Abstract
We examined the mechanisms underlying leukotriene D4- (LTD4) induced constriction of human small (300 – 500 μm i.d.) bronchioles, and the effect of LTD4 on ion currents and Ca2+ transients in smooth muscle cells (SMC) isolated from these bronchioles.
LTD4 caused a concentration-dependent bronchoconstriction with an EC50=0.58±0.05 nM (n=7) which was not easily reversible upon washout. This bronchoconstriction was entirely dependent on extracellular Ca2+.
Blockade of L-type Ca2+ channels with nifedipine (10 μM) reduced LTD4 response by 39±2% (n=8), whilst La3+, Gd3+ and SK&F 96,365 abolished LTD4-induced bronchoconstriction completely and reversibly, suggesting the majority of Ca2+ entry was via non-selective cation channels.
Antagonists of PI-PLC (U73,122 and ET-18-OCH3), PLD (propranolol) and PKC (cheleretrine and Ro31-8220) were without any effect on LTD4-induced bronchoconstriction, whilst the PC-PLC inhibitor D609 caused complete relaxation. Inhibition of protein tyrosine kinase with tyrphostin A23 (100 μM) caused about 50% relaxation, although the inactive analogue tyrphostin A1 was without effect.
In freshly isolated SMC from human small bronchioles LTD4 caused a slow increase of intracellular Ca2+ concentration, with a consequent rise of the activity of large conductance Ca2+-dependent K+ channels and the amplitude of depolarization-induced outward whole-cell current. Again, no effect of LTD4 could be observed in the absence of extracellular Ca2+.
We conclude that LTD4 causes constriction of these small bronchioles primarily by activating Ca2+ entry via non-voltage gated channels, possibly by a PC-PLC mediated pathway.
Keywords: Bronchoconstriction, human, leukotrienes, smooth muscle, PC-PLC, intracellular Ca2+
Introduction
Cysteinyl leukotrienes are potent pro-inflammatory lipid mediators that are produced by many inflammatory cells, and cause smooth muscle constriction, hyper-secretion of mucus, and increased vascular permeability. There is clear evidence that leukotriene D4 (LTD4) plays a major role in the pathophysiology of asthma and other inflammatory diseases (Arm & Lee, 1993). However, the specific receptors and transduction pathways remain poorly understood, particularly in human airways. Moreover, they may differ considerably between different tissues and species (Crooke et al., 1988). It has been suggested that LTD4 induces bronchoconstriction through phosphatidylinositol phospholipase C (PI-PLC) activation, as LTD4 increases inositol polyphosphates in guinea-pig ASM (Dumitriu et al., 1997; Howard et al., 1992), and LTD4-induced contraction of guinea-pig ASM is reduced by PLC inhibition (Salari et al., 1993). However other signal transduction pathways are also possible, involving Gi proteins, phosphatidylcholine phospholipase C (PC-PLC)- and phospholipase D (PLD)-mediated generation of diacylglycerols (DAG), activation of protein kinase C (PKC) and sustained contraction and Ca2+ sensitization (Kim et al., 1998). Another alternative to the IP3-mediated mechanism is one that includes the membrane-delimited conversion of DAG generated by PC-PLC or PLD into phosphatidic acid by DAG kinase, with subsequent Ca2+ release from IP3-insensitive intracellular stores (Camiña et al., 1999).
It has been predicted that much of the resistance to airflow seen during agonist challenge is due to contraction of small (<∼2 mm i.d.) airways (Moreno et al., 1986). However there have been very few in vitro studies on these functionally significant small bronchioles, particularly in man, and the large majority of reports have been on tissue derived from trachea or main bronchus of other species. This may have important consequences, as significant pharmacological and mechanical differences have been reported between airways from different parts of the bronchial tree (Gauthier et al., 1992; Mustafa et al., 1994), including our own studies on small bronchioles of the rat (Chopra et al., 1994; 1997). Moreover we have demonstrated that the electrophysiology of human ASM differs from other species commonly used in airways research (Snetkov et al., 1995; 1998), and more recently that ASM from human small bronchioles (<1 mm i.d.) have a different distribution of ionic channels and currents than ASM from main bronchus (Snetkov & Ward, 1999). This parallels the well known variation in function that occurs between arteries of different calibre within the vascular tree (e.g. Archer, 1996).
The contraction of smooth muscle from different tissues depends to a variable extent on Ca2+ influx across the plasma membrane. Though a number of ion channels have been implicated in such influx, the role of L-type voltage gated Ca2+ channels in human ASM seems to be a relatively minor one. Attention has been recently drawn to store-operated Ca2+ channels (SOC) which are activated upon emptying of IP3-dependent Ca2+ stores (Putney & Mckay, 1999), and to a variety of channels directly activated by second messengers such as Ca2+, IP3, IP4, arachidonic acid and its metabolites (for review see Kiselyov & Muallem, 1999).
The aim of the present study was to establish the role for different sources of Ca2+ in the constrictor response of functionally important human small bronchioles to LTD4, and to elucidate the signalling mechanisms involved.
Methods
Human intralobular bronchioles were dissected from tissue obtained after lobectomies within 90 min of removal from the patient, as approved by the Guy's and St Thomas' Hospital Committee on Ethics. In total 46 lung specimens from patients aged 49 – 88 years have been used.
Contraction measurements
1 – 2 mm segments of epithelium-intact bronchioles (300 – 500 μm i.d.) were cleaned of parenchyma, mounted on a small vessel myograph (Myodata 1200, Denmark), and stretched to 80% of the maximum of the active length – tension curve as previously described (Chopra et al., 1997). The chamber contained physiological saline (PSS; for composition see below), equilibrated with 95% O2/5% CO2 at 37°C. After 45 min preparations were challenged with at least three 4 min exposures to PSS containing 80 mM KCl (KPSS; equimolar substitution for NaCl). Experiments were performed once the response to KPSS was repeatable and stable.
Cells isolation
Epithelium-denuded bronchiole segments were incubated for 60 min at 36°C in 2 ml oxygenated M-199 medium containing 2 mg ml−1 collagenase (Sigma type XI), 1 mg ml−1 papain, 2 mg ml−1 soyabean trypsin inhibitor and 20 μM dithiothreitol. Then tissue was then washed with fresh M-199 medium and kept at 36°C for further 20 min, following which the tissue was triturated gently with a flame-polished Pasteur pipette in 0.5 ml M-199 in order to release single cells. Five to ten min before use a drop of the cell suspension was placed on a alcohol-cleaned glass coverslip. This procedure provided sufficient number of easy identifiable, relaxed smooth muscle cells as well as a number of active ciliated epithelial cells.
