Abstract
Ro60-0175 has been described as a selective agonist at the 5-HT2C receptor, yet it has only 10- fold higher affinity at the 5-HT2C compared to the 5-HT2A subtype, and equivalent affinity for the 5-HT2B receptor.
The selective 5-HT2C receptor antagonist SB242,084 (0.5 mg kg−1 i.p.), blocked the hypoactivity and penile grooming induced by Ro60-0175 (1 mg kg−1 s.c.). The combination of SB242,084 (0.5 mg kg−1 i.p.) and Ro60-0175 (3 – 10 mg kg−1) produced a completely different pattern of behaviours including wet-dog shakes, hyperactivity and back muscle contractions. These latter effects were blocked by the selective 5-HT2A receptor antagonist MDL100,907 (0.5 mg kg−1 i.p.), but not the 5-HT2B receptor antagonist SB215,505 (3 mg kg−1 p.o.).
The indirect 5-HT releaser/reuptake inhibitor dexfenfluramine (1 – 10 mg kg−1 i.p.) produced a mild increase in locomotor activity, penile grooming, and occasional back muscle contractions and wet-dog shakes. Pre-treatment with SB242,084 (0.5 mg kg−1), blocked the incidence of penile grooming, and markedly potentiated both the dexfenfluramine-induced hyperactivity, the incidence of back muscle contractions, and to a lesser extent wet-dog shakes. Some toxicity was also evident in animals treated with dexfenfluramine (10 mg kg−1)/SB242,084 (0.5 mg kg−1), but not in any other treatment groups.
The hyperactivity and toxicity produced by the dexfenfluramine (10 mg kg−1)/SB242,084 (0.5 mg kg−1) combination was replicated in a further study, and hyperthermia was also recorded. Both hyperthermia and toxicity were blocked by MDL100,907 (0.5 mg kg−1) but not SB215,505 (3 mg kg−1). An attenuation of the hyperlocomotor response was also observed following MDL100,907.
These findings suggest that 5-HT2C receptor activation can inhibit the expression of behaviours mediated through other 5-HT receptor subtypes.
Keywords: Ro60-0175; SB242,084; MDL100,907; SB215,505; dexfenfluramine; 5-HT syndrome; 5-HT2C; 5-HT2A
Introduction
There are presently at least 14 distinct 5-HT receptor subtypes which are encoded by distinct genes, and which appear to have functional roles (Boess & Martin, 1994; Hoyer & Martin, 1997; Barnes & Sharp, 1999). The 5-HT2 receptor is comprised of 5-HT2A, 5-HT2B and 5-HT2C receptors. They are grouped according to similar sequence homology and second messenger system, each being positively coupled to phospholipase C (Hoyer & Martin, 1997; Baxter et al., 1995).
Activation of the 5-HT2A receptor has been associated with the hallucinogenic properties of the phenylisopropylamines such as DOI, and the indoleamine, LSD (Aghajanian & Marek, 1999). Upon systemic administration, these drugs also produce specific behaviours which comprise part of the 5-HT syndrome, including wet-dog shakes, back muscle contractions and forepaw treading (Fone et al., 1989; Pranzatelli, 1990; Wettstein et al., 1999; Ouagazzal et al., 2001). In contrast, hypoactivity and penile grooming/erections are characteristic behavioural signs following activation of 5-HT2C receptors by agents such as mCPP and (S)-2-(6-chloro-5-fluoroindol-1-yl)-1-methlethylamine) (Ro60-0175, Millan et al., 1997; Martin et al., 1998). However, neither mCPP nor Ro60-0175 show more than 10 fold selectivity for the 5-HT2C receptor compared to other 5-HT receptor subtypes (Porter et al., 1999), yet surprisingly neither drug seems to elicit other 5-HT receptor-mediated behavioural signs (Millan et al., 1997; Martin et al., 1998).
In a recent study, Tecott and coworkers (Heiser et al., 1999; Heiser & Tecott, 2000) described a paradoxical effect of mCPP in mice deficient in the 5-HT2C receptor (Tecott et al., 1995). More specifically, in 5-HT2C receptor knock-out mice, a hyperactivity was unmasked following mCPP treatment, which was in stark contrast to the hypoactivity typically seen with this drug in non-genetically manipulated rats and mice (Kennett & Curzon, 1988). Furthermore, this hyperactivity was blocked by the 5-HT1B/1D receptor antagonist GR127935 (Clitheroe et al., 1994). These findings are of interest in that they highlight interactions between the 5-HT receptor family, and suggest that 5-HT2C receptor activation can elicit a profound effect on behaviour, which can mask that mediated through other 5-HT receptor subtypes.
It was the purpose of the present series of studies to follow up on these findings. Specifically, we examined the overt behaviours produced by various doses of Ro60-0175 in the presence and absence of the selective 5-HT2C receptor antagonist (6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy) pyridin-3-yl carbamoyl] indoline) (SB242,084; Kennett et al., 1997). In common with Heiser & Tecott (2000), following pre-treatment with SB242,084, we observed a paradoxical hyperactivity to Ro60-0175, and an unmasking of hitherto unseen behaviours including wet-dog shakes and back muscle contractions. These were shown in a subsequent experiment to be completely blocked by the 5-HT2A receptor antagonist MDL100,907 (Kehne et al., 1996). Following this observation, we then studied the indirect 5-HT agonist dexfenfluramine, both in the presence and absence of SB242,084. Again, co-treatment with SB242,084 resulted in a marked change in the behavioural profile of dexfenfluramine, including the emergence of toxicity.
