Abstract
We investigated the mechanism of suppression of inducible nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2) by ergolide, sesquiterpene lactone from Inula britannica.
iNOS activity in cell-free extract of LPS/IFN-γ-stimulated RAW 264.7 macrophages was markedly attenuated by the treatment with ergolide. Its inhibitory effect on iNOS was paralleled by decrease in nitrite accumulation in culture medium of LPS/IFN-γ-stimulated RAW 264.7 macrophages in a concentration-dependent manner. However, its inhibitory effect does not result from direct inhibition of the catalytic activity of NOS.
Ergolide markedly decreased the production of prostaglandin E2 (PGE2) in cell-free extract of LPS/IFN-γ-stimulated RAW 264.7 macrophages in a concentration-dependent manner, without alteration of the catalytic activity of COX-2 itself.
Ergolide decreased the level of iNOS and COX-2 protein, and iNOS mRNA caused by stimulation of LPS/IFN-γ in a concentration-dependent manner, as measured by Western blot and Northern blot analysis, respectively.
Ergolide inhibited nuclear factor-κB (NF-κB) activation, a transcription factor necessary for iNOS and COX-2 expression in response to LPS/IFN-γ. This effect was accompanied by the parallel reduction of nuclear translocation of subunit p65 of NF-κB as well as IκB-α degradation. In addition, these effects were completely blocked by treatment of cysteine, indicating that this inhibitory effect of ergolide could be mediated by alkylation of NF-κB itself or an upstream molecule of NF-κB.
Ergolide also directly inhibited the DNA-binding activity of active NF-κB in LPS/IFN-γ-pretreated RAW 264.7 macrophages.
These results demonstrate that the suppression of NF-κB activation by ergolide might be attributed to the inhibition of nuclear translocation of NF-κB resulted from blockade of the degradation of IκB and the direct modification of active NF-κB, leading to the suppression of the expression of iNOS and COX-2, which play important roles in inflammatory signalling pathway.
Keywords: Ergolide, inducible nitric oxide synthase, cyclo-oxygenase-2, NF-κB, IκB
Introduction
Both nitric oxide (NO) and prostaglandins (PG), and their associated enzymes nitric oxide synthases (NOS) and cyclo-oxygenases (COX) (specifically COX-2) have been implicated in the development of inflammation (Nathan, 1997; Sautebin, 2000). Three isoforms of NOS exist, constitutively expressed neuronal NOS (nNOS) and endothelial NOS (eNOS), which are both Ca2+ calmodulin-dependent, and an inducible isoform (iNOS), which is Ca2+ calmodulin-independent (Michel et al., 1995). Two isoforms of COX exist, constitutively expressed COX-1 and an inducible isoform COX-2 (Smith et al., 1996). iNOS and COX-2 are up-regulated in response to inflammatory and proinflammatory mediators (Fu et al., 1990; Moncada et al., 1991; MacMicking et al., 1997; Sautebin et al., 1998), and their products are known to be an important mediator of acute and chronic inflammation.
Numerous reports focused on the interaction between NO and PG pathway in inflammatory process. It has been reported that NO stimulates the production of PG by two mechanisms: NO has been shown to stimulate COX-2 activity via reaction with the haeme component, which binds to the active site of the COX-2 enzyme (Salvemini et al., 1993; 1994; Corbett et al., 1993; Franchi et al., 1994), and low concentration of NO promotes COX-2 expression in RAW 264.7 macrophages (von knethen & Brune, 1997; von knethen et al., 1999), although its mechanism remains elusive. In contrast to the stimulatory effect of NO on PG production, it has been reported that endogenous NO inhibits PG synthesis in LPS-stimulated macrophages (Habib et al., 1997), and PG downregulates iNOS induction (Tetsuka et al., 1994), thus demonstrating cross-talk between the COX-2 and NOS pathways.
Nuclear factor-κB (NF-κB) is a transcriptional factor that acts as a central mediator of the human immune response and regulates the transcription of various inflammatory cytokines such as IL-1, IL-2, IL-6, IL-8 and tumour necrosis factor-α as well as genes encoding cell adhesion molecules, immunoreceptors, hematopoetic growth factors and growth factor receptors (Baeuerle & Henkel, 1994; Baeuerle & Baltimore, 1996; May & Ghosh, 1998). There are indications that activation of NF-κB is involved in iNOS and COX-2 expression because there is an NF-κB consensus site in the upstream promotor region of the iNOS and COX-2 gene (Yamamoto et al., 1995; Kim et al., 1997; Barnes & Karin, 1997; Crofford et al., 1997). Therefore, iNOS and COX-2 expression, and NF-κB activation have been used as tools for the screening of anti-inflammatory activity.
Using iNOS and COX-2 expression, and NF-κB activation as a molecular target, we screened active compounds from many traditional herbal medicines, which had been used as traditional remedies for inflammatory diseases, and found that ergolide (Figure 1) isolated from the dried flowers of Inula britannica inhibited iNOS activity in RAW 264.7 macrophages in preliminary experiment. Although ergolide was isolated from Inula species (Wang et al., 1996; Park & Kim, 1998), there is little information available on the biological activities of ergolide. Here, in order to elucidate the mechanism of anti-inflammatory effect of ergolide, we investigated ergolide for its ability to inhibit iNOS and COX-2 expression, and NF-κB activation in RAW 264.7 macrophages.
Figure 1.
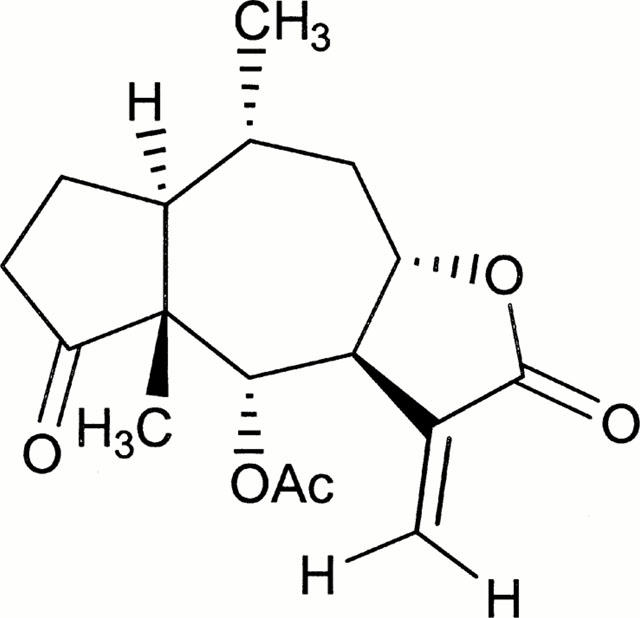
Chemical structure of ergolide isolated from Inula britannica.