Intracellular Ca2+
Isolated cells were incubated with 0.5 μM Fura-PE3/AM for 30 min at room temperature. A drop of cell suspension was placed at the bottom of a recording chamber (RC-26, Warner Instrument Corp.) consisting of a 13 mm glass coverslip (thickness 0), and mounted on the stage of inverted microscope (Nikon Diaphot). Attached cells were visualized with a ×100 oil immersion UV objective, and the ratio of emission intensities at excitation wavelengths 340 and 380 nm were recorded using a microspectrofluorimeter (Cairn Research Ltd., U.K.).
Electrophysiology
Single cells were studied with standard patch-clamp techniques using an Axopatch-1C amplifier (Axon Instruments, Inc., Foster City, CA, U.S.A.), as previously described (Snetkov & Ward, 1999). The experiments were carried out at room temperature. The bath (≈0.5 ml) was continuously superfused (1-2 ml min−1) with external physiological salt solution (PSS, see below). Agonists and drugs were added through the perfusion system. Whole-cell recording was performed in permeabilized patch mode with 150 μg ml−1 nystatin in the pipette. Whole-cells and single channel currents were filtered at 5 or 1 kHz respectively (−3 dB, low pass 80 dB/dec, Bessel type built-in filter) and digitised with a 20 or 5 kHz sampling frequency using a CED 1401 interface and PATCH v.6 software (Cambridge Electronic Design, Cambridge, U.K.). Whole-cell current-voltage relationships were obtained using voltage ramp protocol when holding potential was kept at −60 mV and 0.5 s ramp from −100 mV to +100 mV was applied each 5 s.
Solutions and reagents
PSS contained (in mM): NaCl 118, NaHCO3 24, MgSO4 1, NaH2PO4 0.435, glucose 5.56, Na-pyruvate 5, CaCl2 1.8, KCl 4, pH 7.4 when equilibrated with 95% O2/5% CO2. Ca2+-free solution contained no added Ca2+ and 1 mM EGTA. The pipette solution used both in cell-attached and whole-cell recording contained (in mM): KCl 140, MgCl2 2, EGTA 0.1, HEPES 10, ATP 2, GTP 0.2, pH 7.2 with KOH. The normal bath solution for patch-clamp experiments and intracellular Ca2+ recording contained (in mM): NaCl 135, KCl 5, MgCl2 2, CaCl2 2, glucose 11, HEPES 10, pH 7.4 with NaOH. The same solution was used for contraction experiments involving La3+, Gd3+ and Ni2+ to prevent precipitation. D609, U73,122, SK&F 96,365 and MDL-12,330A were obtained from Calbiochem, Notts., U.K., and 2-aminoethoxydiphenyl borate (2-APB) from Tocris Cookson Ltd., Bristol, U.K. All other drugs and reagents were purchased from Sigma Chemical Company (Poole, U.K.).
Data are presented as mean±s.e.mean, and n is the number of separate preparations. Fitting was performed using the Marquardt-Levenberg algorithm (SigmaPlot v.6, SPSS Inc.)
Results
Agonist-induced bronchoconstriction
In order to characterise the contractile response of these small bronchioles, concentration-response curves were constructed for LTD4, LTE4, carbachol and histamine. LTD4 induced contraction with a threshold concentration of ∼0.03 nM and an EC50=0.58±0.05 nM (n=7), which is smaller than previously reported for larger human bronchi (11 nM: Bourdillat et al., 1987; 3.5 nM: Hay et al., 1993). The maximally effective concentration was 10 nM, giving a contractile response of ∼150% of the response to KPSS. LTE4 was significantly less potent (EC50=4.6±0.3 nM (n=6; P<0.01). The EC50 for carbachol and histamine were 213±17 nM (n=8) and 446±26 nM (n=6) respectively (Figure 1a). Preincubation with 10 μM indomethacin for 30 min had no effect on LTD4-induced contraction (n=4). 3 mM L-cysteine, an inhibitor of the degradation of LTD4 to LTE4 by aminopeptidases (Bourdillat et al., 1987) did not alter the dose-response of LTD4 (n=3). The CysLT1 antagonist ICI 198,615 (0.1 μM) caused complete relaxation of preparations constricted with 10 nM LTD4 (n=6). Prior addition of ICI 198,615 also completely blocked the response to 10 nM LTD4, without having any effect on the response to KPSS or 10 μM carbachol (n=3).
Figure 1.
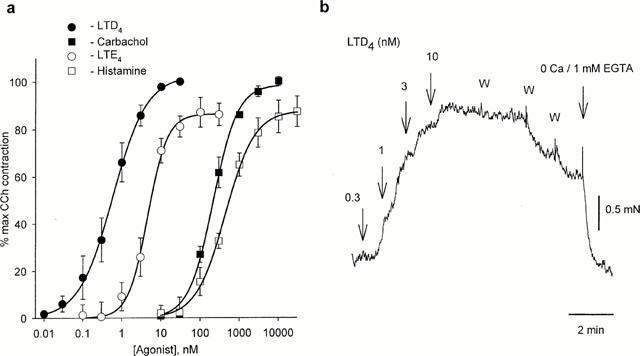
(a) Dose – response for agonist-induced contractions in human small bronchioles (n=6 – 8). (b) Contraction of human small bronchiole induced by increasing concentrations of LTD 4. W indicates repetitive washes with normal PSS.
