Abstract
Shortened, more stable and weakly hydrophobic analogues of melanin-concentrating hormone (MCH) were searched as candidates for radioiodination. Starting from the dodecapeptide MCH6 – 17, we found that: (1) substitution of Tyr13 by a Phe residue; (2) addition of a 3-iodo-Tyr residue at the N-terminus; and (3) addition of a hydrophilic spacer 8-amino-3,6-dioxyoctanoyl between the 3-iodo-Tyr and MCH6 – 17 (compound S36057), led to an agonist more potent than MCH itself in stimulating [35S]-GTPγS binding at membranes from HEK293 cells stably expressing the human MCH receptor.
Specific binding of [125I]-S36057 was found in HEK293 and CHO cell lines stably expressing the human MCH receptor. This radioligand recognized a similar number of binding sites (ca. 800 fmol mg−1) than [125I]-[3-iodo Tyr13]-MCH.
However, the KD for [125I]-S36057 obtained from saturation studies (0.037 nM) or from binding kinetics (0.046 nM) was at least 10 fold higher to that of [125I]-[3-iodo Tyr13]-MCH (0.46 nM).
Affinities determined for a series of MCH analogues were similar with both radioligands, S36057 being the most potent compound tested (Ki=0.053 nM).
Finally, [125I]-S36057 also potently labelled the MCH receptor in membranes from whole rat brain (KD 0.044 nM, Bmax=11 fmol mg−1).
In conclusion, [125I]-S36057 is a more potent and more stable radioligand than [125I]-[3-iodo Tyr13]-MCH that will represent a reliable tool for binding assays in the search of novel MCH ligands. It should also provide great help for autoradiographic studies of the MCH receptor distribution in the central nervous system.
Keywords: [125I]-(3-iodo Tyr13)-MCH binding, [125I]-S36057 binding, MCH, SLC-1 receptor
Introduction
Human melanin-concentrating hormone (MCH) is a cyclic nonadecapeptide, involved in the regulation of feeding behaviour (Qu et al., 1996; Presse et al., 1996; Rossi et al., 1997; Tritos & Maratos-Flier, 1999; Shimada et al., 1998). While MCH has been known for a long time (Kawauchi et al., 1983), its receptor has been cloned only recently by reverse pharmacology (Chambers et al., 1999; Saito et al., 1999; Shimomura et al., 1999; Bachner et al., 1999; Lembo et al., 1999, for review see Saito et al., 2000). The MCH function was assigned to the orphan receptor SLC-1 (Kolakowski et al., 1996; Lakaye et al., 1998), on the basis of the observed inhibition of forskolin-stimulated cyclic AMP production and induction of calcium rise. Indeed, the lack of suitable binding conditions, due mainly to the hydrophobic and sticky nature of MCH (Drozdz & Eberle, 1995a; Kokkotou et al., 2000), was a limiting step for expression cloning. Now that the receptor has been cloned, the distribution of receptor mRNA in the central nervous system has been characterized precisely (Hervieu et al., 2000). However no autoradiographic studies showing the distribution of the receptor protein are yet available. In the past, several attempts failed to find either a tritiated or an iodinated (Drozdz & Eberle, 1995a; Hintermann et al., 1999) potent and stable ligand, although the iodinated analogue [125I]-[Phe13Tyr19]-MCH was shown to detect a potential MCH site in several cell lines and tissues (Drozdz & Eberle, 1995b; Burgaud et al., 1997). However, comparison of the functional and binding data obtained at the recently cloned receptor, with those previously reported in native cell lines, showed that the latter MCH binding sites did not match those at the cloned MCH receptor (Drozdz & Eberle, 1995a; Drozdz et al., 1995; Burgaud et al., 1997; Chambers et al., 1999; Lembo et al., 1999). Therefore, the results obtained in these studies, including the MCH structure-activity relationships, should be interpreted cautiously (Drozdz & Eberle, 1995a; Drozdz et al., 1995). Iodination of the residue Tyr13, inside the Cys – Cys loop, of human MCH has been achieved recently and three groups have reported limited binding data at both cloned and native human MCH receptors (Chambers et al., 1999; Sone et al., 2000; Macdonald et al., 2000). However, the sticky nature of MCH and its sensitivity to proteolysis (Checler et al., 1992; Castrucci et al., 1992; Hintermann et al., 1999; Kokkotou et al., 2000), required the discovery of a shorter, more stable radiolabelled and potentially less hydrophobic ligand.
In a recent and extensive study characterizing 57 MCH analogues for their functional activity at the human cloned MCH receptor to inhibit cyclic AMP formation or alternatively to stimulate [35S]-GTPγS binding, we have shown that the minimal sequence required for a potent MCH agonist activity was the dodecapeptide MCH6 – 17 (Audinot et al., 2001). In the present study, analogues of this peptide were investigated as candidates for iodination. The agonist activity at the human MCH receptor was evaluated through two functional assays: the inhibition of forskolin-stimulated cyclic AMP level and the stimulation of [35S]-GTPγS binding. The strategy involved the substitution of Tyr13 by a Phe residue and the addition of a3-iodo-Tyr residue at the N-terminal position which was linked to Arg6 via a relatively hydrophilic spacer (dioxyoctanoyl) leading to compound S36057. The characterization of [125I]-S3605 binding was compared with that of [125I]-(3-iodo Tyr13)-MCH at the human cloned receptor as well as in rat brain membranes.
