Abstract
We investigated the contribution of neuronal or inducible nitric oxide synthase (nNOS or iNOS) at the rostral ventrolateral medulla (RVLM) to central cardiovascular regulation by endogenous nitric oxide (NO), using Sprague-Dawley rats anaesthetized and maintained with propofol.
Microinjection bilaterally into the RVLM of a NO trapping agent, carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-l-oxy-l-3-oxide (10, 50 or 100 nmoles) resulted in significant hypotension and bradycardia.
Similar application of a selective antagonist of nNOS, 7-nitroindazole (1, 2.5 or 5 pmoles), or selective antagonists of iNOS, aminoguanidine (125, 250 or 500 pmoles), N6-(l-iminoethyl)-L-lysine (250 pmoles) or S-methylisothiourea (250 pmoles), induced respectively a reduction or an enhancement in systemic arterial pressure, heart rate and power density of the vasomotor components in the spectrum of arterial blood pressure signals, the experimental index for sympathetic neurogenic vasomotor tone.
Both hypotension and bradycardia induced by the NO precursor, L-arginine (100 nmoles), were significantly blunted when aminoguanidine (250 pmoles) was co-microinjected bilaterally into the RVLM. On the other hand, co-administered 7-nitroindazole (2.5 pmoles) was ineffective.
Whereas low doses of S-nitro-N-acetylpenicillamine (0.25 or 0.5 nmoles) elicited hypertension and tachycardia, high doses of this non-nitrate NO donor (5 nmoles) induced hypotension and bradycardia.
Reverse transcription – polymerase chain reaction analysis revealed that both iNOS and nNOS mRNA were expressed in the ventrolateral medulla.
We conclude that the prevalence of nNOS over iNOS activity at the RVLM and the associated dominance of sympathoexcitation over sympathoinhibition may underlie the maintenance of sympathetic vasomotor outflow and stable systemic arterial pressure by the endogenous NO.
Keywords: Nitric oxide, neuronal and inducible nitric oxide synthase, rostral ventrolateral medulla, systemic arterial pressure, heart rate, sympathetic vasomotor outflow
Introduction
Nitric oxide (NO) is a diffusible messenger which was initially identified as an endothelial-derived relaxing factor that promotes relaxation of peripheral vasculature (Rees et al., 1989). Recent studies (see Krukoff, 1999; Zanzinger, 1999) further assigns a role to NO in central cardiovascular regulation. As the last area within the brain stem for the integration of sympathetic outflow (Dampney, 1994), the sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM), which provide the major tonic source of excitatory input to sympathetic preganglionic cells in the spinal cord (Guertzenstein & Silver, 1974), is a potential target site for NO to exert its modulatory action on cardiovascular functions. Immunohistochemical (Dun et al., 1994; Simonian & Herbison, 1996) and in situ hybridization (Plochocka-Zulinska & Krukoff, 1997; Iwase et al., 1998) studies demonstrated the presence of nitric oxide synthase (NOS) in the RVLM. However, controversy exists regarding the cardiovascular effects of NO at the RVLM. Application of NO precursor or donor into the RVLM reportedly decreases sympathetic nerve activity and systemic arterial pressure (SAP) (Zanzinger et al., 1995; Tseng et al., 1996; Kagiyama et al., 1997). Other studies (Hakim et al., 1995; Hirooka et al., 1996; Martins-Pinge et al., 1997) demonstrated a sympathoexcitatory and pressor role for NO in the RVLM.
Three isoforms of NOS have so far been identified in a variety of cell types. Neuronal NOS (nNOS) is expressed in neurons and glial cells (Bredt et al., 1990; Forstermann et al., 1995). Inducible NOS (iNOS) is localized in most nucleated cells, including macrophages, smooth muscle cells and glial cells (Murphy et al., 1993). Endothelial NOS (eNOS) is principally present in endothelia, platelets and cardiomyocytes (Forstermann et al., 1995; Szabo & Thiemermann, 1995; Wong et al., 1996). It is generally contended (Murphy et al., 1993; Szabo & Thiemermann, 1995; Wong et al., 1996) that whereas both nNOS and eNOS are expressed constitutively, the activity of iNOS is principally induced by macrophage in response to inflammatory stimuli. Whether this contention is unequivocally appropriate remains unsettled. At the same time, the potential role of individual isoform of NOS at the RVLM in the regulation of sympathetic outflow and SAP by NO has not been determined.
The present study was designed to address two important issues on the role of NO in central cardiovascular regulation. First, does endogenous NO at the RVLM modulate SAP, heart rate (HR) and sympathetic vasomotor outflow. Second, what is the contribution by NOS, in particular nNOS and iNOS, to this modulatory process. Our results suggest that endogenous NO at the RVLM participates actively in the maintenance of SAP and HR. Furthermore, under physiologic conditions, both nNOS and iNOS are active at the levels of functional expression and molecular synthesis. However, the maintenance of sympathetic vasomotor outflow by endogenous NO at the RVLM may result from a prevalence of nNOS over iNOS activities, which are respectively responsible for the sympathoexcitatory and sympathoinhibitory action of NO.
Methods
All experimental procedures were carried out in compliance with the guidelines for the care and use of experimental animals endorsed by our institutional animal care committee. All efforts were made to reduce the number of animals used and to minimize animal suffering during the experiment.
General preparation
Specific pathogen-free adult male Sprague-Dawley rats (250 – 290 g, n=124) were obtained from the Experimental Animal Center of the National Science Council, Taiwan, Republic of China. They were housed in an animal room under temperature control (24 – 25°C) and 12-h light – dark cycle. Standard laboratory rat chow and tap water were available ad libitum.
Animals were anaesthetized initially with pentobarbital sodium (50 mg kg h−1, i.p.) to perform preparatory surgery. This included intubation of the trachea to facilitate ventilation and cannulation of the left femoral artery to measure SAP. Both femoral veins were also cannulated. One of them was used in i.v. infusion of propofol (Zeneca, Macclesfield, U.K.) at 30 mg kg h−1. This scheme (Yang et al., 1995) provided satisfactory anaesthetic maintenance while preserving the capacity of central cardiovascular regulation, based on observations of on-line and real-time changes in the spectrum of SAP signals. Animals also received neuromuscular blockade with i.v. infusion of pancuronium (2 mg kg h−1) via the other femoral vein, and were mechanically ventilated (Harvard 683, South Natik, MA, U.S.A.) to maintain end-tidal CO2 to be within 4 – 5%, as monitored by a capnograph (Datex Normocap, Helsinki, Finland). The head of animals was thereafter fixed to a stereotaxic headholder (Kopf 1430, Tujunga, CA, U.S.A.), and body temperature was maintained at 37°C by a heating pad.