Methods
Reagents
Ergolide (Figure 1) was isolated from dried flowers of Inula britannica L. var chinensis Regel and structural identity of ergolide was determined spectroscopically (1H and 13C NMR, IR, MS) as described previously (Wang et al., 1996). Dulbecco's modified Eagle's medium (DMEM) with high glucose and other reagents for cell culture were obtained from Gibco – BRL Life Biotechnologies (Grand Island, NY, U.S.A.). [2,3,4,5-3H]-L-arginine monohydrochloride (57 Ci mmol−1) was obtained from Amersham International (Amersham, Bucks., U.K.) and [32P]-labelled dATP (3000 Ci mmol−1) was from NEN (NEN Life Science Products, Boston, MA, U.S.A.). Mouse anti-mouse iNOS monoclonal antibody and mouse anti-rat COX-2 antibody were obtained from Transduction Laboratories (Lexington, KY, U.S.A.). Rabbit polyclonal p65 (RelA) antibody was obtained from Calbiochem (U.S.A.), and rabbit polyclonal IκB-α antibody was from New England BioLabs (U.S.A.). LPS (from Escherichia coli, 011:B4), IFN-γ (recombinant mouse, expressed in E. coli), N-(1-naphthyl) ethylenediamine hydrochloride, sulphanilamide, (6R)-5,6,7,8-tetrahydrobiopterin (6R-H4B), aprotinin, leupeptin, pepstatin A, phenylmethylsulphonylfluoride, β-NADPH, dithiothreitol, calmodulin, L-citrulline, Dowex 50WX8-200 resin, FAD, FMN, Igepal CA-630, and L-NG-monomethylarginine (L-NMMA) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Cell culture
The murine macrophage/monocyte cell line RAW 264.7 macrophages (from ATCC) were maintained at 37°C and 5% CO2 in DMEM containing 10% heat-inactivated foetal bovine serum, 2 mM glutamine, 100 μ ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were plated at a density of 1.0×106 cells ml−1, and allowed to attach for 2 h. For stimulation, the medium was replaced with fresh DMEM, and the cells were then stimulated with 1 μg ml−1 of LPS and 10 units ml−1 of IFN-γ in the presence or absence of ergolide for the indicated periods.
Measurement of nitrite production using Griess reagent
NO production in culture supernatant was spectrophotometrically evaluated by measuring nitrite, an oxidative product of NO. Nitrite was determined with the Griess reaction (Green et al., 1982) by mixing 100 μl of culture supernatant with 100 μl of Griess reagent containing equal volumes of 1% (w v−1) sulphanilamide in 5% (w v−1) phosphoric acid and 0.1% (w v−1) of N-(1-naphthyl)ethylenediamine solution. Absorbance was measured in a microplate reader (Bio-Tek EL×800, Bio-Tek Instrument Inc., U.S.A.) at 540 nm against a calibration curve with sodium nitrite standards.
Measurement of NOS activity using [3H]-L-citrulline formation assay
Cell-free extracts as enzyme sources were prepared from the RAW 264.7 macrophages cultured for 16 – 18 h in the presence of ergolide and stimulators, LPS (1 μg ml−1) and IFN-γ (10 unit ml−1), using Hayashi's method with minor modifications (Hayashi et al., 1997). Specific activity of iNOS was measured in cell-free extracts by monitoring the conversion of [3H]-L-arginine to [3H]-L-citrulline (Vodovotz et al., 1993). After preincubation of reaction mixtures which contained 50 mM Tris buffer (pH 7.4), 1 mM NADPH, 20 μM H4B, 5 μM FAD, 5 μM FMN, 1 mM dithiothreitol and 20 – 40 μg of cell lysate protein for 3 – 5 min at 37°C, 10 μl of 500 μM arginine containing [3H]-L-arginine (about 200,000 c.p.m.) was added to the mixture (at a final concentration of 25 μM in 200 μl of total reaction mixture), and was further incubated for 10 – 15 min. The reaction was stopped by the addition of 1 ml ice-cold 20 mM sodium acetate stop buffer (pH 5.5) containing 1 mM citrulline, 2 mM EDTA and 2 mM EGTA. Then the reaction mixtures were applied onto columns (1 cm diameter) containing 1 ml DOWEX 50W-X8 (Na+ form) cation exchange resin, and each column was eluted with 2 ml of water. The radioactivity of [3H]-L-citrulline in the eluates was measured on a liquid scintillation counter (LKB, RackBeta, Finland). For the nNOS assay, fresh rat brain extract as an enzyme source was prepared as previously published (Chan et al., 1997) and 10 μg ml−1 of calmodulin and 1.5 mM of CaCl2 were used in addition to the above reaction mixture described for the iNOS assay.
Western blot analysis
Whole cell extracts containing equal quantities of proteins (30 – 50 μg) were electrophoresed in 10% polyacrylamide gel. Subsequently, the separated proteins were transferred to PVDF membrane using Semi-Dry Transfer Cell (TRANS-BLOT, BIO-RAD, U.S.A.). Briefly, the membrane was blocked for 30 min with blocking buffer (5% skim milk in 50 mM Tris-HCl, 200 mM NaCl, and 0.05% Tween 20, pH 7.5), and was incubated with appropriate dilutions of primary antibodies (against iNOS, COX-2, p65 or IκB-α) for overnight at 4°C. After washing twice with the above buffer, the membrane was further incubated for 2 h with a 1 : 1000 dilution of alkaline phosphatase-conjugated goat anti-rabbit or anti-mouse antibody, and were developed with BCIP (5-bromo-4-chloro-3-indoyl phosphate)/NBT (nitroblue tetrazolium) colour developing solution. In the case of COX-2, an enhanced chemiluminescence (ECL) method was used as Western blot detection system (ECL Plus™, Amersham). As a primary antibody, murine anti-rat COX-2 polyclonal antibody (Transduction Lab) was used at a 1 : 500 dilution. The secondary antibody, horseradish peroxidase-conjugated sheep anti-mouse IgG (Amersham), was diluted 1 : 2000 and the immunoblot was visualized using Amersham ECL-film.