Methods
Animals and housing
Male Sprague-Dawley rats (BRL, Fullinsdorf, body weight 230 – 270 g) were used throughout. Animals were housed in groups of four within a holding room controlled for temperature (22±2°C), and lighting (lights on: 06:00 – 18:00 h). All experiments were conducted during the light phase. Rats received food and water ad-libitum except during periods of behavioural observation. All experiments were conducted in accordance with the relevant local and national guidelines regarding animal experimentation.
Experimental design
In each study a crossover design was used, in that the same rats were run in a locomotor activity (LMA) and visual observation test. Seven days separated each test and the sequence of each was counterbalanced across animals. Treatment designation for individual rats was counterbalanced across the LMA and visual observation experiments.
For the measurement of LMA, the animals were singly placed into activity chambers (36×24×19 cm, L×W×H; Benwick Electronics, U.K.) containing sawdust bedding for a period of 60 min. Locomotor activity was automatically recorded by photocell interruption. The animals were naïve to the test apparatus at the time of the LMA experiment.
For the measurement of behavioural signs, subjects were treated with the appropriate treatment and individually placed in observation chambers (26×10×30 cm, L×W×H), with a mirror placed behind the chamber to allow an all round view of behaviour. Over a 60 min observation period, the following behaviours were scored: penile grooming (number of distinct penile grooming bouts accompanied by an erection), wet-dog shakes (total number) and back muscle contractions (scored as present or absent within 5 min time bins, i.e total possible score=12). The experimenter was unaware of drug treatment at the time of testing.
Experiment 1: Ro60-0175
A total of eight treatment groups were used in the initial part of this study (n=8 rats per group). Ro60-0175 was tested at four doses (vehicle, 1, 3, 10 mg kg−1) in the presence or absence of the 5-HT2C antagonist SB242,084 (0.5 mg kg−1). This dose of SB242,084 was selected on both published and in-house data, confirming 5-HT2C receptor blockade in vivo (Kennett et al., 1997; Grottick et al., 2000). Ro60-0175 was tested at doses previously reported as 5-HT2C selective, and at higher doses likely to be less selective (Millan et al., 1997; Martin et al., 1998; Dekeyne et al., 1999; Kennett et al., 2000).
In a subsequent experiment utilizing a further four groups of experimentally naïve rats (n=8 per group), the effect of the 5-HT2A antagonist MDL100,907 (0.5 mg kg−1) was tested against the hyperlocomotion, wet-dog shakes, and back muscle contractions induced by the Ro60-0175 (10)/SB242,084 (0.5) combination.
A further experiment evaluated the effect of the 5-HT2B preferring antagonist SB215,505 (3 mg kg−1 p.o.) against the hyperlocomotion, wet-dog shakes, and back muscle contractions induced by the Ro60-0175 (10)/SB242,084 (0.5) combination. The dose, route and pre-treatment time was based on published work on this compound (Kennett et al., 1998; Reavill et al., 1999).
Experiment 2: Dexfenfluramine
A total of eight treatment groups were used in this study. Dexfenfluramine was tested at four doses (vehicle, 1, 3, 10 mg kg−1) in the presence or absence of the 5-HT2C antagonist SB242,084 (0.5 mg kg−1). Initial group sizes were n=8, however during the course of this study, some toxicity was evident in the dex (10)/ SB242,084 pre-treated group. Final group sizes reflect this adjustment.
In a separate group of rats, a study was designed to examine the effect of MDL100,907 (0.5 mg kg−1 i.p.) and SB215,505 (3 mg kg−1 p.o.) against the behavioural changes and toxicity seen following dex (10)/SB242,085 (0.5) treatment. For this particular study the animals rectal body temperature was measured immediately pre and 60 min post treatment. During the intervening 60 min period the animals were tested in activity chambers but were not formally assessed for signs of 5-HT behavioural syndrome. A total of 10 rats per group were used for this study.
Drugs and injections
SB242,084 (6-chloro-5-methyl-1-[2-(2-methylpyridyl-3-oxy)-pyrid-5-yl carbomyl] indoline), MDL100,907 ((±)2,3-dimethoxyphenyl-1-[2-4-(piperidine)-methanol]), Ro60-0175 ((S)-2-(chloro-5-fluoro-indol-1-yl)-1-methylethylamine 1 : 1 C4H4O4) and dexfenfluramine were synthesized within the Chemistry Department at Hoffmann-La Roche Ltd., Basel, Switzerland. SB215,505 (6-chloro-5-methyl-1-(5-quinolylcarbamoyl) indoline) was generously provided by SmithKline Beecham Pharmaceuticals, Harlow, U.K. Ro60-0175 was dissolved in 0.9% saline before subcutaneous injection immediately prior to test onset. Dexfenfluramine was dissolved in 0.9% saline solution and injected 10 min prior to test via the intraperitoneal route. SB242,084 was prepared in 0.9% saline solution containing 8% hydroxypropyl-β-cyclodextrin and 25 mM citric acid. SB242,084 was injected by the intraperitoneal route with a pre-treatment time of 30 min. MDL100,907 was dissolved in 0.9% saline solution containing 0.3% Tween and injected s.c. 30 min before test onset. SB215,505 was dissolved in a solution of 0.3% Tween, and injected p.o. 60 min before test at a volume of 5 ml kg−1. All drug doses are expressed as that of the base.
