Abstract
Regional haemodynamic responses to the cannabinoid agonist, WIN 55212-2 (5 – 250 μg kg−1 i.v.) were assessed in conscious, normotensive, Hannover, Sprague-Dawley (HSD) rats, and in hypertensive, transgenic ((mRen-2)27) (abbreviated to TG) rats.
In HSD rats, WIN 55212-2 caused pressor, and renal and mesenteric vasoconstrictor effects, with a hindquarters vasodilator effect occurring only at the highest dose. In TG rats, the effects of the cannabinoid agonist were qualitatively similar to those seen in HSD rats, except there was no hindquarters vasodilatation.
In both strains of rat, in the presence of losartan, pentolinium and a vasopressin (V1-receptor) antagonist, the pressor and vasoconstrictor effects of WIN 55212-2 were abolished, but the hindquarters vasodilator response was enhanced (HSD rats) or was seen only in that circumstance (TG rats). Under these conditions, both strains of rat showed a modest fall in blood pressure, together with mesenteric vasodilatation.
In additional experiments in normotensive SD rats from Charles River (CRSD), it was shown that, in the presence of the V1-receptor antagonist alone, or losartan alone, or the two antagonists together, the cardiovascular effects of WIN 55212-2 (50 or 150 μg kg−1) were not attenuated. Hence, the effects described above were likely due to pentolinium.
There were no consistent differences between HSD and TG rats in their haemodynamic responses to methoxamine or noradrenaline, indicating the two strains were not likely to differ markedly in their responsiveness to any putative sympathetic activation induced by WIN 55212-2.
Collectively, the results indicate that the predominant cardiovascular effects of WIN 55212-2 in conscious HSD and TG rats (i.e., pressor and vasoconstrictor actions) can be attributed largely to indirect, pentolinium-sensitive mechanisms, which appear to differ little in the normotensive and hypertensive state, at least in conscious animals. Under the conditions of our experiments, signs of cannabinoid-induced vasodilatation were modest.
Keywords: WIN 55212-2, cannabinoids, transgenic rats, hypertension, vasodilatation
Introduction
The discovery of the existence of endogenous cannabinoids (see Mechoulam et al., 1994; 1998 for review), and specific receptors for them (see Pertwee, 1997 for review), has stimulated renewed interest in the possible biological role, and therapeutic potential, of drugs derived from these compounds. However, the literature to date amply illustrates the complexity of this area of research, particularly in the context of cardiovascular function (see Compton et al., 1996 for review). For example, the effects of the cannabinoids may be influenced by the nature of the experiment (in vitro vs in vivo), the type of in vitro preparation (e.g., isolated mesenteric vessel vs perfused mesenteric vascular bed), and, for in vivo studies, the state of the experimental animals (pithed, anaesthetized, or conscious), their strain, and the route of administration (central vs peripheral) of the compounds. Further complexity is added by recent observations indicating that endogenous cannabinoids, such as anandamide, may exert effects by interacting, not only with cannabinoid receptors, but also with vanilloid receptors (Zygmunt et al., 1999; see Szallasi & Di Marzo, 2000 for review). Thus, some of the reported differences between anandamide and other cannabinoids could be due to such additional actions of anandamide. However, even synthetic cannabinoids, such as WIN 55212-2 (an aminoalkylindole with affinity for CB1 and CB2 receptors (see Pertwee, 1997)), have complex influences on cardiovascular function. In that context, a recent, elegant study by Niederhoffer & Szabo (1999), identified at least four cardiovascular regulatory mechanisms influenced by WIN 55212-2 in rabbits, namely: prejunctional inhibition of noradrenaline release from postganglionic sympathetic neurones (pithed animals), central sympathoexcitation, and vagal cardiac efferent activation (both at the level of the brain stem following central administration to conscious rabbits), and central sympathoinhibition (following high systemic doses in conscious rabbits). Interestingly, Niederhoffer & Szabo (1999) could find no evidence for cannabinoid-mediated vasodilatation (Randall et al., 1996), as judged by a lack of depressor effect of WIN 55212-2 in pithed, noradrenaline-supported, rabbits, although no direct measures of vascular tone were made in any of their experiments. Furthermore, the question of whether or not cannabinoids have vasodilator actions in other species in vivo is unresolved.
With these various observations as a backdrop, our present objectives were to evaluate the regional haemodynamic actions of a range of systemic doses of WIN 55212-2 in conscious rats. We studied normotensive, Hannover Sprague-Dawley (HSD) rats, and transgenic ((mRen-2)-27) hypertensive (TG) rats (Mullins et al., 1990), our hypothesis being that the altered sympathetic control mechanisms in the latter (Averill et al., 1996) might influence the cardiovascular responses to WIN 55212-2. This seemed feasible, since Lake et al. (1997b) have reported that the cardiovascular effects of anandamide in conscious spontaneously hypertensive rats (SHRs) differed from those seen in the normotensive control rats, and they attributed the difference to the level of pre-existing sympathetic tone. Our experiments were performed before and after inhibition of the major neurohumoral vasoconstrictor systems (sympathoadrenal, renin-angiotensin and vasopressin), using ganglion blockade, and angiotensin (AT1), and vasopressin (V1) receptor antagonism. We did this in an attempt to determine whether or not, in the absence of the major pressor systems, vasodilator effects of the cannabinoid could be demonstrated. Since we observed some differences between the responses to WIN 55212-2 in the HSD and TG rats in the intact state, in a final experiment we compared regional haemodynamic responses to noradrenaline and methoxamine, to discern whether or not the differential effects of WIN 55212-2 might be attributable to differences in responsiveness to sympathoexcitation in the two strains of rat.
Some of the results have been presented to the British Pharmacological Society (Gardiner et al., 1999a; 2000a).
