Abstract
Legume-derived isoflavones such as genistein, diadzein and equol have been associated with a reduction in risk of cardiovascular disease. In the current study, we explore the vascular activity of several isoflavone metabolites namely dihydrodaidzein, cis and trans-tetrahydrodaidzein and dehydroequol for potential cardioprotective properties.
Rat isolated aortic rings were used. 17β-oestradiol, equol, and all four of the metabolites studied significantly antagonized contractile responses to noradrenaline.
The direct vasodilatory action of these compounds were examined and in contrast to 17β-oestradiol, the vasodilatory effect of which was demonstrated to be endothelium independent, the dilatory action of all four compounds could be inhibited by endothelium denudation.
Further, the dilatory action of both dihydrodaidzein and cis-tetrahydrodaidzein were inhibited by the nitric oxide synthase inhibitor, Nω-nitro-L-arginine (NOLA), by the soluble guanylate cyclase inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and by 40 mM KCl. Dilatory responses to dehydroequol and trans-tetrahydrodaidzein, on the other hand, were inhibited by 40 mM KCL but not by NOLA nor ODQ.
Finally, we examined the protective potential of these compounds in inhibiting endothelium damage by oxidized low density lipoprotein (ox-LDL). Trans-tetrahydrodaidzein was at least 10 fold more potent than 17β-oestradiol in protecting against ox-LDL induced damage.
We conclude that the isoflavone metabolites, dihydrodaidzein, cis- and trans-tetrahydrodaidzein and dehydroequol, may potentially represent a novel series of cardioprotective therapeutics.
Keywords: Isoflavone metabolites, vascular, rat, aorta, endothelium
Introduction
Isoflavones are a class of phytoestrogens found predominantly in legumes. Their estrogenic activity has been related to their structural similarity to steroidal estrogens. High dietary intake of isoflavones has been associated with a reduction in the risk of developing osteoporosis (Anderson & Garner, 1998), some forms of cancer (Moyad, 1999) and cardiovascular disease (Clarkson & Anthony, 1998). The cardioprotective ability of these plant products has been attributed, at least in part, to their ability to lower cholesterol (Wong et al., 1998) and blood pressure (Lichtenstein, 1998) and to improve arterial compliance (Nestel et al., 1997).
Endogenous steroidal estrogens are well documented to have cardiovascular protective potential. One mechanism by which ovarian steroids exert these effects may be their ability to regulate vascular activity. 17β-oestradiol is a direct vaso-relaxant (Jiang et al., 1991; Freay et al., 1997). In addition, it attenuates vasoconstrictor activity (Jiang et al., 1992; Ravi et al., 1994; Mugge et al., 1997), protects against vascular injury by oxidized low density lipoproteins (Peng et al., 1996) and prevents early vascular lesion and advance atherosclerosis (Nascimento et al., 1999). Some of these vasoactive properties are mimicked by the isoflavones genistein (Williams et al., 1999) and diadzein (Nevala et al., 1998; Toma et al., 1995).
Isoflavone metabolites such as equol, dihydrodaidzein and tetrahydrodaidzein have been identified in human urine following ingestion of isoflavones (Kelly et al., 1993). In the current paper we investigate the vascular activity of a series of such compounds. Their ability to inhibit vasoconstriction and to protect against endothelium damage by oxidized low density lipoproteins was compared with that of 17β-oestradiol. In addition, the vasodilatory capacity of these compounds and possible mechanisms of action were also explored.
Methods
Isolation of rat thoracic aorta
Male Sprague-Dawley rats (250±50 g) were euthanased with 80% CO2 and 20% O2. The thoracic aorta was excised and quickly mounted in organ-baths as previously described (Lewis et al., 1997).
This study was approved by the Animal Ethics Committee, Baker Medical Research Institute.
Protocol 1: The effect of isoflavone metabolites on contractile curves to noradrenaline
Full concentration-contractile curves were obtained to noradrenaline (0.1 nM – 10 mM) in the absence and presence of equol (0.1, 1 and 10 μg ml−1); β-oestradiol (1 μg ml−1); dehydroequol (1 μg ml−1); dihydrodaidzein (0.1 and 1 μg ml−1); cis-tetrahydrodaidzein (0.1 and 1 μg ml−1); trans-tetrahydrodaidzein (0.1 and 1 μg ml−1) and the vehicle equi-volume DMSO. Only one compound at any one concentration was tested on any one ring from any one animal.