Data analysis
Activity data and scores derived from penile grooming and wet-dog shakes were analysed by one or two way ANOVA for independent groups using Statistica software. Data for back muscle contractions first underwent square root transformation before a similar ANOVA procedure was applied. Tabulated data for back muscle contractions reflect scores prior to transformation. Following a significant main effect or interaction, post-hoc comparisons were carried out using the Neuman-Keuls test. In all cases the accepted level of significance was taken at P<0.05.
Results
Experiment 1: Ro60-0175
In vehicle pre-treated rats, Ro60-0175 (1 – 10 mg kg−1) produced a dose related hypoactivity [F(3,28)=5.5, P<0.01], with a significant decrease compared to controls at 3 – 10 mg kg−1. In contrast, following pre-treatment with SB242,084 (0.5 mg kg−1) a significant hyperactivity emerged [F(3,28)=3.1, P<0.05]. Consequently 2-way ANOVA revealed a significant interaction between Ro60-0175 and SB242,084 pre-treatment [F(3,56)=6.8, P<0.01]. SB242,084 alone did not significantly affect activity, although there was a trend toward an increase (P=0.08) (Figure 1A).
Figure 1.
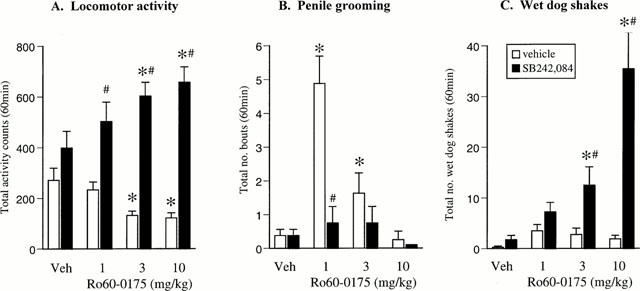
Effect of Ro60-0175 (1–10 mg kg−1) alone and in combination with the selective 5-HT2C receptor antagonist SB242,084 (0.5 mg kg−1) on locomotor activity (A), number of bouts of penile grooming (B) and number of wet-dog shakes (C) recorded over a 60 min test session. n=8 rats per group. *P<0.05 vs respective vehicle group. #P<0.05 vs respective Ro60-0175/veh group (Newman-Keuls test).
SB242,084 (0.5 mg kg−1) pre-treatment alone did not produce any overt behavioural change (Figure 1), but blocked the incidence of penile grooming induced by the lowest dose of Ro60-0175 (1 mg kg−1). Thus a main effect of Ro60-0175 on penile grooming was observed [F(3,56)=10.4, P<0.01] and a significant Ro60-0175×SB242,084 interaction [F(3,56)=17.2, P<0.01] (Figure 1B). Incidence of wet-dog shakes following treatment with Ro60-0175 alone (1 – 10 mg kg−1) did not differ significantly at any dose from controls. However, SB242,084 pre-treatment potentiated Ro60-0175-induced wet-dog shakes [Ro60-0175×SB242,084 interaction: F(3,56)=6.2, P<0.01]. Ro60-0175 alone did not produce a significant number of back muscle contractions [F(3,28)=1.0, NS) however these were increased by SB242,084 pre-treatment [F(3,56)=5.2, P<0.01] (e.g. vehicle=0, Ro60-0175 10 mg kg−1=0, SB242,084=0, Ro60-0175 (10)/SB242,084=3±1).
The effect of MDL100,907 (0.5 mg kg−1) (MDL) and SB215,505 (3 mg kg−1 p.o.) on the hyperactivity, wet-dog shakes and back muscle contractions produced by the Ro60-0175 (10 mg kg−1)/SB242,084 (0.5 mg kg−1) combination was examined. In this study, the Ro60-0175/SB242,084 combination resulted in a significant hyperactivity and an increased incidence of wet-dog shakes and back muscle contractions compared to vehicle controls. MDL pre-treatment alone did not affect either measure, yet completely prevented the Ro60-0175/SB242,084-induced behavioural response (Figure 2A,B). [Ro60-0175/SB242,084×MDL interaction – LMA: F(1,32)=18.4, P<0.01; wet-dog shakes: F(1,28)=29.6, P<0.01; back muscle contractions: F(1,28)=185.6, P<0.01]. In contrast SB215,505 failed to significantly influence the Ro60-0175/SB242,084 response as evidenced by no significant Ro60-0175/SB242,084×SB215,505 interaction for the main parameters measured [LMA: F(1,20)=1.0, NS; wet-dog shakes: F(1,20)=1.9, NS; back muscle contractions: F(1,20)=0.6, NS] (Figure 2C,D).
Figure 2.
