Abstract
The aim of this study was to determine whether acute dual angiotensin-converting enzyme (ACE)/neutral endopeptidase 24-11 (NEP) inhibition could improve whole body insulin-mediated glucose disposal (IMGD) more than ACE inhibition alone and whether this effect was mediated by the kinin-nitric oxide (NO) pathway activation.
We therefore compared in anaesthetized obese (fa/fa) Zucker rats (ZOs) the effects of captopril (2 mg kg−1, i.v.+2 mg kg−1 h−1), retrothiorphan (25 mg kg−1, i.v. +25 mg kg−1 h−1), a selective NEP inhibitor, and mixanpril (25 mg kg−1, i.v.+25 mg kg−1 h−1), a dual ACE/NEP inhibitor, on IMGD using hyperinsulinaemic euglycaemic clamp technique. The role of the kinin-NO pathway in the effects of mixanpril was tested using a bradykinin B2 receptor antagonist (Hoe-140, 300 μg kg−1) and a NO-synthase inhibitor (Nω-nitro-L-arginine methyl ester, L-NAME, 10 mg kg−1 i.v. +10 mg kg−1 h−1) as pretreatments.
Insulin sensitivity index (ISI) was lower in ZO controls than in lean littermates. Increases in ISI were observed in captopril- and retrothiorphan-treated ZOs. In mixanpril-treated ZOs, ISI was further increased, compared to captopril- and retrothiorphan-treated ZOs.
In ZOs, Hoe-140 and L-NAME alone did not significantly alter and slightly reduced the ISI respectively. Hoe-140 and L-NAME markedly inhibited the ISI improvement induced by mixanpril.
These results show that in obese insulin-resistant Zucker rats, under acute conditions, NEP or ACE inhibition can improve IMGD and that dual ACE/NEP inhibition improves IMGD more effectively than does either single inhibition. This effect is linked to an increased activation of the kinin-NO pathway.
Keywords: Obese Zucker rat; insulin resistance; whole body insulin-mediated glucose disposal; angiotensin-converting enzyme inhibitor; neutral endopeptidase 24-11 inhibitor; captopril, retrothiorphan; mixanpril; bradykinin; nitric oxide
Introduction
Resistance to insulin-mediated glucose transport has been identified as the primary mechanism in the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus (for review, see Donnelly & Qu, 1998). Angiotensin-converting enzyme (ACE) inhibition increases insulin sensitivity in animal models of insulin resistance (Henriksen et al., 1996; Nakagawa et al., 1999) and may have similar effects in insulino-resistant patients (Uehara et al., 1994; Galletti et al., 1999).
In both insulin-resistant genetic hypertensive rats (Tomiyama et al., 1994; Nakagawa et al., 1999; Caldiz & De Cingolani, 1999) and obese Zucker rats (Henriksen et al., 1996), the acute ACE inhibitor-induced improvement in insulin action can be inhibited by pre-treatment with bradykinin (BK) B2 receptor antagonists. In addition, the ACE inhibitor captopril has been shown to increase insulin-mediated glucose disposal during an euglycaemic hyperinsulinaemic clamp in Wistar rats but not in Brown-Norway Katholiek kininogen-deficient rats which have low kinin levels (Damas et al., 1999). On the other hand, it has been reported that angiotensin II-receptor antagonists did not affect the glucose requirement for the euglycaemic clamp test (Tomiyama et al., 1994) or increase it to a lesser extent than did ACE inhibition (Nakagawa et al., 1999). In both isolated epitrochlearis muscles from obese Zucker rats (Henriksen et al., 1999) and adipocytes from spontaneously hypertensive rats (Caldiz & De Cingolani, 1999), angiotensin II-receptor antagonists did not modify insulin-stimulated glucose uptake. Collectively, these findings suggest that the acute metabolic effects of ACE inhibitors on insulin-stimulated whole body and skeletal muscle glucose disposal are mediated primarily via the action of BK on B2 receptor with no substantial contribution of the reduction in angiotensin II action.
BK administration has been reported to improve insulin sensitivity in diabetic dogs (Uehara et al., 1994) and potentiated insulin-stimulated glucose uptake in the isolated epitrochlearis muscle from obese Zucker rats (Henriksen et al., 1999). This later effect was abolished by pre-treatment with either the BK B2 receptor antagonist Hoe-140 or the nitric oxide-synthase inhibitor L-NAME, suggesting that the modulation of insulin action by BK is mediated, through kinin B2 receptor activation, by an increase in nitric oxide (NO) production and/or action in skeletal muscle tissue (Henriksen et al., 1999).
In non-diabetic situations, ACE is the primary enzyme responsible for the breakdown of BK in the vascular wall. However, neutral endopeptidase 24-11 (NEP) is another plasma and endothelial membrane-bound zinc metalloprotease that cleaves, like ACE, the nonapeptide BK at the Pro7-Phe8 Bond (for review, see Bhoola et al., 1992), especially when ACE is inhibited (Graf et al., 1993). In the skeletal muscle in which insulin-mediated glucose uptake mainly occurs (Baron et al., 1988), NEP contribution to the breakdown of BK appears to be more consistent. Indeed, 46% of the kininase activity is attributed to ACE and 36% to NEP (Dragovic et al., 1996). So we hypothesized that in insulin-resistant rats, dual ACE/NEP inhibition could improve insulin-mediated glucose disposal more effectively than does ACE inhibition alone by increased activation of the kinin-NO pathway.
We therefore studied the acute effects of dual ACE and NEP 24-11 inhibition on whole body glucose disposal in obese insulin-resistant Zucker rats using a hyperinsulinaemic euglycaemic clamp technique. These effects were compared to those elicited by ACE or NEP inhibition alone. As inhibitors, we used the sulphydryl-containing dual ACE/NEP inhibitor mixanpril (MIX, N-[(2S,3R)-2-[(benzoylthio)methyl]-3-phenylbutanoyl]-L-alanine, Ki=4.2±0.5 nM for ACE and 1.7±0.3 nM for NEP 24-11) (Fournié-zaluski et al., 1994); the potent and selective NEP inhibitor retrothiorphan (RT, Ki=6 nM), which has a very low affinity for ACE (IC50>10 μM) (Roques et al., 1983); and the sulphydryl-containing ACE inhibitor captopril (CAP, Ki=1.7 nM, Cushman et al., 1977). Finally, we assessed the role of the kinin-NO pathway in the effects of MIX by acute pre-administration of the kinin B2 antagonist Hoe-140 or the NO-synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME).
