by the low ability of verapamil or cyclosporin A to reverse the CMT-1- resistance. We previously observed that, in contrast to HL60, HL60R cells did not express Fas and they were resistant towards agonistic anti-Fas monoclonal antibody, such as CH11 (Tolomeo et al., 1999). The hypothesis that the lack of Fas could be implicated, at least in part, in CMT-1 resistance is confirmed by the observation that CMT-1 is effective in terms of apoptosis induction in HUT78 T-cell lymphoma cell line, but is scarcely active in the CH11 resistant subclone HUT78B1. The implication of Fas in the CMT-1-induced programmed cell death is also confirmed by the ability of DX2 to reduce the apoptotic activity of CMT-1 in HUT78 cells. On the contrary, CMT-8 showed in HL60R and HUT78B1 cells a cytotoxic and apoptotic activity similar to that observed in the respective parental cell lines. The activation of caspases, and specifically caspase-9, seem implicated in apoptosis induced by both CMT-1 and CMT-8 as shown by the ability of pan-caspase inhibitor ZVAD-fmk and caspase-9 inhibitor ZLEHD-fmk to decrease the percentage of apoptosis cells induced by these two chemically modified tetracycline.
Concerning the structural features associated with the apoptotic activity of the CMTs described in this study, it appears evident that the absence of the 4-dimethylamino moiety represents an important prerequisite for the apoptotic activity of the compounds. Indeed, removal of the functionality at the C-4 carbon atom clearly proved, when compared with doxycycline, an increased apoptosis activity in CMT-8. Moreover, a potent apoptotic activity was also observed for CMT-1, the dedimethylamino analogue of the natural tetracycline. Interestingly, it is noteworthy to observe that CMT-8 was also able to induce apoptosis in multidrug resistant and apoptosis resistant cell lines: from a structural point of view this activity appears to be due, apart from the absence of the 4-dimethylamino group, to a single structural difference, namely the presence of the hydroxy moiety at the C-5. Therefore, since doxycycline still retain an appreciable cytotoxicity but is not active on HL60R cells and its apoptotic activity is lower that than of CMT-8, we may advance the hypothesis that the C-5 hydroxy moiety may play an important role in conferring the activity in multidrug resistant cells. In addition, as most of the synthetic modifications along the lower peripheral region of tetracyclines have been described to greatly decrease both their antibiotic and non-antibiotic activities, the present results further support this statement: indeed, CMT-5, a compound bearing the pyrazole heterocycle as a bioisosteric substitution of the b-hydroxyketone at the C-11/C-12 carbon atoms, as well as the C-12a deoxy derivative CMT-7, were both poor inducers of apoptosis.
Altogether, these findings seem to support the hypothesis that CMT-8 may represent an interesting lead for the development of a new class of potent apoptotic inducer agents, active in multidrug resistant cells. Therefore, the study of novel 5-oxatetracycline-based compounds may provide and interesting new entry for the development of chemotherapeutic agents useful in malignancies resistant to conventional treatments.
CMT-8 may have important implications in the clinical management of malignancies treatment not only for their ability to kill sensitive and drug resistant neoplastic cells through the activation of programmed cell death, but also for other properties recently described. It has been demonstrated that some tetracyclines, and especially CMT-8, markedly reduced the severity of osteoporosis by enhancing new bone formation as well as reducing bone reabsorption (Sasaki et al., 1998; 1999; Llavaneras et al., 1999). This seems related to the ability of tetracyclines and CMTs to stimulate the collagen production and to increase the alkaline phosphatase activity in osteoblasts; moreover they inhibit selective osteoblast ontogeny or development also inducing their apoptosis (Sorsa et al., 1998; Vernillo & Rifkinn, 1998). These properties make CMT-8 potentially useful for the treatment of malignances endowed with bone tropism.