Methods
Experiments were carried out on male, inbred, Hannover, Sprague-Dawley rats (abbreviated to SD rats), or male, age-matched, heterozygous, transgenic ((mRen-2)-27) rats (abbreviated to TG rats), bred in Nottingham from animals originally supplied by Professor J.J. Mullins (University of Edinburgh). The heterozygous, TG rats were produced by mating homozygous, male, TG rats with female HSD rats. Homozygous TG rats were maintained on chronic captopril treatment (50 mg l−1 in the drinking water (Mullins et al., 1990)), but the heterozygous animals used in the experiments were untreated; all animals were 3 – 4 months old at the time of study. Some additional experiments were performed in normotensive SD rats obtained from Charles River (CRSD).
Surgical preparation and cardiovascular recordings
For the instrumentation of HSD and TG rats surgery was performed in two stages, under sodium methohexitone anaesthesia (Brietal, Lily, 40 – 60 mg kg−1 i.p., supplemented as required) with post-operative analgesia (buprenorphine hydrochloride, Vetergesic, Reckitt & Colman, 10 μg kg−1 i.m.). Initially, miniaturized pulsed Doppler flow probes were implanted around the left renal and superior mesenteric arteries and the distal abdominal aorta (to monitor hindquarters flow), as described previously (Gardiner et al., 1995; 2000c). Subsequently, and at least 14 days after probe placement, catheters were implanted in the distal abdominal aorta (via the caudal artery) to monitor arterial blood pressure and heart rate, and in the right jugular vein for drug administrations. Experiments began 24 h after catheterization, when the animals were fully conscious and freely-moving. Food and water were available ad libitum throughout the experiments.
Blood pressures were measured using a fluid-filled pressure transducer (Bell & Howell, type 4-442) with a modified, low volume displacement dome (Ardill et al., 1968), connected via a Gould transducer amplifier (model 13-4615-50) to a custom-designed, computer based system (Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastrict, The Netherlands). The characteristics of the catheter-transducer and recording system were such that it was capable of faithfully following frequencies of up to 40 Hz (Gardiner & Bennett, 1980) and was, therefore, suitable for accurate arterial pressure recording in rats (Geddes, 1970). The data acquisition system also derived instantaneous heart rate and processed the Doppler shift signals. Raw data were sampled by HDAS every 2 ms, averaged every cardiac cycle, and stored to disc at 5 s intervals. Off-line, data were analysed using customized data-analysis software (Datview, University of Limburn, Maastrict, The Netherlands), which interfaced with HDAS.
Cardiovascular responses to WIN 55212-2 in HSD and TG rats
On the first experimental day, nine HSD rats and nine TG rats were given i.v. bolus doses of WIN 55212-2 (5, 50 and 250 μg kg−1) in ascending order, separated by at least 60 min. A bolus injection of vehicle (0.1 ml of 20% 2-hydroxypropyl-β-cyclodextrin in 5% sterile dextrose) was given either before or after the doses of WIN 55212-2.
On the second experimental day, the same animals were given an angiotensin (AT1) receptor antagonist (losartan, 10 mg kg−1; Batin et al., 1991a,1991b) followed, 60 min later, by a ganglion blocker (pentolinium, 5 mg kg−1; 5 mg kg−1 h−1; Gardiner & Bennett, 1985) and, after a further 30 min, a vasopressin (V1) receptor antagonist (d(CH2)5-O-Me-Tyr-AVP (abbreviated to AVPX) 10 μg kg−1; 10 μg kg−1 h−1; Gardiner & Bennett, 1985). Starting 30 min after the onset of administration of the V1-receptor antagonist, bolus doses of WIN 55212-2 and vehicle were administered, as above. There is good evidence that the antagonists employed are highly selective (see Gardiner & Bennett, 1985; Batin et al., 1991b).
Since the protocol above did not allow us to determine if losartan alone, or the V1-receptor antagonist alone, or the two drugs together, influenced responses to WIN 55212-2, we carried out additional experiments to address this point in normotensive CRSD rats. Due to the unavailability of sodium methohexitone, these animals were anaesthetized with fentanyl and meditomidine (300 μg kg−1 of each, i.p.) reversed with nalbuphine and atipamezole (1 mg kg−1 of each, s.c.). Instrumentation for regional haemodynamic monitoring was as described above. Experiments were performed on four consecutive days. On day 1, animals were given the V1-receptor antagonist (dose as above; n=7) or vehicle (sterile saline; n=7) and, 30 min later, WIN 55212-2 was administered at a dose of 50 μg kg−1; after a further 60 min, WIN 55212-2 was given at a dose of 150 μg kg−1. On the second experimental day, the animals that had received the V1-receptor antagonist on day 1 were given losartan (dose as above) and were challenged with WIN 55212-2 at doses of 50 and 150 μg kg−1 30 min and 90 min later, respectively. Prior to the administration of losartan, two of these animals were challenged with AVP (7.5 ng rat−1) to ensure their responses to the peptide had returned to normal. The animals that had received vehicle on the first day were given vehicle again on the second day and were re-challenged with WIN 55212-2 (as above). On day 3, the animals that had had active treatment were given the V1-receptor antagonist and losartan together (doses as above) before being given WIN 55212-2 (as above). The vehicle group were also re-challenged with WIN 55212-2 (as above). On the fourth experimental day the animals receiving active treatment were given the combination of V1-receptor antagonist, losartan and pentolinium (as above) and were subsequently challenged with WIN 55212-2 (as above). The vehicle-treated animals were re-challenged with WIN 55212 in the presence of vehicle once again.
Cardiovascular responses to noradrenaline or methoxamine in HSD and TG rats
In separate groups of animals, bolus i.v. doses of noradrenaline (75, 250 and 750 ng kg−1) and methoxamine (6, 20 and 60 μg kg−1), were administered in ascending order, alternately, at 15 min intervals. Ten rats of each strain received noradrenaline, but, due to problems with achieving the appropriate doses of methoxamine, only six rats of each strain received the chosen doses of this agonist.