Methods
Experimental animals
Male obese (fa/fa) Zucker rats and their lean (Fa/Fa, Fa/fa) littermates (12 – 13 weeks old) were purchased from two different breeding centres: from Iffa Credo (L'Arbresle, France) and from the Institut National de la Santé et de la Recherche Médicale (U465 INSERM, Centre des Cordeliers, Paris, France). The Zucker rats purchased from U465 INSERM were obtained by breeding heterozygous females and obese males and the initial breeding pairs were from Harriet G. Bird, Memorial Hospital, Stow, MA, U.S.A. Animals were housed four per cage under controlled temperature conditions (21±1°C) in a conventional animal room, with free access to standard rat chow (aliment UAR AO4, Villemoisson, France) and water until the evening before the experiment (see below).
Animal preparation
Experiments were performed after an overnight period of food restriction (4 g of chow was provided at 18 00 h the evening before the experiment, according to Henriksen et al. (1999). Experiments started at 09 00 h. Animals were anaesthetized with a mixture of chloralose (100 mg kg−1) and urethan (450 mg kg−1) administered i.p. Anaesthesia was maintained throughout the experiment by subcutaneous injections of one eighth of the initial dose every 30 – 40 min. Animals breathed room air spontaneously via a tracheal cannula. Body temperature was monitored and maintained at 37°C by a heating pad (Ellab instruments, Carrieri, Paris, France). Catheters filled with heparin-saline (50 UI heparin/ml saline) were inserted in the pudendal and right internal jugular veins to allow insulin and glucose infusions respectively. The right femoral vein and the right common carotid were catheterized for administration of drugs and to collect arterial blood samples respectively.
Euglycaemic hyperinsulinaemic clamp
Highly purified human neutral insulin (Actrapid, Novo Nordisk, France) was diluted in 0.9% NaCl, 1% BSA and infused via a Precidor pump (Infors, Pfersal, Switzerland) at 15 mu kg−1 min−1 for 2 h (1 ml h−1). A variable glucose infusion (10% glucose for the obese and 20% for the lean rats purchased from Iffa Credo; 20% for the obese and 30% for the lean rats purchased from U465 INSERM) was immediately delivered, then the rate was constantly adjusted throughout the experiment to clamp blood glucose at basal concentration. Blood glucose concentrations were determined from tail capillary blood samples (25 μl) using a glucometer (‘One Touch Profile', Life Scan Company, Paris, France), before and at 5-min intervals during the first 70 min of the clamp and at 10-min intervals during the last 50 min of the clamp.
Just before the beginning of the clamp, a carotid arterial blood sample (0.3 ml, 1.5 mg ml−1 EDTA) was collected, centrifuged, and the plasma aliquoted and frozen for later determinations of plasma glucose and insulin concentrations. Steady-state plasma glucose and insulin concentrations were determined from two other carotid arterial blood samples (0.15 ml, 1.5 mg ml−1 EDTA) at 100 and 120 min. Rats were sacrificed by an i.v. overdose of sodium pentobarbitone (180 mg kg−1, i.v.). All procedures adopted for the care and euthanasia of the rats were in compliance with the European Community Standards on the care and use of laboratory animals (Ministère de l'Agriculture, France: authorization n°00.860).
Experimental protocols
Rats were allowed to stabilize for approximately 30 min after surgery. Two types of experiments were then carried out.
Experiment 1: Effects of captopril, retrothiorphan and mixanpril on whole body insulin-mediated glucose uptake
The obese and lean Zucker rats were purchased from Iffa Credo. Three groups of five obese Zucker rats received respectively CAP, RT or MIX. One group of obese (n=6) and one group of lean (n=5) control Zucker rats received the vehicle (0.9% NaCl). The vehicle and the inhibitors were administered as follows: Isotonic saline was injected as a priming bolus (0.5 ml kg−1, i.v.), followed by a sustaining infusion (0.5 ml kg−1 h−1). CAP was injected at the dose of 2 mg kg−1, i.v. bolus, followed by a sustaining infusion of 2 mg kg−1 h−1 until the end of the experiment. RT (Pham et al., 1992) and MIX (Gonzales Vera et al., 1995) were both injected at the dose of 25 mg kg−1, i.v. bolus (1 ml kg−1), followed by a sustaining infusion of 25 mg kg−1 h−1 until the end of the experiment. Infusions of the inhibitors were set at 0.5 ml h−1. At the dose used, MIX has been shown to induce supra-maximal ACE functional inhibition during 4 h in the rat (Gonzales Vera et al., 1995). CAP induces supra-maximal ACE functional inhibition at the dose of 2 mg kg−1 i.v bolus (Muller et al., 1990) and more than 70% of the angiotensin I pressor response were still inhibited 150 min after an i.v. bolus of 1 mg kg−1 of CAP (Anastasopoulos et al., 1998). The ratio between the dose of CAP and MIX was in agreement with the study of Anastasopoulos et al. (1998) showing that CAP is about 10 fold more potent than MIX as an ACE inhibitor in vivo. Therefore, it appears that during the euglycaemic hyperinsulinaemic clamp, complete inhibition of the ACE activity was achieved after administration of CAP and MIX at the doses used. On the other hand, kidney NEP activity has been shown to be completely inhibited by mixanpril 3 h after acute oral administration of the inhibitor at doses ⩾2.5 mg kg−1 (Gonzales Vera et al., 1995) and by retrothiorphan at the dose of 25 mg kg−1 i.v. (Pham et al., 1993).