CMT-8 and other tetracyclines show also strong inhibition of MMP activity in a variety of tissues, with little systemic toxicity at therapeutically effective doses. MMPs play a significant role in tumour cell invasion and metastasis. It is known that aggressive cancer cells secrete a large excess of MMPs and low levels of endogenous inhibitors (e.g. TIMPSs); this imbalance represents a causative factor for invasion and metastasis (Lokeshwar et al., 1993). The ability of tetracyclines and CMTs to inhibit the MMPs activity may be useful to decrease the incidence of metastasis (Lokeshwar et al., 1998; Lokeshwar et al., 1998; Selzer et al., 1999). Moreover, the ability of CMTs to inhibit cell motility, as a consequence of the lack of mitochondrial function, can contribute to reducing the invasive activity of malignant cells. Experiences by Lokeshwar et al. (1996) confirm in vivo the property of doxycycline and CMT-3 to inhibit tumour incidence and metastatic foci formation.
In conclusion, we have demonstrated that some CMTs can act as antitumoural agents by induction of programmed cell death; the CMT-induced apoptotic pathway can be related to the activation of Fas, but can also be independent from the activation of this receptor. CMT-8 represents the most interesting drug tested in this study. For its ability to act in MDR and apoptosis resistant cell lines it may be useful in the treatment of malignancies resistant to the conventional chemotherapeutic drugs.
Acknowledgments
We thank Dr Brian Gallagher for the critical reading of the manuscript. CMTs were kindly supplied by CollaGenex Pharmaceuticals, Inc. (Newtown, PA, U.S.A.).
Abbreviations
- Ac-DEVD-CHO
acetyl-Asp-Glu-Val-Asp-aldehyde
- c-YVAD-CHO
acetyl-Tyr-Val-Ala-Asp-CHO
- Ac-ZLEHD-fmk
acetyl-Z-leu-Glu(Ome)-His-Asp(Ome)-fmk
- Ac-ZVAD-fmk
acetyl-Tyr-Val-Ala-Asp-chloromethyl ketone
- CMT
chemically modified tetracycline
- DMSO
dimethylsulphoxide
- ECM
extracellular matrix
- MDR
multidrug resistance
- MMP
matrix metalloproteinase
- MoAb
monoclonal antibody
- Pgp
P-glycoprotein
References
- BROWN P.B. Matrix metalloproteinase inhibitors: a novel class of anticancer drugs. Adv. Enzyme Res. 1995;35:293–301. doi: 10.1016/0065-2571(94)00022-u. [DOI] [PubMed] [Google Scholar]
- CILLARI E., MILANO S., D'AGOSTINO P., DI BELLA G., LA ROSA M., BARBERA C., FERLAZZO V., CAMMARATA G., GRIMAUDO S., TOLOMEO M., FEO S. Modulation of nitric oxide production by tetracyclines and chemically modified tetracyclines. Adv. Dent. Res. 1998;12:126–130. doi: 10.1177/08959374980120010701. [DOI] [PubMed] [Google Scholar]
- COCKETT M.I., BIRCH M.L., MURPHY G., HART I.R., DOCHERTY A.J. Metalloproteinase domain structure, cellular invasion and metastasis. Biochem. Soc. Trans. 1994;22:55–57. doi: 10.1042/bst0220055. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO P., ARCOLEO F., BARBERA C., DI BELLA G., LA ROSA M., MISIANO G., MILANO S., BRAI M., CAMMARATA G., FEO S., CILLARI E. Tetracycline inhibits the nitric oxide synthase activity induced by endotoxin in cultured murine macrophages. Eur. J. Pharmacol. 1998a;346:283–290. doi: 10.1016/s0014-2999(98)00046-6. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO P., LA ROSA M., BARBERA C., ARCOLEO F., DI BELLA G., MILANO S., CILLARI E. Doxycycline reduces mortality to lethal endotoxemia by reducing nitric oxide synthesis via an interleukin-10 independent mechanism. J. Infect. Dis. 1998b;177:489–492. doi: 10.1086/517383. [DOI] [PubMed] [Google Scholar]