Effect of Ca2+ removal
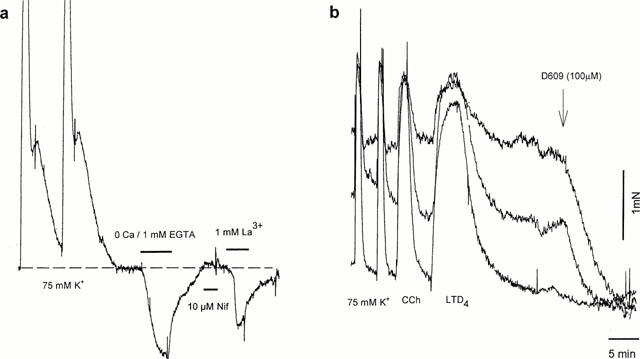
In marked contrast to the response to such agonists as carbachol (10 μM) and histamine (50 μM), LTD4-induced contraction was not readily reversible and was sustained after several washes. However, replacement of PSS with a Ca2+-free solution containing 1 mM EGTA led to immediate relaxation (n=11) (Figure 1b). This was reversible upon restoration of extracellular Ca2+. In Ca2+-free solution even high concentrations of LTD4 (10 nM) were without effect (n=7). However, reintroduction of Ca2+ following challenge with LTD4 induced a maximal response, even after prolonged washing with Ca2+-free solution (Figure 2). As this effect was observed even when the LTD4 antagonist ICI 198,615 was present during washout, it was not due to continued binding of LTD4 to its receptor.
Figure 2.
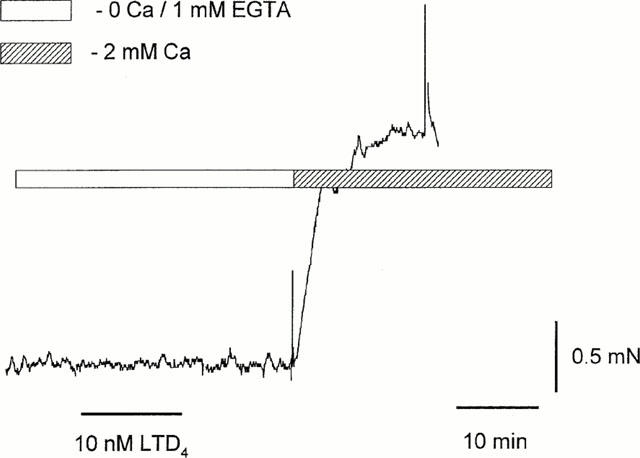
LTD4 does not contract bronchiole in Ca-free solution; reintroduction of normal Ca2+ induced a contractive response.
Mechanisms of Ca2+ entry
To investigate possible pathways of Ca2+ entry we studied the effects of various Ca2+ channels blockers on the sustained contraction induced with LTD4. Nifedipine (10 μM), a blocker of L-type voltage-gated Ca2+ channels, produced only partial relaxation of bronchioles preconstricted with 10 nM LTD4 (39±2% initial tension, n=8) (Figure 3a). Consistent with this, preincubation with nifedipine reduced the subsequent response to LTD4 to 62±3% of control (n=7). Blockade of Ca2+ influx with La3+ (1 mM, n=7) or SK&F 96,365 (100 μM, n=6) caused complete relaxation of bronchioles constricted with 10 nM LTD4 (Figure 3b,c respectively). Lower concentrations of both La3+ and SK&F 96,365 were less effective and more variable in effect between preparations; 100 μM La3+ caused only 25 – 30% relaxation (n=7), whereas 10 μM SK&F 96,365 caused only ∼15% relaxation. Gd3+ (100 μM and 1 mM) had the same effect as La3+, while Ni2+ (1 mM) was without any effect (n=4). MDL-12,330A, otherwise used as an adenylyl cyclase inhibitor, has been reported to block Ca2+ entry in a variety of cell types in a similar but more reproducible fashion to SK&F 96,365, independently of its effect on adenylate cyclase (Van rossum et al., 2000). In these human bronchioles it blocked 35% of the LTD4 (10 nM) response at 10 μM and 90% at 100 μM (n=3). 2-APB, a membrane-permeable IP3 receptor antagonist, has been shown to prevent activation of both endogenous SOC and transfected TRP3 in HEK293 cells (Ma et al., 2000). 2-APB at 75 μM had no effect on LTD4 (10 nM) induced bronchoconstriction (n=4), although this concentration was sufficient to abolish SOC in pulmonary arteries and human cultured ASM cells (Snetkov, unpublished observations).
Figure 3.
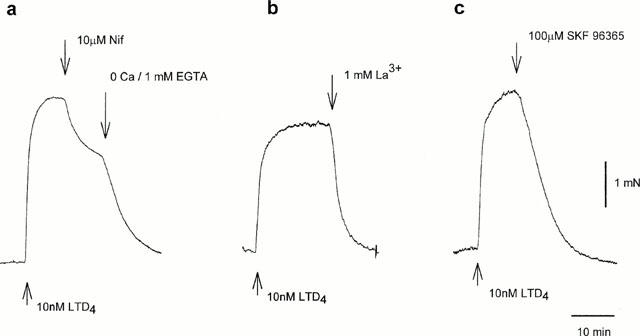
Nifedipine blocks LTD4-induced contraction only partially (a), while La3+ (b) and SK&F96,365 (c) relax bronchioles completely.
Signal transduction
Neither inhibition of PI-PLC with U73,122 (100 μM, n=5) or ET-18-OCH3 (100 μM, n=3), or PLD with propranolol (100 μM, n=4) had any effect on LTD4-induced bronchoconstriction. In contrast, the PC-PLC antagonist D609 (1 – 100 μM) relaxed the LTD4-induced constriction in a concentration-dependent manner with a IC50 ≈ 5 μM. At 100 μM it often produced depression below the baseline, with return to baseline tone after washout of D609 (n=8) (Figure 4). However the response to 10 μM carbachol was only reduced by D609 to 57±5% (n=6) even at 100 μM, whilst the response to KPSS was not affected (n=14). Preincubation with the PKC inhibitors cheleretrine (10 μM, n=7) or Ro31-8220 (10 μM, n=3) had no effect on LTD4-induced constriction. In contrast the protein tyrosine kinase inhibitor tyrphostin A23 (100 μM for 10 min) reduced the LTD4 response to 49±11% (n=4); the inactive analogue tyrphostin A1 was however without effect. The effects of inhibition of the various phospholipases and protein kinases are summarized in Figure 5.
Figure 4.