Methods
Peptides
Most of the natural and modified peptides were custom-synthesized from Neosystem, Strasbourg, France, and checked by HPLC and mass spectrometry. Analytical data of the peptides are given in Table 1.
Table 1.
Analytical data of the peptides

Iodination of [125I]-S36057
S36057 (50 μg; 1.0 mg ml−1) in phosphate buffer (0.2 M; pH 7.4; 300 μl) in an Eppendorf tube was mixed with sodium [125I]-iodide (IMS30; 249 MBq; 7 mCi; 70 μl; Amersham Pharmacia Biotech, England). Reaction was initiated by addition of lactoperoxidase (25 IU ml−1; 50 μl) and hydrogen peroxide (0.003%; 50 μl) and allowed to react for 20 min. The reaction mixture was loaded onto a Jupiter C-5 RP-HPLC column (250×4.6 mm; Phenomenex, U.K.) and purified to obtain the mono-iodinated product (74 TBq mmol−1, 2000 Ci mmol−1) using a linear gradient with water, acetonitrile and trifluoracetic acid. The product was diluted with a sodium phosphate (50 mM; pH 7.4), lactose (5%), bovine serum albumin (0.25%; RIA grade), L-methionine (0.1%) and aprotinin (0.3 TIU ml−1) buffer to obtain 100 μCi ml−1 in the buffer. The product was freeze-dried overnight and stored at 4°C.
Establishment of stable cell lines
HEK293 (HEK) or CHO cells grown in DMEM medium supplemented with 10% foetal calf serum, 2 mM glutamine, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin were seeded at 5×106 cells in a T75 cm2 culture flask. Twenty-four hours later, they were transfected with 10 μg of the pcDNA3.1 (Invitrogen, Groningen, The Netherlands) containing the human MCH receptor using lipofectamine (Life Technologies, Cergy Pontoise, France) as previously described (Audinot et al., 2001). The day following transfection, cells were trypsinized, resuspended in complete DMEM medium containing 800 μg ml−1 of active geneticin and seeded at different dilutions in 96-well plates which were kept 2 – 3 weeks in a humidified CO2 incubator. At the end of this selection period, isolated clones were picked, amplified and further characterized by cyclic AMP experiments. For each cell line, one positive clone was subcloned before being used for all the cyclic AMP, [35S]-GTPγS and receptor binding experiments. The stable HEK293 and CHO cell lines expressing the human MCH receptor were, respectively, named HEK-hMCH-R and CHO-hMCH-R.
Intracellular cyclic AMP assay
Intracellular cyclic AMP was determined using the Flashplate technology (SMP004 New England Nuclear, Les Ulis, France). Briefly, forskolin (15 μM) and test peptides diluted in 0.1% BSA were added into 96-well flashplates and incubation was started with the addition of HEK-hMCH-R cells stably expressing the human or rat MCH receptor (35,000 cells per well). After 15 min at 37°C, incubation was stopped by the addition of the revelation mix and 2 h later, plates were counted on a TopCount (Packard, Meriden, CT, U.S.A.).
Membrane preparations of cell lines
Cell lines stably expressing the human MCH receptor were grown to confluency, harvested in PBS buffer containing 2 mM EDTA and centrifuged at 1000×g for 5 min (4°C). The resulting pellet was suspended in 20 mM HEPES buffer (pH 7.5), containing 5 mM EGTA and homogenized using a Kinematica polytron. The homogenate was then centrifuged (95,000×g, 30 min, 4°C) and the resulting pellet suspended in 50 mM HEPES buffer (pH 7.5), containing 10 mM MgCl2 and 2 mM EGTA. Aliquots of membrane preparations were stored at −80°C until use.
Rat brain membrane preparation
Frozen whole rat brains (Iffa Credo, L'Arbresle, France) were suspended using a Kinematica polytron in 50 mM TRIS buffer (pH 7.4), containing (in mM): NaCl 120, KCl 5, MgCl2 1 and CaCl2 2.5. The suspension was centrifuged for 15 min at 50,000×g. The resulting pellet was resuspended in the same buffer and centrifuged again. Aliquots of membrane preparations were stored at −80°C until use.
[35S]-GTPγS binding
Membranes and peptides were diluted in 50 mM HEPES buffer (pH 7.4), containing 100 mM NaCl, 3 μM GDP, 5 mM MgCl2, 0.1% BSA, and 10 μg ml−1 saponin. Incubation was started by the addition of 0.2 nM [35S]-GTPγS (1000 Ci mmol−1, Amersham Pharmacia Biotech, Orsay, France) to membranes (25 μg ml−1) and test compounds, and continued for 45 min at room temperature. Non-specific binding was defined using nonradiolabelled GTPγS (10 μM). Reaction was stopped by rapid filtration through GF/B unfilters followed by three successive washes with ice cold buffer. Data were analysed by non-linear regression using the program Prism (GraphPad Software Inc., San Diego, CA, U.S.A.), to yield EC50 (Effective Concentration50).