Protocol 2a: The vasodilatory capacity of isoflavone metabolites
A full concentration response to noradrenaline was obtained. From this the concentration producing a submaximal contraction of approximately 80% was selected (0.03 – 0.3 μM). Full concentration-relaxation curves were then obtained to β-oestradiol, dehydroequol, dihydrodaidzein, cis-tetrahydrodaidzein, trans-tetrahydrodaidzein and the vehicle DMSO (at equi-volume). The molecular structure of the isoflavone metabolites studied are shown in Figure 1. Only one compound was studied with any one ring from any one animal.
Figure 1.
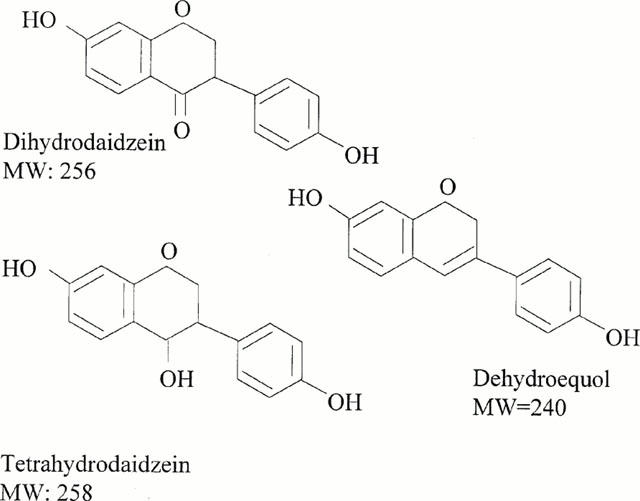
Shows the structure and molecular weights of the main isoflavone metabolites examined. The molecular weight (MW) of β-oestradiol is 272.
Protocol 2b: The vasodilatory capacity of isoflavone metabolites: mechanism of action
Full relaxation curves were obtained to dihydrodaidzein, cis-tetrahydrodaidzein, trans-tetrahydrodaidzein, dehydroequol and β-oestradiol in the absence and presence of the endothelium (rings were endothelially denuded by gentle rotation of the lumen against a rough surface). Successful endothelium denudation was confirmed by the total lack of response to acetylcholine (10 μM) on noradrenaline-preconstricted vessels (results not shown). Where denudation inhibited the vasorelaxing effects of the compounds, the effect of inhibition with nitro-L-arginine (NOLA 10 μM; with the exception of trans-tetrahydrodaidzein due to limited supply of the compound), KCl 40 mM, indomethacin (10 μM) and the soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μM) were examined. Only one compound with only one intervention was tested on any one ring from any one animal.
Protocol 3: Protective effect of isoflavone metabolites against endothelium damage induced by oxidized low density lipoprotein
Full concentration response curves to phenylephrine were constructed. Tissues were then constricted with phenylephrine at a submaximal (80%) concentration and allowed to plateau. Phenylephrine, rather than noradrenaline was used since a sustained plateau vasoconstrictory response to noradrenaline could not be obtained in the presence of ox-LDL. Full concentration dilatory curves were constructed to acetylcholine. Following this, 0.1% antifoam B (SIGMA), demonstrated in preliminary experiments not to affect responses to acetylcholine, was added to the bath. Full concentration response curves to acetylcholine were then repeated following a 1 h incubation with oxidized low density lipoprotein (ox-LDL: 0.3 mg protein/ml) in the absence and added presence of β-oestradiol (10 μg ml−1), dihydrodaidzein (300 ng ml−1), cis-tetrahydrodaidzein (1 μg ml−1), trans-tetrahydrodiadzein (3 μg ml−1) or dehydroequol (3 μg ml−1). The concentrations were chosen based on neg log EC45 – EC50 from the experiments performed in protocol 2a. Low density lipoprotein were prepared as previously described (Lewis et al., 1997) and oxidized by a 2 h incubation with CuSO4 5 μM.
Data presentation and statistical analysis
Results are analysed by two-way repeated measure analysis of variance where appropriate followed by post-hoc t-tests with corrections using Sigmastat Statistical software (Jandel Scientific, San Rafael, CA, U.S.A.). P<0.05 was taken as a measure of statistical significance.
Results
Protocol 1: The effect of isoflavone metabolites on contractile curves to noradrenaline
Figure 2 shows the effects of β-oestradiol, equol, the isoflavone metabolites and the vehicle used on responses to noradrenaline. Table 1 lists the maximal force obtained to noradrenaline in the absence and presence of the compounds used. All the active compounds studied had a significant antagonistic effect on noradrenaline-induced contractions. To obtain an index of the order of potency of the compounds studied, the difference in maximal response to noradrenaline (the maximal response in the absence minus the maximal response in the presence of compounds), as well as the per cent reduction in maximal response to noradrenaline was calculated for each compound (Table 1).