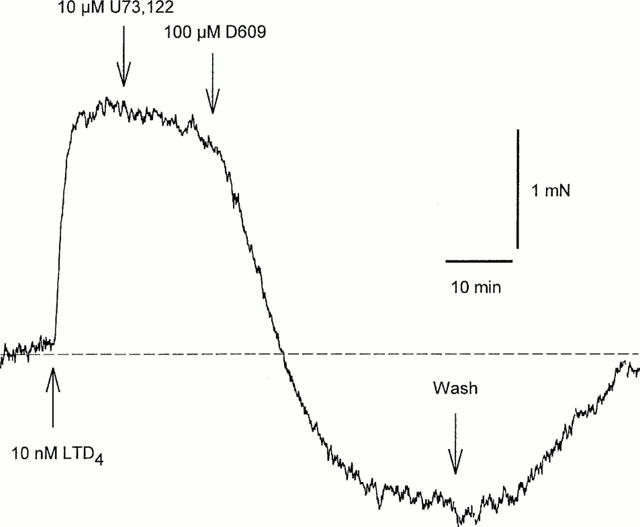
PI-PLC inhibitor U73,122 has no effect on LTD4-induced contraction, while PC-PLC inhibitor D609 causes complete relaxation.
Figure 5.
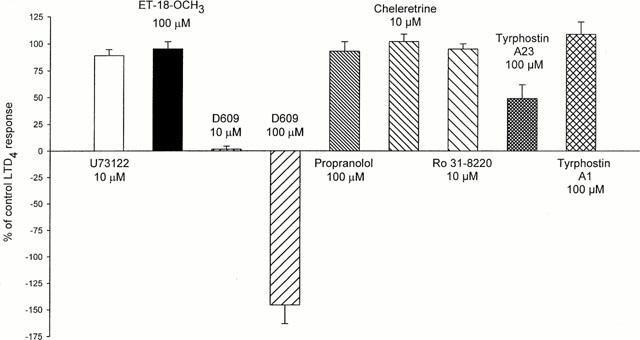
Effects of inhibitors of PI-PLC (U73,122 and ET-18-OCH3), PC-PLC (D609), PLD (propranolol), PKC (cheleretrine and Ro31 – 8220) and PTK (tyrphostin A23) on LTD4-induced contraction. Inactive tyrphostin A1 was used as control. n=4 – 8.
Basal tone
Human small bronchioles mounted on the myograph often possessed a variable basal tone which could be reversible abolished by removal of extracellular Ca2+ (n=14), or addition of 1 mM La3+ (n=4) or 100 μM SK&F 96,365 (n=4) (Figure 6a). 10 μM nifedipine had no significant effect on basal tone (n=12). D609 (100 μM) also caused complete relaxation of basal tone (n=4) (Figure 6b), while U73,122 and ICI 198,615 were without effect (n=5). It should be noticed that the preparations with the highest Ca-dependent basal tone demonstrated the smallest response to LTD4, and vice versa.
Figure 6.
(a) Basal tone in bronchiole could be abolished by Ca2+ removal or La3+, but not with nifedipine. (b) Variable amount of basal tone present in bronchioles dissected from the same specimen is blocked by PC-PLC inhibitor D609.
Intracellular stores
To study a role for intracellular Ca2+ stores, bronchioles were challenged with 10 μM cyclopiazonic acid (CPA) which induces Ca2+ release by inhibiting sarcoplasmic Ca-ATPase. CPA induced a maximal response in normal PSS, but did not cause any constriction in the absence of extracellular Ca2+ (n=3). However, restoration of normal Ca2+ after CPA had been washed out with a Ca2+-free solution caused a substantial constriction, similar to that seen for LTD4. No constriction was ever observed with caffeine (5 mM, n=7) or ryanodine (20 μM, n=2); in fact caffeine commonly caused a small fall in baseline tone.
Intracellular Ca2+ and electrophysiology
10 nM LTD4 caused visible shortening of the freshly isolated human bronchiole SMC loaded with Fura PE-3, accompanied with a slower rise in intracellular Ca2+ concentration, though this was much smaller than that induced with either caffeine, KPSS or CPA (Figure 7). Consistent with this rise in intracellular Ca2+, 10 nM LTD4 considerably increased the open state probability of large conductance, Ca2+-dependent K+-channels recorded in cell-attached configuration (n=6). LTD4 also greatly enhanced the activity of small conductance (∼25 pS) channels present in some patches (Figure 8a), which persisted long after the activity of large conductance K+ channels returned to control levels. In perforated patch whole-cell recording LTD4 enhanced an outward whole-cell current induced with depolarisation beyond +50 mV (n=5). Such enhancement persisted during prolonged washout of agonist, and could be abolished quickly and reversibly with the removal of extracellular Ca2+ or addition of 100 μM La3+ (Figure 8b), or 10-100 μM SK&F 96,365 (n=5, data not shown). In current-clamp mode 10 nM LTD4 failed to induce any change of the resting membrane potential (−40.−50 mV). At higher concentrations (>50 nM) LTD4 evoked Ca2+ transients in Fura PE-3 loaded cells, as well as a transient inward Cl− current, similar to the effects observed with caffeine (5 mM) when applied to the same cells (Figure 9).
Figure 7.
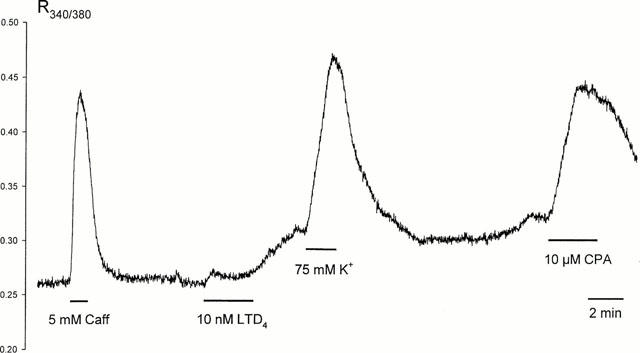
Changes in intracellular Ca2+ concentration measured in single smooth muscle cell isolated from human bronchiole. Data presented as Fura PE-3 emission ratio at excitation wavelengths of 340 and 380 nm.
Figure 8.