[125I]-(3-iodo Tyr13)MCH and [125I]-S36057 binding
Cell lines membranes (10 – 25 μg ml−1) or rat brain membranes (50 μg ml−1) were incubated for 90 min at room temperature in 25 mM HEPES buffer (pH 7.4), containing 1 mM CaCl2, 5 mM MgCl2, 0.5% BSA, in a final volume of 250 μl containing, except where stated otherwise, 0.02 nM of [125I]-(3-iodo Tyr13)-MCH (2000 Ci mmol−1, NEN, Les Ulis, France) or [125I]-S36057 (vide supra, 2000 Ci mmol−1) and test peptides. Non-specific binding was defined with 1 μM MCH. The reaction was stopped by rapid filtration through GF/B unifilters presoaked with polyethyleneimine 0.5%, followed by three successive washes with ice cold buffer. Data were analysed by non-linear regression using the program Prism. For displacement experiments, inhibition constants (Ki) were calculated according to the Cheng-Prusoff equation: Ki=IC50/[1+(L/KD)], where IC50 is the Inhibitory Concentration50, L is the concentration and KD the dissociation constant of the radioligand.
Results
Design of a shortened iodinated MCH analogue
In both tests performed on human MCH receptors expressed in HEK293 cells (HEK-hMCH-R), MCH6 – 17 was less potent than MCH: by ca. 10 fold in the cyclic AMP assay and only by 2 fold in the [35S]-GTPγS binding assay (Table 2, Figure 1). However, addition of a terminal Tyr residue in position 5, bearing (compounds 2, 4 and S36057) or not (compounds 1, 3 and 5) an iodo group restored or even increased potencies as compared to MCH6 – 17 in both tests (Table 2). Substitution of Tyr13 by a Phe residue did not significantly alter the potency (compounds 3, 4, 5 and S36057). Addition of different spacers: Gly – Gly (compounds 1, 2, 3 and 4), 8-amino-3,6-dioxyoctanoyl (ADO, compounds 5 and S36057) between a N-terminal tyrosine and MCH6 – 17 did not strongly alter the activity (Table 2). The final choice of the candidate for radioiodination was for compound 5 which was more potent than MCH at least in the [35S]-GTPγS binding assay (Table 2, Figure 1). Interestingly, S36057 was more stable towards proteolysis than MCH, in protease-rich media (M. Bertrand, 2001, personal communication).
Table 2.
Functional potencies of MCH analogues to inhibit forskolin-induced cyclic AMP level in HEK-hMCH-R cells or to stimulate [35S]-GTPγS binding to membranes from HEK-hMCH-R cells

Figure 1.
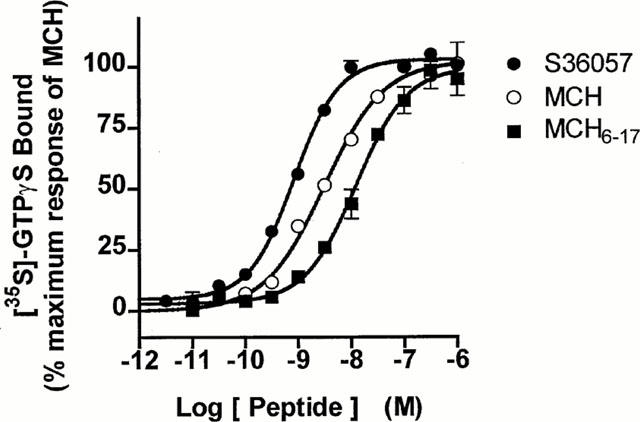
Dose-dependent stimulation of [35S]-GTPγS binding by S36057, MCH or MCH6–17 to HEK-hMCH-R membranes. Results are expressed as percentage of effect versus the maximal effect of MCH 1 μM (=100%). Basal binding level corresponded to 2736±180 d.p.m. and the MCH maximal response (1 μM) to 11750±936 d.p.m. (corresponding to a 4.3 fold stimulation above basal). Points shown are from representative experiments performed in triplicates and repeated at least three times.
[125I]-S36057 selectively binds to the human MCH receptor
The ability of [125I]-S36057 (0.02 nM) to selectively label the MCH receptor expressed either in HEK or CHO cells (CHO-hMCH-R) was evaluated (Figure 2). A saturable protein dose-dependent binding was observed in both transfected cell lines with a similar plateau, which may be indicative of a similar number of sites in both preparations. In contrast, no specific binding was observed on membranes from the corresponding native cells nor from the SVK14 cell line, even at a high membrane concentration (Figure 2). Similar observations were made with [125I]-(3-iodo Tyr13)-MCH (not shown). Specific binding represented typically 70 – 75% of total binding for both radioligands for a protein concentration of 10 – 25 μg ml−1, which was then routinely used.
Figure 2.

Specific binding of [125I]-S36057 (0.02 nM) to membranes from HEK-hMCH-R or CHO-hMCH-R cells stably transfected with the human SLC-1 receptors, and from native HEK, CHO and SVK14 cells. The protein concentrations tested ranged from 0 to 200 μg/ml.