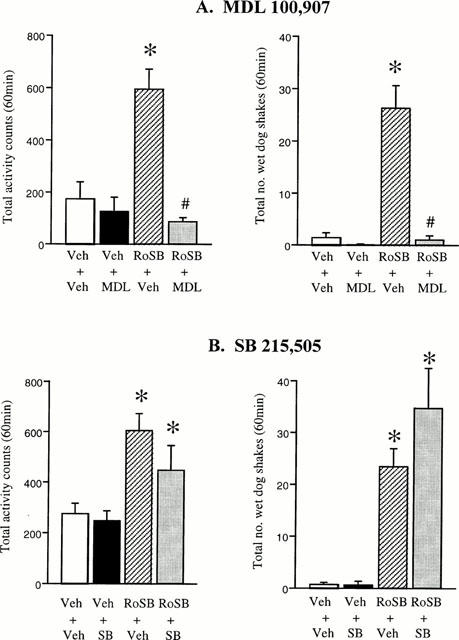
Effect of the 5-HT2A receptor antagonist MDL100,907 (0.5 mg kg−1; A) and the 5-HT2B receptor antagonist SB215,505 (3 mg kg−1; B) against the hyperactivity and wet-dog shakes produced by the combination of Ro60-0175 (10 mg kg−1) and SB242,084 (0.5 mg kg−1) (RoSB group). n=6–8 rats per group. *P<0.05 vs vehicle/vehicle group. #P<0.05 vs RoSB/veh group (Newman-Keuls test).
Experiment 2: Dexfenfluramine
Dexfenfluramine (1 – 10 mg kg−1) pre-treatment produced a modest hyperactivity [F(3,28)=9.1, P<0.01], that was significantly different from controls at 10 mg kg−1. In combination with SB242,084 (0.5 mg kg−1) a marked increase in this measure was observed [DEX×SB242,084 interaction: F(3,56)=28.9, P<0.01], with SB242,084 potentiating the locomotor change produced by each dose of dexfenfluramine (Figure 3A). In this experiment, SB242,084 pre-treatment alone did not affect activity.
Figure 3.
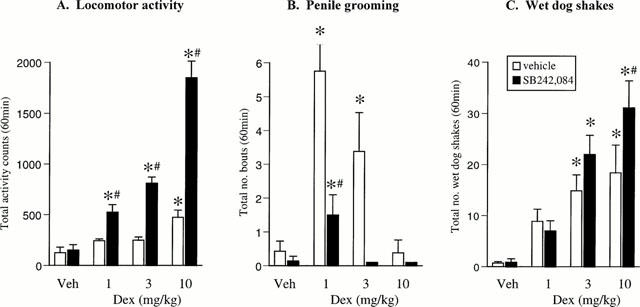
Effect of dexfenfluramine (1–10 mg kg−1) alone and in combination with the selective 5-HT2C receptor antagonist SB242,084 (0.5 mg kg−1) on locomotor activity (A), number of bouts of penile grooming (B) and number of wet-dog shakes (C) recorded over a 60-min test session. *P<0.05 vs respective vehicle group. #P<0.05 vs respective dexfenfluramine/veh group (Newman-Keuls test).
Dexfenfluramine induced penile grooming [F(3,27)=4.7, P<0.01] and a dose related incidence of wet-dog shakes [F(3,27)=11.7, P<0.01]. Similar to Experiment 1, SB242,084 (0.5 mg kg−1) pre-treatment inhibited the incidence of penile grooming [DEX×SB242,084 interaction – penile grooming (F(3,53)=5.9, P<0.01)]. A significant interaction term was not observed for wet-dog shakes [F(3,53)=1.9, NS], although post-hoc analyses revealed a significant increase in the dexfenfluramine (10 mg kg−1) group following SB242,084 pre-treatment (Figure 3B,C). Dexfenfluramine also induced back muscle contractions at the 10 mg kg−1 dose [F(3,27)=13.5, P<0.01], whilst SB242,084 treatment alone did not influence this parameter. However, the combination of dexfenfluramine and SB242,084 produced a marked increase in the incidence of back muscle contractions [DEX×SB242,084 interaction: F(3,53)=3.3, P<0.05] (Table 1).
Table 1.
Effect of dexfenfluramine and SB242,084 on the incidence of back muscle contractions
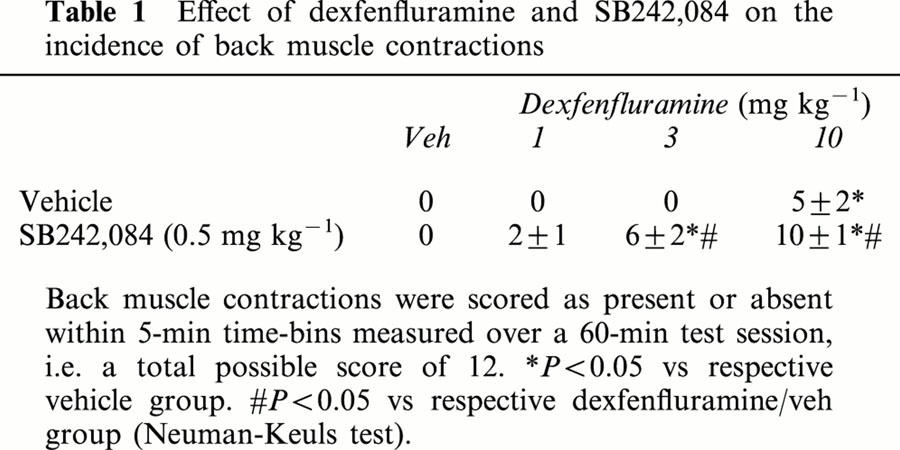
A notable finding from this study was the toxicity that emerged following the combination of dexfenfluramine 10 mg kg−1 and SB242,084 0.5 mg kg−1. Six animals out of 16 treated with this combination (38%), died within 24 h of treatment. Animals who survived this treatment appeared normal at the time of retest 7 days later. No toxicity was evident in any of the other treatment groups.