Figure 2.
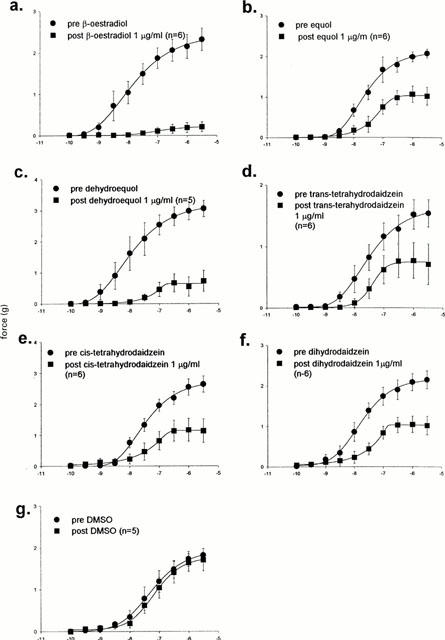
Effects of β-oestradiol, equol, dehydroequol, trans-tetrahydrodaidzein, cis-tetrahydrodaidzein, dihydrodaidzein (1 μg ml−1 30 min) and DMSO on full concentration-contractile responses to noradrenaline.
Table 1.
Maximal force (y max) generated by noradrenaline in the absence and presence of β-oestradiol, equol and the isoflavone metabolites used

Protocol 2a: The vasodilatory capacity of isoflavone metabolites: mechanisms of action
Figure 3 depicts the concentration-dilatory effects of the compounds studied. Dilatory responses to dihydrodaidzein, cis-tetrahydrodaidzein and dehydroequol were significantly greater than those to β-oestradiol. The mechanism by which these compounds exerted the dilatory effect was further examined using specific antagonists.
Figure 3.
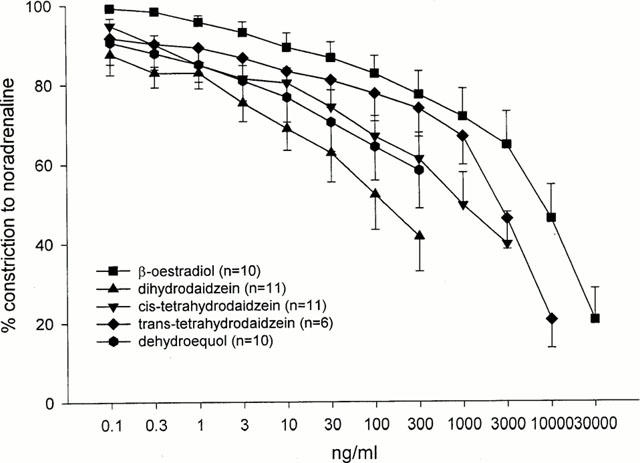
Concentration-dilatory responses to β-oestradiol, dihydrodaidzein, cis- and trans-tetrahydrodaidzein and dehydroequol were constructed on rat aortic rings pre-contracted with a sub-maximal concentration of noradrenaline. Dilatory responses to dihydrodaidzein, cis-tetrahydrodaidzein and dehydroequol were significantly greater than those to β-oestradiol. Analysis was by two-way ANOVA.
β-oestradiol
Vasodilatory responses to β-oestradiol were not significantly inhibited by removal of the endothelium (Figure 4a). No time dependent changes were observed (Figure 4b).
Figure 4.
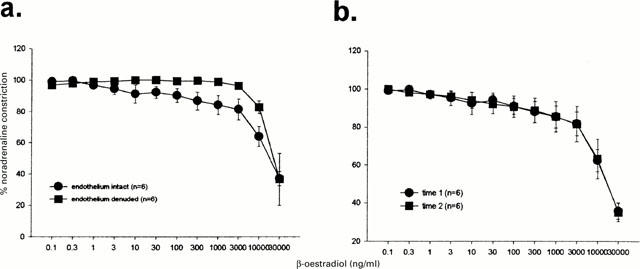
Dilatory responses to β-oestradiol were not significantly affected by endothelium denudation (a) nor by 30 min time-control (b).
Dihydrodaidzein
Vasodilatory responses to dihydrodaidzein were inhibited by removal of the endothelium (Figure 5a), incubation with nitro-L-arginine (Figure 5b), high KCl levels (Figure 5c) and ODQ (Figure 5d). Indomethacin did not influence the dilatory effects of this steroid (Figure 5e) and no time dependent changes were observed (Figure 5f).