Northern blot analysis
Total cellular RNA was extracted with TRIZOL® reagent (Gibco) according to the manufacturer's instructions. Twenty micrograms of total RNA were separated on 1.0% (w v−1) agarose gels containing 1.8% formaldehyde and were transferred to a positively charged-nylon membrane (Boehringer Mannheim GmbH, Mannheim, Germany) via downward capillary action. After u.v. cross-linking and prehybridization for 3 h at 45°C in 20 ml of hybridization solution (DIG Easy Hyb®, BM), the filters were hybridized to a digoxigenin-dUTP-labelled iNOS probe and digoxigenin-dUTP-labelled glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probe overnight at 45°C in hybridization incubator. Purified PCR products of iNOS (ca 500 bps) and GAPDH (ca 1000 bps) were used as DNA probes after labelling with digoxigenin-11-dUTP by random priming using a Dig-DNA labelling kit (BM). RNA blots hybridized with digoxigenin-labelled DNA probes were detected by EIA with luminescence using Dig-luminescent detection kit (BM) according to the manufacturer's instructions. Briefly, after hybridization and thorough washing, the membrane was blocked with non-fat milk for 30 min and then incubated for 30 min in 20 ml of antibody solution containing polyclonal sheep anti-digoxigenin Fab fragments conjugated to alkaline phosphatase (BM). Alkaline phosphatase activity also was detected by the ECL system (BM) using Amersham ECL-film.
Measurement of COX-2 activity by enzyme immunoassay
PGE2 production was measured in culture medium in order to determine COX-2 activity. For the assay of COX-2 induction, RAW 264.7 macrophages were plated in 24-well plates at a density of 5×105 cells well−1 in 1 ml of DMEM and treated with 500 μM acetylsalicylic acid for COX inactivation. After a 2-h incubation, culture media were replaced with fresh DMEM containing 5% foetal bovine serum. The cells were stimulated with LPS (1 μg ml−1) and IFN-γ (10 units ml−1), and incubated in the presence of ergolide for 16 h at 37°C. The culture supernatants were immediately used for PGE2 determination or stored at −70°C until measurement. For the assay of intrinsic COX-2 activity, the cells incubated and pretreated with acetylsalicylic acid by the same protocol as for the induction were stimulated with LPS (1 μg ml−1) and IFN-γ (10 units ml−1) in the absence of ergolide. After a 16-h incubation and wash, the media were replaced with 0.9 ml of fresh media with or without ergolide. The cells were incubated for 20 min, and 100 μl of 100 μM arachidonic acid (final concentration, 10 μM) was then added to each well and the incubation was continued for another 12 – 13 min. The culture supernatants were used immediately or stored at −70°C until PGE2 determination. PGE2 concentration was measured using a commercial competitive enzyme immunoassay kit (EIA, Caymann Chem., Co., Ann Arbor, MI, U.S.A.) according to the manufacturer's protocol.
Electrophoretic mobility shift assay (EMSA) of NF-κB
Nuclear proteins were extracted by using a modification of Andrew's method (Andrews & Faller, 1991). All the procedures for nuclear protein extraction were performed at 0°C to 4°C with ice-cold reagents. Scrapped and pelleted cells were resuspended in 1 ml of ice-cold lysis buffer (10 mM Tris-HCl, pH 7.4, 3 mM CaCl2, 2 mM MgCl2, 1% Igepal CA-630, 0.5 mM phenylmethylsulphonylfluoride, and 5 μg ml−1 of leupeptin, pepstatin, aprotinin, respectively) and incubated for 15 min on ice with occasional vortexing. After centrifugation and washing of the nuclei pellet, 30 – 50 μl of ice-cold hypertonic extraction buffer [20 mM HEPES-KOH, pH 7.9, 25% (w v−1) glycerol, 420 mM NaCl, 0.2 mM EDTA, 1.5 mM MgCl2, 0.5 mM dithiothreitol and protease inhibitors] was added and incubated at 4°C for 40 min with constant shaking. Nuclear extracts were isolated by centrifugation at 14,000 r.p.m. for 30 min and the protein content in aliquots was determined by Bradford assay (Bradford, 1976). Nuclear extracts were stored at −70° until use for EMSA. The oligonucleotide probe used for EMSA contained the NF-κB consensus sequence. Double-stranded NF-κB consensus sequence was obtained from Bioneer Corp. (Chungbuk, Korea) and used for radioactive labelling after annealing. The sequences of probes used in this work are shown as follows (binding site underlined).
NF-κBU 5′-AGC-TTG-GGG-ACT-TTC-C-3′
NF-κBL 3′-C-CCC-TGA-AAG-GTC-GGC-5′
One nanomole of each oligonucleotide was annealed by heating at 95° for 5 min, cooled slowly to 30°C, and diluted to 1.75 pmol μl−1. Oligonucleotide probe was labelled with α-[32P]dATP using Klenow fragment (BM). The total volume of the labelling mixture was 25 μl and the composition of the labelling mixture was as follows: 7 pmol oligomer (DNA probe), 0.4 mM dNTPs (w/o dATP), labelling buffer (50 mM Tris-HCl, pH 8.0, 50 mM NaCl and 10 mM MgCl2), 4 μl of α-[32P]dATP (>3000 Ci mmol−1, 10 Ci μl−1), and 1 μl of Klenow fragment (1 unit μl−1). The labelling reaction was performed for 40 min at 37°C and the labelled probes were purified by Sephadex G-25 spin-column chromatography.
Binding reactions were performed at room temperature for 30 min with 5 – 10 μg of nuclear protein in 20 μl of binding buffer (10 mM HEPES-KOH, pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 1 mM dithiothreitol, 10% glycerol, and 1 μg ml−1 of leupeptin, pepstatin and aprotinin) containing 1 μg of Poly[dI-dC·dI-dC] and 100,000 c.p.m. [32P]-labelled probe. The specificity of the binding reaction was confirmed by competition assay with a 100 fold molar excess of unlabelled oligonucleotide probe. DNA-protein complex was separated from unbound probe on native 6% polyacrylamide gels in 0.25X-TBE running buffer. After electrophoresis, the gel was vacuum-dried and analysed by Fluorescent Image Analyzer FLA-2000 (Fujifilm, Japan).
Statistical analysis
Results are expressed means±s.d., and an analysis of variance (ANOVA) with Bonferroni's test was used for the statistical analysis of multiple comparisons of data. P-values less than 0.05 were considered statistically significant.
Results
Ergolide inhibits LPS/IFN-γ-mediated production of nitrite, but does not directly inhibit catalytic activity of NOS
In order to evaluate the iNOS inhibitory capacity of ergolide, nitrite accumulation and citrulline formation were examined in LPS/IFN-γ-stimulated RAW 264.7 macrophages. Ergolide was added to the culture media immediately prior to stimulation of RAW 264.7 macrophages with LPS/IFN-γ and the cells were incubated for 18 h. Thereafter, iNOS activity in cell-free extract and nitrite accumulation in the culture medium were determined. As shown in Figure 2, ergolide inhibited nitrite accumulation and [3H]-L-citrulline formation in a concentration-dependent manner. Furthermore, the inhibitory capacity determined by citrulline formation was a little higher than that determined by nitrite accumulation (Figure 2B vs 2A). IC50 value of ergolide is 1.95 μM in nitrite accumulation and less than 1.5 μM in citrulline formation assay. Ergolide had no effect on the cell viability at concentration of 1.5 – 10 μM (data not shown), assessed by MTT method. Thus, these inhibitory effects were not the result of cytotoxicity.