Data analysis
Elsewhere (Gardiner et al., 1999b), we have discussed the problems associated with interpreting data, of the sort dealt with here, when baseline conditions differ between the strains of rat studied. In that paper, we argued the case for presenting the haemodynamic data in terms of vascular conductance (rather than resistance), and expressing any changes both in absolute and percentage terms (Gardiner et al., 1999b); for the reasons given previously we have used the same mode of data presentation here.
Within-group analysis of data was by Friedman's test (Theodorsson-Norheim, 1987). Between-group comparisons were made using the Mann – Whitney U-test or Kruskal – Wallis test, as appropriate, applied to integrated responses (areas under or over curves) for experiments involving WIN 55212-2, or to values measured at the peak of the pressor responses (15 s) to noradrenaline or methoxamine. A P value <0.05 was taken as significant, with the raw P values being adjusted by the Holm – Bonferroni procedure (Ludbrook, 1998).
Materials
When available, sodium methohexitone was obtained from Eli Lilly. Fentanyl citrate was obtained from Martindale; meditomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were obtained from Pfizer; nalbuphine hydrochloride (Nubain) was obtained from Du Pont; WIN 55212-2 (R(+)-[2,3-dihydro-5-methyl-30-[(morpholinyl) methyl] pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate) was obtained from RBI (Sigma, U.K.); noradrenaline bitartrate, methoxamine hydrochloride and pentolinium tartrate were obtained from Sigma (U.K.); losartan was a gift from Dr R.D. Smith (DuPont, U.S.A.), and (+)-(CH2)5-O-Me-Tyr-AVP was obtained from Bachem (U.K.). Drugs were prepared fresh daily and dissolved in sterile saline, with the exception of WIN 55212-2, which was solubilized in 20% 2-hydroxypropyl-β-cyclodextrin in 5% sterile dextrose. Bolus injections were given in a volume of 0.1 ml, and infusions were at a rate of 0.4 ml h−1.
Results
Resting cardiovascular variables
Prior to administration of WIN 55212-2 on the first experimental day, TG rats had significantly (P<0.05) higher mean arterial blood pressures (166±3 vs 107±2 mmHg), lower heart rates (347±8 vs 382±14 beats min−1), and lower conductances in all three vascular beds (renal 50±6 vs 88±5, mesenteric 37±3 vs 96±5, hindquarters 26±3 vs 46±2 (kHz mmHg−1] 103) than HSD rats.
Cardiovascular responses to WIN 55212-2 in HSD and TG rats in the absence of losartan, pentolinium and AVPX
In HSD rats, following vehicle administration, there were no changes in blood pressure, heart rate or hindquarters vascular conductance, but there were significant (P<0.05) falls in vascular conductance in the renal and mesenteric vascular beds (Figure 1). The cardiovascular effects of the lowest dose of WIN 55212-2 (5 μg kg−1) in HSD rats did not differ from those of the vehicle (Figure 1). Higher doses of WIN 55212-2 caused increases in blood pressure, and significantly (P<0.05) greater renal and mesenteric vasoconstrictions than vehicle (Figure 1). The middle dose of WIN 55212-2 (50 μg kg−1) did not affect hindquarters vascular conductance, but the highest dose (250 μg kg−1) caused significant (P<0.05) vasodilatation (Figure 1). The highest dose of WIN 55212-2 also caused bradycardia; a reduction in heart rate was not seen at any other dose (Figure 1).
Figure 1.
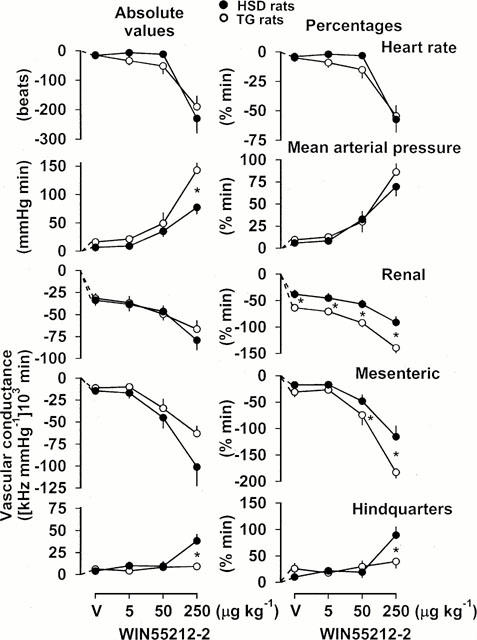
Integrated (areas under or over curves 0 – 5 min) cardiovascular changes (absolute values and percentages) in HSD rats (n=9) and TG rats (n=9) in response to vehicle (V) or increasing bolus, i.v. doses of WIN 55212-2. For clarity, the statistics relating to the effects of these interventions are given in the text, and only the between-strain differences are indicated. Values are mean, and vertical bars show s.e.mean; *P<0.05 for change in HSD rats vs TG rats (Mann – Whitney U-test).
In TG rats, vehicle administration, and the lowest dose of WIN 55212-2 (5 μg kg−1), had effects qualitatively similar to those seen in HSD rats (Figure 1), although the percentage falls in renal vascular conductances were greater. The effects of the middle dose of WIN 55212-2 (50 μg kg−1) were also qualitatively similar in TG and HSD rats (Figure 1), but the former showed significantly (P<0.05) greater percentage falls in renal, and in mesenteric vascular conductances (Figure 1). The highest dose of WIN 55212-2 caused a significantly (P<0.05) greater absolute pressor response, and greater percentage falls in renal and mesenteric vascular conductances, in TG rats than in HSD rats (Figure 1). Most notably, there was no hindquarters vasodilatation following administration of WIN 55212-2 in TG rats, which differed significantly (P<0.05) from the response seen in the HSD rats (Figure 1). The changes in heart rate following WIN 55212-2 were similar in TG and HSD rats (Figure 1).