Figure 5.
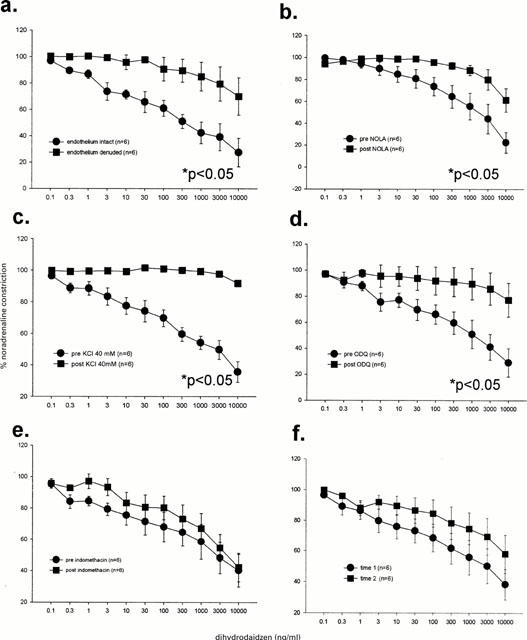
Dilatory responses to dihydrodaidzein were significantly inhibited by endothelium denudation (a), incubation with and Nω-nitro-L-arginine (b; NOLA), KCl (c) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (d; ODQ) but not by indomethacin (e) nor time (f). Analysis was by two-way repeated measures ANOVA.
Cis-tetrahydrodaidzein
Vasodilatory responses to cis-tetrahydrodaidzein were inhibited by removal of the endothelium (Figure 6a), high KCl levels (Figure 6c) and ODQ (Figure 6c). There was a trend towards inhibition by NOLA but this was not statistically significant. Indomethacin did not influence the dilatory effects of this steroid (Figure 6d) and no time dependent changes were observed (Figure 6e).
Figure 6.
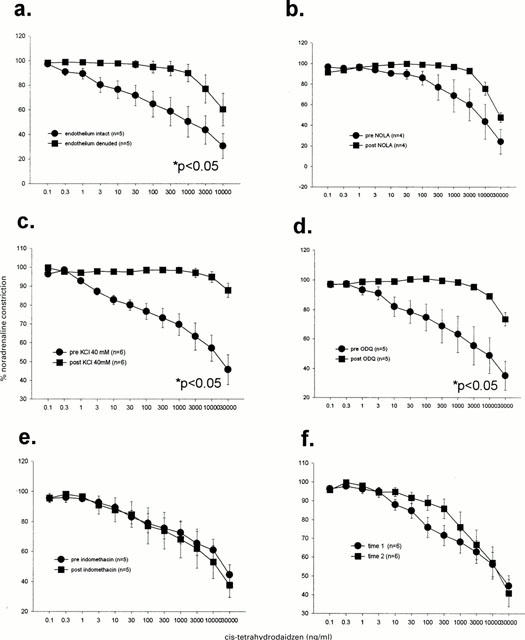
Dilatory responses to cis-tetrahydrodaidzein were significantly inhibited by endothelium denudation (a), KCl (c) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; d) but not by Nω-nitro-L-arginine (NOLA; b), indomethacin (e) nor time (f). Analysis was by two-way repeated measures ANOVA.
Trans-tetrahydrodaidzein
Vasodilatory responses to trans-tetrahydrodaidzein were inhibited by removal of the endothelium (Figure 7a), high KCl levels (Figure 7b) but not by ODQ (Figure 7c). Indomethacin potentiated the inhibitory effects of trans-tetrahydrodaidzein (Figure 7d). No time dependent changes were observed (Figure 7e).
Figure 7.