In a final study, the effect of MDL100,907 and SB215,505 against the hyperactivity and toxicity produced by the dexfenfluramine (10 mg kg−1) and SB242,084 (0.5 mg kg−1) combination (Dex/SB242,084) was examined. Body temperature assessment was also included in this study (see Discussion). Again the combination of Dex/SB242,084 resulted in a significant hyperactivity (Dex/SB242,084 main effect F(1,54)=426.1; P<0.01), which was attenuated by MDL100,907 (0.5 mg kg−1 i.p.) but not SB215,505 (3 mg kg−1 p.o.) (Figure 4A). Dex/SB242,084 also produced a hyperthermic response (+3°C) (Dex/SB242,084 main effect: F(1,54)=8.8, P<0.01), which was completely reversed by MDL100,907 but not SB215,505 (Figure 4B). MDL100,907 also appeared to prevent the toxicity produced by the Dex/SB242,084 combination recorded up to 24 h post dosing (Dex/SB242,084+vehicle: 3/10 deaths; Dex/SB242,084+SB215,505: 4/10 deaths; Dex/SB242,084+MDL100,907: 0/10 deaths).
Figure 4.
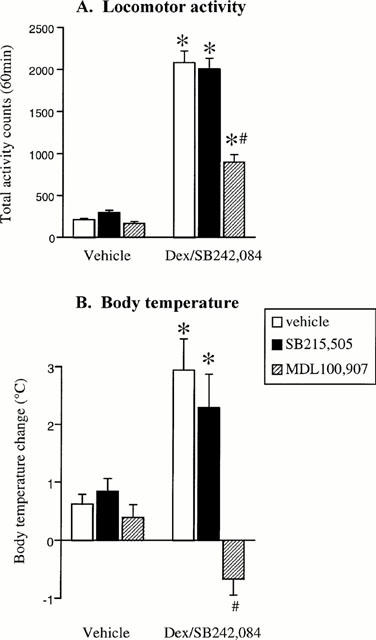
Effect of vehicle, SB215,505 (3 mg kg−1), MDL100,907 (0.5 mg kg−1) against the hyperactivity (A) and hyperthermia (B) produced the combination of dexfenfluramine (10 mg kg−1) and SB242,084 (0.5 mg kg−1). *P<0.05 vs respective vehicle group. #P<0.05 vs dexfenfluramine/SB242,084/veh group (Newman-Keuls test).
Discussion
Ro60-0175 has been described in the literature as a 5-HT2C receptor preferring agonist (Millan et al., 1997). When tested in vivo this compound seems to satisfy this claim, producing behaviours characteristic of 5-HT2C receptor activation including hypoactivity, penile grooming and hypophagia (Millan et al., 1997; Martin et al., 1998; Kennett et al., 2000), but not behaviours mediated through other 5-HT (notably 5-HT2A) receptors. Ro60-0175 also produces an interoceptive cue that is most likely 5-HT2C receptor mediated (Dekeyne et al., 1999). However, in vitro Ro60-0175 has reasonable affinity and efficacy at human 5-HT2A and particularly human (and rat) 5-HT2B receptors (Martin et al., 1998; Porter et al., 1999). The findings from Experiment 1 suggest that the apparent in vivo selectivity of this drug is because its 5-HT2C agonist properties inhibit the expression of 5-HT2A receptor-mediated behaviours, most notably wet-dog shakes, back muscle contractions and hyperactivity. The doses of Ro60-0175 producing these effects (3 – 10 mg kg−1) were approximately 10 fold higher than the doses required to elicit 5-HT2C related behaviours (0.3 – 1 mg kg−1; Millan et al., 1997; Martin et al., 1998; Kennett et al., 2000), consistent with the relative potencies of Ro60-0175 at human 5-HT2A and 5-HT2C receptors (Porter et al., 1999).
Therefore the results from Experiment 1 are similar to the observations of Heiser & Tecott (2000), who described a paradoxical hyperactivity following another 5-HT2C preferring agonist mCPP in a line of 5-HT2C receptor k.o. mice (Tecott et al., 1995). Pre-treatment with the selective 5-HT2B/2C receptor antagonist, SB206,553 (Kennett et al., 1996), resulted in a similar hyperactivity manifest in wild type mice following mCPP treatment, confirming that these effects were not simply a non-specific consequence of changes brought about by the gene targeting technique (Heiser & Tecott, 2000). Furthermore, during the course of this work we became aware of similar findings reported in preliminary form by Vickers et al. (2000). Taken together with the present data, these findings demonstrate that 5-HT2C receptor activation can markedly influence behaviour, and inhibit the expression of behaviours mediated through other 5-HT receptor subtypes.