Recording and power spectral analysis of SAP signals
The arterial catheter was connected to a pressure transducer (Gould P23ID, Valley View, OH, U.S.A.; frequency range: DC to 200 Hz) and in turn to a pressure processor amplifier (Gould G-20-4615-52) via which SAP signals were amplified and filtered (frequency range: DC to 100 Hz). The catheter-transducer system has a damped natural frequency of 40 Hz, and showed a flat amplitude response with no phase shift to 20 Hz. HR was determined by a biotachometer (Gould G-20-4615-66) triggered by the arterial pulses. Pulsatile and mean arterial blood pressure (MSAP), as well as HR were recorded on a polygraph (Gould RS 3400).
The SAP signals were simultaneously subjected to on-line power spectral analysis as detailed previously (Kuo & Chan, 1993; Yang et al., 1995; Kuo et al., 1997). The power density of each spectral component of SAP signals was estimated based on fast Fourier transform. The resultant two-dimensional spectrogram and values of each spectral components were output either graphically or numerically on a printer. By repeating these procedures continuously, we were able to examine the spectral changes of SAP signals over time in a real-time manner. We were particularly interested in the very low-frequency (0 – 0.25 Hz) and low-frequency (0.25 – 0.8 Hz) components of SAP signals. Our laboratory demonstrated (Kuo et al., 1997) that these spectral components of SAP signals may take origin from the RVLM, and reflect the prevalence of sympathetic neurogenic vasomotor tone.
Microinjection of test agents into the RVLM
Microinjection bilaterally of test agents into the RVLM, at a volume of 50 nl, was carried out stereotaxically and sequentially with a glass micropipette (tip diameter: 50 – 80 μm) connected to a 0.5-μl Hamilton (Reno, NV, U.S.A.) microsyringe. The coordinates were: 4.5 – 5 mm posterior to lambda, 1.8 – 2.1 mm lateral to midline, and 8.1 – 8.4 mm below the dorsal surface of cerebellum (Chan et al., 2001). Functional location of RVLM neurons on either side was carried out at the beginning of each experiment by the elicitation of transient increase in SAP (25 – 30 mmHg) on microinjection of glutamate (2 nmoles). The time between injections from one side of the RVLM to the other was 5 min. Subsequent microinjections of test agents were delivered to the identified pressor loci.
All microinjection solutions contained 1% Evans blue to aid in subsequent histologic verification of the injection site. Possible volume effect of microinjection was controlled by injecting the same amount of artificial cerebrospinal fluid (aCSF) or the appropriate solvent. To avoid confounding effects of drug interactions, each animal received only one test agent or vehicle, which was microinjected to the RVLM 10 min after the completion of glutamate application. This time lag was adopted to ensure a complete recovery from the glutamate-induced pressor response, and a stable basal MSAP and HR before microinjection bilaterally into the RVLM of other test agents or vehicle. Effect of various test agents on basal MSAP, HR or the power density of the vasomotor components of SAP spectrum was evaluated for 60 min post-treatment.
Test agents
Test agents used in this study included a NO precursor, L-arginine (L-Arg) (Szabo, 1996); a non-nitrate NO donor, S-nitro-N-acetylpenicillamine (SNAP) (Harrison & Bates, 1993); a selective nNOS inhibitor (Moore et al., 1993; Moore & Handy, 1997; Osborne & Coderre, 1999), 7-nitroindazole (7-NI); three selective iNOS inhibitor, aminoguanidine (AG) (Moore & Handy, 1997; Mattson et al., 1998; Osborne & Coderre, 1999), S-methylisothiourea (SMT) (Southan et al., 1995) or N6-(l-iminoethyl)-L-lysine (L-NIL) (Moore et al., 1994; Connor et al., 1995); and a NO trapping agent, carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide (carboxy-PTIO) (Yoshida et al., 1994; Rand & Li, 1995). With the exception of SMT and L-NIL, which were obtained from Tocris Cookson (Bristol, U.K.), test agents were purchased from RBI (Natik, MA, U.S.A.). All chemicals were freshly prepared immediately before use. They were dissolved in aCSF, with the exception of 7-NI, which was dissolved in 3% methanol.
Histology
The brain stem was removed after each experiment and was fixed in 30% sucrose in 10% formaldehyde-saline solution for at least 72 h. Histologic verification of the microinjection site was carried out in 20-μm frozen sections stained with Neutral Red.
Isolation of total RNA and reverse transcription – polymerase chain reaction
Isolation and extraction of total RNA from the ventrolateral part of medulla oblongata, at the level of RVLM (0.5 – 2.5 mm rostral to the obex), and reverse transcription – polymerase chain reaction (RT – PCR) analysis of iNOS, nNOS or β-actin mRNA was carried out as reported previously (Chan et al., 2001). Medullary tissues from 5 – 6 rats under the same experimental condition were pooled for this purpose. The predominant cDNA amplification product predicted for iNOS, nNOS and β-actin was respectively 317, 345 and 440 bp in length. The amount of mRNA products for iNOS or nNOS was analysed by ImageMaster VDS analysis software (Amersham Pharmacia Biotech, Piscataway, NJ, U.S.A.), and was expressed as the ratio to β-actin mRNA product.
Statistical analysis
All values are expressed as mean±s.e.mean. The averaged value of MSAP or HR calculated every 10 min after microinjection of a test agent or vehicle, and the sum total of power density for the vasomotor components (0 – 0.8 Hz) of the SAP spectra over 10 min, were used for statistical analysis. The temporal effects of various treatments on MSAP, HR or vasomotor components of SAP signals were assessed using two-way analysis of variance (ANOVA) with repeated measures for group difference. They were followed by the Scheffé multiple range test for post hoc comparison of individual means. P<0.05 was considered to be statistically significant.
Results
Temporal effects of microinjection bilaterally into the RVLM of carboxy-PTIO on SAP or HR
Our first series of experiments was designed to establish the participation of endogenous NO at the RVLM in cardiovascular regulation. Microinjection bilaterally into the RVLM of carboxy-PTIO (10, 50 or 100 nmoles) resulted in a significant reduction in MSAP or HR. These cardiovascular suppressive effects were dose-related, and persisted the entire 60-min observation period (Figure 1).