Figure 2.
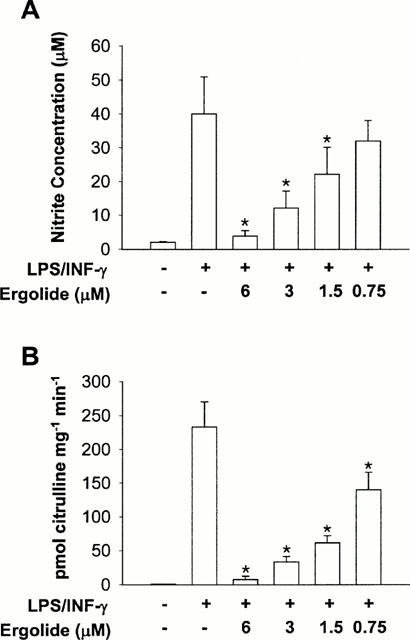
Effects of ergolide on nitrite accumulation and iNOS activity in RAW 264.7 macrophages stimulated with LPS/IFN-γ. Ergolide was added to RAW 264.7 cells in 100 mm-culture dishes immediately prior to the addition of 1 μg ml−1 LPS/10 units ml−1 IFN-γ, and the cells were incubated for 18 h. Nitrite accumulation (A) was determined by Griess reaction in culture medium, and iNOS activity (B) was measured by [3H]-L-citrulline formation with cell-free extracts. Data are expressed as mean±s.d. from three separate experiments in duplicate. Significant differences between control and ergolide-treated groups are indicated by *P<0.05.
Since the result described in Figure 2 reflects the cumulative consequence of iNOS expression and its activity, we investigated whether the inhibition by ergolide of iNOS was due to a direct effect on the enzyme catalytic activity itself or not. We, therefore, directly assayed NOS activity by citrulline formation with cell-free extracts of RAW 264.7 cells stimulated with LPS (1 μg ml−1) and IFN-γ (10 units ml−1) for 18 h in the absence of the inhibitors. Unlike L-NMMA which is a well-known NOS inhibitor, ergolide did not show any significant inhibitory effect on iNOS activity (Figure 3A). Also, ergolide did not inhibit rat brain NOS activity as in the case of iNOS (Figure 3B).
Figure 3.
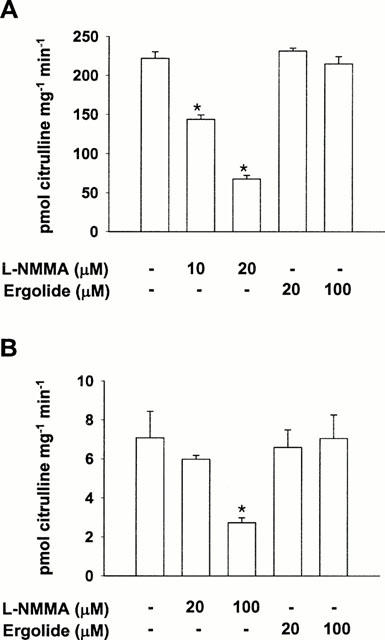
Effects of ergolide on iNOS activity once expressed in RAW 264.7 macrophages pretreated with LPS/IFN-γ and on nNOS from rat brain. (A) Cell lysates were prepared from RAW 264.7 macrophages stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for 18 h. iNOS activity in the lysates was measured using [3H]-L-citrulline formation with or without the addition of L-NMMA or ergolide. (B) nNOS from rat brain was prepared as described in ‘Methods.' nNOS activity was measured using L-[3H]-citrulline formation with or without the addition of L-NMMA or ergolide. Data are expressed as mean±s.d. from three separate experiments in duplicate. Significant differences between control and ergolide-treated groups are indicated by *P<0.05.
Ergolide inhibits LPS/IFN-γ-mediated iNOS protein and mRNA expression
Since ergolide inhibited nitrite production when present together with stimulants, but had no inhibitory effect on iNOS or nNOS in vitro, we investigated its effect on iNOS protein and mRNA expression in order to elucidate the mechanism of inhibition of ergolide (Figure 4). Although hardly detected in unstimulated RAW 264.7 macrophages by Western blot analysis, iNOS protein was sufficiently expressed after stimulation with LPS/IFN-γ for 18 h, and the presence of ergolide in LPS/IFN-γ-stimulated cell cultures markedly decreased iNOS expression in a concentration-dependent manner (Figure 4A). We then performed Northern blot analysis and found that ergolide decreased concentration-dependently the expression level of iNOS mRNA (Figure 4B). These data demonstrate that the decreased activity of iNOS by ergolide could have resulted from the inhibition of iNOS protein and mRNA expression in RAW 264.7 macrophages treated with LPS/IFN-γ.
Figure 4.
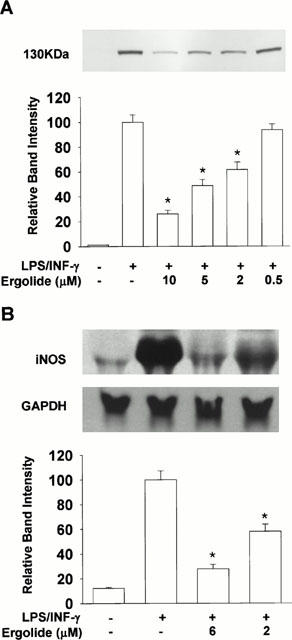
Effects of ergolide on the expression of iNOS. RAW 264.7 macrophages were stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ and incubated for 18 h in the presence or absence of ergolide. Western blot analysis (A) and Northern blot analysis (B) were carried out as described under ‘Methods'. Results show a representative blot and the mean±s.d. of the corresponding band intensities from three separate experiments. Significant differences between control and ergolide-treated groups are indicated by *P<0.05.