Saturation studies: [125I]-S36057 is a highly potent radioligand
As shown in Figure 3 and in Table 3, [125I]-S36057 and [125I]-(3-iodo Tyr13)-MCH labelled a similar number of sites at HEK-hMCH-R membranes. However, when comparing the KD values [125I]-S36057 was a more potent radioligand than [125I]-(3-iodo Tyr13)-MCH, by a factor of 10 (Table 3). [125I]-S36057 labelled a similar number of sites in both HEK-hMCH-R and CHO-hMCH-R membranes as proven by their similar Bmax (Table 3) and as expected from the similar plateau obtained in the dose-dependent protein binding saturation (Figure 2). In both cell lines, the KD values of [125I]-S36057 were similar (Table 3).
Figure 3.
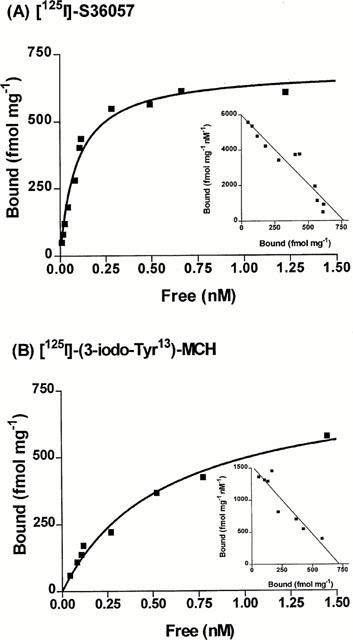
Saturation binding experiments to HEK-hMCH-R membranes. Specific binding is represented. Inset, Scatchard plot of the specific binding. Points shown are from representative experiments performed in triplicates and repeated at least three times.
Table 3.
KD and Bmax from saturation analysis of [125I]-S 36057 and [125I]-(3-iodo-Tyr13)-MCH binding to HEK-hMCH-R and CHO-hMCH-R membranes

Association and dissociation kinetics of [125I]-S36057 binding to HEK-hMCH-R membranes
Binding association of [125I]-S36057 was achieved in 60 min and stable for at least 160 min while dissociation was completely achieved at 3 h (Figure 4). The k+1obs of association was 0.027±0.003 min−1 (n=3) and the k−1 for dissociation was 0.018±0.001 min−1 (n=2). According to the equation k+1=(k+1obs−k−1)/L where L is the concentration of radioligand (0.02 nM), k+1 was equal to 0.39 min−1 nM−1. Calculation of the dissociation constant of [125I]-S36057 as the ratio of k−1 vs k+1 gave a value of 0.046 nM similar to the KD obtained from saturation experiments (Table 3).
Figure 4.
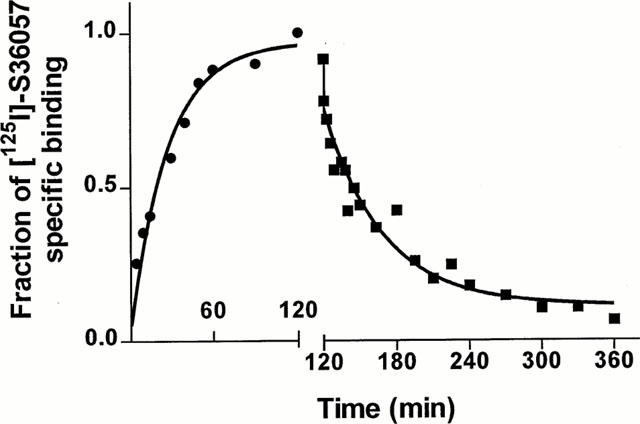
Association (•) and dissociation (▪) kinetics of 0.02 nM [125I]-S36057 specific binding to HEK-hMCH-R membranes. Both association and dissociation were monophasic. The apparent association rate constant (kobs) was 0.027±0.003 (n=3) min−1. Dissociation rate constant was determined after an incubation of 120 min by the addition of 1 μM MCH, giving the dissociation rate constant of 0.018±0.001 min−1 (n=2). The calculated association rate constant was 0.39 min−1 nM−1 and the derived KD was 0.046 nM. Points shown are from a representative experiment.
Pharmacological characterization of [125I]-S36057 binding to HEK-hMCH-R membranes
Inhibition binding isotherms of [125I]-S36057 (0.02 nM) in the presence of MCH analogues were monophasic (Figure 5A, B). MCH, salmon MCH and the human analogue [Phe13,Tyr19]-MCH exhibited subnanomolar affinities (Table 4). The fragment MCH6 – 17 and (3-iodo-Tyr13)-MCH retained potent binding affinity (Figure 5B, Table 4). It is noteworthy that compound S36057 was the most potent agonist tested (Figure 5B, Table 4). The linear analogue of MCH6 – 17 obtained by replacement of the two Cys by two Ser residues, compound S36077 (Table 1), led to a compound far less potent than MCH6 – 17 (Figure 5B, Table 4). The three compounds S36541, S36539 and S36540 (Table 1), also derived from MCH6 – 17, substituted with non natural amino acids and with the replacement of the cystine bridge by an amide bond – previously described as MCH antagonists (Audinot et al., 2001) – showed relatively potent affinities (Figure 5B, Table 4). Similar affinities were obtained using [125I]-(3-iodo Tyr13)-MCH as a radioligand (Table 4), and this was demonstrated by the significant correlation (r=0.992, P<0.0001, n=10) between affinities determined either with [125I]-S36057 or [125I]-(3-iodo Tyr13)-MCH (Figure 6).