Figure 1.
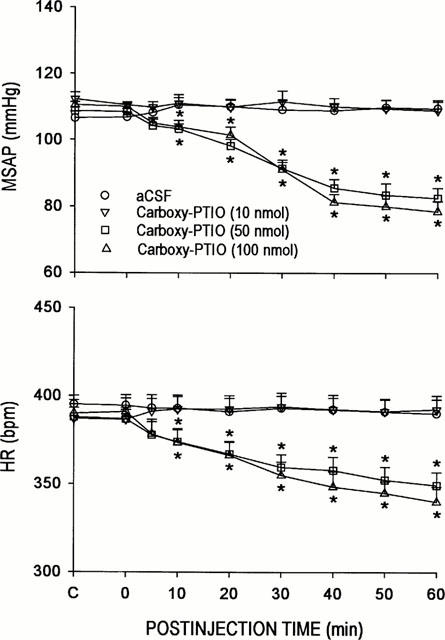
Time-course changes in mean arterial blood pressure (MSAP) of heart rate (HR) in rats that received microinjection bilaterally into the RVLM of carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide (carboxy-PTIO) or aCSF. Values are mean±s.e.mean, n=6–7 animals per experimental group. *P<0.05 vs aCSF group in the Scheffé multiple-range test.
Temporal effects of microinjection bilaterally into the RVLM of nNOS or iNOS antagonists on SAP, HR or vasomotor components of SAP spectrum
Our second series of experiments expounded on the role of nNOS and iNOS in the implied participation of endogenous NO at the RVLM in cardiovascular regulation. Microinjection bilaterally of 7-NI (1, 2.5 or 5 pmoles) into the RVLM resulted in progressive and significant hypotension or bradycardia. These effects of 7-NI were dose-related (Table 1), and lasted for the entire 60-min observation period (Figure 2). On the other hand, microinjection bilaterally of AG (125, 250 or 500 pmoles) into the RVLM promoted a significant increase in MSAP or HR. These effects of AG were again dose-dependent (Table 1), but endured only 20 – 30 min (Figure 2). Local application of 3% methanol (solvent for 7-NI) into the RVLM, similar to aCSF (solvent for AG), effected minimal changes in MSAP or HR (Table 1).
Table 1.
Dose-related effects of blockade of iNOS or nNOS in the RVLM on mean systemic arterial pressure (MSAP) and heart rate (HR)
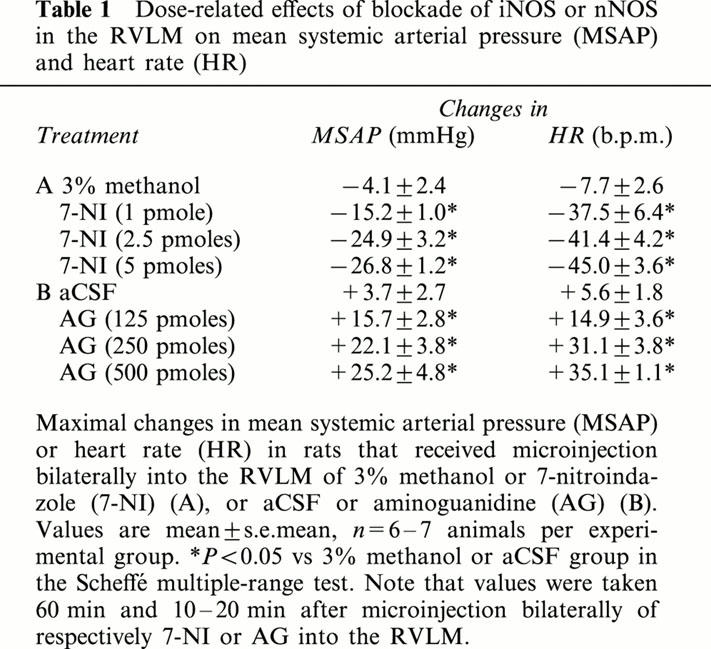
Figure 2.
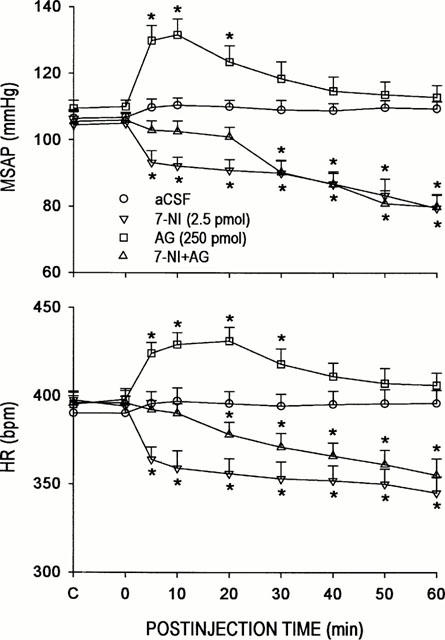
Time-course changes in mean arterial blood pressure (MSAP) or heart rate (HR) in rats that received microinjection bilaterally into the RVLM of aminoguanidine (AG) or 7-nitroindazole (7-NI), given alone or together; or aCSF. Values are mean±s.e.mean, n=6–7 animals per experimental group. *P<0.05 vs aCSF group at corresponding time-points in the Scheffé multiple-range test.
It is interesting to note that co-administration of 7-NI (2.5 pmoles) and AG (250 pmoles) into the RVLM elicited changes in MSAP or HR (Figure 2) that approximated the algebraic sum of their individual actions. Furthermore, both the time-course and magnitude of these cardiovascular alterations greatly resembled those elicited by carboxy-PTIO (50 or 100 nmoles) (Figure 1).
We also investigated whether alterations in sympathetic vasomotor outflow to the blood vessels may underlie the cardiovascular changes elicited by 7-NI or AG. Figure 3 illustrates that microinjection bilaterally of 7-NI (1, 2.5 or 5 pmoles) into the RVLM resulted in a significant and dose-related reduction in the power density of the vasomotor components of SAP spectrum that persisted 60 min. On the other hand, local application of AG (125, 250 or 500 pmoles) into the RVLM promoted a 30-min period of significant and dose-dependent elevation in the power density of the same spectral components of SAP signals. It is interesting to note that the time course of decrease or increase in the suggested sympathetic neurogenic vasomotor outflow induced by 7-NI or AG paralleled that observed in the reduction or elevation in MSAP elicited by the same test chemicals (Figure 2).