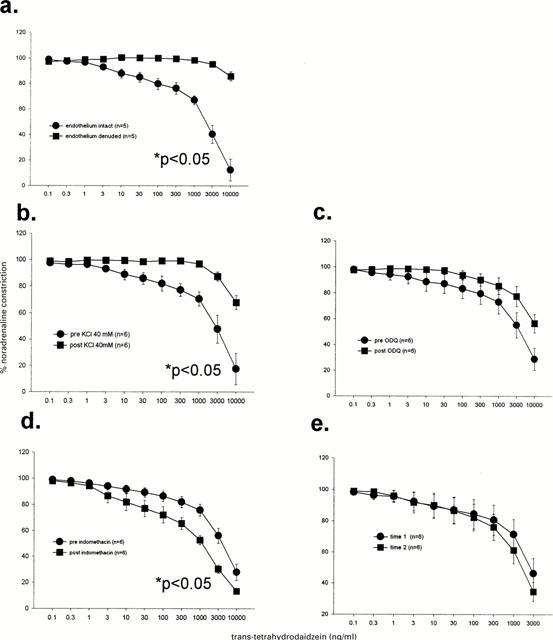
Dilatory responses to trans-tetrahydrodaidzein were significantly inhibited by endothelium denudation (a) and KCl (b) but not by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; c) nor time (e). These responses were significantly augmented with indomethacin (d). Analysis was by two-way repeated measures ANOVA.
Dehydroequol
The vasodilatory effects to dehydroequol were inhibited by removal of the endothelium (Figure 8a) and high KCl levels (Figure 8c) but not by NOLA (Figure 8b) or ODQ (Figure 8d) nor indomethacin (Figure 8e). No time dependent changes were observed (Figure 8f).
Figure 8.
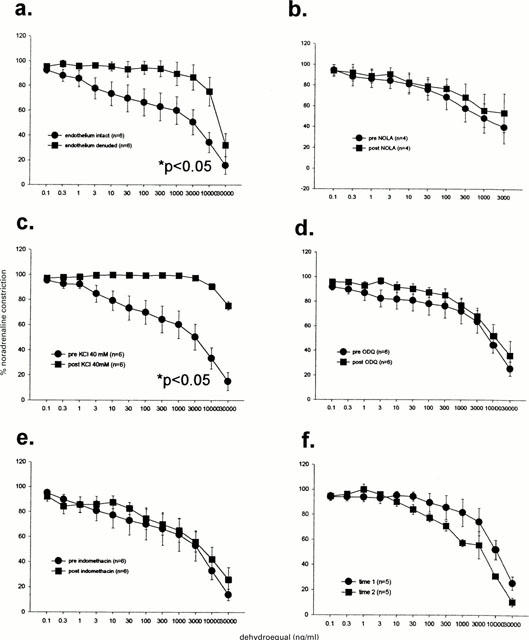
Dilatory responses to dehydroequol were significantly inhibited by endothelium denudation (a) and KCl (b) but not by Nω-nitro-L-arginine (NOLA; b), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; c), indomethacin (e) nor time (e). Analysis was by two-way repeated measures ANOVA.
Protocol 3: Protective effect of isoflavone metabolites against endothelium damage induced by oxidized low-density lipoprotein
Responses to acetylcholine were significantly attenuated by incubation with ox-LDL (Figure 9a). Co-incubation of ox-LDL with trans-tetrahydrodaidzein diminished the inhibitory effect of ox-LDL (Figure 9b). Further, the maximal response obtained to acetylcholine in the presence of co-incubation with this compound was significantly less than with sole incubation with ox-LDL (Figure 9c). Co-incubation of ox-LDL with β-oestradiol and the other compounds diminished the effect of ox-LDL such that the significant difference between the maximal dilatation obtained before and after ox-LDL incubation was no longer apparent. However maximal dilatation to acetylcholine obtained in the presence of the co-incubation was no different to that obtained in the presence of only ox-LDL (Figure 9c).
Figure 9.
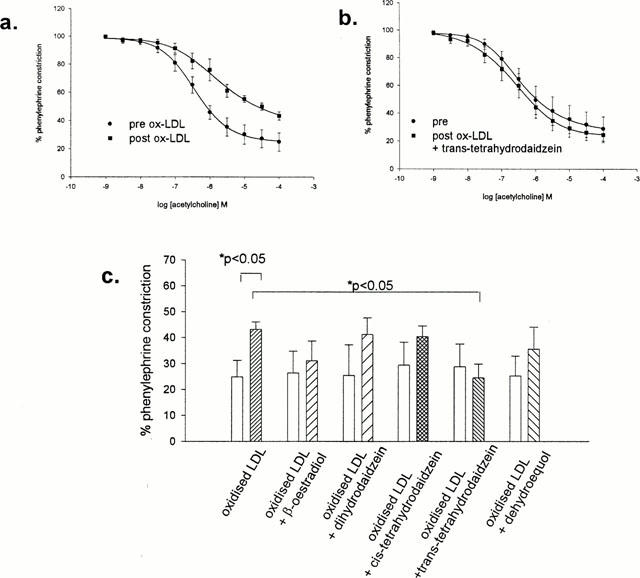
Dilatory responses to acetylcholine were significantly attenuated by incubation with ox-LDL (a). This inhibitory effect was no longer apparent when ox-LDL was co-incubated with trans-tetrahydrodaidzein. (b). Histograms (c) depict maximal responses to acetylcholine±oxidized LDL±metabolites. Analysis was by ANOVA.