Ergolide decreases LPS/IFN-γ-induced PGE2 production in RAW 264.7 macrophages, but does not inhibit COX-2 activity
PGE2 in the supernatant of cultured RAW 264.7 macrophages stimulated with LPS (1 μg ml−1) and IFN-γ (10 units ml−1) for 16 h in the presence of ergolide was measured. As shown in Figure 5A, while unstimulated cells synthesized 3.8 ng ml−1 of PGE2, LPS/IFN-γ-stimulated cells had about 16 folds increased PGE2 synthesis. Since the cells had been pretreated with acetylsalicylic acid to irreversibly inactivate COX by acetylation, this increased PGE2 production indicates increased de novo synthesis of COX-2 enzyme. However, ergolide inhibited PGE2 synthesis in LPS/IFN-γ-stimulated RAW 264.7 macrophages concentration-dependently, with IC50 value of 3 μM. Indomethacin, a potent competitive inhibitor of COX at 0.03 μM, also blocked the PGE2 production in response to LPS/IFN-γ. To test whether inhibitory effect of ergolide on PGE2 production is due to its direct inhibition of catalytic activity of COX-2, the cells were treated with ergolide after being fully stimulated with LPS/IFN-γ. Posttreatment with ergolide did not cause any significant decrease in PGE2 produced by COX-2, while indomethacin inhibited PGE2 production in a concentration-dependent manner as expected (Figure 6). This result demonstrates that ergolide does not inhibit COX-2 activity itself.
Figure 5.
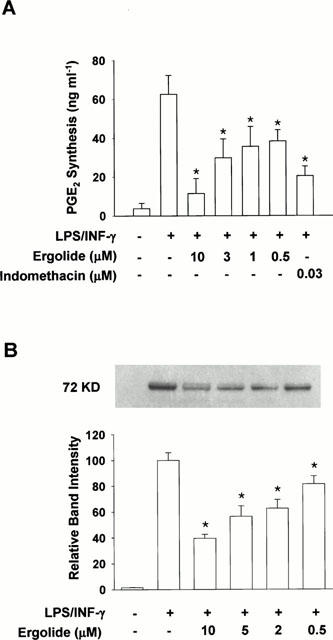
Effects of ergolide on COX-2 activity and COX-2 protein in RAW 264.7 macrophages treated with LPS/IFN-γ. (A) The cells were first treated with acetylsalicylic acid (500 μM) to inactivate COX followed by replacement of the medium with fresh medium together with 1 μg ml−1 LPS/10 units ml−1 IFN-γ. The cells were further incubated in the presence or absence of ergolide. Detailed procedures for cell culture and COX-2 assay are described under ‘Methods'. (B) To measure the level of COX-2 protein, the cells were stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for 18 h in the presence or absence of ergolide and analysed by Western blot analysis as described under ‘Methods'. Results show a representative blot and the mean±s.d. of the corresponding band intensities from three separate experiments. Significant differences between control and ergolide-treated groups are indicated by *P<0.05.
Figure 6.
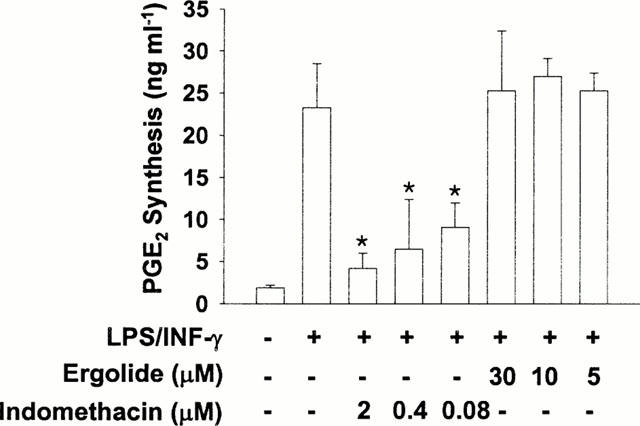
Effects of ergolide on COX-2 activity once expressed by pretreatment with LPS/IFN-γ in RAW 264.7 macrophages. RAW 264.7 macrophages were stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for 16 h. After washing and replacement with fresh medium, ergolide or indomethacin was added and equilibrated for 20 min. The cells were further incubated with 10 μM arachidonic acid for 13 min, and then PGE2 content was determined in culture medium. Data are expressed as mean±s.d. from three separate experiments in duplicate. Significant differences between control and treated groups are indicated by *P<0.05.
Ergolide inhibits COX-2 protein expression
To assess whether de novo protein synthesis is involved in the inhibition of COX-2 by ergolide, Western blot analysis was performed with RAW 264.7 macrophages treated with ergolide together with LPS/IFN-γ stimulation for 18 h. Figure 5B shows that ergolide inhibits LPS/FN-γð-induced COX-2 protein expression in a concentration-dependent manner. This result is in good agreement with the above results that ergolide decreased PGE2 production by LPS/IFN-γ, but did not inhibit COX-2 activity.
Ergolide inhibits LPS/IFN-γ-mediated nuclear translocation of NF-κB
Since the activation of NF-κB is critical for the induction of iNOS or COX-2 by LPS or other inflammatory cytokines, EMSA was performed in order to examine whether ergolide may suppress NF-κB activation. Thus, nuclear binding assay of NF-κB was carried out with nuclear extracts obtained from RAW 264.7 macrophages stimulated with LPS (1 μg ml−1)/IFN-γ (10 U ml−1) for 1 h in the presence or absence of ergolide. Treatment with LPS/IFN-γ for 1 h markedly increased the DNA binding activity of NF-κB (Figure 7A). DNA binding of NF-κB was completely blocked by the addition of 100 fold molar excess of unlabelled oligonucleotide NF-κB DNA probes, which indicated the specificity of the binding reaction (data not shown). Treatment with ergolide, for 1 h before treatment with LPS/IFN-γ, inhibited the DNA binding of NF-κB. To prove that NF-κB inhibition is due to irreversible alkylation of free sulfhydryls on cysteine residues, an excess of thiol cysteine (1 mM) was added 1 h before LPS/IFN-γ stimulation. If ergolide modifies NF-κB by alkylation, an excess of cysteine should prevent this reaction, since ergolide would react with the large quantities of free sulfhydryls in the cysteine instead of reacting with NF-κB. Indeed, addition of cysteine completely suppressed the inhibitory effect of ergolide on the DNA binding activity of NF-κB. Cysteine itself did not affect the binding activity of NF-κB.
Figure 7.
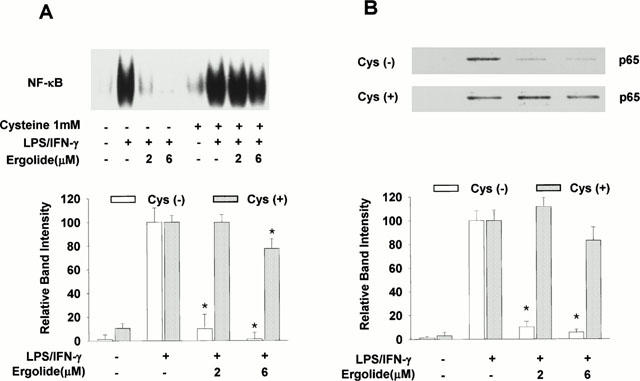
Effects of ergolide on the activation of NF-κB in RAW 264.7 macrophages treated with LPS/IFN-γ. The cells were stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for 1 h after 1 h-pretreatment of ergolide in the presence or absence of cysteine 1 mM. (A) Nuclear extracts were prepared as described under ‘Methods' and DNA-binding activity of NK-κB was determined by EMSA. (B) The nuclear extracts prepared from the experiments in (A) were analysed by Western blotting with an anti-p65 polyclonal antibody. Results show a representative blot and the mean±s.d. of the corresponding band intensities from three separate experiments. Significant differences between control and ergolide-treated groups are indicated by *P<0.05.