Figure 3.
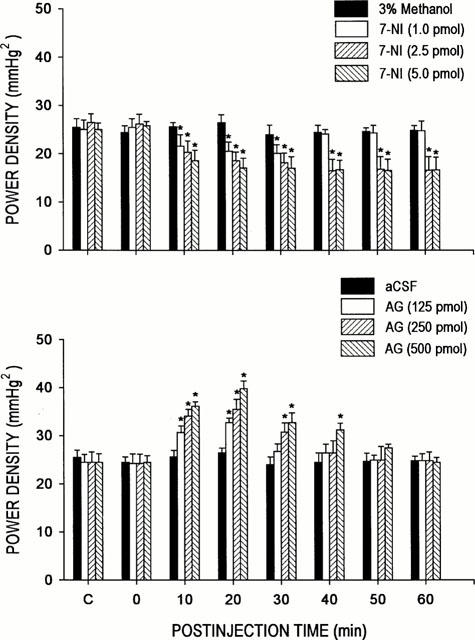
Time-course changes in total power density of the vasomotor components (0–0.8 Hz) in the systemic arterial pressure spectrum from rats that received microinjection bilaterally into the RVLM of 3% methanol or 7-NI (upper panel), or aCSF or AG (lower panel). Values are mean±s.e.mean, n=6–7 animals per experimental group. *P<0.05 vs 3% methanol or aCSF group at corresponding time-points in the Scheffé multiple-range test.
To further confirm that the cardiovascular responses to AG were due to its selective blockade of iNOS activity, two other iNOS inhibitors, SMT or L-NIL, were microinjected bilaterally into the RVLM (Figure 4). On an equimolar basis (250 pmol), we found that both selective iNOS inhibitors elicited hypertension, tachycardia or increase in the power density of the vasomotor components of SAP spectrum that was comparable in magnitude and time-course to treatment with AG.
Figure 4.
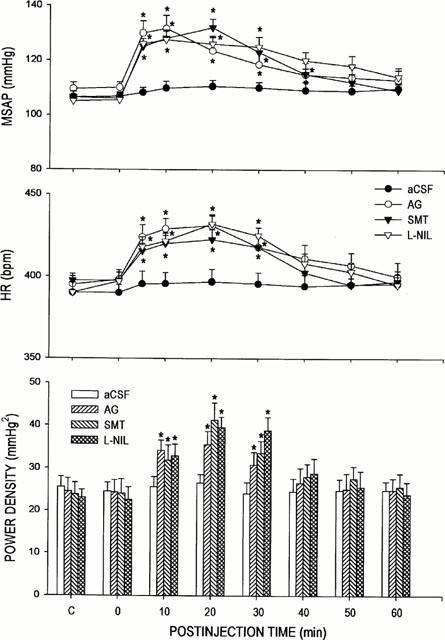
Time-course changes in mean arterial blood pressure (MSAP), heart rate (HR) or total power density of the vasomotor components (0–0.8 Hz) in the systemic arterial pressure spectrum in rats that received microinjection bilaterally into the RVLM of equimolar doses (250 pmoles) of S-methylisothiourea (SMT) or N6-(1-iminoethyl)-L-lysine (L-NIL); or aCSF. Data on AG from Figures 2 and 3 were duplicated for comparison. Values are mean±s.e.mean, n=6–7 animals per experimental group. *P<0.05 vs aCSF group at corresponding time-points in the Scheffé multiple-range test.
Differential effects of 7-NI or AG on cardiovascular actions of endogenous NO at the RVLM
The differential role of nNOS and iNOS in the engagement of endogenous NO at the RVLM in central cardiovascular regulation was further elucidated in our third series of experiments by examining the effects of 7-NI or AG on circulatory changes induced by L-Arg. Microinjection bilaterally into the RVLM of L-Arg (100 nmoles) resulted in a significant decrease in MSAP or HR that endured 40 – 60 min (Figure 5). Interestingly, co-microinjection of AG (250 pmoles) into the RVLM (Figure 5) blunted significantly the bradycardia induced by L-Arg (100 nmoles). Furthermore, AG not only antagonized the hypotension induced by the NO precursor, but reversed it to an increase in MSAP (Figure 5) that resembled qualitatively that promoted by the iNOS inhibitor alone (Figure 2). On the other hand (Figure 5), co-administration of 7-NI (2.5 pmoles) did not induce further augmentation of the cardiovascular suppressive effects of L-Arg (100 nmoles).
Figure 5.
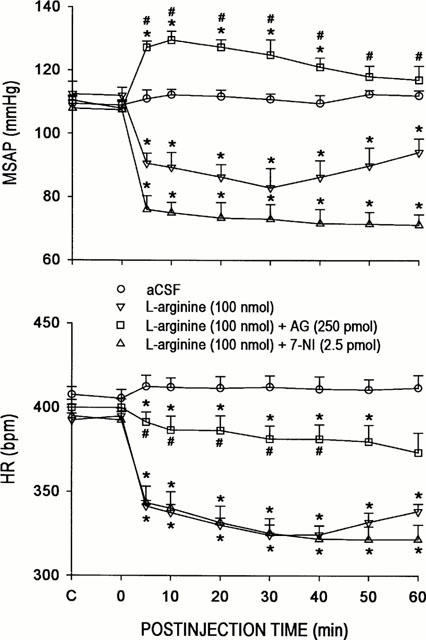
Time-course changes in mean arterial blood pressure (MSAP) or heart rate (HR) in rats that received microinjection bilaterally into the RVLM of L-arginine, given alone or together with AG or 7-NI; or aCSF. Values are mean±s.e.mean, n=6–8 animals per experimental group. *P<0.05 vs aCSF group and #P<0.05 vs L-arginine group at corresponding time-points in the Scheffé multiple-range test.
Dose-dependency of differential cardiovascular actions of exogenous NO at the RVLM
It has been suggested that iNOS differs from nNOS by producing larger amounts of NO upon activation (Szabo & Thiemermann, 1995; Szabo, 1996). Thus, NO may exert a differential action on cardiovascular regulation that is dependent on the amount present in the RVLM. Our fourth series of experiments was designed to evaluate this notion, using SNAP. Microinjection bilaterally of SNAP (0.25, 0.5, 1 or 5 nmoles) into the RVLM promoted differential changes in MSAP or HR that were dose dependent (Figure 6). At the two lower doses (0.25 or 0.5 nmoles) used, SNAP elicited significant hypertension and tachycardia. This gave way to minor alterations in MSAP or HR when the NO donor was increased to 1 nmoles. Significant hypotension and bradycardia were observed when SNAP was microinjected to the RVLM at 5 nmoles.