Ro60-0175 has affinity and efficacy at 5-HT2B receptors seemingly equivalent to that at the 5-HT2C receptor (Martin et al., 1998; Porter et al., 1999). However the involvement of this receptor subtype on the repertoire of behaviours produced by this drug seems minimal given the lack of effect of SB215,505 against the Ro60-0175/SB242,084 treatment combination. SB215,505 has recently been described in the literature as a 5-HT2B receptor antagonist with moderate selectivity over 5-HT2A and 5-HT2C receptors (pKi h5-HT2B 8.3±0.1, h5-HT2A 6.8±0.2, h5-HT2C 7.7±0.1; see Table 1 from Reavill et al., 1999). Indeed Kennett et al. (1998) reported antagonism of an anticonflict effect of BW-723C86 following SB215,505 (3 mg kg−1 p.o.) pre-treatment, suggesting the dose and route of administration used in the present study to be pharmacologically relevant.
In the second part of this study, we examined the 5-HT reuptake inhibitor/releaser dexfenfluramine, both alone, and in combination with SB242,084. Despite producing a generalized facilitation of 5-HT function, dexfenfluramine produces some behavioural effects indicative of 5-HT2C activation (e.g. hypophagia and penile grooming) (Berendsen & Broekkamp, 1987; Bickerdike et al., 1999). Indeed, each of these effects are blocked by antagonists at the 5-HT2C receptor, confirming that this receptor is a principal mediator of at least some of dexfenfluramine's effects on behaviour (Hartley et al., 1995; Bickerdike et al., 1999; present study). Again, the combination of dexfenfluramine with SB242,084 resulted in a change in behavioural profile, including potentiation of both an existing hyperactivity and of 5-HT-mediated behavioural signs. The most striking observation from this experiment was the emergence of toxicity in animals treated with the dexfenfluramine (10 mg kg−1)/SB242,084 combination. We speculated that this toxicity may be associated with the hyperthermia which has been noted following central 5-HT2A receptor agonist treatment (Gudelsky et al., 1986), exacerbated perhaps by the intense hyperactivity seen in this treatment group. Accordingly, in a follow-up study we confirmed that the Dex/SB242,084 treatment combination resulted in a marked hyperthermic response that was blocked by MDL100,907. Toxicity was also eliminated by this pre-treatment combination. These studies identify a striking adverse interaction between dexfenfluramine and a 5-HT2C receptor antagonist that should be recognised for its potential clinical implication.
In some respects, the behavioural profile of dexfenfluramine and Ro60-0175 were similar, most notably in the induction of penile grooming, with a maximal incidence at the lowest dose tested. At higher doses the frequency of this behaviour was reduced. A similar biphasic effect of 5-HT2C agonists on penile grooming/erection has been reported by others, and the decline may relate to the induction of alternative and competing behaviours (e.g. wet-dog shakes/hypolocomotion), probably in some cases related to interactions with other 5-HT receptor subtypes (Berendsen & Broekkamp, 1990; Millan et al., 1997; Martin et al., 1998). In contrast to the hypolocomotor effects of Ro60-0175, dexfenfluramine produced a mild increase in activity, which was greatly potentiated by SB242,084 – considerably more so than the Ro60-0175/SB242,084 combination. This hyperactivity was only partially blocked by MDL100,907 pre-treatment. Notwithstanding the limited dose ranges studied, this difference may relate to additional 5-HT receptors activated by dexfenfluramine, such as 5-HT1B/1D which additionally influence locomotor activity in the rodent (Oberlander et al., 1983; Cheetham & Heal, 1993). Selective antagonists at the various 5-HT receptors would be useful tools for the pharmacological dissection of this effect.
A final consideration is that the interactions identified in these experiments do not reflect receptor mediated events, but rather a pharmacodynamic or pharmacokinetic interaction between SB242,084 and Ro60-0175 or dexfenfluramine. We feel that this is unlikely for the following reasons. Firstly, in the Ro60-0175 study, the treatment combination of Ro60-0175 with SB242,084 resulted in a completely distinct pattern of behaviour; whereas a metabolic interaction might be expected to potentiate an existing behavioural pattern. Secondly these behavioural effects were completely blocked by pre-treatment with MDL100,907, consistent with it being a 5-HT2A receptor mediated response (Martin et al., 1998; Porter et al., 1999). Thirdly, SB242,084 has been reported to have extremely low inhibitory activity at cytochrome P450 enzymes (IC50's>100 μM; Bromidge et al., 1997) making it unlikely to interact with the metabolism of either dexfenfluramine or Ro60-0175, at least at the relatively low doses used in the present study. However, the availability of plasma levels of Ro60-0175 and dexfenfluramine with and without SB242,084 pretreatment, would have been a useful addition to supplement these conclusions.