Figure 5.
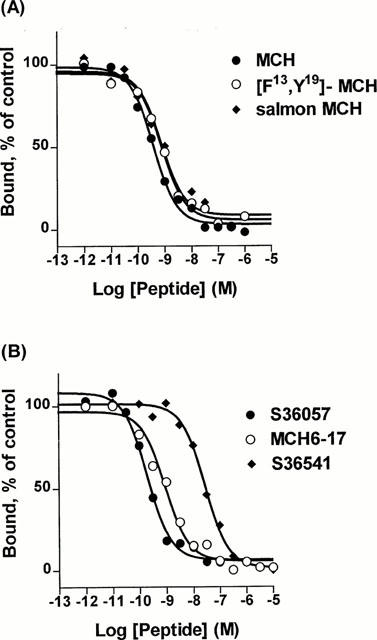
Concentration isotherms of [125I]-S36057 specific binding to HEK-hMCH-R membranes. Points shown are from a representative experiment performed in triplicates and repeated at least three times.
Table 4.
Potency of compounds to inhibit [125I]-S 36057 and [125I]-(3-iodo-Tyr13)-MCH binding to HEK-hMCH-R membranes
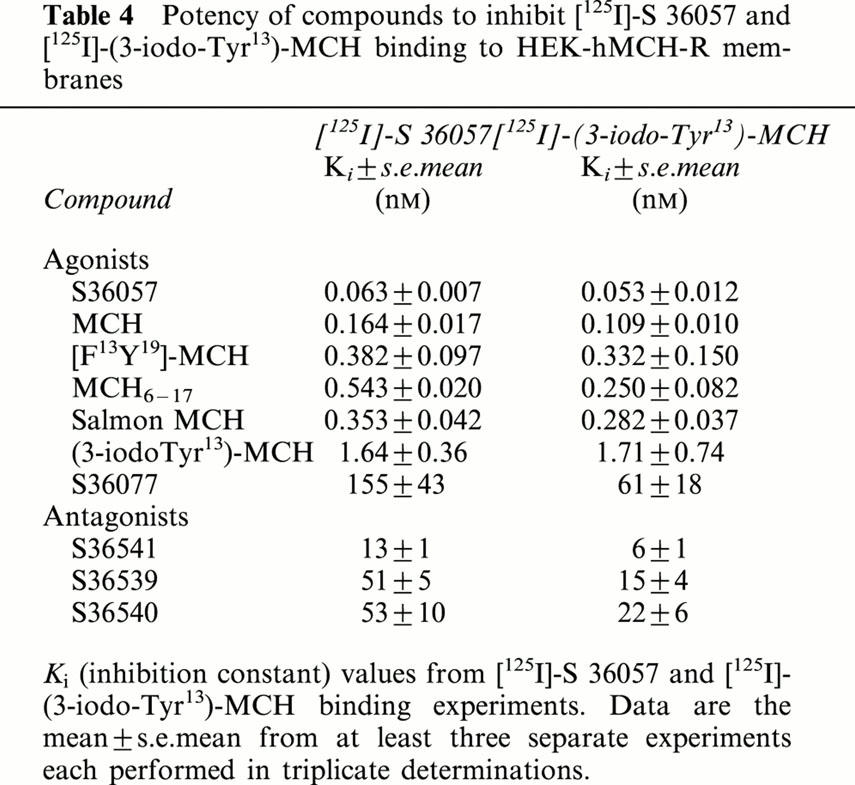
Figure 6.
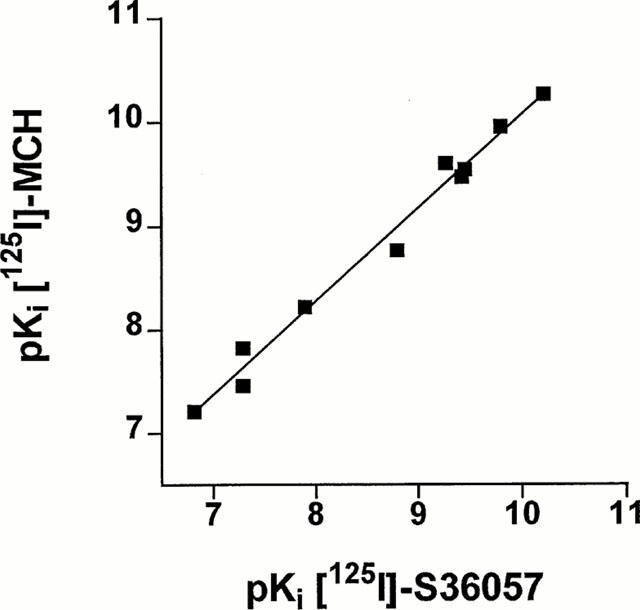
Correlation plot of binding affinities (expressed as pKi=−log Ki) determined either with [125I]-S36057 or with [125I]-(3-iodo Tyr13)-MCH at HEK-hMCH-R membranes. Data were calculated from Table 4. The correlation coefficient was 0.992 (P<0.0001, n=10).