Figure 6.
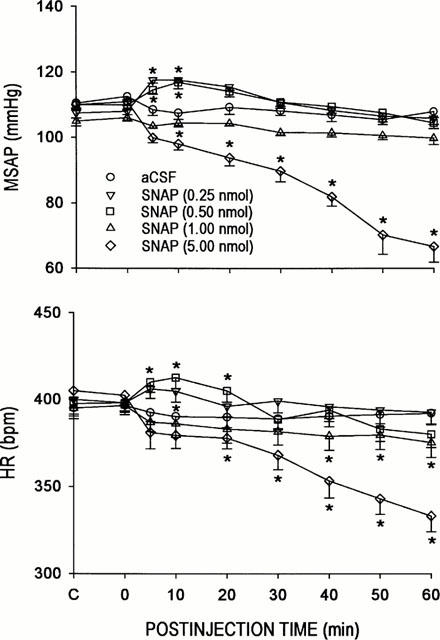
Time-course changes in mean arterial blood pressure (MSAP) or heart rate (HR) in rats that received microinjection bilaterally into the RVLM of aCSF or S-nitro-N-acetylpenicillamine (SNAP). Values are mean±s.e.mean, n=6–7 animals per experimental group. *P<0.05 vs aCSF group at corresponding time-points in the Scheffé multiple-range test.
Microinjection sites
Histologic verification of locations of the tip of microinjection pipettes (Figure 7) confirmed that all observations were made from animals that received local administration of the test drugs within the confines of the RVLM.
Figure 7.
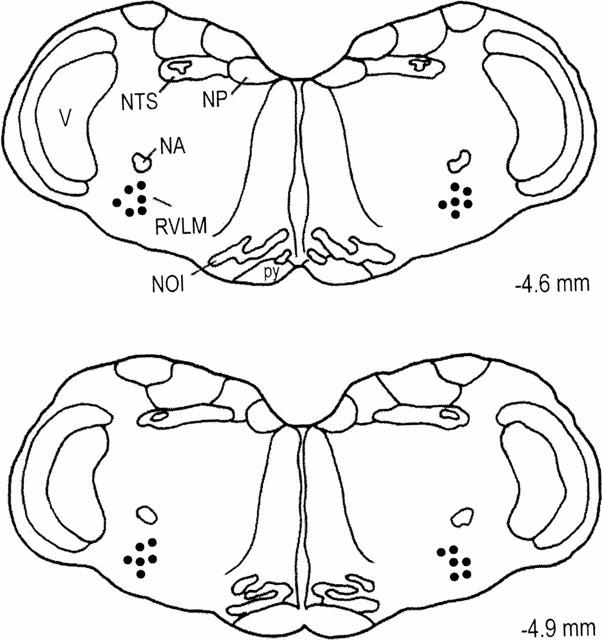
Diagrammatic representation of the medulla oblongata showing the bilateral location of the tip of the microinjection pipettes within the core of the RVLM. For clarity, only 10% of the total microinjection sites are included. Numbers indicate distance from lambda. NA, nucleus ambiguus; NP, nucleus prepositus; NTS, nucleus tractus solitarius; NOI, nucleus olivaris inferior; RVLM, rostral ventrolateral medulla; V, nucleus and tractus trigemini spinalis; py, tractus pyramidalis.
Basal expression of nNOS and iNOS mRNA at the RVLM
Our fifth series of experiments provides crucial supporting evidence for the contention that both nNOS and iNOS in the RVLM are active under the present experimental conditions. Results from RT – PCR analysis (Figure 8) indicated the presence of nNOS mRNA in the ventrolateral medulla. Intriguingly, under the same experimental condition, we also detected discernible expression of iNOS mRNA in this part of the medulla oblongata. We further validated the specificity of our RT – PCR products by demonstrating a significant surge in the level of iNOS mRNA 60 min after administration (30 mg kg−1, i.v.) of E. coli lipopolysaccharide (Chan et al., 2001). The level of nNOS mRNA, on the other hand, remained essentially unchanged.
Figure 8.
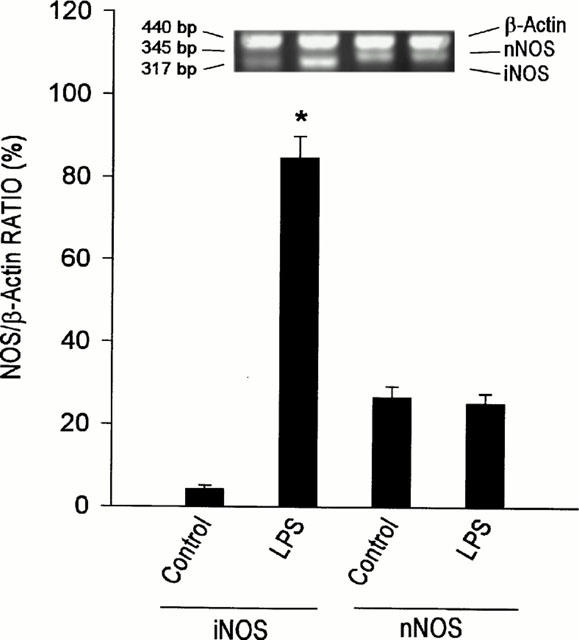
Representative gels for RT–PCR products (upper panel), or amount of neuronal or inducible nitric oxide synthase (nNOS or iNOS) mRNA relative to β-actin mRNA, detected from the ventrolateral medulla under basal experimental conditions (control) or 60 min after i.v. administration of E. coli lipopolysaccharide (30 mg kg−1). Values are mean±s.e.mean of triplicate analysis, n=5–6 animals per experimental group. *P<0.05 vs control group in the Scheffé multiple-range test.
Discussion
By demonstrating significant hypotension and bradycardia on microinjection bilaterally into the RVLM of a NO trapping agent, carboxy-PTIO, the present study provided the crucial experimental evidence that establishes a sympathoexcitatory role for the endogenous NO at the RVLM in the maintenance of SAP and HR. We also found that the time-course and magnitude of reduction in MSAP and HR elicited by coadministration into the RVLM of the nNOS and iNOS blocker, 7-NI and AG, resembled those induced by carboxy-PTIO. As such, it is likely that nNOS and iNOS jointly contribute to the cardiovascular actions of the endogenous NO in the RVLM.