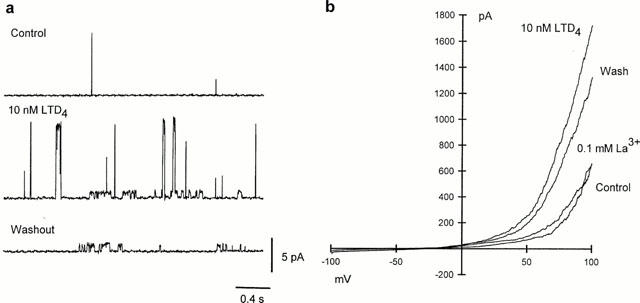
(a) LTD4 induces rise of activity of Ca2+-dependent large conductance K+ channels and smaller non-selective cation channels in cell-attached patch from single smooth muscle cell isolated from human bronchiole. Bath and pipette solution contained 140 mM KCl; membrane potential +60 mV. (b) Whole-cell responses to voltage ramp from −100 mV to +100 mV (holding potential between ramps −60 mV). Bath solution 5 mM KCl, pipette solution 140 mM KCl.
Figure 9.
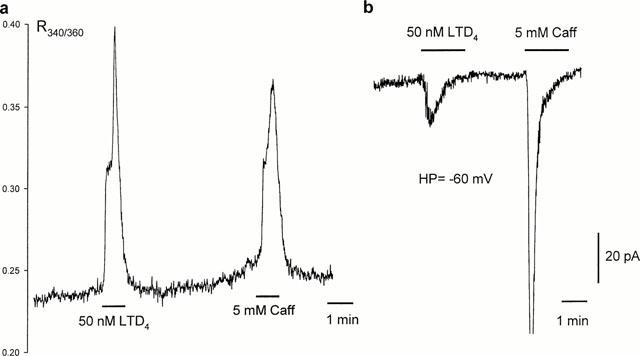
High concentration of LTD4 evokes Ca2+ transients in single Fura PE-3-loaded myocytes similar to that induced with caffein (a), as well as transient Cl− current in whole-cell voltage clamp condition (b).
Discussion
The results of the present study suggest that the contractile response to LTD4 in human small bronchioles is mediated by the CysLT1 receptor, which has recently been cloned (Lynch et al., 1999), and is dependent on a sustained influx of extracellular Ca2+. It has been proposed that LTD4 primarily induces bronchoconstriction in airways via IP3-mediated release from Ca2+ stores. However, removal of external Ca2+ has been shown to substantially reduce or abolish ASM contraction (Black et al., 1986; Matran et al., 1988). We also found that removal of external Ca2+ abolished LTD4-induced bronchoconstriction (Figures 1b and 2), as well as the responses to histamine and carbachol. The LTD4-induced increase of Ca2+-dependent K+ channels activity, which can be used to assess subplasmalemmal concentration of free Ca2+, was also abolished in Ca2+-free solution.
The above results do not necessarily rule out a role for IP3 generation and Ca2+ release, as PI-PLC is Ca2+-sensitive, and IP3 formation has been reported to be dependent on external Ca2+ (Dumitriu et al., 1997). Indeed, we have found that at very high concentrations (>50 nM) LTD4 could induce Ca2+ release (Figure 9). However, neither inhibition of PI-PLC with U73,122 or ET-18-OCH3 nor application of the IP3 receptor antagonist 2-APB had any effect on bronchoconstriction induced by more physiologically relevant concentrations of LTD4 (10 nM), which was in any case sufficient to induce maximum bronchoconstriction (Figure 1) in these small bronchioles. This is consistent with one other report on guinea-pig trachea, that suggested that Ca2+ release from stores makes little direct contribution to LTD4-induced contraction of ASM (Cuthbert et al., 1994). Indeed, even though caffeine (5 mM) induced a massive release of Ca2+ from intracellular stores followed by a transient inward Cl− current (Figures 7 and 9) and a longer lasting increase in Ca2+-dependent outward current, it did not cause any contraction in human small bronchioles. Moreover, it has been reported that in rat neonatal cardiac myocytes IP3 generation results rather from the rise of intracellular Ca2+ than from the cleavage of PIP2 (Matkovich & Woodcock, 2000). Wang & Kotlikoff (2000) have also reported that although U73,122 abolished histamine- or methacholine-induced Cl− and cationic currents in equine tracheal myocytes, simultaneous application of caffeine with these agonists restored the currents in the presence of U73,122. Thus it seems unlikely that Ca2+ release from IP3-sensitive stores is directly involved in activation of the contractile machinery.
Our results provide good evidence that the primary action of LTD4 is to induce Ca2+ influx across the cell membrane. In many types of smooth muscle voltage-operated Ca2+ channels (VOC) are clearly involved in Ca2+ entry, but although influx of Ca2+ via VOCs has been implicated in guinea-pig ASM, their role in human ASM remains controversial, especially in relation to the LTD4 response. The VOC agonist Bay K 8644 did not significantly modify the EC50 of acetylcholine or histamine on human main bronchus, but potentiated the effects of KCl on that preparation (Advenier et al., 1986). In human bronchial muscle preparations preincubated for 30 min with 10 μM diltiazem, the LTD4 cumulative concentration – effect curves following these drug treatments were similar to controls (Bourdillat et al., 1987). In human bronchial rings (2 – 5 mm) nifedipine had no effect on the LTD4 dose – response curve or on the maximal LTD4 contraction (Gorenne et al., 1998). On the other hand, Kohrogi et al. (1985) have reported that nifedipine decreased or reversed LTD4-induced contraction of human bronchial strips. We found that in human small bronchioles nifedipine could only partially block or reverse LTD4-induced contraction (Figure 3a). The possible involvement of T-type channels, which are highly sensitive to Ni2+, seems unlikely, as 1 mM Ni2+ did not affect contraction.
An alternative path for sustained extracellular Ca2+ entry is through Ca2+-permeable non-selective cation channels (NSCC) as proposed for muscarinic stimulation of equine ASM (Fleischmann et al., 1997), histamine in cultured human ASM (Murray & Kotlikoff, 1991), and specifically LTD4 in guinea-pig and human ASM (Gorenne et al., 1998; Cuthbert et al., 1994). These channels could be blocked with some selectivity with La3+ and SK&F 96,365. Indeed we have found that La3+, Gd3+ and SK&F 96,365 abolished LTD4-induced constriction in human bronchioles (Figure 3b,c), and MDL-12,330A, which has been shown to block NSCC and SOC in variety of cell types (Van rossum et al., 2000), was also an efficient inhibitor. These findings are consistent with the observation that SK&F 96,365 significantly reduced the nifedipine-resistant response to LTD4 in human bronchial rings (Gorenne et al., 1998), and together lend support to the hypothesis that LTD4-mediated Ca2+ entry is via NSCCs. We have previously characterized NSCCs in ASM from human foetal trachea and adult bronchioles (Snetkov et al., 1998; Snetkov & Ward, 1999), which have very similar properties to the channels activated by LTD4 and shown in Figure 8a.