Experiment 2: Effects of the NO-synthase inhibitor, Nω-nitro-L-arginine methyl ester, and of the B2 receptor antagonist, Hoe-140, on the responses to the dual ACE/NEP inhibitor mixanpril
For this study, the obese and lean Zucker rats were purchased from the U465 INSERM. One group of obese Zucker rats received MIX (n=7) as described above. Two groups of obese Zucker rats received as pre-treatment either (1) the BK B2 receptor antagonist Hoe-140 (300 μg kg−1, i.v., bolus), injected 10 min before MIX (n=6) or (2) the NO-synthase inhibitor L-NAME (10 mg kg−1, i.v., bolus, followed by a sustaining infusion of 10 mg kg−1 h−1 until the end of the clamp), injected 15 min before MIX (n=6). In preliminary studies, we had checked that the effect on femoral blood flow of 1 nmol kg−1 BK i.v. bolus, which is totally B2 receptor dependent and partially NO dependent (Gardiner et al., 1990), was totally inhibited by Hoe-140 (+0.63±0.22 ml min−1 before administration vs +0±0 ml min−1 after 2 h, P=0.023, n=6) and significantly blocked by L-NAME (+0.50±0.13 ml min−1 before administration vs +0.25±0.10 ml min−1 after 2 h, P=0.0007, n=5). Three other groups of obese Zucker rats received respectively (1) CAP (n=6) as described in the first experiment, (2) Hoe-140 alone (300 μg kg−1, i.v., bolus, n=5), and (3) L-NAME alone (10 mg kg−1, i.v., bolus+10 mg kg−1 h−1 until the end of the clamp, n=7). Finally, one group of obese (n=8) and one group of lean (n=6) control Zucker rats received the vehicle as described in the first experiment.
In experiments 1 and 2, the euglycaemic hyperinsulinaemic clamp was started 20 min after the i.v. bolus injection of peptidase inhibitors or isotonic saline.
Biochemical measurements
Capillary blood glucose concentrations were determined using a glucometer (‘One Touch Profile', Life Scan company, Paris, France). Plasma glucose was measured by a glucose oxidase method using the diagnostic kit from Sigma Chemical Co. (St Quentin-Fallavier, France). Plasma glucose concentrations were substantially higher (about 2 fold) than whole blood glucose due to the difference in methods (Weitgasser et al., 1999) and to the difference in the haematocrit between arterial blood and capillary blood (in the rat: 36 and 40% respectively). Plasma insulin was assayed with a radioimmunoassay kit (INSULIN-CT kit, CIS bio international, Gif-Sur-Yvette, France), using rat insulin standard (from U465 INSERM, Paris, France). In this test, the antiserum used shows a cross reactivity of 100 and 89.5% for human and rat insulin respectively. Preliminary experiments have shown that the values of steady-state plasma insulin concentrations were underestimated by 15% in these conditions.
Result expression and data analysis
Glucose infusion rates (GIR90 – 120 min, mg kg−1 min−1) were determined at steady-state during the last 30 min of the clamp. An insulin sensitivity index (ISI) was calculated according to Damas et al. (1999): GIR90 – 120 min (mg kg−1 min−1)/steady-state plasma insulin (μu ml−1)×1000. Experiments in which the coefficient of variation for steady-state blood glucose concentrations exceeded 10% were excluded. Data are presented as means±s.e.mean. Results were compared using one-way analysis of variance (ANOVA) followed by Newman-Keuls test. Since standard deviations for basal plasma insulin concentrations and GIR90 – 120 min were different among lean and obese populations in the first experiment, data were transformed in log before analysis. In other cases, data were compared by unpaired or paired Student's t-test. A P value <0.05 was considered as significant.
Drugs
Captopril, BSA and Nω-nitro-L-arginine methyl ester were purchased from Sigma Chemical Co. (St Quentin-Fallavier, France), D-Arg-(Hyp3,Thi5,D-Tic7,Oic8)-bradykinin (Hoe-140) from Hoechst-Marion Roussel (Frankfurt, Germany), chloralose, urethan and glucose from Prolabo (Paris, France). Mixanpril and retrothiorphan were synthesized in our laboratory (Laboratoire de Pharmacochimie moléculaire, INSERM U266, UMR 8600, CNRS). All drugs were dissolved in 0.9% NaCl. Mixanpril and retrothiorphan were dissolved using 1 mol l−1 CO3Na2 and the pH was adjusted to 7.4 with 1 mol l−1 HCl. Injections were given as 0.5 ml kg−1 unless otherwise precise and flushed with 0.05 ml of isotonic saline.
Results
Characteristics of animals
Obese Zucker rats had higher body weight than age-matched lean Zucker rats: 480±10 g vs 283±13 g, P<0.001 in the first experiment; 344±17 g vs 262±11 g, P<0.001 in the second experiment. Before the clamp, vehicle-treated obese rats had similar plasma glucose and higher plasma insulin concentrations compared to vehicle-treated lean rats (Table 1).
Table 1.
Metabolic characteristics of the lean and obese Zucker rats

Effect of the various types of peptidase inhibition on whole body glucose disposal (experiment 1)
Twenty minutes after the start of CAP, RT or MIX infusion, plasma glucose and insulin concentrations were not modified when compared to vehicle-treated obese rats (Table 1). There was no significant difference between plasma glucose concentrations before and at the steady-state of the clamp among the different groups (Table 1). Plasma insulin concentrations achieved at the steady-state were higher in vehicle-treated obese rats compared to vehicle-treated lean rats. No significant difference was observed between the steady-state plasma insulin concentrations in vehicle-, CAP-, RT- and MIX-treated obese rats (Table 1).
During the insulin infusion, the glucose infusion rates required for maintaining euglycaemia reached a steady-state at 80 – 90 min in all groups of rats (Figure 1). The GIR90 – 120 min averaged over the last 30 min of glucose infusion was reduced by 78% in vehicle-treated obese rats when compared to vehicle-treated lean rats (5.5±0.9 vs 25.5±1.7 mg kg−1 min−1, P<0.001). CAP or RT increased about 2 fold the GIR90 – 120.min (12.5±2.1 mg kg−1 min−1 in CAP-treated obese rats and 10.5±1.0 mg kg−1 min−1 in RT-treated obese rats vs 5.5±0.9 mg kg−1 min−1 in vehicle-treated obese rats, P<0.01 and P<0.05 respectively). In contrast, MIX elicited a 4 fold increase in GIR90 – 120 min (24.5±1.6 mg kg−1 min−1 in MIX-treated obese rats vs 5.5±0.9 mg kg−1 min−1 in vehicle-treated obese rats, P<0.001). The MIX-induced increase in GIR90 – 120 min was significantly higher than that induced by CAP or RT alone (P<0.001 vs CAP- or RT-treated obese rats).
Figure 1.