The present studies therefore provide some explanation for the apparent paradox that while Ro60-0175 (and mCPP; see Kennett & Curzon, 1988) seem to have little selectivity for the 5-HT2C receptor in vitro (Porter et al., 1999), in vivo both drugs seem to function as selective agonists at this receptor. Preferential activation of this receptor would appear to inhibit the expression of other non-5-HT2C receptor mediated effects. The converse may also be true for 5-HT2A receptor agonists, such as LSD and DOI, which again appear to have little selectivity over other members of the 5-HT2 receptor subclass in vitro (Egan et al., 1998; Porter et al., 1999). In vivo both LSD and DOI induce wet-dog shakes, back muscle contractions and hyperactivity, effects likely attributable to 5-HT2A receptor agonism (Wettstein et al., 1999; Ouagazzal et al., 2001). However, at doses which should achieve appreciable activation of 5-HT2C receptors, no signs of 5-HT2C-mediated behaviour are apparent. It is quite possible that these interactions extend to higher CNS functions such as perception and mood, and perhaps explain why drugs such as LSD are hallucinogenic while pharmacologically similar (but not identical) drugs such as lisuride are not (Egan et al., 1998). The present studies also indicate that the combination of dexfenfluramine and a 5-HT2C antagonist can elicit a severe 5-HT syndrome, including toxicity.
Acknowledgments
We thank Drs Mark Duxon and Derek Middlemiss of SB Pharmaceuticals, Harlow, U.K. for the generous supply of SB215,505 and also to Dr Graham Riley (SB Pharmaceuticals, Harlow, U.K.) for the provision of binding affinity data for this drug. We would also like to thank Drs Juergen Wichmann and Phillippe Guerry of Hoffmann-La Roche, Basel for the synthesis of SB242,084 and MDL100,907 respectively.
Abbreviations
- ANOVA
analysis of variance
- DOI
(±)-2-dimethoxy-4-iodoamphetamine hydrochloride
- LMA
locomotor activity
- LSD
lysergic acid diethylamide
- mCPP
m-(chlorophenyl)piperazine
- Ro60-0175
(S)-2-(6-chloro-5-fluoroindol-1-yl)-1-methlethylamine)
- SB242,084
(6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy) pyridin-3-yl carbamoyl] indoline)
References
- AGHAJANIAN G.K., MAREK G.J. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BAXTER G.S., KENNETT G.A., BLANEY F., BLACKBURN T. 5-HT2 receptor subtypes: a family reunited. TipS. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- BERENDSEN H.G., BROEKKAMP C.L.E. Drug-induced penile erections in rats: indications of serotonin1B receptor mediation. Eur. J. Pharmacol. 1987;135:279–287. doi: 10.1016/0014-2999(87)90676-5. [DOI] [PubMed] [Google Scholar]
- BERENDSEN H.G., BROEKKAMP C.L.E. Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br. J. Pharmacol. 1990;101:667–673. doi: 10.1111/j.1476-5381.1990.tb14138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BICKERDIKE M.J., VICKERS S.P., DOURISH C.T. 5-HT2C receptor modulation and the treatment of obesity. Diabetes, Obesity Metab. 1999;1:207–214. doi: 10.1046/j.1463-1326.1999.00037.x. [DOI] [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- BROMIDGE S.M., DUCKWORTH M., FORBES I.T., HAM P., KING F.D., THEWLIS K.M., BLANEY F.E., NAYLOR C.B., BLACKBURN T.P., KENNETT G.A., WOOD M.D., CLARKE S.E. 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-indoline (SB-242084): The first selective and brain penetrant 5-HT2C receptor antagonist. J. Med. Chem. 1997;40:3494–3496. doi: 10.1021/jm970424c. [DOI] [PubMed] [Google Scholar]
- CHEETHAM S.C., HEAL D.J. Evidence that RU 24969-induced locomotor activity in C57/Bl/6 mice is specifically mediated by the 5-HT1B receptor. Br. J. Pharmacol. 1993;110:1621–1629. doi: 10.1111/j.1476-5381.1993.tb14010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLITHEROE J.W., SCOPES D.I.C., SKINGLE M., JORDAN C.C., FENIUK W., CAMPBELL I.B., CARTER M.C., COLLINGTON E.W., CONNOR H.E., HIGGINS G.A., BEATTIE D.T., KELLY H.A., MITCHELL W.L., OXFORD A.W., WADSWORTH A., TYERS M.B. Evolution of a novel series of benzanilides as the first selective 5-HT1D antagonists. J. Med. Chem. 1994;37:2253–2257. doi: 10.1021/jm00041a001. [DOI] [PubMed] [Google Scholar]
- DEKEYNE A., GIRARDON S., MILLAN M.J. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, Ro60-0175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- EGAN C.T., HERRICK-DAVIS K., MILLER K., GLENNON R.A., TITELER M. Agonist activity of LSD and lisuride at cloned 5-HT2A and 5-HT2C receptors. Psychopharmacology. 1998;136:409–414. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- FONE K.C., JOHNSON J.V., BENNETT G.W., MARSDEN C.A. Involvement of 5-HT2 receptors in the behaviours produced by intrathecal administration of selected 5-HT agonists and the TRH analogue (CG 3509) to rats. Br. J. Pharmacol. 1989;96:599–608. doi: 10.1111/j.1476-5381.1989.tb11858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROTTICK A.J., FLETCHER P.J, HIGGINS G.A. Studies to investigate the role of 5-HT2C receptors on cocaine and food maintained behaviour. J. Pharmacol. Exp. Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- GUDELSKY G.A., KOENIG J.I., MELTZER H.Y. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat: evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- HARTLEY J.E., BROWN G., FLETCHER A., DOURISH C.T. Evidence for the involvement of 5-HT2B/2C receptors in mediating fenfluramine-induced anorexia in rats. Br. J. Pharmacol. 1995;114:373P. [Google Scholar]