Another important contribution of the present study is to unveil a physiologic role for iNOS at the RVLM in NO-promoted cardiovascular regulation. We observed that selective blockade of nNOS or iNOS activity in the RVLM resulted respectively in a reduction or an enhancement of SAP, HR and the power density of vasomotor components in the SAP spectrum, the experimental index for sympathetic vasomotor outflow (Kuo et al., 1997). We further noted that co-administration of 7-NI and AG into the RVLM elicited changes in MSAP and HR that approximated the algebraic sum of their individual effects. These findings suggest that nNOS may be essential for the sympathoexcitatory, and iNOS may be responsible for the sympathoinhibitory, action of NO at the RVLM. In addition, by eliciting selective inhibition on nNOS activity, 7-NI also unmasked the contingent cardiovascular actions of iNOS in the RVLM. Similarly, a selective blockade of iNOS activity unveiled the coincident circulatory actions of nNOS. As such, regulation of sympathetic vasomotor outflow by the endogenous NO at the RVLM may be determined by a balance between sympathoinhibition and sympathoexcitation induced by the tonically active iNOS and nNOS in this medullary site.
Activated nNOS releases short ‘puffs' of NO, and iNOS produces long-lasting generation of NO (Green et al., 1991; Nathan, 1992). It follows that the relative prevalence of nNOS and iNOS activities, which in turn regulates the amount of NO present in the RVLM, plays a crucial role in cardiovascular regulation. This notion is supported by our results from experiments that manipulated the amount of NO in the RVLM. We observed that, superimposed on the endogenous release, the addition of a small amount of NO provided non-enzymatically by low doses of SNAP elicited a transient increase in MSAP and HR. On the other hand, progressive and long-lasting hypotension and bradycardia were induced by microinjection bilaterally of high doses of this NO donor to the RVLM.
Our results from treatments with L-Arg provide further insights to the relative prevalence of nNOS and iNOS at the RVLM under physiologic conditions. As a NO precursor, L-Arg generates NO in a NOS-dependent manner (Szabo, 1996). Furthermore, nNOS is responsive to an increase in intracellular Ca2+ and subsequent binding of Ca2+ to calmodulin (Bredt & Snyder, 1990; Mayer et al., 1990). In contrast, iNOS is not regulated by intracellular Ca2+ and is under constant activation (Yui et al., 1991). It is therefore reasonable to suggest that iNOS is responsible for the generation of a larger amount of NO by L-Arg in the RVLM, resulting in our observed cardiovascular depression. It also accounts for our observation that both 7-NI and L-Arg induced hypotension and bradycardia. However, that blockade of nNOS by 7-NI did not further augment the cardiovascular suppressive effects of the NO precursor implies that despite its presence, iNOS in the RVLM may not be prevalent under physiologic conditions. This notion is also supported by our observation that, in the presence of AG, the hypotensive action elicited by bilateral microinjection of L-Arg into the RVLM was reversed to an increase in MSAP that resembled qualitatively that promoted by the iNOS inhibitor alone. Our RT – PCR analysis further demonstrated that whereas both iNOS and nNOS mRNA were expressed in the ventrolateral medulla under physiologic conditions, the amount of iNOS mRNA was again less than that of nNOS mRNA. It is only under conditions such as administration of E. coli lipopolysaccharide when we observed a dramatic surge in iNOS mRNA.
The notion that iNOS is functionally active at the RVLM under physiologic conditions, while novel, seemingly contradicts the general contention (Szabo & Thiemermann, 1995; Szabo, 1996) that iNOS is induced only by proinflammatory stimuli. We noted, however, that a physiologic role for iNOS has been reported in the regulation of arterial pressure via an action on renal tubules (Mattson et al., 1998). Several studies (Murphy et al., 1993; Wong et al., 1996; Kitamura et al., 1998) also indicate that NO may be generated in the CNS by iNOS present in microglia or astrocytes. Although expressed in very low level, basal iNOS immunoreactivity is detected in culture microglia and astrocytes (Boje & Arora, 1992; Murphy et al., 1993), and in glial cells from brain tissue (Weldon et al., 1998). Whether the stipulated sympathoinhibition exerted by iNOS may take origin from these glial cells in the RVLM remains to be clarified.
We are aware that the selectivity of our test agents may affect the interpretation of our results. For example, in addition to nitrogen radicals, it is possible that our results with carboxy-PTIO may also arise from its ability to scavenge reactive oxygen radicals (Aoyagi et al., 1999). This possibility is deemed minimal because the time-course and magnitude of cardiovascular depression induced by co-administration of 7-NI and AG into the RVLM greatly resembled those elicited by carboxy-PTIO. AG has been reported to be 26 times more potent in inhibiting iNOS than nNOS activity (Moore & Handy, 1997). In addition, calcium-dependent NOS activity is not significantly altered by AG (Mattson et al., 1998). The doses of AG we used have been demonstrated to effectively inhibit iNOS, but not nNOS, activity evoked by LPS in the RVLM (Chan et al., 2001). That comparable results were obtained from treatments with two other selective iNOS antagonists, SMT (Southan et al., 1995) and L-NIL (Moore et al., 1994; Connor et al., 1995), further validated a functional role for iNOS in the RVLM. Handy & Moore (1998) commented that, on the balance of evidence presently available and until even more selective antagonists are available, 7-NI is a useful experimental tool to study the roles of neuronally derived NO. That AG, SMT or L-NIL and 7-NI produced opposing effects in the present study also pointed to the differentiating capability of these iNOS and nNOS antagonists. Several reports (Zagvazdin et al., 1996; Reiner & Zagvazdin, 1998) suggest that 7-NI may also inhibit the activity of eNOS in vivo. Thus, the possibility that both nNOS and eNOS in the RVLM are involved in the pressor and tachycardiac effects of endogenous NO cannot be excluded. However, since NO derived from nNOS increases SAP in eNOS null mutant mice (Huang et al., 1995; Kurihara et al., 1998), the role of eNOS in central circulatory control remains to be delineated. Likewise, the contribution of, e.g., diamine oxidase, which is also inhibited by AG (Holt & Baker, 1995), to the regulation of sympathetic outflows from the RVLM requires further elucidation.