Cardiovascular effects of losartan, pentolinium and AVPX in HSD and TG rats
At the start of the second experimental day, as on the first day, TG rats had higher resting arterial blood pressures, lower heart rates, and lower vascular conductances than HSD rats (Figure 2). Sixty minutes following administration of losartan, there was a fall in blood pressure in TG rats, but not in HSD rats, although there was tachycardia and renal vasodilatation in both strains (Figure 2). Pentolinium caused a marked fall in blood pressure in HSD and TG rats, associated with vasodilatation in the renal and hindquarters vascular beds, but only in the TG rats did pentolinium cause sustained mesenteric vasodilatation (Figure 2). The hypotensive and vasodilator effects recorded 30 min after administration of pentolinium were significantly greater in TG than in HSD rats (Figure 2). Administration of AVPX caused further falls in blood pressure in both strains, associated with mesenteric and hindquarters vasodilatation (Figure 2). In the presence of pentolinium, losartan and AVPX together, resting arterial blood pressures, and renal and hindquarters vascular conductances were similar in the two strains of rat, but mesenteric vascular conductance was still significantly lower in TG, than in HSD, rats (Figure 2).
Figure 2.
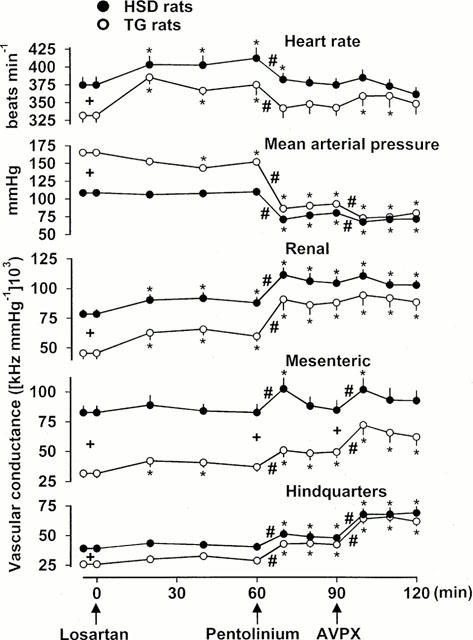
Cardiovascular variables in HSD rats (n=9) and TG rats (n=9) under resting conditions, and following sequential administration of losartan (10 mg kg−1 bolus), pentolinium (5 mg kg−1 bolus, 5 mg kg−1 h−1 infusion) and a vasopressin, V1-receptor antagonist (AVPX) (10 μg kg−1 h−1 infusion). Values are mean and vertical bars show s.e.mean; +P<0.05 for differences between resting values in HSD rats and TG rats (Mann – Whitney U-test); *P<0.05 for differences from original baseline (Friedman's test); #P<0.05 for initial response to pentolinium or AVPX (Friedman's test).
Cardiovascular responses to WIN 55212-2 in HSD and TG rats in the presence of losartan pentolinium and AVPX
In HSD and TG rats, in the presence of losartan, pentolinium and AVPX, the pressor and renal and mesenteric vasoconstrictor responses to all three doses of WIN 55212-2 were not significantly different from those seen following administration of vehicle (Figure 3). However, unlike the vehicle, the highest dose of WIN 55212-2 (250 μg kg−1) caused hindquarters vasodilatation in both strains of rat (Figure 3). In HSD rats, the hindquarters vasodilator response to WIN 55212-2 (250 μg kg−1) in the presence of losartan, pentolinium and AVPX was significantly (P<0.05) greater than that seen in the absence of those drugs (Figures 1 and 3) whereas in TG rats, a hindquarters vasodilator response was observed only under those conditions (Figure 3). In both strains of rat, the bradycardic response to the highest dose of WIN 55212-2 in the presence of losartan, pentolinium and AVPX was significantly smaller than in the absence of the antagonists (Figures 1 and 3).
Figure 3.
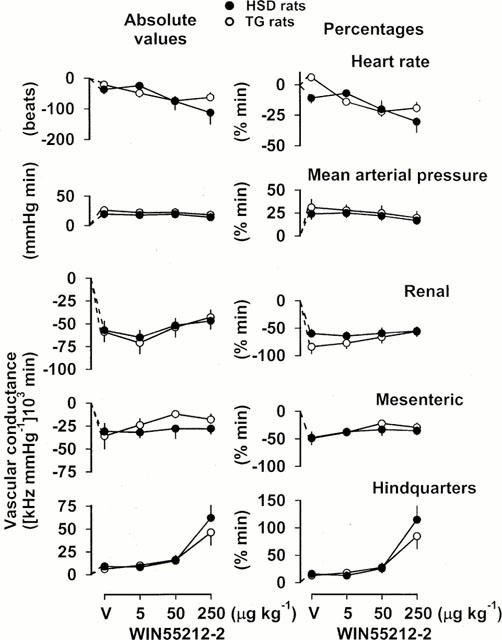
Integrated (areas under or over curves 0 – 5 min) cardiovascular changes (absolute values and percentages) in HSD rats (n=9) and TG rats (n=9) in response to vehicle (V) or increasing bolus, i.v. doses of WIN 55212-2 in the presence of losartan, pentolinium and a V1-receptor antagonist. For clarity, the statistics relating to the effects of these interventions are given in the text, and only the between-strain differences are indicated. Values are mean, and vertical bars show s.e.mean; *P<0.05 for change in HSD rats vs TG rats (Mann – Whitney U-test).