- HEISER L.K., BALWA P., TECOTT L.H.5-HT2C receptor null mice display paradoxical hyperactivity following mCPP Soc. Neurosci. Abs. 199925483.14 [Google Scholar]
- HEISER L.K., TECOTT L.H.A paradoxical locomotor response in serotonin 5-HT2C receptor mutant mice J. Neurosci. 200020RC71 (1-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., MARTIN G. 5-HT receptor classification and nomenclature: Towards a harmonisation with the human genome. Neuropharmacology. 1997;36:419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- KEHNE J.H., BARON B., CARR A.A., CHANEY S.F., ELANDS J., FELDMAN D.J., FRANK R.A., GIERSBERGEN P.L.M., MCCLOSKY T.C., JOHNSON M.P., MCCARTY D.R., POIROT M., SENYAH Y., SIEGEL B.W., WIDMAIER C. Preclinical characterisation of the potential of the putative atypical antipsychotic MDL100,907 as a potent 5-HT2A antagonist with a favourable CNS safety profile. J. Pharm. Exp. Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- KENNETT G.A., CURZON G. Evidence that mCPP may have behavioural effects mediated by central 5-HT1C receptors. Br. J. Pharmacol. 1988;94:137–147. doi: 10.1111/j.1476-5381.1988.tb11508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNETT G.A., LIGHTOWLER S., TRAIL B., BRIGHT F., BROMIDGE S. Effects of Ro60-0175, a 5-HT2C receptor agonist, in three animal models of anxiety. Eur. J. Pharmacol. 2000;387:197–204. doi: 10.1016/s0014-2999(99)00706-2. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., TRAIL B., BRIGHT F. Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B receptor mediated. Neuropharmacology. 1998;37:1603–1610. doi: 10.1016/s0028-3908(98)00115-4. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., CILIA J., PIPER D.C., GAGER T., THOMAS D., BAXTER G.S., FORBES I.T., HAM P., BLACKBURN T.P. In vitro and in vivo profile of SB 206,553, a potent 5-HT2C/5-HT2B receptor antagonist, with anxiolytic-like properties. Br. J. Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., TRAIL B., RILEY G., HOLLAND V., AVENELL K.Y., STEAN T., UPTON N., BROMIDGE S., FORBES I.T., BROWN A.M., MIDDLEMISS D.N., BLACKBURN T.P. SB242,084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- MARTIN J.R., BOS M., JENCK F., MOREAU J-L., MUTEL V., SLEIGHT A.J., WICHMANN J., ANDREWS J.S., BERENDSEN H.H.G., BROEKKAMP C.L.E., RUIGHT G.S.F., KOHLER C., van DELFT A.M.L. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- MILLAN M.J., PEGLION J-L., LAVIELLE G., PERRIN-MONNEYRON S. 5-HT2C receptors mediate penile erections in rats: actions of novel and selective agonists and antagonists. Eur. J. Pharmacol. 1997;325:9–12. doi: 10.1016/s0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- OBERLANDER C. Effects of a potent 5-HT agonist, RU 24969, on the mesocortico-limbic and nigrostriatal dopamine systems. Br. J. Pharmacol. 1983;80:675P. [Google Scholar]
- OUAGAZZAL A.-M., GROTTICK A.J., MOREAU J.-L., HIGGINS G.A.Effect of LSD on prepulse inhibition and spontaneous behaviour in the rat: a pharmacological analysis and comparison between two rat strains Neuropsychopharmacology 2001(in press) [DOI] [PubMed]
- PORTER R.H.P., BENWELL K.R., LAMB H., MALCOLM C.S., ALLEN N.H., REVELL D.F., ADAMS D.R., SHEARDOWN M.J. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRANZATELLI M.R. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioural effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropanr)-2 (DOI) Neurosci. Lett. 1990;115:74–80. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- REAVILL C., KETTLE A., HOLLAND V., RILEY G., BLACKBURN T. Attenuation of haloperidol-induced catalepsy by a 5-HT2C receptor antagonist. Br. J. Pharmacol. 1999;126:572–574. doi: 10.1038/sj.bjp.0702350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TECOTT L.H., SUN L.M., AKANA S.F., STRACK A.M., LOWENSTEIN D.H., DALLMAN M.F., D JULIUS D. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- VICKERS S.P., BASS C.A., KENNETT G.A. Interaction of the 5-HT2A and 5-HT2C receptors in the mediation of head shake behaviour in rats. Br. J. Pharmacol. 2000;129:152P. [Google Scholar]
- WETTSTEIN J.G., HOST M., HITCHCOCK J.M. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioural effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog. NeuroPsychopharmacol. Biol. Psychiat. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]