As the premotor sympathetic neurons, the RVLM is believed to maintain basal levels of SAP (Ross et al., 1984; Hakim et al., 1995) by providing tonic excitation to preganglionic sympathetic neurons of the spinal cord. Our present results revealed that, under physiologic conditions, both nNOS and iNOS in the RVLM are active at the levels of functional expression and molecular synthesis. We further showed that the prevalence of nNOS over iNOS activity and the associated dominance of sympathoexcitation over sympathoinhibition may underlie the maintenance of sympathetic vasomotor outflow and stable SAP by endogenous NO in the RVLM.
Acknowledgments
This study was supported in part by research grants NSC-88-2314-B075B-016 to J.Y.H. Chan and NSC-89-2320-B-110-012 to S.H.H. Chan from the National Science Council, VGHKS88-35 and VGHKS90-05 to J.Y.H. Chan from the Kaohsiung Veterans General Hospital, NHRI-GT-RX89B922L to S.H.H. Chan from the National Health Research Institutes, and Academic Excellence Program (89-B-FA08-1-4) to S.H.H. Chan and J.Y.H. Chan. It was carried out during the tenure of S.H.H. Chan as the National Chair Professor of Neuroscience appointed by the Ministry of Education, Taiwan, Republic of China.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AG
aminoguanidine
- carboxy-PTIO
carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide
- eNOS
endothelial nitric oxide synthase
- HR
heart rate
- iNOS
inducible nitric oxide synthase
- MSAP
mean systemic arterial pressure
- L-Arg
L-arginine
- L-NIL
N6-(l-iminoethyl)-L-lysine
- mRNA
messenger ribonucleic acid
- 7-NI
7-nitroindazole
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- PCR
polymerase chain reaction
- RT
reverse transcription
- RVLM
rostral ventrolateral medulla
- SAP
systemic arterial pressure
- SMT
S-methylisothiourea
- SNAP
S-nitro-N-acetylpenicillamine
References
- AOYAGI K., AKIYAMA K., SHAHRZAD S., TOMIDA C., HIRAYAMA A., NAGASE S., TAKEMURA K., KOYAMA A., OHBA S., NARITA M. Formation of guanidinosuccinic acid, a stable nitric oxide mimic, from argininosuccinic acid and nitric oxide-derived free radicals. Free Radical Res. 1999;31:59–65. doi: 10.1080/10715769900300601. [DOI] [PubMed] [Google Scholar]
- BOJE K.M., ARORA P.K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Isolation of nitric oxide synthase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREDT D.S., HWANG P.M., SNYDER S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- CHAN J.Y.H., WANG S.H., CHAN S.H.H. Differential roles of iNOS and nNOS at rostral ventrolateral medulla during experimental endotoxemia in the rat. Shock. 2001;15:65–72. doi: 10.1097/00024382-200115010-00011. [DOI] [PubMed] [Google Scholar]
- CONNOR J.R., MANNING P.T., SETTLE S.L., MOORE W.M., JEROME G.M., WEBBER R.K., TJOENG F.S., CURRIE M.G. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur. J. Pharmacol. 1995;273:15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- DAMPNEY R.A.L. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog. Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- DUN N.J., DUN S.L., FORSTERMANN U. Nitric oxide synthase immunoreactivity in rat pontine medullary neurons. Neuroscience. 1994;59:429–445. doi: 10.1016/0306-4522(94)90607-6. [DOI] [PubMed] [Google Scholar]
- FORSTERMANN U., KLEINHERT H., GATH I., SCHWARZ P., CLOSS E.I., DUN N.J. Expression and expressional control of nitric oxide synthases in various cell types. Adv. Pharmacol. 1995;34:171–186. doi: 10.1016/s1054-3589(08)61085-6. [DOI] [PubMed] [Google Scholar]
- GREEN S.J., NACY C.A., MELTZER M.S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J. Leuk. Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- GUERTZENSTEIN P.G., SILVER A. Fall in blood pressure produced from discrete regions of the ventral surface of the medulla by glycine and lesions. J. Physiol. 1974;242:489–503. doi: 10.1113/jphysiol.1974.sp010719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKIM M.A., HIROOKA Y., COLEMAN M.J., BENNETT M.R., DAMPNEY R.A.L. Evidence for a critical role of nitric oxide in the tonic excitation of rabbit renal sympathetic preganglionic neurones. J. Physiol. 1995;482:401–407. doi: 10.1113/jphysiol.1995.sp020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANDY R.L.C., MOORE P.K. Handy and Moore reply. Trends Pharmacol. Sci. 1998;19:350. [Google Scholar]
- HARRISON D.G., BATES J.N. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87:1461–1467. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- HIROOKA Y., POLSON J.W., DAMPNEY R.A.L. Pressor and sympathoexcitatory effects of nitric oxide in the rostral ventrolateral medulla. J. Hypertens. 1996;14:1317–1324. doi: 10.1097/00004872-199611000-00010. [DOI] [PubMed] [Google Scholar]
- HOLT A., BAKER G.B. Metabolism of agmatine (clonidine-displacing substance) by diamine oxidase and the possible implications for studies of imidazoline receptors. Prog. Brain Res. 1995;106:187–197. doi: 10.1016/s0079-6123(08)61215-7. [DOI] [PubMed] [Google Scholar]
- HUANG P.L., HUANG Z., MASHIMO H., BLOCH K.D., MOSKOWITZ M.A., BEVAN J.A., FISHMAN M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- IWASE K., IYAMA K., AKAGI K., YANO S., FUKUNAGA K., MIYAMOTO E., MORI M., TAKIGUCHI M. Precise localization of neuronal nitric oxide synthase mRNA in the rat brain revealed by nonradioisotopic in situ hybridization. Mol. Brain Res. 1998;53:1–12. doi: 10.1016/s0169-328x(97)00139-3. [DOI] [PubMed] [Google Scholar]
- KAGIYAMA S., TSUCHIHASHI T., ABE I., FUJISHIMA M. Cardiovascular effects of nitric oxide in the rostral ventrolateral medulla of rats. Brain Res. 1997;757:155–158. doi: 10.1016/s0006-8993(97)00336-3. [DOI] [PubMed] [Google Scholar]
- KITAMURA Y., MATSUOKA Y., NOMURA Y., TANIGUCHI T. Induction of inducible nitric oxide synthase and heme oxygenase-1 in rat glial cells. Life Sci. 1998;62:1717–1721. doi: 10.1016/s0024-3205(98)00134-9. [DOI] [PubMed] [Google Scholar]