The only molecular entities presently characterized functionally as channels responsible for receptor-mediated Ca2+ influx in vertebrate and invertebrate cells are transient receptor potential (TRP) protein homologues. Of the seven mammalian TRP homologues currently cloned, TRP3, TRP6 and TRP7 have been reported to be activated by DAG (Kiselyov & Muallem, 1999). The fact that D609 abolished LTD4-induced constriction in these small bronchioles could imply that LTD4 stimulates PC-PLC mediated production of DAG, and thus activation of plasmalemmal TRP channels, inducing influx of extracellular Ca2+. Although non-specific effects of D609 cannot be entirely ruled out, it should be noted that we found its EC50 for inhibition of LTD4-induced bronchoconstriction to be ≈5 μM, which is equivalent to the Ki for PC-PLC inhibition in vitro (5 – 10 μM). As PKC antagonists were without effect (Figure 5), it seems reasonable to assume that any stimulation of PKC by DAG plays little part in LTD4-induced bronchoconstriction.
Spontaneous activity of TRP3 and TRP7 channels in transfected cells has been reported to give rise to a background non-selective current and Ca2+ permeability (Zhu et al., 1998; Okada et al., 1998). Such activity could underlie the Ca2+-dependent but nifedipine resistant basal tone we have observed in human small bronchioles (Figure 6). It is notable that in contrast to the study on human bronchial strips of Fujiwara et al. (1993), the basal tone in these small bronchioles was apparently not related to basal release of leukotrienes, as it was not affected by ICI 198,615.
The fact that emptying of intracellular stores with CPA does not produce contraction per se, but apparently activates subsequent Ca2+ influx, may be interpreted as evidence for store-activated rather than second messenger-activated channels. Specifically, TRP4 channels have been suggested as a part of a native Ca2+ release activated channels in adrenal cells (Philipp et al., 2000). However, Schaefer et al. (2000) have reported that the properties of murine TRP4 suit this role considerably less well than reported for its bovine counterpart, and in vascular smooth muscle TRP1 has been suggested to fulfil a similar role (Xu & Beech, 2001). While the effect of CPA shows that SOC may well be present in human small bronchioles, the lack of any significant effect of low micromolar concentrations of trivalent cations or 2-APB on the LTD4-induced bronchoconstriction, both of which are reported to block SOC, tends to suggest that SOC is not the major Ca2+ entry pathway during LTD4 stimulation of human small bronchioles. Moreover, the ability of the PC-PLC antagonist D609 to block the LTD4 response implies involvement of second-messenger operated channels such as TRP3 or TRP6.
Recent data suggest that there might be a direct molecular interaction between components of the intracellular stores (such as the IP3 receptor itself) and plasmalemmal TRP channels (see Putney & Mckay, 1999). However, there is the possibility that Ca2+ released by CPA could activate phospholipid hydrolysis and produce second messenger(s) (Matkovich & Woodcock, 2000). It is very probable that more than one type of TRP channel is expressed in ASM. Indeed, both TRP6 and TRP7 have been found in lung (Boulay et al., 1997; Moore et al., 1998; Okada et al., 1999), while TRP1 has been shown to regulate endothelial permeability in the pulmonary vasculature (Moore et al., 1998).
In our experiments the PTK inhibitor tyrphostin A23 significantly suppressed LTD4-induced contraction. This is in agreement with previous findings that PTK inhibition reduces carbachol and serotonin-induced constriction of rat airways, particularly in small bronchioles (Chopra et al., 1997), and suppresses LTD4-induced bronchoconstriction in guinea-pig (Wong et al., 1997). Gronroos et al. (1995) have also demonstrated that tyrosine phosphorylation of PLC as well as other downstream targets modulates LTD4-mediated Ca2+ influx in human epithelial cells, and in human fibroblasts PTK inhibition blocked Ca2+ influx induced by bradykinin and thapsigargin (Lee et al., 1993). Taken together, these data allow us to consider a possible role for tyrosine phosphorylation in mediating Ca2+ influx in human bronchioles.
In conclusion, we have found that the constrictor effect of LTD4 in human small bronchioles is mediated by CysLT1 receptors, requires Ca2+ entry across the sarcolemma, and seems to be independent of PI-PLC and PKC. Ca2+ released from intracellular stores is unable to induce contraction directly, though store emptying could be inducing Ca2+ influx through TRP channels. It would appear that PC-PLC plays an important role in LTD4-induced bronchoconstriction in this tissue. This apparently does not involve activation of PKC by DAG, but we can speculate that DAG generated by this pathway is activating TRP channels and therefore Ca2+ influx.