The inhibitory effect of ergolide on DNA binding activity of NF-κB might be due to either the direct modification of NF-κB or the inhibition of upstream events leading to NF-κB activation. We performed Western blot analysis to determine whether the translocation of the subunit p65 of heterodimer NF-κB was associated with inhibition of DNA binding of NF-κB by ergolide. As shown in Figure 7B, LPS/IFN-γ treatment caused the translocation of the subunit p65 into the nucleus. However, the amount of p65 translocated into the nucleus in response to LPS/IFN-γ was significantly reduced in the presence of ergolide, and this effect was blocked by the addition of excess of cysteine. We next determined the effect of ergolide on LPS/IFN-γ-mediated degradation of IκB-α by Western blots analysis using polyclonal antibody. Treatment with LPS/IFN-γ led to a rapid disappearance of immunoreactive IκB-α band within 30 min, which was returned to basal levels within 60 min (Figure 8A). However, LPS/IFN-γ-induced degradation of IκB-α was attenuated in the presence of ergolide, and this effect was blocked by the addition of excess of cysteine (Figure 8B). These results suggest that suppression of iNOS and COX-2 gene expression by ergolide might be due to the attenuation of nuclear translocation of NF-κB by inhibiting degradation of IκB-α.
Figure 8.
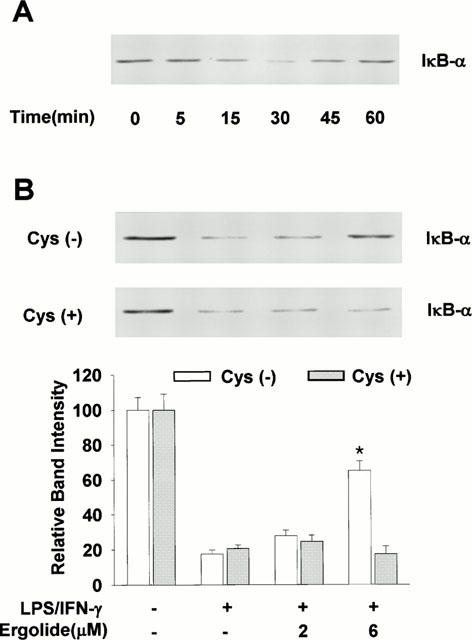
Effects of ergolide on the degradation of IκB-α in RAW 264.7 cells treated with LPS/IFN-γ. (A) The cells were stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for indicated times. Cellular extracts were analysed by Western blotting with an anti-IκB-α polyclonal antibody. (B) After treating with ergolide for 1 h in the presence or absence of cysteine 1 mM, cells were stimulated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for 30 min. Cellular extracts were analysed by Western blotting with an anti-IκB-α polyclonal antibody. Results show a representative blot and the mean±s.d. of the corresponding band intensities from three separate experiments. Significant differences between control and ergolide-treated groups are indicated by *P<0.05.
Ergolide directly interferes with DNA binding of activated NF-κB
Next, we determined whether DNA binding activity of active NF-κB could be directly modified by ergolide in vivo. RAW 264.7 macrophages were pretreated with LPS/IFN-γ for 30 min. Subsequently, ergolide was added at 6 μM concentration. Cell extracts were prepared after indicated times and analysed for NF-κB DNA binding by EMSA. DNA binding of NF-κB in response to LPS/IFN-γ was sustained until 120 min, but addition of ergolide caused inhibition of DNA binding of NF-κB (Figure 9). This result indicates that ergolide could inhibit the DNA binding of NF-κB resulted from a direct modification of active NF-κB in the nucleus as in the case of helenalin (Lyss et al., 1998).
Figure 9.
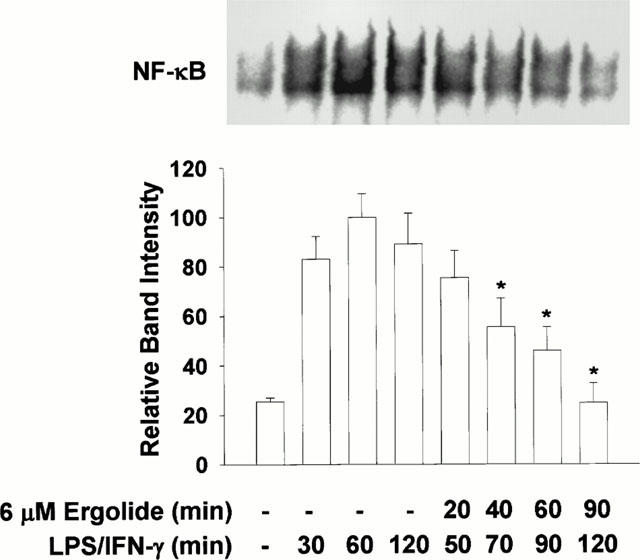
Effects of ergolide on the DNA-binding of NF-κB in LPS/IFN-γ pretreated cells. RAW 264.7 macrophages were pretreated with 1 μg ml−1 LPS/10 units ml−1 IFN-γ for 30 min. Subsequently, 6 μM ergolide was added and nuclear extracts were prepared 20, 40, 60 and 90 min later, and DNA-binding activity of NK-κB was determined by EMSA. Results show a representative blot and the mean±s.d. of the corresponding band intensities from three separate experiments. Significant differences between LPS/IFN-γ-treatment group (60 min) and ergolide-treated groups are indicated by *P<0.05.