Following the two lower doses of WIN 55212-2, cardiovascular variables had generally returned to baseline after 5 min (data not shown). However, between 5 and 10 min after the highest dose of WIN 55212-2, in the presence of losartan, pentolinium and AVPX, there were modest falls in blood pressure in both strains (−6±2 mmHg in HSD rats, −10±2 mmHg in TG rats after 10 min), associated with increases in mesenteric vascular conductance +11±4 [kHz mmHg−1] 103 in HSD rats, +13±4 [kHz mmHg−1] 103 in TG rats after 10 min). These changes were significantly different from the increases in blood pressure (+10±2 mmHg in HSD rats, +19±3 mmHg in TG rats) and reductions in mesenteric vascular conductance [−17±4 (kHz mmHg−1)] 103 in HSD rats, −12±1 [kHz mmHg−1] 103 in TG rats), measured 10 min following the highest dose of WIN 55212-2 in the absence of the losartan, pentolinium and AVPX.
Cardiovascular responses to WIN 55212-2 in CRSD rats in the presence of AVPX alone, or losartan alone, or AVPX and losartan together, or AVPX, losartan and pentolinium
Resting haemodynamic variables and responses to WIN 55212-2 (150 μg kg−1) in the animals given active treatment or vehicle are summarized in Table 1. For the sake of clarity the data for WIN 55212-2 at a dose of 50 μg kg−1 have been omitted. Resting cardiovascular status and responses to WIN 55212-2 showed no significant differences across the four experimental days in the animals given vehicle (Table 1).
Table 1.
Resting cardiovascular variables and responses to WIN 55212-2 (150 μg kg−1; 0 – 5 min areas) in conscious CRSD rats (n=7 in each group)
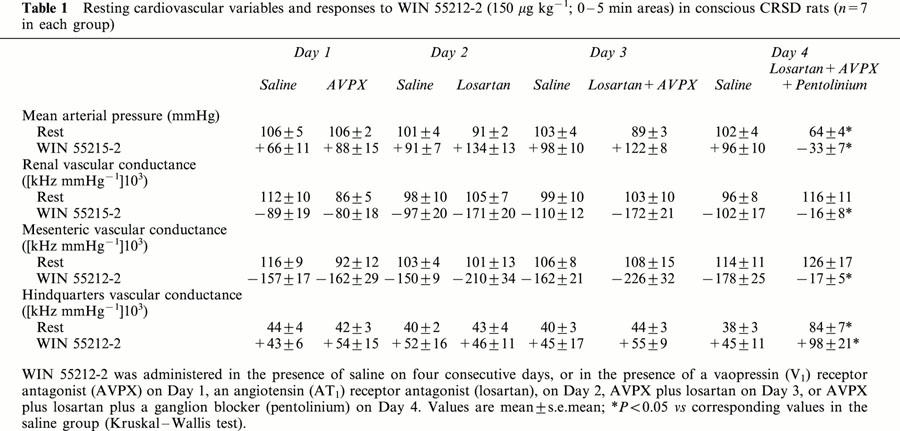
In the presence of AVPX, or of losartan, or of both antagonists together, neither resting cardiovascular status nor responses to WIN 55212-2 were different from the corresponding values in the vehicle treated group (Table 1). It was only when pentolinium was added to the latter combination that the pressor and vasoconstrictor responses to WIN 55212-2 were abolished (Table 1). The hindquarters vasodilator effect of WIN 55212-2 was enhanced in the presence of all three antagonists (Table 1), as in the HSD and TG rats (see above), but the depressor effect was already apparent within the first 5 min (Table 1).
Cardiovascular responses to noradrenaline and methoxamine in HSD and TG rats
In both strains of rat, i.v. administration of noradrenaline (Figure 4) caused dose-dependent increases in blood pressure and vasoconstrictions in renal, mesenteric and hindquarters vascular beds. Responses to noradrenaline were associated with bradycardia in HSD rats, but not in TG rats (Figure 4). The absolute pressor response to the highest dose of noradrenaline was significantly (P<0.05) greater in TG than in HSD rats, but when expressed as percentage changes, the pressor responses were smaller in the former strain (significant (P<0.05) at the middle dose only). The renal and mesenteric vasoconstrictor responses to noradrenaline in TG rats were significantly (P<0.05) less than those in HSD rats in absolute, but not in percentage, terms, whereas the hindquarters vasoconstrictor responses were generally reduced, both in absolute and percentage terms (Figure 4).
Figure 4.
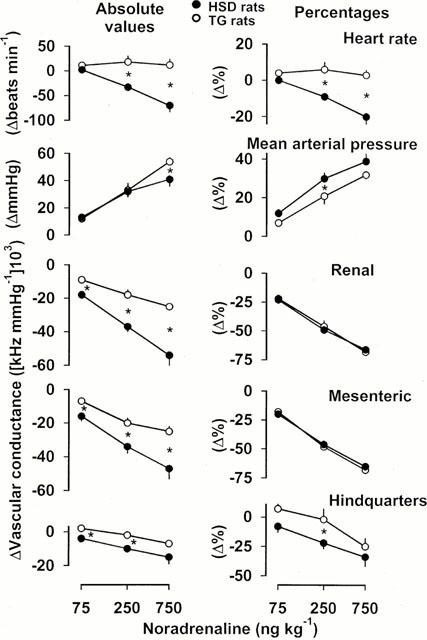
Cardiovascular changes (absolute values and percentages) in HSD rats (n=10) and TG rats (n=10) in response to noradrenaline in increasing bolus i.v. doses. For clarity, the statistics relating to the effects of these interventions are given in the text, and only the between-strain differences are indicated. Values are mean, and vertical bars show s.e.mean; *P<0.05 for change in HSD rats vs TG rats (Mann – Whitney U-test).
Methoxamine caused dose-dependent increases in blood pressure and vasoconstriction in all three vascular beds in both strains of rat (Figure 5). There was a bradycardic response to methoxamine in both strains of rat, but, at the highest dose tested, the fall in heart rate was significantly (P<0.05) smaller in TG, than in HSD, rats (Figure 5). Absolute pressor responses to the two higher doses of methoxamine were greater in TG rats than in HSD rats, and the associated renal and mesenteric vasoconstrictions were smaller. However, these differences were not seen when the data were expressed in percentage terms (Figure 5). Hindquarters vasoconstrictor responses to methoxamine were not different in TG and HSD rats (Figure 5).