- DARZIYNKIEWICK Z., BRUNO S., DEL BINO G., GORCZYCA W., HOLZ M.A., LASSOTA P., TRAGANOS F. Features of apoptosis cells measured by flow cytometry. Cytometry. 1992;13:795–798. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- DUKE R.C., COHEN J.J.Morphological and biochemical assays of apoptosis Current Protocols in Immunology 1992New York: John Wiley & Sons; eds Coligan, J.E. & Kruisbeak, A.M. p. 3.17.1 [Google Scholar]
- EKLUND K.K., SORSA T. Tetracycline derivtive CMT-3 inhibits cytokine production, degranulation, and proliferation in cultured mouse and human mast cells. Ann. N.Y. Acad. Sci. 1999;878:689–691. doi: 10.1111/j.1749-6632.1999.tb07763.x. [DOI] [PubMed] [Google Scholar]
- EISCHEN C.M., KOTTKE T.J., MARTINS L.M, , BASI G.S., TUNG J.S., EARNSHOW W.C., LEIBSON P.J., KAUFMANN S.H. Comparison of apoptosis in wild-type and Fas-resistant cells: Chemotherapy-induced apoptosis is not dependent on Fas/Fas Ligand interactions. Blood. 1997;90:935–943. [PubMed] [Google Scholar]
- ELATTAR T.M.A., LIN H.S., SCHULTZ R.J. Effect of minocycline on prostaglandin formation in gengival fibroblast. J. Periodont. Res. 1988;23:285–286. doi: 10.1111/j.1600-0765.1988.tb01418.x. [DOI] [PubMed] [Google Scholar]
- FIFE R., SLEDGE G. Effects of doxycycline on in vitro growth, migration, and gelatinase activity of breast carcinoma cells. J. Lab. Clin. Med. 1995;125:407–411. [PubMed] [Google Scholar]
- FIFE R.S., ROUGRAFF B.T., PROCTOR C., SLEDGE G.W. Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J. Lab. Clin. Med. 1997;130:530–534. doi: 10.1016/s0022-2143(97)90130-x. [DOI] [PubMed] [Google Scholar]
- FIFE R.S., SLEDGE G.W., ROTH B.J., PROCTOR C. Effects of doxycycline on human prostate cancer cells in vitro. Cancer Lett. 1998;127:37–41. doi: 10.1016/s0304-3835(98)00003-2. [DOI] [PubMed] [Google Scholar]
- FIFE R.S., SLEDGE G.W. , JR Effects of doxycycline on cancer cells in vitro and in vivo. Adv. Dent. Res. 1998;12:94–96. doi: 10.1177/08959374980120012801. [DOI] [PubMed] [Google Scholar]
- FRIESEN C., HERR I., KRAMMER P.H., DEBATIN K.-M. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med. 1996;5:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- FULDA S., SIEVERTS H., FRIESEN C., HERR I., DEBATIN K.M. The CD95 (APO-/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 1997;57:3823–3829. [PubMed] [Google Scholar]
- HANEMAAIJER R., VISSER H., KOOLWIJK P., SORSA T., SALO T., GOLUB L.M., VAN HINSBERGH V.W. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv. Dent. Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- HIMRLSTEIN B.P., CANETE-SOLAR R., BERNHARD E.J., DILKS D.W., MUSCHEL R.J. Metalloproteinases in tumor progression: the contribution of MMP-9. Inv. Metast. 1994;14:246–258. [PubMed] [Google Scholar]
- KOHN E.C., LIOTTA L.A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- KROON A.M., DONTJE B.H.J., HOLTROP M., VAN DEN BOGERT C. The mitochondrial genetic system as a target for chemotherapy: tetracycline as cytostatics. Cancer Lett. 1984;25:33–40. doi: 10.1016/s0304-3835(84)80023-3. [DOI] [PubMed] [Google Scholar]