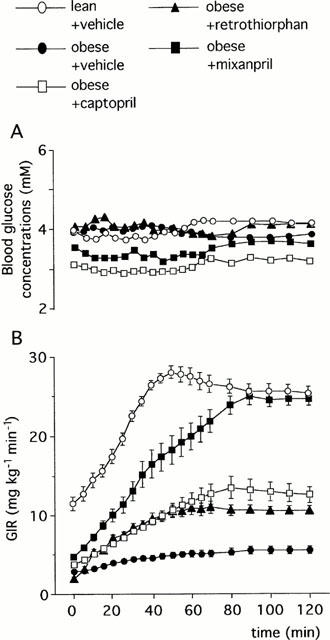
Blood glucose concentrations (A) and Glucose Infusion Rate (GIR, B) required for maintaining euglycaemia during glucose clamp study at insulin infusion rate of 15 mu kg−1 min−1 in obese and lean Zucker rats: Effects of captopril (2 mg kg−1, i.v. bolus+2 mg kg−1 h−1 infusion), retrothiorphan (25 mg kg−1, i.v. bolus+25 mg kg−1 h−1 infusion) and mixanpril (25 mg kg−1, i.v. bolus+25 mg kg−1 h−1 infusion) in the obese Zucker rats (experiment 1). In A, s.e.mean were not shown for clarity and ranged from 1.8–9.8%. In B, each point represent the mean value of n rats (n=5–6) and the vertical T bar the s.e.mean.
The Insulin Sensitivity Index (ISI) was reduced by 87% in vehicle-treated obese rats when compared to vehicle-treated lean rats (Figure 2). In obese rats, both CAP and RT increased the ISI (+141% and +53% respectively, Figure 2). MIX further increased the ISI (+80% vs CAP and +184% vs RT, Figure 2).
Figure 2.
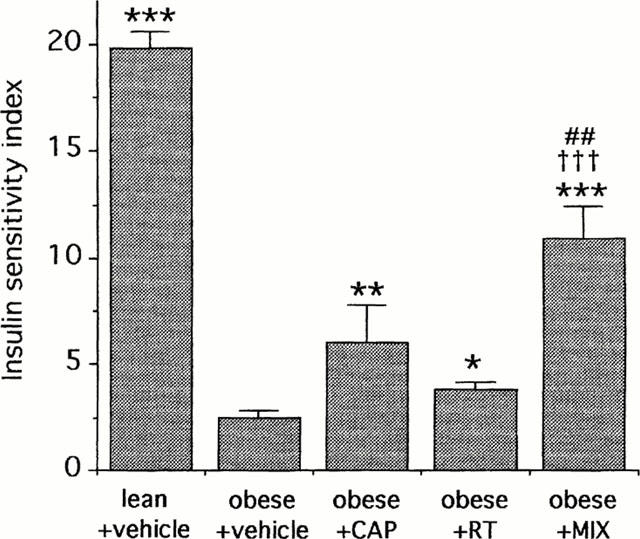
Insulin Sensitivity Index (ISI, mg glucose kg−1 min−1 μu insulin−1 ml−1×1000) at the steady-state of the 2-h euglycaemic hyperinsulinaemic clamp (15 mu kg−1 min−1) in obese and lean Zucker rats. Effects of captopril (CAP, 2 mg kg−1, i.v. bolus+2 mg kg−1 h−1 infusion), retrothiorphan (RT, 25 mg kg−1, i.v. bolus+25 mg kg−1 h−1 infusion) and mixanpril (MIX, 25 mg kg−1, i.v. bolus+25 mg kg−1 h−1 infusion) in the obese Zucker rats (experiment 1). For each rat, ISI was determined from the ratio: GIR90–120 min (mg kg−1 min−1)/steady-state plasma insulin (μu ml−1) ×1000. Each column represents the mean value of n rats (n=5–6) and the vertical bar the s.e.mean. *P<0.05, **P<0.01 and ***P<0.01 vs obese+vehicle; ##P<0.01 vs obese+captopril, †††vs obese+retrothiorphan (ANOVA followed by Newman-Keuls test).
Effect of the NO-synthase inhibitor, Nω-nitro-L-arginine methyl ester, and of the B2 receptor antagonist, Hoe-140, on the responses to mixanpril (experiment 2)
Plasma glucose and insulin concentrations measured before the clamp were not different among the different groups of treated obese rats (Table 1). Plasma glucose concentrations before and at the steady-state of the clamp were not different among the different groups of rats (Table 1). Compared to vehicle-treated lean rats, steady-state plasma insulin concentrations were higher in vehicle-treated obese rats (Table 1). No significant difference was observed between the steady-state plasma insulin concentrations among the different groups of the treated obese rats (Table 1).
Glucose infusion rates required for maintaining euglycaemia during the insulin infusion reached a steady-state at 80 – 90 min in all groups of rats (Figure 3). The GIR90 – 120 min averaged over the last 30 min of glucose infusion was reduced by 62% in vehicle-treated obese rats when compared to vehicle-treated lean rats (14.4±0.7 vs 37.7±2.1 mg kg−1 min−1, P<0.001). The CAP-treated obese rats showed a 36% increase in GIR90 – 120 min (19.6±0.6 mg kg−1 min−1 in CAP-treated obese rats vs 14.4±0.7 mg kg−1 min−1 in vehicle-treated obese rats, P<0.001) but the MIX-treated obese rats showed an 83% increase in GIR90 – 120 min (26.3±0.8 mg kg−1 min−1 in MIX-treated obese rats vs 14.4±0.7 mg kg−1 min−1 in vehicle-treated obese rats, P<0.001). As in the first experiment, the MIX-induced increase in GIR90 – 120 min was significantly higher than that induced by CAP alone (+34%, P<0.01).
Figure 3.
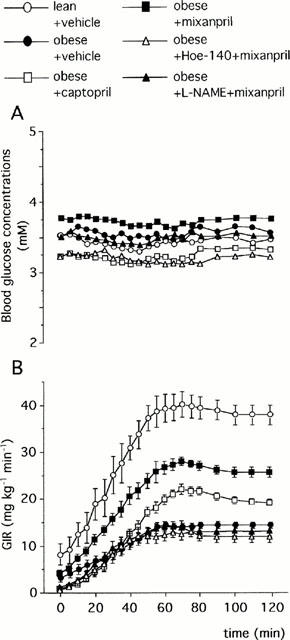
Blood glucose concentrations (A) and Glucose Infusion Rate (GIR, B) required for maintaining euglycaemia during glucose clamp study at insulin infusion rate at 15 mu kg−1 min−1 in obese and lean Zucker rats: Effects of captopril (2 mg kg−1, i.v. bolus+2 mg kg−1 h−1 infusion), mixanpril (25 mg kg−1, i.v. bolus+25 mg kg−1 h−1 infusion), Hoe-140 (300 μg kg−1) in combination with mixanpril and L-NAME (10 mg kg−1, i.v. bolus+10 mg kg−1 h−1 infusion) in combination with mixanpril in the obese Zucker rats (experiment 2). In A, s.e.mean were not shown for clarity and ranged from 0.4–8.8%. Each column represents the mean value of n rats (n=5–6) and the vertical bar the s.e.mean.