Discussion
Our study shows that ergolide, sesquiterpene lactone isolated from dried flowers of I. britannica, does not directly influence the catalytic activity of NOS and COX-2 that are responsible for the synthesis of NO and PGE2, respectively. Neither NOS nor COX-2 activity in RAW 264.7 macrophages was inhibited at 100 μM or 20 μM concentrations. Nevertheless, low concentrations (<10 μM) of ergolide inhibited the production of NO and PGE2 in LPS/IFN-γ-stimulated RAW 264.7 macrophages. These inhibitory effects of ergolide were accompanied by the decrease in expression of iNOS and COX-2 protein, and iNOS mRNA in a concentration-dependent manner. The IC50 value (1.95 μM) of ergolide for nitrite accumulation in RAW 264.7 macrophages is comparable with that of dehydrocostus lactone (3 μM) obtained in LPS-activated RAW 264.7 macrophages (Lee et al., 1999), and lower than that of andalusol, oroxylin A, pharthenolide and isohelenalin (Chen et al., 2000; de las Heras et al., 1999; Wong & Menendez, 1999). These data demonstrated that iNOS and COX-2 gene expression in RAW 264.7 macrophages were suppressed by ergolide, and the inhibition of iNOS and COX-2 gene expression was evident by reductions of inducible NO and PGE2. This suppression was in parallel to the decrease in the association of NF-κB with consensus oligonucleotide and the degradation of IκB.
The present study aimed to investigate the mechanisms by which ergolide abrogated the induction of iNOS and COX-2. Our data show an important effect of ergolide on the inhibition of NF-κB activation. Since NF-κB participates in the regulation of the expression of multiple immediate early genes involved in the immune and inflammatory responses, the effect of ergolide on this target might explain part of the anti-inflammatory actions of this molecule (Ghosh et al., 1998; Baeuerle & Baichwal, 1997).
NF-κB has been shown to be essential for the expression of iNOS and COX-2 (Xie et al., 1994; Barnes & Karin, 1997; Roshak et al., 1996). In the majority of cell types NF-κB is composed of a p50 and p65 subunit. It is retained in an inactive cytoplasmic complex by binding to a group of inhibitory proteins belonging to the IκB family (Baeuerle & Baltimore, 1988). A large variety of inflammatory conditions, such as bacterial and viral infections as well as inflammatory cytokines, rapidly induce NF-κB activity. The phosphorylation of serine residues 32 and 36 in IκB-α and residues 19 and 23 in IκB-β by IKK-α and IKK-β trigger the degradation of IκB and the dissociation of NF-κB from IκB. This causes NF-κB to translocate into the nucleus and interact with the NF-κB binding motif in the promoters of target genes (Baeuerle & Henkel, 1994; Mercurio et al., 1997; Didonato et al., 1997). The present study demonstrated that the block of NF-κB activation caused by ergolide accounted for the inhibition of the induction of iNOS and COX-2. The inhibitory effect of ergolide on PGE2 production, therefore, might be mediated through interfering directly with expression of COX-2 rather than with cross-talk between NO and COX-2 pathways (Salvemini et al., 1993; von knethen & Brune, 1997).
Regarding the mechanism of inhibition of NF-κB by ergolide, two possibilities can be considered. First, ergolide may inhibit the translocation of NF-κB into the nucleus via stabilizing the NF-κB/IκB complex or interfering with the degradation of IκB family. Second, ergolide may exert its effect by directly modifying the active NF-κB as in the case of helenalin (Lyss et al., 1998). Therefore, we first examined the amount of p65 translocated into the nucleus and the degradation of IκB-α by Western blot analysis. Our data showed that the amount of p65 translocated into the nucleus was significantly attenuated by treatment with ergolide in RAW 264.7 macrophages. This result agrees with that observed by Western blotting of IκB-α. Ergolide prevented the degradation of IκB-α from the cytosolic fraction inhibiting the transcription of genes dependent on NF-κB activity. Then, we determined whether DNA binding activity of active NF-κB could be directly modified by ergolide in vivo. Our data showed that ergolide directly inhibited the DNA binding of active NF-κB, which is translocated into the nucleus by treatment with LPS/IFN-γ. This result is similar to that of helenalin (Lyss et al., 1998). Taken together, these results indicate that ergolide has a dual effect, the inhibition of nuclear translocation and the direct modification of active NF-κB, on the inhibition of NF-κB.
Exomethylene group in sesquiterpene lactones react with nucleophiles, especially sulfhydryl groups of NF-κB, by a Michael-type addition. Recently, based on computer molecular modelling, Rüngeler et al. (1999) proposed how helenalin, sesquiterpene lactone, may alkylate p65 subunit of NF-κB. It may crosslink two cysteine residues, Cys 38 being located within the DNA binding domain and Cys 120 being found in a proximal loop, thereby inhibiting DNA binding of NF-κB. The inhibition of DNA binding activity of NF-κB by ergolide might be due to irreversible alkylation of free sulfhydryls on cysteine residues because the addition of 1 mM cysteine abolished the inhibitory effect of ergolide, although we cannot specify precisely where ergolide alkylation occurs. Furthermore, the addition of cysteine completely blocked the inhibitory effect on p65 translocation and IκB degradation of ergolide. These results indicate that ergolide irreversibly alkylates the cysteine residues of p65 and then stabilizes the NF-κB/IκB complex, thereby inhibiting the translocation of NF-κB into the nucleus. However, because ergolide may inhibit a step leading to the activation of the IκB kinase or affecting the functioning of 26 S proteosome, further work is required to account for this question.
In conclusion, these data described herein suggest that ergolide from I. britannica suppress NO and PGE2 synthesis in RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression, respectively. And, the inhibition of iNOS and COX-2 gene expression is, in large part, associated with the inhibition of nuclear translocation of NF-κB through stabilizing the NF-κB/IκB complex as well as the direct modification of active NF-κB. Furthermore, the ability to inhibit iNOS and COX-2 gene expression may account, in part, for the anti-inflammatory properties of ergolide.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea. (HMP-98-D-4-0041).