Figure 5.
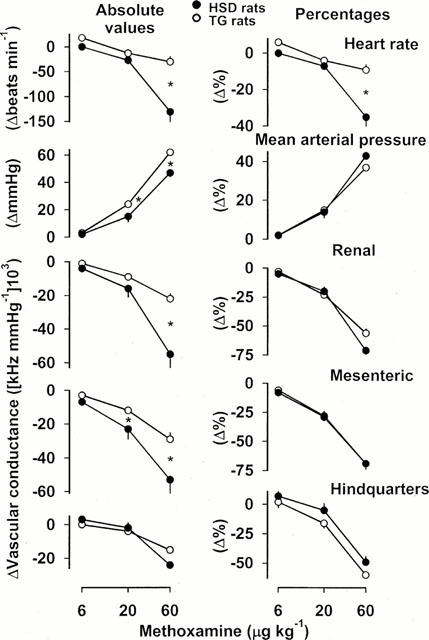
Cardiovascular changes (absolute values and percentages) in HSD rats (n=6) and TG rats (n=6) in response to methoxamine in increasing bolus i.v. doses. For clarity, the statistics relating to the effects of these interventions are given in the text, and only the between-strain differences are indicated. Values are mean, and vertical bars show s.e.mean; *P<0.05 for change in HSD rats vs TG rats (Mann – Whitney U-test).
Discussion
The aim of the present study was to profile the regional haemodynamic effects of a range of doses of the cannabinoid, WIN 55212-2, in conscious rats. Since Niederhoffer & Szabo (1999; 2000) had provided convincing evidence for an element of sympathoexcitation in the responses to WIN 55212-2 in rabbits, we speculated that inhibition of the major neurohumoral vasoconstrictor systems (Gardiner & Bennett, 1985) might optimise our chances of seeing vasodilator responses to the cannabinoid. Studies were performed in normotensive rats, and in hypertensive transgenic ((mRen-2)-27) rats, to determine whether or not the altered sympathetic control mechanisms in the latter (Averill et al., 1996), influenced cardiovascular responses to the cannabinoid. In the light of some of our findings, additional experiments were designed so as to give us information about any putative contribution from vasopressin and/or angiotensin II to the effects of WIN 55212-2 in normotensive rats.
Collectively, our results show that, in intact HSD and TG rats, the predominant effect of WIN 55212-2 was to cause a rise in systemic arterial blood pressure and vasoconstriction in the renal and mesenteric vascular beds. Interestingly, there was a vasodilatation only in the hindquarters vascular bed which, in the intact state, occurred only in the HSD rats. This hindquarters vasodilatation was enhanced in the presence of neurohumoral blockade. Furthermore, in the TG rats it was only in the latter condition that a hindquarters vasodilator response to WIN 55212-2 was observed. In addition, inhibition of the major vasoconstrictor systems revealed a modest hypotensive and mesenteric vasodilator effect of WIN 55212-2 in both strains of rat. Some possible mechanisms underlying these events are discussed below.
In contrast to our present results, Lake et al. (1997a) reported only dose-dependent depressor effects of WIN 55212-2 in urethane-anaesthetized normotensive rats, with no pressor effect under any circumstance. Those studies did not include measurements of vascular tone, and thus, the cause of the fall in blood pressure was not determined. However, it has been shown that the cardiovascular effects of the endocannabinoid, anandamide, are different in anaesthetized and conscious normotensive rats, with the slower-onset, secondary depressor response being absent in the conscious state (Lake et al., 1997b), or only apparent at a particular dose of anandamide (i.e., not dose-related (Stein et al., 1996)). Our present results indicate that the cardiovascular effects of the synthetic cannabinoid, WIN 55212-2, may also differ between anaesthetized and conscious rats, although it is notable that this compound caused bradycardia in our conscious rats, as in the experiments of Lake et al. (1997a) and Niederhoffer & Szabo (1999). The clear dissociation between the effects of WIN 55212-2 on arterial blood pressure and heart rate are consistent with the bradycardia being centrally-mediated (Niederhoffer & Szabo, 2000).
The pressor and vasoconstrictor effects of WIN 55212-2 in HSD and TG rats were abolished by neurohumoral blockade. Clearly, our experimental protocol in HSD and TG rats did not permit us to ascertain which of the vasoconstrictor systems was responsible, but, on the basis of the findings of Niederhoffer & Szabo (1999; 2000), and the results of our additional experiments in CRSD rats, it is most likely that sympathoexcitation was involved.
Thus, in CRSD rats, we found no attenuation of the pressor or vasoconstrictor effects of WIN 55212-2 in the presence of AVPX or losartan, alone, or in combination. It was only in the additional presence of ganglion blockade that those effects of WIN 55212-2 were abolished. Interestingly, Niederhoffer & Szabo (1999) recorded an increase in renal sympathetic nerve activity following i.v. administration of WIN 55212-2 to conscious rabbits. However, from our results, it appears that such an effect, if present in rats, does not result in an involvement of the renin-angiotensin system in the cardiovascular effects of WIN 55212-2.
We found greater pressor effects of WIN 55212-2 in TG than in HSD rats, but only when the data were expressed in absolute terms. Similar results were obtained with noradrenaline and methoxamine, for which any apparent greater pressor sensitivity in TG rats was not seen when the data were normalized (see later). Similarly, there were no consistent differences in the renal or mesenteric vasoconstrictor responses to WIN 55212-2, noradrenaline, or methoxamine between HSD and TG rats. However, a clear strain difference in the regional haemodynamic responses to the highest dose of WIN 55212-2 was apparent in that hindquarters vasodilatation occurred in HSD (and CRSD), but not in TG, rats in the unblocked state. Whether or not the vasodilatation was cannabinoid receptor-mediated cannot be determined from this study, but since it occurred to a greater, rather than a lesser, extent in the presence of ganglion blockade, we clearly cannot invoke sympathetically-mediated adrenal medullary adrenaline release as a mechanism (Gardiner et al., 1988). The possible involvement of cannabinoid receptor-mediated adrenaline release from chromaffin cells, for example, requires further investigation.