Discussion
For the first time, we report on the vascular properties of the isoflavone metabolites dehydroequol, dihydrodaidzein, cis- and trans-tetrahydrodaidzein and demonstrate that these compounds: (a) inhibit vasoconstrictive responses to noradrenaline; (b) are vasodilatory; and (c) that trans-tetrahydrodaidzein significantly protects against endothelium damage by oxidized LDL. The in vitro vascular profile of some of these compounds is comparable to, and in some cases more effective than, that of the ovarian steroid 17β-oestradiol. These compounds may hence be responsible for the cardioprotective effects attributed to high isoflavone diets but more importantly they may be useful as potential cardioprotective agents especially if administered in doses greater than that achieved from normal dietary isoflavone intake.
The ability of the ovarian steroid 17β-oestradiol to inhibit the vasoconstrictor effects of endothelin-1 in rabbit coronary arteries (Jiang et al., 1992) and histamine, serotonin and angiotensin II responses in human internal mammary arteries (Mugge et al., 1997) has previously been documented. In this paper, we demonstrate that it effectively inhibits responses to noradrenaline in the rat isolated aorta. In addition, we demonstrate a direct vasodilatory effect of 17β-oestradiol that is independent of the endothelium. Our findings support those of Jiang et al. (1992) in that the effect of 17β-oestradiol on responses to endothelin-1 was independent of an intact endothelium. Further, since 17β-oestradiol also antagonized contractions induced by calcium and BAY K 8644 (a specific agonist of voltage-dependent calcium channels) in a comparable manner and time course to verapamil, this antagonistic effect of 17β-oestradiol can, at least partially, be attributed to an inhibitory effect on calcium influx. Inhibition of calcium influx by 17β-oestradiol may be the mechanism by which this steroid produced the vasodilatory responses observed in this paper.
The antagonistic action of the isoflavones genistein and diadzein and the metabolite equol have previously been reported (Nevala et al., 1998; Toma et al., 1995; Gimenez et al., 1997). Similar to the effects of 17β-oestradiol, vasodilatory responses to genistein and diadzein are endothelium independent (Nevala et al., 1998) and there is direct evidence that the tyrosine kinase inhibitor genistein, reduces fura-2 measured intracellular calcium activity in arteries stimulated with noradrenaline (Toma et al., 1995). However since the structurally similar but tyrosine kinase inactive compound diadzein also exhibits vasodilatory capacity, it is clear that some of these effects must be unrelated to tyrosine kinase inhibition. In this study equol was demonstrated to dose-dependently inhibit the contractile responses to noradrenaline.
Dihydrodaidzein, cis- and trans-tetrahydrodaidzein and dehydroequol were first examined for their ability to antagonize the contractile effects of noradrenaline. All were active to differing degrees, such that dehydroequol was most effective and comparable to 17β-oestradiol in this application. Dehydroequol was more potent than equol. Dihydrodaidzein and cis-tetrahydrodaidzein appeared equi-potent to each other but less potent than dehydroequol. They were followed in potency by equol and finally by trans-tetrahydrodaidzein. The antagonizing action of equol, dihydrodaidzein and cis- and trans-tetrahydrodaidzein were dose dependent. Neither the dose-dependency of dehydroequol nor β-oestradiol was examined. At equi-volume, the vehicle (DMSO) was ineffective at inhibiting responses to noradrenaline.