The ISI was highly reduced in vehicle-treated obese rats when compared to vehicle-treated lean rats (Figure 4). The CAP-treated obese rats showed a 53% increase in ISI but the MIX-treated obese rats showed a 101% increase in ISI (Figure 4). As in the first experiment, the MIX-induced increase in ISI was significantly higher than that induced by CAP alone (Figure 4).
Figure 4.
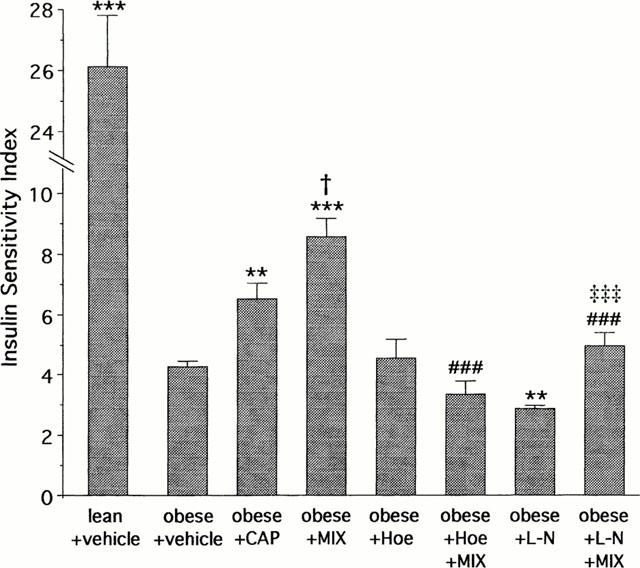
Insulin Sensitivity Index (ISI, mg glucose kg−1 min−1 μu insulin−1 ml−1×1000) at the steady-state of the 2-h euglycaemic hyperinsulinaemic clamp (15 mu kg−1 min−1) in obese Zucker rats. Effects of captopril (CAP, 2 mg kg−1, i.v. bolus+2 mg kg−1 h−1 infusion), mixanpril (MIX, 25 mg kg−1, i.v. bolus+25 mg kg−1 h−1 infusion), Hoe-140 alone (Hoe, 300 μg kg−1) or in combination with MIX and L-NAME (L-N, 10 mg kg−1, i.v. bolus+10 mg kg−1 h−1 infusion) alone or in combination with MIX in the obese Zucker rats (experiment 2). For each rat, ISI was determined from the ratio: GIR90–120 min (mg kg−1 min−1)/steady-state plasma insulin (μu ml−1)×1000. Results are expressed as mean values of n rats (n=5–8)±s.e.mean. **P<0.01 and ***P<0.01 vs obese+vehicle; †P<0.05 vs obese+captopril; ###P<0.001 vs obese+mixanpril; ‡‡‡P<0.001 vs obese+L-N (ANOVA followed by Newman-Keuls test).
Although Hoe-140 alone did not significantly alter the GIR90 – 120 min (12.6±0.8 mg kg−1 min−1 in Hoe-140-treated rats vs 14.4±0.7 mg kg−1 min−1 in vehicle-treated obese rats, P>0.05), it abolished the increase in GIR90 – 120 min induced by MIX (12.3±1.2 mg kg−1 min−1 in Hoe-140+MIX-treated obese rats vs 26.3±0.8 mg kg−1 min−1 in MIX-treated obese rats, P<0.001, Figure 3).
L-NAME alone slightly reduced the GIR90 – 120 min (11.0±0.6 mg kg−1 min−1 in L-NAME-treated obese rats vs 14.4±0.7 mg kg−1 min−1 in vehicle-treated obese rats, P<0.05) and abolished the increase in GIR90 – 120 min induced by MIX (13.0±1.1 mg kg−1 min−1 in L-NAME+MIX-treated obese rats vs 26.3±0.8 mg kg−1 min−1 in MIX-treated obese rats, P<0.001, Figure 3).
In obese rats, Hoe-140 alone did not significantly alter the ISI but abolished the increase in ISI induced by MIX (Figure 4). L-NAME alone slightly reduced the ISI and significantly reduced the increase in ISI induced by MIX (Figure 4).
Discussion
The present study shows that the acute dual ACE/NEP inhibition can increase whole body insulin-mediated glucose disposal more effectively than does ACE inhibition alone in the obese insulin-resistant Zucker rat. Since both drugs were dissolved in the same vehicle and given by primed, continuous i.v. infusion throughout the study, it is unlikely that differences in pharmacokinetics are responsible for the results obtained. In addition, it is unlikely that a lesser inhibition of ACE by CAP than by MIX underlies their differential effects on glucose infusion rate or insulin sensitivity index since the dose of CAP is supra-maximal for functional ACE inhibition (Muller et al., 1990; Anastasopoulos et al., 1998).
In agreement with previous results of hyperglycaemic euglycaemic clamp experiments performed in insulin-resistant rats (Tomiyama et al., 1994; Nakagawa et al., 1999), we observed that Hoe-140 alone in pre-treatment did not affect whole body insulin-mediated glucose disposal in the obese Zucker rat. More importantly, the acute effect of the dual ACE/NEP inhibitor MIX on whole body insulin-mediated glucose disposal was abrogated by pre-treatment with Hoe-140. This result indicates that in the obese Zucker rat, the effect of the dual ACE/NEP inhibition on insulin sensitivity is mediated by a BK B2 receptor activation.