[125I]-S36057 binding at the rat MCH receptor
[125I]-S36057 binding at the rat cloned MCH receptor expressed in HEK293 cells showed similar binding characteristics (Suply et al., 2001, unpublished results) than those obtained with HEK-hMCH-R. In particular, a similar potent dissociation constant was observed (KD=0.029±0.002 nM, Bmax=1608±123 fmol/mg, n=4). Binding to rat brain membranes was also performed. Specific binding of [125I]-S36057 and [125I]-MCH only represented ca. 30% of total binding. Attempts to reduce the non-specific binding failed to succeed (data not shown). Nevertheless, it was possible to perform [125I]-S36057 binding saturation experiments which gave a saturable specific binding (Figure 7). The number of binding sites was 11±4 fmol/mg and the dissociation constant was 0.044±0.007 nM (n=4).
Figure 7.
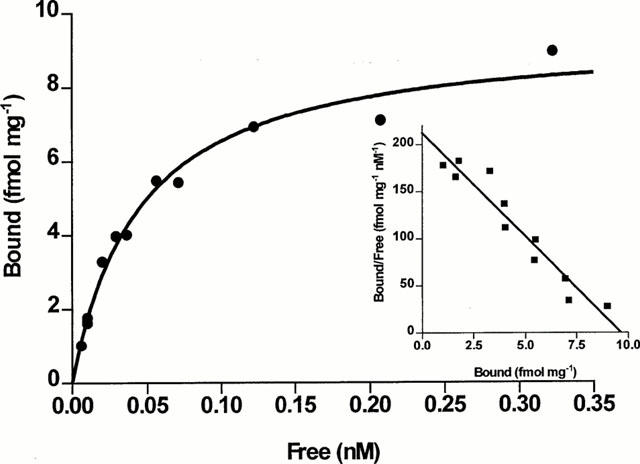
Saturation binding experiments of [125I]-S36057 to rat brain membranes. Specific binding is represented. Inset, Scatchard plot of the specific binding. Points shown are from representative experiments performed in triplicates and repeated four times. In this experiment, the KD and Bmax were respectively 0.045 nM and 9.6 fmol mg−1.
Discussion
In this study a new highly potent radioligand was described, which mimics the structure and function of MCH and which could be used for the characterization of MCH receptors. Starting from the dodecapeptide MCH6 – 17, which was described as the minimal sequence to retain potent agonistic activity (Audinot et al., 2001), several modifications were performed. Interestingly, the addition of a tyrosine residue at the N-terminus of MCH6 – 17 over either the Gly – Gly or the nine-atom linear spacer ADO, even improved its potency as detected through inhibition of cyclic AMP production or [35S]-GTPγS binding. Further, neither the iodination of this residue nor the Phe substitution in position 13 were deleterious to agonist activity. Compound S36057, which was chosen for radioiodination, was even more potent than MCH itself. This new ligand is both shorter and, due to the presence of the ADO spacer, more hydrophilic – and thus less sticky – than MCH. Shortening of fish MCH has previously been shown to protect against proteolysis as compared to full-length MCH (Checler et al., 1992; Castrucci et al., 1992) as was also the case for S36057. Indeed, [125I]-S36057 was, in our hands, more stable as a radioligand than [125I]-(3-iodo Tyr13)-MCH. A similar observation was made comparing [125I]-[Phe13,3-iodo Tyr19]-MCH and [125I]-(3-iodo Tyr13)-MCH (Kokkotou et al., 2000), thus suggesting that iodination of the Tyr13 residue inside the Cys-loop could be deleterious to activity.
The radioligand [125I]-S36057 specifically recognized binding sites on membranes from the HEK293 and CHO cell lines stably transfected with the human MCH receptor. In contrast and as expected, no specific binding was found in native cells. Similarly, no specific binding of [125I]-S36057 was observed on membranes from the SVK14 cell line. Previous studies in this cell line as well as in others (Burgaud et al., 1997) have reported the existence of a putative MCH binding site using [125I]-[Phe13,3-iodo Tyr19]-MCH as a radioligand. However, these binding sites are not likely to represent the MCH receptor (at least the SLC-1 form) since: (1) in our experimental binding conditions, which can detect the MCH receptors specifically expressed in the transfected cell lines, no binding was observed; (2) no messenger mRNA encoding for the MCH receptor was found in the SVK14 cell line (M. Rodriguez, 2001 unpublished results); and (3) there are strong discrepancies between binding affinities reported in the SVK14 cells and functional activities in transfected cells especially for salmon MCH and other peptides such as ANF (Burgaud et al., 1997; Chambers et al., 1999, Lembo et al., 1999; Audinot et al., 2001). Moreover, Kokkotou et al. (2000) have recently reported in several cell lines, the existence of a non-specific MCH binding site which was not associated with the plasma membrane. These particular binding sites thus need to be further characterized.