The vasodilatory effects of the isoflavone metabolites were at least as potent as, if not more potent than, 17β-oestradiol. The mechanism of action of the four metabolites studied however differed from 17β-oestradiol in that the dilatory action of all four metabolites diminished upon removal of the endothelium. None of the compounds were affected by indomethacin suggesting that prostacyclin is unlikely to play a substantial role in the vasodilatory capacity of these compounds. Indomethacin did however potentiate the effects of trans-tetrahydroequol such that an increase in vasodilatory capacity was observed suggesting that trans-tetrahydrodaidzein increased the release of a vasoconstrictory prostanoid, such as thromboxane, the removal of which led to the increased dilatation observed. The dilatory responses of both dihydrodaidzein and cis-tetrahydrodaidzein were inhibited by high levels of KCl and ODQ and NOLA. The dilatory action of both these compounds can therefore be associated with the release of an endothelium derived relaxing factor, probably nitric oxide activating soluble guanylate cyclase activity. The inhibition by KCl suggests that an endothelium derived hyperpolarizing factor is also released. It is recognized that there are limitations to the use of KCl in isolating EDHF responses, and that further investigation is warranted with the use of specific K+ channel inhibitors such as apamin and charybdotoxin. Given the limitations, in this study, there is no evidence to suggest that the hyperpolarizing factor released by dihydrodaidzein and cis-tetrahydrodaidzein is not nitric oxide. The dilatory effects of dehydroequol and trans-tetrahydrodaidzein, on the other hand, can be totally attributed to EDHF which in this instance is clearly not nitric oxide and not activated via soluble guanylate cyclase. It would thus appear that the mechanism of action by which these metabolites cause vasodilatation differs not only from the ovarian steroid β-oestradiol and the parent compounds genistein and daidzein, but also from each other.
In the final series of experiments the protective effect of these compounds against endothelium damage induced by oxidized LDL was examined. In this paper oxidized LDL inhibited the vasodilatory capacity of the endothelium dependent vasodilator acetylcholine substantiating previous work (Lewis et al., 1997; Jacobs et al., 1990; Plane et al., 1992). While all the compounds studied diminished the significant difference in responses to acetylcholine due to ox-LDL, co-incubation with trans-tetrahydroequol was the only compound which could be demonstrated to have a significant effect on responses to acetylcholine in direct comparison with sole incubation with ox-LDL. That 17β-oestradiol can protect against endothelial damage by ox-LDL has previously been demonstrated (Peng et al., 1996). From the current data, it would appear that the protective effect of trans-tetrahydrodaidzein in this context is at least 10 times more potent. Since trans-tetrahydrodaidzein is not the most potent compound in the other protocols i.e. in either antagonizing noradrenaline nor in its direct vasodilatory capacity, the mechanism of the cardioprotective action of this compound is likely to be different to the others tested and may lie in its anti-oxidant capacity.
Conclusion
In conclusion, we report that the isoflavone metabolites dihydrodaidzein, cis- and trans- tetrahydrodaidzein and dehydroequol have vascular regulatory capacity that, while comparable to the ovarian steroid 17β-oestradiol, and to the plant-derived parent compounds genistein, daidzein and equol, also appear to be unique in their mechanism of action. These compounds are capable of antagonizing contractile activity, direct vasodilatation and protecting against endothelium damage by oxidized low density lipoprotein and as such potentially represent a novel series of cardioprotective therapeutics.
Acknowledgments
This study was funded by Novogen Ltd, Sydney, Australia.
Abbreviations
- NOLA
Nw-nitro-L-argine
- ox-LDL
oxidized low density lipoprotein
References
- ANDERSON J., GARNER S. Phytoestrogens and bone. Baillieres Clin. Endocrinol. Metab. 1998;12:543–557. doi: 10.1016/s0950-351x(98)80003-7. [DOI] [PubMed] [Google Scholar]
- CLARKSON T., ANTHONY M. Phytoestrogens and coronary heart disease. Baillieres Clin. Endocrinol. Metab. 1998;12:589–604. doi: 10.1016/s0950-351x(98)80006-2. [DOI] [PubMed] [Google Scholar]