We studied the effects of WIN 55212-2 in conscious hypertensive TG rats to test the hypothesis that elevated sympathetic tone in these animals (Averill et al., 1996) might unmask a delayed hypotensive response to the cannabinoid, as seen in conscious SHRs when challenged with anandamide (Lake et al., 1997b). The explanation offered by Lake et al. (1997b) was that this hypotensive response was due to withdrawal of sympathetic tone, which was higher in anaesthetized compared to conscious normotensive rats, and higher in SHR compared to normotensive (SD) rats. Our finding that the hindquarters vasodilator effects of WIN 55212-2 were enhanced under conditions of ganglion blockade rules out sympathetic withdrawal as a possible mechanism. It is feasible that WIN 55212-2 exerts direct and/or endothelial-mediated vasodilator effects (e.g., Randall et al., 1996), although it is surprising that any such effects were not seen in all vascular beds, and were more apparent in the hindquarters than in the mesenteric vascular bed, since, in experiments assessing regional haemodynamic effects of NO donors in conscious rats, the mesenteric vascular bed usually shows the most marked vasodilator response (e.g., Gardiner et al., 1990; 1991).
The inclusion of an assessment of regional haemodynamic responses to noradrenaline and methoxamine in our study allowed us, incidentally, to show that the only apparent differences between the responses in HSD and TG rats were that the bradycardic effect associated with administration of noradrenaline or methoxamine was absent or diminished, respectively, in TG compared to HSD rats, and the hindquarters vasoconstrictor response to noradrenaline in TG rats was generally smaller than in HSD rats. These findings are consistent with those of Jacinto et al. (1999), who reported similar blood pressure and renal vascular responses to i.v. administration of noradrenaline in anaesthetized TG and HSD rats, although they showed reduced renal vascular responses to intrarenal arterial administration of the catecholamine, possibly due to desensitization of the α-adrenoceptor signal transduction pathway in TG rats (Schnabel et al., 1997). If such an abnormality has functional significance, however, it is difficult to explain why systemic responses to i.v. administration of the α-adrenoceptor agonist, methoxamine, were not affected in our study.
The occurrence of a bradycardic, rather than a tachycardic, response to noradrenaline indicates that the baroreflex over-rides any direct positive chronotropic effects of the catecholamine. Elsewhere (Gardiner et al., 2000c), we have reported diminished baroreflex sensitivities in TG, compared to HSD, rats, particularly in response to rises in blood pressure. Thus, the smaller bradycardic responses to noradrenaline and methoxamine reported here, in TG rats, are consistent with that finding. For a given change in blood pressure, the bradycardic response was larger to methoxamine than to noradrenaline in HSD rats, probably because the bradycardia was being opposed by a direct positive chronotropic effect of noradrenaline. The absence of any significant heart rate change in TG rats in response to noradrenaline might be explained by a combination of cardiac β-adrenoceptor downregulation (Böhm et al., 1994) together with a diminished cardiac baroreflex response to the noradrenaline-induced rise in blood pressure (Gardiner et al., 2000c).
Here we confirmed the marked, acute antihypertensive action of i.v. losartan in conscious TG rats (Gardiner et al., 1995), and extended those results by showing that subsequent treatment with pentolinium caused a greater fall in blood pressure in TG than in HSD rats. It could be argued that the hypotension caused by prior treatment with losartan triggered sympathetic activation, but we have evidence to suggest that, even in the absence of losartan, the hypotensive effect of pentolinium is substantially greater in TG than in HSD rats (unpublished observations). Thus, our findings may be taken as support for enhanced sympathetic activity in TG rats (Averill et al., 1996).
It is notable that, in the presence of losartan, pentolinium and the vasopressin antagonist, mean blood pressures were similar in HSD and TG rats, as were renal and hindquarters vascular conductances. In this condition, the lower conductance in the mesenteric vascular bed of TG, compared to HSD, rats may have been due to endothelin (Gardiner et al., 1995), and/or to structural changes in the former (Dunn & Gardiner, 1997).
In conclusion, the present work in conscious HSD and TG rats has shown that the haemodynamic responses to the cannabinoid agonist, WIN 55212-2, are generally pressor and vasoconstrictor, and these effects are absent when the major pressor systems are pharmacologically blocked. In the intact state, only normotensive rats showed a vasodilator effect of WIN 55212-2, and this was confined to the hindquarters. Even in the presence of neurohumoral blockade, responses to WIN 55212-2 were relatively modest and were confined to hindquarters and mesenteric vascular beds. Taken together with our findings of an absence of acute vasodilator responses to anandamide or methanandamide in conscious rats (Gardiner et al., 2000b; Ralevic et al., 2000) it is clear there is little direct evidence for cannabinoids exerting substantial vasodilator effects in vivo. Thus, the potential of cannabinoid receptor agonists as putative antihypertensive drugs is not obvious.
Acknowledgments
This work was supported by the BHF (Grant PG 99/063).