Acknowledgments
Supported by Wellcome Trust grants 58166 (V.A. Snetkov) and 52411 (K.J. Hapgood) and the National Asthma Campaign; C.G. McVicker holds an MRC PhD studentship.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- ASM
airway smooth muscle
- DAG
diacylglycerols
- IP3
inositol 1,4,5-trisphosphate
- KPSS
physiological salt solution containing 80 mM KCl (equimolar substitution for NaCl)
- LTD4
leukotriene D4
- NSCC
non-selective cation channels
- PC-PLC
phosphatidylcholine phospholipase C
- PI-PLC
phosphatidylinositol phospholipase C
- PKC
protein kinase C
- PLD
phospholipase D
- PSS
physiological salt solution
- SOC
store-operated channels
- VOC
voltage-operated Ca2+ channels
References
- ADVENIER C., NALINE E., RENIER A. Effects of Bay K 8644 on contraction of the human isolated bronchus and guinea-pig isolated trachea. Br. J. Pharmacol. 1986;88:33–39. doi: 10.1111/j.1476-5381.1986.tb09468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCHER S.L. Diversity of phenotype and function of vascular smooth muscle cells. J. Lab. Clin. Med. 1996;127:524–529. doi: 10.1016/s0022-2143(96)90142-0. [DOI] [PubMed] [Google Scholar]
- ARM J.P., LEE T.H. Sulphidopeptide leukotrienes in asthma. Clin. Sci. 1993;84:501–510. doi: 10.1042/cs0840501. [DOI] [PubMed] [Google Scholar]
- BLACK J., ARMOUR C., JOHNSON P., VINCENC K. The calcium dependence of histamine, carbachol and potassium chloride-induced contraction in human airways in vitro. Eur. J. Pharmacol. 1986;125:159–168. doi: 10.1016/0014-2999(86)90023-3. [DOI] [PubMed] [Google Scholar]
- BOULAY G., ZHU X., PEYTON M., JIANG M., HURST R., STEFANI E., BIRNBAUMER L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- BOURDILLAT B., HAYE-LEGRAND I., LABAT C., RAFFESTIN B., NOREL X., BENVENISTE J., BRINK C. Effects of various pharmacological agents on isolated human bronchial and pulmonary arterial and venous muscle preparations contracted by leukotriene D4. Fundam. Clin. Pharmacol. 1987;1:433–444. doi: 10.1111/j.1472-8206.1987.tb00576.x. [DOI] [PubMed] [Google Scholar]
- CAMIÑA J.P., CASABIELL X., CASANUEVA F.F. Inositol 1,4,5-trisphosphate-independent Ca2+ mobilization triggered by a lipid factor isolated from vitreous body. J. Biol. Chem. 1999;274:28134–28141. doi: 10.1074/jbc.274.40.28134. [DOI] [PubMed] [Google Scholar]
- CHOPRA L.C., HUCKS D., TWORT C.H., WARD J.P.T. Effects of protein tyrosine kinase inhibitors on contractility of isolated bronchioles of the rat. Am. J. Respir. Cell Mol. Biol. 1997;16:372–378. doi: 10.1165/ajrcmb.16.4.9115747. [DOI] [PubMed] [Google Scholar]
- CHOPRA L.C., TWORT C.H., WARD J.P.T. Differences in sensitivity to the specific protein kinase C inhibitor RO31-8220 between small and large bronchioles of the rat. Br. J. Pharmacol. 1994;113:1237–1242. doi: 10.1111/j.1476-5381.1994.tb17130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOKE S.T., MONG S., SARAU H.M., WINKLER J.D., VEGESNA V.K. Mechanisms of regulation of receptors and signal transduction pathways for the peptidyl leukotrienes. Ann. N.Y. Acad. Sci. 1988;524:153–161. doi: 10.1111/j.1749-6632.1988.tb38538.x. [DOI] [PubMed] [Google Scholar]
- CUTHBERT N.J., GARDINER P.J., NASH K., POLL C.T. Roles of Ca2+ influx and intracellular Ca2+ release in agonist-induced contractions in guinea pig trachea. Am. J. Physiol. 1994;266:L620–L627. doi: 10.1152/ajplung.1994.266.6.L620. [DOI] [PubMed] [Google Scholar]
- DUMITRIU D., PRIE S., BERNIER S.G., GUILLEMETTE G., SIROIS P. Mechanism of action of leukotriene D4 on guinea pig tracheal smooth muscle cells: roles of Ca++ influx and intracellular Ca++ release. J. Pharmacol. Exp Ther. 1997;280:1357–1365. [PubMed] [Google Scholar]
- FLEISCHMANN B.K., WANG Y.X., KOTLIKOFF M.I. Muscarinic activation and calcium permeation of nonselective cation currents in airway myocytes. Am. J. Physiol. 1997;41:C341–C349. doi: 10.1152/ajpcell.1997.272.1.C341. [DOI] [PubMed] [Google Scholar]
- FUJIWARA H., KURIHARA N., OHT A., HIRATA K., MATSUSHITA H., KANAZAWA H., TAKEDA T. Effect of a new leukotriene receptor antagonist, ONO-1078, on human bronchial smooth muscle in vitro. Prostaglandins Leukot. Essent. Fatty Acids. 1993;48:241–246. doi: 10.1016/0952-3278(93)90092-b. [DOI] [PubMed] [Google Scholar]
- GAUTHIER S.P., WOLFSON M.R., DEORAS K.S., SHAFFER T.H. Structure-function of airway generations 0 to 4 in the preterm lamb. Pediatr. Res. 1992;31:157–162. doi: 10.1203/00006450-199202000-00013. [DOI] [PubMed] [Google Scholar]
- GORENNE I., LABAT C., GASCARD J.P., NOREL X., NASHASHIBI N., BRINK C. Leukotriene D4 contractions in human airways are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. J. Pharmacol. Exp. Therap. 1998;284:549–552. [PubMed] [Google Scholar]
- GRONROOS E., SCHIPPERT A., ENGSTROM M., SJOLANDER A. The regulation of leukotriene D4-induced calcium influx in human epithelial cells involves protein tyrosine phosphorylation. Cell Calcium. 1995;17:177–186. doi: 10.1016/0143-4160(95)90032-2. [DOI] [PubMed] [Google Scholar]
- HAY D.W., HUBBARD W.C., UNDEM B.J. Endothelin-induced contraction and mediator release in human bronchus. Br. J. Pharmacol. 1993;110:392–398. doi: 10.1111/j.1476-5381.1993.tb13822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD S., CHANYEUNG M., MARTIN L., PHANEUF S., SALARI H. Polyphosphoinositide hydrolysis and protein kinase C activation in guinea pig tracheal smooth muscle cells in culture by leukotriene D4 involve a pertussis toxin sensitive G-protein. Eur. J. Pharmacol. 1992;227:123–129. doi: 10.1016/0922-4106(92)90119-g. [DOI] [PubMed] [Google Scholar]
- KIM N., CAO W., SONG I.S., KIM C.Y., SOHN U.D., HARNETT K.M., BIANCANI P. Leukotriene D4-induced contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Gastroenterology. 1998;115:919–928. doi: 10.1016/s0016-5085(98)70264-1. [DOI] [PubMed] [Google Scholar]