- FREAY A., CURTIS S., KORACH K., RUBANYI G. Mechanism of vascular smooth muscle relaxation by estrogen in depolarized rat and mouse aorta. Role of nuclear estrogen receptor and Ca2+ uptake. Circ. Res. 1997;81:242–248. doi: 10.1161/01.res.81.2.242. [DOI] [PubMed] [Google Scholar]
- GIMENEZ I., LOU M., VARGAS F., ALVAREZ-GUERRA M., MAYORAL J., MARTINEZ R., GARAY R., ALDA J. Renal and vascular actions of equol in the rat. J. Hypertens. 1997;15:1303–1308. doi: 10.1097/00004872-199715110-00015. [DOI] [PubMed] [Google Scholar]
- JACOBS M., PLANE F., BRUCKDORFER K.R. Native and oxidized low-density lipoproteins have different inhibitory effects on endothelium-derived relaxing factor in the rabbit aorta. Br. J. Pharmacol. 1990;100:21–26. doi: 10.1111/j.1476-5381.1990.tb12045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG C., SARREL P., LINDSAY D., POOLE-WILSON P., COLLINS P. Endothelium-independent relaxation of rabbit coronary artery by 17β-oestradiol in vitro. Br. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG C., SARREL P., POOLE-WILSON P., COLLINS P. Acute effect of 17b-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am. J. Physiol. 1992;263:H271–H275. doi: 10.1152/ajpheart.1992.263.1.H271. [DOI] [PubMed] [Google Scholar]
- KELLY G., NELSON C., WARING M., JOANNOU G., REEDER A. Metabolites of dietray (soya) isoflavones in human urine. Clin. Chim. Acta. 1993;223:9–22. doi: 10.1016/0009-8981(93)90058-c. [DOI] [PubMed] [Google Scholar]
- LEWIS T., DART A., CHIN-DUSTING J. Non-specific inhibition by human lipoproteins of endothelium dependent relaxation in rat aorta may be attributed to lipoprotein phospholipids. Cardiovasc. Res. 1997;34:590–596. doi: 10.1016/s0008-6363(97)00061-8. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN A. Soy protein, isoflavones and cardiovascular disease risk. J. Nutr. 1998;128:1589–1592. doi: 10.1093/jn/128.10.1589. [DOI] [PubMed] [Google Scholar]
- MOYAD M. Soy, disease prevention, and prostate cancer. Semin. Urol. Oncol. 1999;17:97–102. [PubMed] [Google Scholar]
- MUGGE A., BARTON M., FIEGUTH H., RIEDEL M. Contractile responses to histamine, serotonin, and angiotensin II are impaired by 17 beta-estradiol in human internal mammary arteries in vitro. Pharmacology. 1997;54:162–168. doi: 10.1159/000139483. [DOI] [PubMed] [Google Scholar]
- NASCIMENTO C., KAUSER K., RUBANYI G. Effect of 17 beta-estradiol in hypercholesterolemic rabbits with severe endoethelial dysfunction. Am. J. Physiol. 1999;276:H1788–H1794. doi: 10.1152/ajpheart.1999.276.5.H1788. [DOI] [PubMed] [Google Scholar]
- NESTEL P., POMEROY S., SASAHARA T., YAMASHITA T., LIANG T., LIANG Y., DART A., JENNINGS G., ABEY M., CAMERON J. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler. Thromb. Vasc. Biol. 1997;17:1163–1170. doi: 10.1161/01.atv.17.6.1163. [DOI] [PubMed] [Google Scholar]
- NEVALA R., KORPELA R., VAPAATALO H. Plant derived estrogens relax rat mesenteric artery in vitro. Life Sc. 1998;63:95–100. doi: 10.1016/s0024-3205(98)00300-2. [DOI] [PubMed] [Google Scholar]
- PENG C., LI Y., DENG H., XIONG X. Protective effects of 17 beta-estradiol on endothelial function injured by oxidized low-density lipoproteins. Chung Kuo. Yao. Li. Hsueh Pao. 1996;17:252–255. [PubMed] [Google Scholar]
- PLANE F., BRUCKDORFER R., KERR P., STEUER A., JACOBS M. Oxidative modification of low-density lipoproteins and the inhibition of relaxations mediated by endothelium-derived nitric oxide in rabbit aorta. Br. J. Pharmacol. 1992;105:216–222. doi: 10.1111/j.1476-5381.1992.tb14237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVI J., MANTZOROS C., PRABHU A., RAM J., SOWERS J. In vitro relaxation of phenylephrine and angiotensin II- contracted aortic rings by beta-estradiol. Am. J. Hypertens. 1994;94:1065–1069. doi: 10.1093/ajh/7.12.1065. [DOI] [PubMed] [Google Scholar]
- TOMA C., JENSEN P., PRIETO D., HUGHES A., MULVANY M., AALKJAER C. Effects of tyrosine kinase inhibitors on the contractility of rat mesenteric resistance arteries. Br. J. Pharmacol. 1995;114:1266–1272. doi: 10.1111/j.1476-5381.1995.tb13342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS K., WINSTON-SALEM N., HODGIN J., SMITHIES O., HILL C., KORACH K. The relative role of ER-α and ER-β in modulating the effects of estradiol and genistein on constrictor and dilator responses of arteries. Circ. 1999. pp. I–219.
- WONG W., SMITH E., STUFF J., HACHEY D., HEIRD W., POWNELL H. Cholesterol-lowering effect of soy protein in normocholesterolemic and hypercholesterolemic men. Am. J. Clin. Nutr. 1998;68:1385S–1389S. doi: 10.1093/ajcn/68.6.1385S. [DOI] [PubMed] [Google Scholar]