In the obese Zucker rat, we observed that acute treatment with CAP resulted in significant increase in whole body insulin-mediated glucose disposal. This finding confirms several previous investigations performed in insulin-resistant rats (Tomiyama et al., 1994; Henriksen et al., 1996; Nakagawa et al., 1999) and in hypertensive and/or diabetic humans (Uehara et al., 1994). One conclusion of these investigations has been that the elevation in endogenous BK concentrations, resulting from the ACE inhibition, underlies the beneficial metabolic effects of the ACE inhibitors (Tomiyama et al., 1994; Uehara et al., 1994; Henriksen et al., 1996; Nakagawa et al., 1999). Since CAP and MIX inhibited ACE activity to the same extent in our study (Anastasopoulos et al., 1998) and since BK appears to be the main mediator of the effects of the two peptidase inhibitors, it appears likely that the higher potency of MIX to increase insulin sensitivity, when compared to CAP, result from a better protection of endogenous BK.
In vivo, ACE is the primary enzyme responsive for BK breakdown (Bhoola et al., 1992; Dragovic et al., 1996). However, increasing evidence indicates that NEP is also involved in this catabolism (Dragovic et al., 1996), particularly during ACE inhibition (Graf et al., 1993). Indeed, we have shown that the combined ACE/NEP inhibition corrects impaired femoral vascular conductance in streptozotocin-treated rats more effectively than ACE inhibition alone by a better protection of endogenous BK (Arbin et al., 2000). Besides kinin breakdown, NEP is also concerned with the breakdown of other peptides such as endothelin and atrial natriuretic peptide (Erdos & Skidgel, 1989). However, we observed here that the effect of MIX was totally inhibited by Hoe-140, suggesting that BK was only concerned in this insulin sensitivity improvement. We also observed that RT administration increased whole body insulin-mediated glucose disposal. We did not address directly the role of BK in this effect but it is likely that it was associated to an increase in BK concentrations resulting from NEP inhibition. In our conditions, RT at a dose which blocks the NEP enzyme activity, improved whole body insulin-mediated glucose disposal to a lesser extent than did CAP at a dose which achieved complete inhibition of ACE activity, but the difference was not significant. This implies that the two protease inhibitors elicited almost similar protection of endogenous BK. This was unexpected since it has been found in endothelial cells (Graf et al., 1992) or in vascular wall (Dendorfer et al., 1997; Griswold et al., 1998) that the ACE pathway is the preponderant mechanism for BK degradation. So it appears likely that in addition to the endothelial enzyme inhibition, blockade of NEP activity in other tissues may contribute to increased local BK concentrations. Myocytes are plausible candidates since in rat skeletal muscle, 36% of the kininase activity should be due to NEP (Dragovic et al., 1996).
In our study, L-NAME alone reduced slightly but significantly the insulin sensitivity in obese rats. This observation is in agreement with previous studies showing that NO-synthase blockade reduces whole body insulin-mediated glucose disposal during euglycaemic hyperinsulinaemic clamp in normal rats (Baron et al., 1995; Roy et al., 1998). Moreover, L-NAME pre-treatment markedly reduced the increased whole body glucose disposal observed after MIX administration. This suggests that an activation of the kinin-NO pathway contributes to the effect of MIX on whole body insulin-mediated glucose disposal. These results are coherent with the observations of Henriksen et al. (1999) showing that exogenous BK can enhance insulin action on glucose uptake via an increased activation of the B2 receptors and via an increase in NO production and/or action in skeletal muscle tissue.
Where this NO production occurs cannot be determined by our experimental design. Acute administration of ACE inhibitors has been shown to modulate the early steps of insulin signalling in the liver and muscle of obese Zucker rats (Carvalho et al., 1998; Nawano et al., 1999). These effects may be simulated by the administration of exogenous bradykinin (Carvalho et al., 1997). Bradykinin B2 receptors are expressed in endothelium and in skeletal muscle (Rabito et al., 1996) and NO can be synthesized by endothelium, skeletal muscle (Balon & Nadler, 1994) and hepatocytes (Zhang et al., 1997). In addition, several investigators have partly attributed the influence of ACE inhibitors on glucose disposal to improved skeletal capillary blood flow and an accompanying increased delivery of insulin and glucose to the muscle (Kodama et al., 1990; Nawano et al., 1999). Combined ACE/NEP inhibition has been shown to vasodilate the hindquarters vascular bed in hypertensive rats (Gardiner et al., 1997) and to increase the femoral blood flow in streptozotocin-induced diabetic rats via an increased activation of the kinin-NO pathway (Arbin et al., 2000). Therefore, a contribution of the haemodynamic influence of MIX on whole body glucose disposal in vivo cannot be ruled out. In contrast, NEP inhibitors have been shown not to modify basal vascular (Gardiner et al., 1992) and arteriolar skeletal muscle haemodynamics (Peyroux et al., 1995), suggesting that RT can improve whole body glucose uptake in obese Zucker rat without modifying glucose and insulin delivery to the muscle.
In conclusion, this study shows that in the obese insulin-resistant Zucker rat, under acute conditions, NEP or ACE inhibition can improve whole body insulin-mediated glucose disposal. Moreover, the dual ACE/NEP inhibition by mixanpril increases whole body insulin-mediated glucose disposal and insulin sensitivity to a greater extent than does either single peptidase inhibition. This beneficial effect of mixanpril is essentially linked to an increased activation of the kinin-NO pathway, which seems to result from a better protection of endogenous BK. These results show that the role of endogenous bradykinin and kinin-NO pathway in the resistance to insulin-mediated glucose transport merits further consideration.
Acknowledgments
We thank Dr K.J. Wirth from Hoechst-Marion Roussel (Frankfurt, Germany) for kindly supplying us with D-Arg-(Hyp3,Thi5,D-Tic7,Oic8)-bradykinin (Hoe-140), Dr M. Guerre-Millo and J. André from U465 INSERM (Paris, France) for their supply of rat insulin standards and their help for plasma insulin measurement, Pr M. Sternberg for useful suggestions in the preparation of the manuscript.