- KISELYOV K., MUALLEM S. Fatty acids, diacylglycerol, Ins(1,4,5)P3 receptors and Ca2+ influx. Trends Neurosci. 1999;22:334–337. doi: 10.1016/s0166-2236(99)01426-5. [DOI] [PubMed] [Google Scholar]
- KOHROGI H., HORIO S., ANDO M., SUGIMOTO M., HONDA I., ARAKI S. Nifedipine inhibits human bronchial smooth muscle contractions induced by leukotrienes C4 and D4, prostaglandin F2α, and potassium. Am. Rev. Respir. Dis. 1985;132:299–304. doi: 10.1164/arrd.1985.132.2.299. [DOI] [PubMed] [Google Scholar]
- LEE K.M., TOSCAS K., VILLEREAL M.L. Inhibition of bradykinin- and thapsigargin- induced Ca2+ entry by tyrosine kinase inhibitors. J. Biol. Chem. 1993;268:9945–9948. [PubMed] [Google Scholar]
- LYNCH K.R., O'NEILL G.P., LIU Q., IM D.S., SAWYER N., METTERS K.M., COULOMBE N., ABRAMOVITZ M., FIGUEROA D.J., ZENG Z., CONNOLLY B.M., BAI C., AUSTIN C.P., CHATEAUNEUF A., STOCCO R., GREIG G.M., KARGMAN S., HOOKS S.B., HOSFIELD E., WILLIAMS D.L.J., FORD-HUTCHINSON A.W., CASKEY C.T., EVANS J.F. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- MA H.T., PATTERSON R.L., VAN ROSSUM D.B., BIRNBAUMER L., MIKOSHIBA K., GILL D.L. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- MATKOVICH S.J., WOODCOCK E.A. Ca2+-activated but not G protein-mediated inositol phosphate responses in rat neonatal cardiomyocytes involve inositol 1,4,5-trisphosphate generation. J. Biol. Chem. 2000;275:10845–10850. doi: 10.1074/jbc.275.15.10845. [DOI] [PubMed] [Google Scholar]
- MATRAN R., NALINE E., ADVENIER C., LOCKHART A., TRICOT J.F., REGOLI D. Role of extracellular calcium in the effects of substance P and neurokinin A on guinea pig trachea and human bronchus. Fundam. Clin. Pharmacol. 1988;2:47–55. doi: 10.1111/j.1472-8206.1988.tb00620.x. [DOI] [PubMed] [Google Scholar]
- MOORE T.M., BROUGH G.H., BABAL P., KELLY J.J., LI M., STEVENS Store-operated calcium entry promotes shape change in pulmonary endothelial cells expressing Trp1. Am. J. Physiol. 1998;275:L574–L582. doi: 10.1152/ajplung.1998.275.3.L574. [DOI] [PubMed] [Google Scholar]
- MORENO R.H., HOGG J.C., PARE P.D. Mechanics of airway narrowing. Am. Rev. Respir. Dis. 1986;133:1171–1180. doi: 10.1164/arrd.1986.133.6.1171. [DOI] [PubMed] [Google Scholar]
- MURRAY R.K., KOTLIKOFF M.I. Receptor-activated calcium influx in human airway smooth muscle cells. J. Physiol. 1991;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSTAFA S., THULESIUS L., THULESIUS O. The contractile response of thiopental in large and small ovine airways. Acta Anaesthesiol. Scand. 1994;38:499–504. doi: 10.1111/j.1399-6576.1994.tb03936.x. [DOI] [PubMed] [Google Scholar]
- OKADA T., INOUE R., YAMAZAKI K., MAEDA A., KUROSAKI T., YAMAKUNI T., TANAKA, SHIMIZU S., IKENAKA K., IMOTO K., MORI Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. J. Biol. Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- OKADA T., SHIMIZU S., WAKAMORI M., MAEDA A., KUROSAKI T., TAKADA N., IMOTO K., MORI Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J. Biol. Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- PHILIPP S., TROST C., WARNAT J., RAUTMANN J., HIMMERKUS N., SCHROTH G., KRETZ O., NASTAINCZYK W., CAVALIE A., HOTH M., FLOCKERZI V. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+ - like channels in adrenal cells. J. Biol. Chem. 2000;275:23965–23972. doi: 10.1074/jbc.M003408200. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W.J., MCKAY R.R. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- SALARI H., BRAMLEY A., LANGLANDS J., HOWARD S., CHAN-YEUNG M., CHAN H., SCHELLENBERG R. Effect of phospholipase C inhibitor U-73122 on antigen-induced airway smooth muscle contraction in guinea pigs. Am. J. Respir. Cell Mol. Biol. 1993;9:405–410. doi: 10.1165/ajrcmb/9.4.405. [DOI] [PubMed] [Google Scholar]
- SCHAEFER M., PLANT T.D., OBUKHOV A.G., HOFMANN T., GUDERMANN T., SCHULTZ G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- SNETKOV V.A., HIRST S.J., TWORT C.H., WARD J.P.T. Potassium currents in human freshly isolated bronchial smooth muscle cells. Br. J. Pharmacol. 1995;115:1117–1125. doi: 10.1111/j.1476-5381.1995.tb15926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNETKOV V.A., PANDYA H., HIRST S.J., WARD J.T. Potassium channels in human fetal airway smooth muscle cells. Pediatric Research. 1998;43:548–554. doi: 10.1203/00006450-199804000-00019. [DOI] [PubMed] [Google Scholar]
- SNETKOV V.A., WARD J.P. Ion currents in smooth muscle cells from human small bronchioles: presence of an inward rectifier K+ current and three types of large conductance K+ channel. Exp. Physiol. 1999;84:835–8460. [PubMed] [Google Scholar]
- VAN ROSSUM D.B., PATTERSON R.L., MA H.T., GILL D.L. Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels. J. Biol. Chem. 2000;275:28562–28568. doi: 10.1074/jbc.M003147200. [DOI] [PubMed] [Google Scholar]
- WANG Y.X., KOTLIKOFF M.I. Signalling pathway for histamine activation of non-selective cation channels in equine tracheal myocytes. J. Physiol. 2000;523:131–138. doi: 10.1111/j.1469-7793.2000.t01-3-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG W.S., KOH D.S., KOH A.H., TING W.L., WONG P.T. Effects of tyrosine kinase inhibitors on antigen challenge of guinea pig lung in vitro. J. Pharmacol. Exp. Therap. 1997;283:131–137. [PubMed] [Google Scholar]
- XU S.Z., BEECH D.J. TrpC1 is a membrane-spanning subunit of store-operated Ca(2+) channels in native vascular smooth muscle cells. Circ. Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- ZHU X., JIANG M., BIRNBAUMER L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK) 293 cells. J. Biol. Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]