The two ligands [125I]-(3-iodo Tyr13)-MCH and [125I]-S36057 recognized a similar number of binding sites on membranes from the HEK-hMCH-R cell line (Table 3). The dissociation constant showed [125I]-S36057 to be 10 fold more potent than [125I]-(3-iodo Tyr13)-MCH (KD 0.037 and 0.46 nM, respectively). This highly potent KD for [125I]-S36057 was also confirmed in the CHO cell line stably expressing a similar number of binding sites than the HEK transfected cell line. In order to confirm the value of the dissociation constant of [125I]-S36057 determined through saturation experiments, the KD was also calculated from the parameters of binding kinetics and a similar value was observed (Table 3). These results confirmed [125I]-S36057 as a 10 fold more potent radioligand than [125I]-(3-iodo Tyr13)-MCH.
S36057 was also more potent than MCH in competition experiments either using [125I]-S36057 or [125I]-(3-iodo Tyr13)-MCH as radioligand. For the seven agonist peptides tested, the rank order of binding affinity was comparable with their functional potencies (this study and Audinot et al., 2001). Three potent antagonists also derived from MCH6 – 17 but extensively substituted with non natural amino acids and with the replacement of the cystine bridge by an amide bond have been reported (Audinot et al., 2001). Significantly these compounds – S36541, S36539 and S36540 – showed potent binding affinities (Figure 5B, Table 4). Therefore, these compounds may represent a useful tool for in vitro studies of MCH function. For the 10 peptides tested, there was an excellent correlation between the affinities determined with the two radioligands.
Finally, the fact that [125I]-S36057 binding to either HEK cells stably expressing the rat MCH receptor, or to rat brain membranes gave a potent KD, similar to the KD of the human counterpart (as well as a comparable pharmacological profile, Suply et al., 2001, unpublished results), is of importance in view of the high homology of the two species receptors and of the necessary use of rat in both in vivo animal models and autoradiographic studies. In our hands, however, a high level of non-specific binding was observed in rat brain membranes both with [125I]-(3-iodo Tyr13)-MCH and [125I]-S36057. Such a high level of non-specific binding has also been mentionned by Sone et al. (2000) using [125I]-(3-iodo Tyr13)-MCH in rat brain. This non-specific binding however might be diminished using brain structures enriched in the MCH receptor (Hervieu et al., 2000).
In conclusion, we have designed, synthesized and used in binding studies [125I]-S36057, a shorter, more potent, more stable and more hydrophilic radioligand than the corresponding [125I]-(3-iodo Tyr13)-MCH. The availability of both this ligand and the cloned receptor provides MCH research with a reliable binding assay suitable for testing large numbers of potential MCH ligands. In addition, the new compound may represent a radioligand of choice for future autoradiographic studies of the MCH receptor distribution.
Abbreviations
- CHO
Chinese hamster ovary
- [35S]-GTPγS
guanosine-5′-O-(3-[35S]-thio-triphosphate)
- HEK
human embryonic kidney
- MCH
melanin concentrating hormone (human, rat, mouse)
- SLC-1
somatostatin-like receptor 1
References
- AUDINOT V., BEAUVERGER P., LAHAYE C., SUPLY T., RODRIGUEZ M., OUVRY C., LAMAMY V., IMBERT J., RIQUE H., NAHON J.-L., GALIZZI J.-P., CANET E., LEVENS N., FAUCHERE J.-L., BOUTIN J.A.Structure activity studies of MCH-related peptide ligands at SLC-1, the human melanin-concentrating hormone receptor J. Biol. Chem. 2001. in press [DOI] [PubMed]
- BACHNER D., KREIENKAMP H.J., WEISE C., BUCK F., RICHTER D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- BURGAUD J.L., POOSTI R., FEHRENTZ J.A., MARTINEZ J., NAHON J.L. Melanin-concentrating hormone binding sites in human SVK14 keratinocytes. Biochem. Biophys. Res. Commun. 1997;241:622–629. doi: 10.1006/bbrc.1997.7849. [DOI] [PubMed] [Google Scholar]
- CASTRUCCI A.M., VISCONTI M.A., MATSUNAGA T.O., HADLEY M.E., HRUBY V.J. Enzymological studies of melanin concentrating hormone (MCH) and related analogues. Comp. Biochem. Physiol. (B). 1992;130:317–320. doi: 10.1016/0305-0491(92)90298-6. [DOI] [PubMed] [Google Scholar]
- CHAMBERS J., AMES R.S., BERGSMA D., MUIR A., FITZGERALD L.R., HERVIEU G., DYTKO G.M., FOLEY J.J., MARTIN J., LIU W.S., PARK J., ELLIS C., GANGULY S., KONCHAR S., CLUDERAY J., LESLIE R., WILSON S., SARAU H.M. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- CHECLER F., DAUCH P., BARELLI H., NAHON J.L., VINCENT J.P. Hydrolysis of rat melanin-concentrating hormone by endopeptidase 24.11 (neutral endopeptidase) Biochem. J. 1992;286:217–221. doi: 10.1042/bj2860217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROZDZ R., EBERLE A.N. Synthesis and iodination of human (Phenylalanine13, Tyrosine19) melanin-concentrating hormone for radioreceptor assay. J. Pept. Sci. 1995a;1:58–65. doi: 10.1002/psc.310010108. [DOI] [PubMed] [Google Scholar]