- LLAVANERAS A., GOLUB L.M., RIFKIN B.R., HEIKKILA P., SORSA T., TERONEN O., SALO T., LIU Y., RYAN M.E., RAMAMURTHY N.S. CMT-8/clodronate combination therapy synergistically inhibits alveolar bone loss in LPS-induced periodontitis. Ann. N.Y. Acad. Sci. 1999;878:671–674. doi: 10.1111/j.1749-6632.1999.tb07758.x. [DOI] [PubMed] [Google Scholar]
- LOKESHWAR B.L., HOUSTON-CLARK H.L., SELZER M.G., BLOCK N.L., GOLUB L.M. Potential application of a chemically modified non-antimicrobial tetracycline (CMT-3) against metastatic prostate cancer. Adv. Dent. Res. 1998;12:97–102. doi: 10.1177/08959374980120012901. [DOI] [PubMed] [Google Scholar]
- LOKESHWAR B.L., SELZER M.G., BLOCK N.L., GOLUB L.M. Inhibition of tumor growth and metastasis by a non-antimicrobial tetracycline analogue in a prostate cancer model (abstract) Proc. Am. Assoc. Cancer Res. 1998;38:2868. [Google Scholar]
- LOKESHWAR B.L., SELZER M.G., BLOCK N.L., GUNJA-SMITH Z. Secretion of matrix metalloproteinase and their inhibitors (TIMPs) by human prostate in explant cultures: reduced tissue inhibitor of metalloproteinase secretion by malignant tissues. Cancer Res. 1993;53:4493–4498. [PubMed] [Google Scholar]
- MAISI P., KIILI M., RAULO S.M., PIRILA E., SORSA T. MMP inhibition by chemically modified tetracycline-3 (CMT-3) in equine pulmonary epithelial lining fluid. Ann. N.Y. Acad. Sci. 1999;878:675–677. doi: 10.1111/j.1749-6632.1999.tb07759.x. [DOI] [PubMed] [Google Scholar]
- MAKELA M., SORSA T., UITTO V.J., SALO T., TERONEN O., LARJAVA H. The effects of chemically modified tetracyclines (CMTs) on human keratinocyte proliferation and migration. Adv. Dent. Res. 1998;12:131–135. doi: 10.1177/08959374980120010801. [DOI] [PubMed] [Google Scholar]
- MILANO S., ARCOLEO F., D'AGOSTINO P., CILLARI E. Intraperitoneal injection of tetracyclines protect mice from lethal endotoxemia down-regulating inducible nitric oxide synthase in various organs and cytokines and nitrate secretion in the blood. Antimicrob. Agents Chemo. 1997;41:117–121. doi: 10.1128/aac.41.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA S., GOLSTEIN P. The Fas death factor. Science. 1995;267:1449–1455. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- NELSON M.L. Chemical and biological dynamics of tetracyclines. Adv. Dent. Res. 1998;12:5–11. doi: 10.1177/08959374980120011901. [DOI] [PubMed] [Google Scholar]
- PATEL R.N., ATTUR M.G., DAVE M.N., PATEL I.V., STUCHIN S.A., ABRAMSON S.B., AMIN A.R. A novel mechanism of action of chemically modified tetracyclines: inhibition of COX-2-mediated prostaglandin E2 production. J. Immunol. 1999;163:3459–3467. [PubMed] [Google Scholar]
- RAMAMURTHY N.S., MCCLAIN S.A., PIRILA E., MAISI P., SALO T., KUCINE A., SORSA T., VISHRAM F., GOLUB L.M. Wound healing in aged normal and ovariectomozed rats: effects of chemically modified doxycycline (CMT-8) on MMP expression and collagen synthesis. Ann. N.Y. Acad. Sci. 1999;878:720–723. doi: 10.1111/j.1749-6632.1999.tb07772.x. [DOI] [PubMed] [Google Scholar]