Abbreviations
- (CRSD)
Charles River Sprague-Dawley rats
- (HSD)
Hannover Sprague-Dawley rats
- (TG)
hypertensive transgenic ((mRen-2)27) rats
References
- ARDILL B.L., FENTEM P.H., WELLARD M.J. A design for the ‘dome' of a medical pressure transducer. Biomed. Eng. 1968;3:160–162. [Google Scholar]
- AVERILL D.B., MATSUMURA K., GANTEN D., FERRARIO C.M. Role of area postrema in transgene hypertension. Hypertension. 1996;27:591–597. doi: 10.1161/01.hyp.27.3.591. [DOI] [PubMed] [Google Scholar]
- BATIN P., GARDINER S.M., COMPTON A.M., BENNETT T. Differential regional haemodynamic effects of the non-peptide angiotensin II antagonist, DuP 753, in water-replete and water-deprived Brattleboro rats. Life Sci. 1991a;48:733–739. doi: 10.1016/0024-3205(91)90087-r. [DOI] [PubMed] [Google Scholar]
- BATIN P., GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Cardiac haemodynamic effects of the non-peptide, angiotensin II-receptor antagonist, DuP 753, in conscious Long Evans and Brattleboro rats. Br. J. Pharmacol. 1991b;103:1585–1591. doi: 10.1111/j.1476-5381.1991.tb09831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÖHM M., MOLL M., SCHMID B., PAUL M., GANTEN D., CASTELLANO M., ERDMANN E. β-adrenergic neuroeffector mechanisms in cardiac hypertrophy of renin transgenic rats. Hypertension. 1994;24:653–662. doi: 10.1161/01.hyp.24.6.653. [DOI] [PubMed] [Google Scholar]
- COMPTON D.R., HARRIS L.S., LICHTMAN A.H., MARTIN B.R.Marihuana Pharmacological Aspects of Drug Dependence. Handbook of Experimental Pharmacology 1996Heidelberg: Springer; 83–158.Ed. Schuster, C.R. & Kuhar, M.J. pp [Google Scholar]
- DUNN W.R., GARDINER S.M. Differential alteration in vascular structure of resistance arteries isolated from the cerebral and mesenteric vascular beds of transgenic [(mRen-2)27], hypertensive rats. Hypertension. 1997;29:1140–1147. doi: 10.1161/01.hyp.29.5.1140. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T. Systemic arterial hypertension in rats exposed to short-term isolation; intra-arterial systolic and diastolic blood pressure and baroreflex sensitivity. Med. Biol. 1980;58:232–239. [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T. Interactions between neural mechanisms, the renin-angiotensin system and vasopressin in the maintenance of blood pressure during water deprivation: studies in Long Evans and Brattleboro rats. Clin. Sci. 1985;68:647–657. doi: 10.1042/cs0680647. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T., COMPTON A.M. Regional haemodynamic effects of neuropeptide Y, vasopressin and angiotensin II in conscious, unrestrained, Long Evans and Brattleboro rats. J. Auton. Nerv. Syst. 1988;24:15–27. doi: 10.1016/0165-1838(88)90131-2. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br. J. Pharmacol. 1990;101:632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br. J. Pharmacol. 1991;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Regional haemodynamic effects of the cannabinoid agonist, WIN 55212-2, in conscious rats. Br. J. Pharmacol. 1999a;128:81P. doi: 10.1038/sj.bjp.0704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Cardiovascular responses to angiotensins I and II in normotensive and hypertensive rats; effects of NO synthase inhibition or ET receptor antagonism. Br. J. Pharmacol. 1999b;128:1795–1803. doi: 10.1038/sj.bjp.0702978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Regional haemodynamic responses to ganglion blockade in conscious, hypertensive transgenic rats. Br. J. Pharmacol. 2000a;129:204P. doi: 10.1038/sj.bjp.0704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Comparative haemodynamic effects of i.p. administration of anandamide or CGRP in conscious rats. Br. J. Pharmacol. 2000b;129:5P. [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Cardiovascular effects of endothelin-1 and endothelin antagonists in conscious hypertensive ((mRen-2)27) rats. Br. J. Pharmacol. 2000c;131:1732–1738. doi: 10.1038/sj.bjp.0703767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., MULLINS J.J., BENNETT T. Haemodynamic effects of losartan and the endothelin antagonist, SB 209670, in conscious, transgenic ((mRen-2)27), hypertensive rats. Br. J. Pharmacol. 1995;116:2237–2244. doi: 10.1111/j.1476-5381.1995.tb15059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEDDES L.A. The Direct and Indirect Measurement of Blood Pressure. Chicago: Year Book Medial Publishers Inc; 1970. [Google Scholar]
- JACINTO S.M., MULLINS J.J., MITCHELL K.D. Enhanced renal vascular responsiveness to angiotensin II in hypertensive ren-2 transgenic rats. Am. J. Physiol. 1999;276:F315–F322. doi: 10.1152/ajprenal.1999.276.2.F315. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- LAKE K.D., MARTIN B.R., KUNOS G., VARGA K. Cardiovascular effects of anandamide in anaesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- LUDBROOK J. Multiple comparison procedures update. Clin. Exp. Pharmacol. Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E., DI MARZO V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., HANUS L., MARTIN B.R. Search for endogenous ligands of the cannabinoid receptor. Biochem. Pharmacol. 1994;48:1537–1544. doi: 10.1016/0006-2952(94)90197-x. [DOI] [PubMed] [Google Scholar]
- MULLINS J.J., PETERS J., GANTEN D. Fulminant hypertension in transgenic rats harvouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Effect of the cannabinoid receptor agonist WIN 55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., BENNETT T., GARDINER S.M. Comparison of the effects of anandamide and methanandamide in bioassays. Br. J. Pharmacol. 2000;129:141P. [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- SCHNABEL P., NOHR T., NICKENIG G., PAUL M., BÖHM M. α-adrenergic signal transduction in renin transgenic rats. Hypertension. 1997;30:1356–1361. doi: 10.1161/01.hyp.30.6.1356. [DOI] [PubMed] [Google Scholar]
- STEIN E.A., FULLER S.A., EDGEMOND W.S., CAMPBELL W.B. Physiological and behavioral effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br. J. Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., DI MARZO V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- THEODORSSON-NORHEIM E. Friedman and Quade tests: BASIC computer program to perform non-parametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput. Biol. Med. 1987;17:5–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG J., SØRJÅRD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediates the vasodilator action of anandamide. Nature. 1999;400:452–453. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