Abbreviations
- CAP
captopril
- GIR
glucose infusion rate
- IMGD
whole body insulin-mediated glucose disposal
- MIX
mixanpril
- NEP
neutral endopeptidase 24-11
- RT
retrothiorphan
- ZO
obese Zucker rat
References
- ANASTASOPOULOS F., LEUNG R., KLADIS A., JAMES G.M., BRISCOE T.A., GORSKI T.P., CAMBELL D.J. Marked difference between angiotensin-converting enzyme and neutral endopeptidase inhibition in vivo by a dual inhibitor of both enzymes. J. Pharmacol. Exp. Ther. 1998;284:799–805. [PubMed] [Google Scholar]
- ARBIN V., CLAPERON N., FOURNIÉ-ZALUSKI M-C., ROQUES B.P., PEYROUX J. Effects of combined neutral endopeptidase 24-11 and angiotensin-converting enzyme inhibition on femoral vascular conductance in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2000;130:1297–1304. doi: 10.1038/sj.bjp.0703442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALON T., NADLER J.L. Nitric oxide is present from incubated skeletal muscle. J. Appl. Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- BARON A.D., BRECHTEL G., WALLACE P., EDELMAN SV. Rate and tissue sites of non insulin and insulin mediated glucose uptake in humans. Am. J. Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- BARON A.D., ZHU J.S., MARSHALL S., IRSULA O., BRECHTEL G., KEECH C. Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats. Am. J. Physiol. 1995;269:E709–E715. doi: 10.1152/ajpendo.1995.269.4.E709. [DOI] [PubMed] [Google Scholar]
- BHOOLA K.D., ERGUEROS C.D., WORTHY K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- CALDIZ C.I., DE CINGOLANI G.E. Insulin resistance in adipocytes from spontaneously hypertensive rats: effect of long-term treatment with enalapril and losartan. Metabolism. 1999;48:1041–1046. doi: 10.1016/s0026-0495(99)90203-2. [DOI] [PubMed] [Google Scholar]
- CARVALHO C., THIRONE A., GONTIJO J., VELLOSO L., SAAD M. Effect of captopril, losartan and bradykinin on early steps of insulin action. Diabetes. 1997;46:1950–1957. doi: 10.2337/diab.46.12.1950. [DOI] [PubMed] [Google Scholar]
- CARVALHO D.S., VILLACA V., BRENELLI S.L., CARVALHO C.R., SAAD M. Angiotensin-converting enzyme inhibitor increases insulin-induced pp 185 phosphorylation in liver and muscle of obese rats. Biochem. Mol. Biol. Int. 1998;46:259–266. doi: 10.1080/15216549800203772. [DOI] [PubMed] [Google Scholar]
- CUSHMAN D.W., CHEUNG H.S., SABO E.F., ONDETTI M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977;16:5484–5491. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- DAMAS J., BOURDON V., LEFEBVRE P.J. Insulin sensitivity, clearance and release in kininogen-deficient rats. Exp. Physiol. 1999;84:549–557. doi: 10.1111/j.1469-445x.1999.01812.x. [DOI] [PubMed] [Google Scholar]
- DENDORFER A., WOLFRUM S., WELHÖNER P., KORSMAN K., DOMINIAK P. Intravascular and interstitial degradation of bradykinin in isolated perfused rat heart. Br. J. Pharmacol. 1997;122:1179–1187. doi: 10.1038/sj.bjp.0701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNELLY R., QU X. Mechanisms of insulin resistance and new pharmacological approaches to metabolism and diabetic complications. Clin. Exp. Pharmacol. Physiol. 1998;25:79–87. doi: 10.1111/j.1440-1681.1998.tb02181.x. [DOI] [PubMed] [Google Scholar]
- DRAGOVIC T., MINSHALL R., JACKMAN H.L., WANG L-X., ERDÖS E.G. Kininase II-Type Enzymes. Their putative role in muscle energy metabolism. Diabetes. 1996;45 Suppl. 1:S34–S37. doi: 10.2337/diab.45.1.s34. [DOI] [PubMed] [Google Scholar]
- ERDOS E.G., SKIDGEL R.A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. Faseb J. 1989;3:145–151. [PubMed] [Google Scholar]
- FOURNIÉ-ZALUSKI M.C., GONZALEZ W., TURCAUD S., PHAM I., ROQUES B.P., MICHEL J.B. Dual inhibition of angiotensin-converting enzyme and neutral endopeptidase by the orally active inhibitor mixanpril: a potential therapeutic approach in hypertension. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4072–4076. doi: 10.1073/pnas.91.9.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLETTI F., STRAZZULLO P., CAPALDO B., CARRETTA R., FABRIS F., FERRARA L.A., GLORIOSO N., SEMPLICINI A., MANCINI M. Controlled study of the effect of angiotensin converting enzyme inhibition versus calcium-entry blockade on insulin sensitivity in over-weight hypertensive patients: Trandolapril Italian Study (TRIS) J. Hypertens. 1999;17:439–445. doi: 10.1097/00004872-199917030-00018. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of Nω-nitro-L-arginine methyl ester. Br. J. Pharmacol. 1990;101:632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of the neutral endopeptidase inhibitor SQ 28,603, on regional haemodynamic responses to atrial natriuretic peptide or proendothelin-1 [1-38] in conscious rats. Br. J. Pharmacol. 1992;106:180–186. doi: 10.1111/j.1476-5381.1992.tb14312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BRUNNER-FERBER F., BENNETT T. Comparative effects of the dual metallopeptidase inhibitor, MDL 100,240 and of enalaprilat on regional and on cardiac haemodynamics in conscious, hypertensive, transgenic ((mRen-2)27) rats. Br. J. Pharmacol. 1997;122:1694–1701. doi: 10.1038/sj.bjp.0701551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ VERA W., FOURNIÉ-ZALUSKI M.C., PHAM I., LABOULANDINE I., ROQUES B.P., MICHEL J.B. Hypotensive and natriuretic effects of RB 105, a new dual inhibitor of angiotensin converting enzyme and neutral endopeptidase in hypertensive rats. J. Pharmacol. Exp. Ther. 1995;272:343–351. [PubMed] [Google Scholar]