- DROZDZ R., EBERLE A.N. Binding sites for melanin-concentrating hormone (MCH) in brain synaptosomes and membranes from peripheral tissues identified with highly tritiated MCH. J. Recept. Signal Transduct. Res. 1995b;15:487–502. doi: 10.3109/10799899509045235. [DOI] [PubMed] [Google Scholar]
- DROZDZ R., SIEGRIST W., BAKER B.I., CHLUBA-DE TAPIA J., EBERLE A.N. Melanin-concentrating hormone binding to mouse melanoma cells in vitro. FEBS Lett. 1995;359:199–202. doi: 10.1016/0014-5793(95)00043-9. [DOI] [PubMed] [Google Scholar]
- HERVIEU G.J., CLUDERAY J.E., HARRISON D., MEAKIN J., MAYCOX P., NASIR S., LESLIE R.A. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur. J. Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- HINTERMANN E., DROZDZ R., TANNER H., EBERLE A.N. Synthesis and characterization of new radioligands for the mammalian melanin-concentrating hormone (MCH) receptor. J. Recept. Signal Transduct. Res. 1999;19:411–422. doi: 10.3109/10799899909036661. [DOI] [PubMed] [Google Scholar]
- KAWAUCHI H., KAWAZOE I., TSUBOKAWA M., KISHIDA M., BAKER B.I. Characterisation of MCH in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- KOKKOTOU E., MASTAITIS J.W., QU D., HOERSCH D., SLIEKER L., BONTER K., TRITOS N.A., MARATOS-FLIER E. Characterization of [Phe13,Tyr19]-MCH analog binding activity to the MCH receptor. Neuropeptides. 2000;34:240–247. doi: 10.1054/npep.2000.0821. [DOI] [PubMed] [Google Scholar]
- KOLAKOWSKI L.F., JUNG B.P., NGUYEN T., JOHNSON M.P., LYNCH K.R., CHENG R., HENG H.H.Q., GEORGE S.R., O'DOWD B.F. Characterisation of a human gene related to genes encoding somatostatin receptors. FEBS Lett. 1996;398:253–258. doi: 10.1016/s0014-5793(96)01160-x. [DOI] [PubMed] [Google Scholar]
- LAKAYE B., MINET A., ZORZI W., GRISAR T. Cloning of the rat brain cDNA encoding for the SLC-1 G protein coupled receptor reveals the presence of an intron in the gene. Biochim. Biophys. Acta. 1998;1401:216–220. doi: 10.1016/s0167-4889(97)00135-3. [DOI] [PubMed] [Google Scholar]
- LEMBO P.M., GRAZZINI E., CAO J., HUBATSCH D.A., PELLETIER M., HOFFERT C., ST-ONGE S., POU C., LABRECQUE J., GROBLEWSKI T., O'DONNELL D., PAYZA K., AHMAD S., WALKER P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat. Cell. Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- PRESSE F., SOROKOVSKY I., MAX J.P., NICOLAIDIS S., NAHON J.L. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71:735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- MACDONALD D., MURGOLO N., ZHANG R., DURKIN J.P., YAO X., STRADER C.D., GRAZIANO M.P. Molecular characterization of the melanin-concentrating hormone/receptor complex: identification of critical residues involved in binding and activation. Mol. Pharmacol. 2000;58:217–225. doi: 10.1124/mol.58.1.217. [DOI] [PubMed] [Google Scholar]
- QU D., LUDWIG D.S., GAMMELTOFT S., PIPER M., PELLEYMOUNTER M.A., CULLEN M.J., FOULDS MATHES W., PRZYPEK J., KANAREK R., MARATOS-FLIER E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- ROSSI M., CHOI S.J., O'SHEA D., MIYOSHI T., GHATEI M.A., BLOOM S.R. MCH acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- SAITO Y., NOTHACKER H.P., WANG Z., LIN S.H.S., LESLIE F., CIVELLI O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- SAITO Y., NOTHACKER H.P., CIVELLI O. Melanin-concentrating hormone receptor: an orphan receptor fits the key. Trends Endocrinol. Metab. 2000;11:299–303. doi: 10.1016/s1043-2760(00)00290-3. [DOI] [PubMed] [Google Scholar]
- SHIMADA M., TRITOS N.A., LOWELL B.B., FLIER J.S., MARATOS-FLIER E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA Y., MORI M., SUGO T., ISHIBASHI Y., ABE M., KUROKAWA T., ONDA H., NISHIMURA O., SUMINO Y., FUJINO M. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem. Biophys. Res. Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- SONE M., TAKAHASHI K., MURAKAMI O., TOTSUNE K., ARIHARA Z., SATOH F., SASANO H., ITO H., MOURI T. Binding sites for melanin-concentrating hormone in the human brain. Peptides. 2000;21:245–250. doi: 10.1016/s0196-9781(99)00206-5. [DOI] [PubMed] [Google Scholar]
- TRITOS N.A., MARATOS-FLIER E. Two important systems in energy homeostasis: melanocortins and melanin-concentrating hormone. Neuropeptides. 1999;33:339–349. doi: 10.1054/npep.1999.0055. [DOI] [PubMed] [Google Scholar]