Abbreviations
- COX-2
cyclo-oxygenase-2
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- IFN-γ
interferon-γ
- iNOS
inducible nitric oxide synthase
- IκB
inhibitor of nuclear factor-κB
- L-NMMA
L-NG-monomethylarginine
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- PGE2
prostaglandin E2
References
- ANDREWS N.C., FALLER D.V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acid Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAEUERLE P.A., BAICHWAL V.R. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immuno. 1997;65:111–137. [PubMed] [Google Scholar]
- BAEUERLE P.A., BALTIMORE D. IκB: a specific inhibitor of the NF-κB transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- BAEUERLE P.A., BALTIMORE D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- BAEUERLE P.A., HENKEL T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHAN M.M., FONG D., HO C.T., HUANG H.I. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem. Pharmacol. 1997;54:1281–1286. doi: 10.1016/s0006-2952(97)00504-2. [DOI] [PubMed] [Google Scholar]
- CHEN Y., YANG L., LEE T.J. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 2000;59:1445–1457. doi: 10.1016/s0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- CORBETT J.A., KWON G., TURK J., MCDANIEL M.L. IL-1 beta induces the coexpression of both nitric oxide synthase and cyclooxygenase by islets of Langerhans: activation of cyclooxygenase by nitric oxide. Biochemistry. 1993;32:13767–13770. doi: 10.1021/bi00213a002. [DOI] [PubMed] [Google Scholar]
- CROFFORD L.J., TAN B., MCCARTHY C.J., HLA T. Involvement of nuclear factor-κB in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 1997;40:226–236. doi: 10.1002/art.1780400207. [DOI] [PubMed] [Google Scholar]
- DE LAS HERAS B., NAVARRO A., DIAZ-GUERRA M.J., BERMEJO P., CASTRILLO A., BOSCA L., VILLAR A. Inhibition of NOS-2 expression in macrophages through the inactivation of NF-κB by andalusol. Br. J. Pharmacol. 1999;128:605–612. doi: 10.1038/sj.bjp.0702844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIDONATO J.A., HAYAKAWA M., ROTHWARF D.M., ZANDI E., KARIN M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- FRANCHI A.M., CHAUD M., RETTORI V., SUBURO A., MCCANN S.M., GIMENO M. Role of nitric oxide in eicosanoid synthesis and uterine motility in estrogen-treated rat uteri. Proc. Natl. Acad. Sci. U.S.A. 1994;91:539–543. doi: 10.1073/pnas.91.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FU J.Y., MASFERRER J.L., SEIBERT K., RAZ A., NEEDLEMAN P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- GHOSH S., MAY M.J., KOPP E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L, , WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate, nitrite, and [15N]-nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HABIB A., BERNARD C., LEBRET M., CREMINON C., ESPOSITO B., TEDGUI A., MACLOUF J. Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J. Immunol. 1997;158:3845–3851. [PubMed] [Google Scholar]
- HAYASHI T., ABE E., YAMATE T., TAGUCHI Y., JASIN H.E. Nitric oxide production by superficial and deep articular chondrocytes. Arthritis Rheum. 1997;40:261–269. doi: 10.1002/art.1780400210. [DOI] [PubMed] [Google Scholar]
- KIM Y.M., LEE B.S., YI K.Y., PAIK S.G. Upstream NF-κB site is required for the maximal expression of mouse inducible nitric oxide synthase gene in interferon-gamma plus lipopolysaccharide-induced RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 1997;236:655–660. doi: 10.1006/bbrc.1997.7031. [DOI] [PubMed] [Google Scholar]
- LEE H.J., KIM N.Y., JANG M.K., SON H.J., KIM K.M., SOHN D.H., LEE S.H., RYU J.H. A sesquiterpene, dehydrocostus lactone, inhibits the expression of inducible nitric oxide synthase and TNF-α in LPS-activated macrophages. Planta Med. 1999;65:104–108. doi: 10.1055/s-1999-13968. [DOI] [PubMed] [Google Scholar]
- LYSS G., KNORRE A., SCHMIDT T.J., PAHL H.L., MERFORT I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- MACMICKING J., XIE Q.W., NATHAN C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- MAY M.J., GHOSH S. Signal transduction through NF-κB. Immunol. Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- MERCURIO F., ZHU H., MURRAY B.W., SHEVCHENKO A., BENNETT B.L., LI J., YOUNG D.B., BARBOSA M., MANN M., MANNING A., RAO A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- MICHEL T., XIE Q.W., NATHAN C.Molecular biological analysis of nitric oxide synthases Methods in Nitric Oxide Research 1995New York: John Wiley & Sons; 161–175.eds Feelisch, M. & Stamler, J.S. pp [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NATHAN C. Inducible nitric oxide synthase: what difference does it make. J. Clin. Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK E.J. &, KIM J. Cytotoxic sesquiterpene lactones from Inula britannica. Planta Med. 1998;64:752–754. doi: 10.1055/s-2006-957573. [DOI] [PubMed] [Google Scholar]
- ROSHAK A.K., JACKSON J.R., MCGOUGH K., CHABOT-FLETCHER M., MOCHAN E., MARSHALL L.A. Manipulation of distinct NFκB proteins alters interleukin-1β-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J. Biol. Chem. 1996;271:31496–31501. doi: 10.1074/jbc.271.49.31496. [DOI] [PubMed] [Google Scholar]
- RÜNGELER P., CASTRO V., MORA G., GOREN N., VICHNEWSKI W., PAHL H.L., MERFORT I., SCHMIDT T.J. Inhibition of transcription factor NF-κB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg. Med. Chem. 1999;7:2343–2352. doi: 10.1016/s0968-0896(99)00195-9. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., SEIBERT K., MASFERRER J.L., MISKO T.P., CURRIE M.G., NEEDLEMAN P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J. Clin. Invest. 1994;93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAUTEBIN L. Prostaglandins and nitric oxide as molecular targets for antiinflammatory therapy. Fitoterapia. 2000;71:S48–S57. doi: 10.1016/s0367-326x(00)00181-7. [DOI] [PubMed] [Google Scholar]
- SAUTEBIN L., IALENTI A., IANARO A., DI ROSA M. Relationship between nitric oxide and prostaglandins in carrageenin pleurisy. Biochem. Pharmacol. 1998;55:1113–1117. doi: 10.1016/s0006-2952(97)00530-3. [DOI] [PubMed] [Google Scholar]
- SMITH W.L., GARAVITO R.M., DEWITT D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- TETSUKA T., DAPHNA-IKEN D., SRIVASTAVA S.K., BAIER L.D., DUMAINE J., MORRISON A.R. Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12168–12172. doi: 10.1073/pnas.91.25.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VODOVOTZ Y., BOGDAN C., PAIK J., XIE Q.W., NATHAN C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor-β. J. Exp. Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KNETHEN A, BRUNE B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- VON KNETHEN A., CALLSEN D., BRUNE B. NF-κB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol. Biol. Cell. 1999;10:361–372. doi: 10.1091/mbc.10.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q., ZHOU B.N., ZHANG R.W., LIN Y.Y., LIN L.Z., GIL R.R., CORDELL G.A. Cytotoxicity and NMR spectral assignments of ergolide and bigelovin. Planta Med. 1996;62:166–168. doi: 10.1055/s-2006-957843. [DOI] [PubMed] [Google Scholar]
- WONG H.R., MENENDEZ I.Y. Sesquiterpene lactones inhibit inducible nitric oxide synthase gene expression in cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 1999;262:375–380. doi: 10.1006/bbrc.1999.1207. [DOI] [PubMed] [Google Scholar]
- XIE Q.W., KASHIWABARA Y., NATHAN C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- YAMAMOTO K., ARAKAWA T., UEDA N., YAMAMOTO S. Transcriptional roles of nuclear factor-κB and nuclear factor-interleukin-6 in the tumor necrosis factor α-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]