- SALVESEN G.S., DIXIT V.M. Caspases: Intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- SASAKI T., OHYORI N., DEBARI K., RAMAMURTHY N.S., GOLUB L.M. Effects of chemically modified tetracycline, CMT-8, on bone loss and osteoclast structure and function in osteoporotic states. Ann. N.Y. Acad. Sci. 1999;878:347–360. doi: 10.1111/j.1749-6632.1999.tb07694.x. [DOI] [PubMed] [Google Scholar]
- SASAKI T., RAMAMURTHY N.S., GOLUB L.M. Long-term therapy with a new chemically modified tetracycline (CMT-8) inhibits bone loss in femurs of ovariectomized rats. Adv. Dent. Res. 1998;12:76–81. doi: 10.1177/08959374980120012501. [DOI] [PubMed] [Google Scholar]
- SELZER M.G., ZHU B., BLOCK N.L., LOKESHWAR B.L. CMT-3, a chemically modified tetracycline, inhibits bon metastases and delays the development of paraplegia in a rat model of prostate cancer. Ann. N.Y. Acad. Sci. 1999;878:678–682. doi: 10.1111/j.1749-6632.1999.tb07760.x. [DOI] [PubMed] [Google Scholar]
- SIMONI D., INVIDIATA F.P., RONDANIN R., GRIMAUDO S., CANNIZZO G., BARBUSCA E., PORRETTO F., D'ALESSANDRO N., TOLOMEO M. Structure-activity relationship studies of novel heteroretinoids: induction of apoptosis in the Hl-60 cell line by a novel isoxazole-containing heteroretinoid. J. Med. Chem. 1999;42:4961–4969. doi: 10.1021/jm991059n. [DOI] [PubMed] [Google Scholar]
- SORSA T., RAMAMURTHY N.S., VERNILLO A.T., ZHANG X., KONTTINEN Y.T., RIFKIN B.R., GOLUB L.M. Functional sites of chemically modified tetracyclines: inhibition of the oxidative activation of human neutrophil and chicken osteoclast pro-matrix metalloproteinases. J. Rheumatol. 1998;25:975–982. [PubMed] [Google Scholar]
- TOLOMEO M., DUSONCHET L., MELI M., GRIMAUDO S., D'ALESSANDRO N., PAPOFF G., RUBERTI G., RAUSA L. The CD95/CD95 ligand system is not the major effector in anticancer drug-mediated apoptosis. Cell Death Differ. 1998;5:735–742. doi: 10.1038/sj.cdd.4400406. [DOI] [PubMed] [Google Scholar]
- TOLOMEO M., GRIMAUDO S., CANNIZZO G., BARBUSCA E., PORRETTO M., MUSSO M., DUSONCHET L., MELI M., ABBADESSA V., PERRICONE R., CAJOZZO A. Implication of Fas/APO-1 and NF-kB in resistance to drug induced apoptosis in MDR leukemic cells. Haematologica (Abstract) 1999;84:291. [Google Scholar]
- VADAS P., GREENWALD R.A., STREET R.T., PRUZANSKI W. Inhibition of synovial fluid phospholipase A2 activity by two tetracycline derivatives, minocycline and doxycycline. Arth. Rheum. 1991;34:160–164. [Google Scholar]
- VERNILLO A.T., RIFKINN B.R. Effects of tetracyclines on bone metabolism. Adv. Dent. Res. 1998;12:56–62. doi: 10.1177/08959374980120012101. [DOI] [PubMed] [Google Scholar]
- VIAL J.P., BELLOC F., DUMAIN P., BESNARD S., LACOMBE F., BOISSEAU M.R., REIFFERS J., BERNARD P. Study of the apoptosis induced in vitro by antitumoral drugs on leukaemic cells. Leukemia Res. 1997;21:163–172. doi: 10.1016/s0145-2126(96)00102-6. [DOI] [PubMed] [Google Scholar]
- VILLUNGER A., EGLE A., KOS M., HARTMANN B.L., GELEY S., KOFLER R., GREIL R. Drug-induced apoptosis is associated with enhanced Fas (Apo-1/CD95) ligand expression but occurs independently of Fas (Apo-/CD95) signaling in human T-acute lymphatic leukemia cells. Cancer Res. 1997;57:3331–3334. [PubMed] [Google Scholar]
- WALKER P.R., SMITH C., YOUDALE T., LEBLANC J., WHITFIELD J.F., SIKORSKA M. Topoisomerase II-reactive drugs induce apoptosis in thymocytes. Cancer Res. 1991;51:1078–1084. [PubMed] [Google Scholar]