- GRAF K., GRÄFE M., AUCH-SCHWELK W., BAUMGARTEN C.R., BOSSALLER C., FLECK E. Bradykinin degrading activity in cultured human endothelial cells. J. Cardiovasc. Pharmacol. 1992;20 Suppl. 9:S16–S20. [PubMed] [Google Scholar]
- GRAF K., GRÄFE M., BOSSALLER C., NIEHUS J., SCHULZ K.D., AUCH-SCHWELK W., FLECK E. Degradation of bradykinin by neutral endopeptidase (EC 3.4.24.11) in cultured human endothelial cells. Eur. J. Clin. Chem. Clin. Biochem. 1993;31:267–272. doi: 10.1515/cclm.1993.31.5.267. [DOI] [PubMed] [Google Scholar]
- GRISWOLD J.A., BAKER C.R., JR, LITTLE D.T., LITTLE G.H., BEHAL F.J. Bradykinin metabolism in rat hindlimbs. Shock. 1998;10:146–152. doi: 10.1097/00024382-199808000-00011. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN E.J., JACOB S., AUGUSTIN H.J., DIETZE G.J. Glucose transport activity in insulin-resistant rat muscle. Effects of angiotensin-converting enzyme inhibitors and bradykinin antagonism. Diabetes. 1996;45:S125–S128. doi: 10.2337/diab.45.1.s125. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN E.J., JACOB S., KINNICK T.R., YOUNGBLOOD E.B., SCHMIT M.B.ACE inhibition and glucose transport in insulin-resistant muscle. Role of bradykinin and nitric oxide Am. J. Physiol. 1999277R332–R336.et al [DOI] [PubMed] [Google Scholar]
- KODAMA J., KATAYAMA S., TANAKA K., ITABASHI A., KAWAZU S., ISHII J. Effect of captopril on glucose concentration. Possible role of augmented postprandial forearm blood flow. Diabetes Care. 1990;13:1109–1111. doi: 10.2337/diacare.13.11.1109. [DOI] [PubMed] [Google Scholar]
- MULLER A.F., GARDINER S.M., COMPTON A.M., BENNETT T. Regional haemodynamic effects of captopril, enalaprilat and lisinopril in conscious water-replete and water-deprived Brattleboro rats. Clin. Sci. 1990;79:393–401. doi: 10.1042/cs0790393. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA H., DAIHARA M., TAMAKAWA H., NOZUE T., KAWAHARA K. Effects of quinapril and losartan on insulin sensitivity in genetic hypertensive rats with different metabolic abnormalities. J. Cardiovasc. Pharmacol. 1999;34:28–33. doi: 10.1097/00005344-199907000-00005. [DOI] [PubMed] [Google Scholar]
- NAWANO M., ANAI M., FUNAKI M., KOBAYASHI H., KANDA A., FUKUSHIMA Y., INUKAI K., OGIHARA T., SAKODA H., ONISHI Y., KIKUCHI M., YASAKI Y., OKA Y., ASANO T. Imidapril, an angiotensin-converting enzyme inhibitor, improves insulin sensitivity by enhancing signal transduction via insulin receptor substrate proteins and improving vascular resistance in the Zucker fatty rat. Metabolism. 1999;48:1248–1255. doi: 10.1016/s0026-0495(99)90263-9. [DOI] [PubMed] [Google Scholar]
- PEYROUX J., BESLOT F., CLAPERON N., FOURNIÉ-ZALUSKI M-C., ROQUES B.P. Effect of endopeptidase 24-11 inhibitors and C-ANP receptor ligand on responses evoked in arterioles of rat cremaster muscle by atrial natriuretic peptide. Br. J. Pharmacol. 1995;116:3117–3124. doi: 10.1111/j.1476-5381.1995.tb15113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAM I., EL AMRANI A.-I. K., FOURNIÉ-ZALUSKI M.-C., CORVOL P., ROQUES B.P., MICHEL J.-B. Effects of the selective NEP inhibitor, retrothiorphan, on renal function and blood pressure in conscious normotensive Wistar and hypertensive DOCA-salt rats. J. Cardiovasc. Pharmacol. 1992;20:847–857. doi: 10.1097/00005344-199212000-00001. [DOI] [PubMed] [Google Scholar]
- PHAM I., GONZALES W., EL AMRANI A.-I. K., FOURNIÉ-ZALUSKI M.-C., LABOULANDINE I., ROQUES B.P., MICHEL J.-B. Effects of converting enzyme inhibitor and neutral endopeptidase inhibitor on blood pressure and renal function in experimental hypertension. J. Pharmacol. Exp. Thera. 1993;265:1339–1347. [PubMed] [Google Scholar]
- RABITO S.F., MINSHALL R.D., NAKAMURA F., WANG L.X. Bradykinin B2 receptors on skeletal muscle are coupled to inositol 1,4,5-triphosphate formation. Diabetes. 1996;45 Suppl. 1:S29–S33. doi: 10.2337/diab.45.1.s29. [DOI] [PubMed] [Google Scholar]
- ROQUES B.P., LUCAS-SORACA E., CHAILLET P., COSTENTIN J., FOURNIÉ-ZALUSKI M.C. Complete differentiation between enkephalinase and angiotensin-converting enzyme inhibition by retrothiorphan. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3178–3182. doi: 10.1073/pnas.80.11.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY D., PERREAULT M., MARETTE A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am. J. Physiol. 1998;274:E692–E688. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- TOMIYAMA H., KUSHIRO T., ABETA H., ISHII T., TAKAHASHI A., FURUKAWA L., ASAGAMI T., HINO T., SAITO F., OTSUKA V. Kinins contribute to the improvement of insulin sensitivity during treatment with angiotensin-converting enzyme inhibitor. Hypertension. 1994;23:450–455. doi: 10.1161/01.hyp.23.4.450. [DOI] [PubMed] [Google Scholar]
- UEHARA M., KISHIKAWA H., ISAMI S., KISANUKI K., OHKUBO Y., MIYAMURA N., MIYATA T., YANO T., SHICHIRI M. Effect on insulin sensitivity of angiotensin-converting enzyme inhibitors with or without a sulphydryl group: bradykinin may improve insulin resistance in dogs and humans. Diabetologia. 1994;37:300–307. doi: 10.1007/BF00398058. [DOI] [PubMed] [Google Scholar]
- WEITGASSER R., DAVALLI A.M., WEIR G.C. Measurement of glucose concentrations in rats: differences between glucose meter and plasma laboratory results. Diabetologia. 1999;42:256. doi: 10.1007/s001250051147. [DOI] [PubMed] [Google Scholar]
- ZHANG B., BORDERIE D., SOGNI P., SOUBRANE O., HOUSSIN D., CALMUS Y. NO-mediated vasodilatation in the rat liver. Role of hepatocytes and liver endothelial cells. J. Hepatol. 1997;26:1348–1355. doi: 10.1016/s0168-8278(97)80471-0. [DOI] [PubMed] [Google Scholar]