- KRUKOFF T.L. Central actions of nitric oxide in regulation of autonomic functions. Brain Res. Rev. 1999;30:52–65. doi: 10.1016/s0165-0173(99)00010-7. [DOI] [PubMed] [Google Scholar]
- KUO T.B.J., CHAN S.H.H. Continuous, on-line, real-time spectral analysis of systemic arterial pressure signals. Am. J. Physiol. 1993;264:H2208–H2213. doi: 10.1152/ajpheart.1993.264.6.H2208. [DOI] [PubMed] [Google Scholar]
- KUO T.B.J., YANG C.C.H., CHAN S.H.H. Selective activation of vasomotor component of SAP spectrum by nucleus reticularis ventrolateralis in rats. Am. J. Physiol. 1997;272:H458–H492. doi: 10.1152/ajpheart.1997.272.1.H485. [DOI] [PubMed] [Google Scholar]
- KURIHARA N., ALFIE M.E., SIGMON D.H., RHALEB N.E., SHESELY E.G., CARRETERO O.A. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32:856–861. doi: 10.1161/01.hyp.32.5.856. [DOI] [PubMed] [Google Scholar]
- MARTINS-PINGE M.C., BARALDI-PASSY I., LOPES O.U. Excitatory effects of nitric oxide within the rostral ventrolateral medulla of freely moving rats. Hypertension. 1997;30:704–707. doi: 10.1161/01.hyp.30.3.704. [DOI] [PubMed] [Google Scholar]
- MATTSON D.L., MAEDA C.Y., BACHMAN T.D., COWLEY A.W., JR Inducible nitric oxide synthase and blood pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- MAYER B., JOHN M., BOHME E. Purification of a calcium/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor role of tetrahydrobiopterin. FEBS Lett. 1990;277:215–219. doi: 10.1016/0014-5793(90)80848-d. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., HANDY R.L.C. Selective inhibitors of neuronal nitric oxide synthase–Is no NOS really good NOS for the nervous system. Trends Pharmacol. Sci. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., WALLACE P., GAFFEN Z., HART S.L., BABBEDGE R.C. Characterization of the novel nitric oxide synthase inhibitor 7-nitroindazole and related indazoles: antinociceptive and cardiovascular effects. Br. J. Pharmacol. 1993;110:219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE W.M., WEBBER R.K., JEROME G.M., TJOENG F.S., MISKO T.P., CURRIE M.G. L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J. Med. Chem. 1994;37:3886–3888. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- MURPHY S., SIMMONS M.L., AGULLO L., GARCIA A., FEINSTEIN D.L., GALEA E., REIS D.J., MINC-GOLOMB D., SCHWARTZ J.P. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 1993;16:323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- NATHAN C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- OSBORNE M.G., CODERRE T.J. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br. J. Pharmacol. 1999;126:1840–1846. doi: 10.1038/sj.bjp.0702508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOCHOCKA-ZULINSKA D., KRUKOFF T.L. Increased gene expression of neuronal nitric oxide synthase in brain of adult spontaneously hypertensive rats. Mol. Brain Res. 1997;48:291–297. doi: 10.1016/s0169-328x(97)00101-0. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br. J. Pharmacol. 1995;116:1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., MONCADA S. Role of endothelium derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINER A., ZAGVAZDIN Y. On the selectivity of 7-nitroindazole as an inhibitor of neuronal nitric oxide synthase. Trends Pharmacol. Sci. 1998;19:348–350. doi: 10.1016/s0165-6147(98)01194-8. [DOI] [PubMed] [Google Scholar]
- ROSS C.A., RUGGIERO D.A., PARK D.H., JOH T.H., SVED A.F., FERNAN DEZ-PARDAL J., SAAVEDRA J.M., REIS D.J. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J. Neurosci. 1984;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMONIAN S.X., HERBISON A.E. Localization of neuronal nitric oxide synthase-immunoreactivity within sub-populations of noradrenergic A1 and A2 neurons in the rat. Brain Res. 1996;732:247–252. doi: 10.1016/0006-8993(96)00687-7. [DOI] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABO C., THIEMERMANN C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO C. Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Res. Bull. 1996;41:131–141. doi: 10.1016/0361-9230(96)00159-1. [DOI] [PubMed] [Google Scholar]
- SZABO C., THIEMERMANN C. Regulation of the expression of the inducible isoform of nitric oxide synthase. Adv. Pharmacol. 1995;34:113–153. doi: 10.1016/s1054-3589(08)61083-2. [DOI] [PubMed] [Google Scholar]
- TSENG C.J., LIU H.Y., LIN H.C., GER L.P., TUNG C.S., YEN M.H. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- WELDON D.T., ROGERS S.D., GHILARDI J.R., FINKE M.P., CLEARY J.P., O'HARE E., ESLER W.P., MAGGIO J.E., MANTYH P. W. Fibrillar β-amyloid induces microgial phagocytosis, expression of inducible nitric oxide synthases, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 1998;18:2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG M.L., RETTORI V., AL-SHEKHLEE A., BONGIORNO P.B., CANTEROS G., MCCANN S.M., GOLD P.W., LICINIO J. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nature Med. 1996;2:581–584. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]
- YANG C.H., SHYR M.H., KUO T.B.J., TAN P.P.C., CHAN S.H.H. Effects of propofol on nociceptive response and power spectra of electroencephalographic and systemic arterial pressure signals in the rat: correlation with plasma concentration. J. Pharmacol. Exp. Ther. 1995;275:1568–1574. [PubMed] [Google Scholar]
- YOSHIDA M., AKAIKE T., WADA Y., SATO K., IKEDA K., UEDA S., MAEDA H. Therapeutic effects of imidazolineoxyl N-oxide against endotoxin shock through its direct nitric oxide-scavenging activity. Biochem. Biophys. Res. Commun. 1994;202:923–930. doi: 10.1006/bbrc.1994.2018. [DOI] [PubMed] [Google Scholar]
- YUI Y., HATTORI R., KOSUGA K., EIZAWA H., HIKI K., KAWAI C. Purification of nitric oxide synthase from rat macrophages. J. Biol. Chem. 1991;266:12544–12547. [PubMed] [Google Scholar]
- ZAGVAZDIN Y., SANCESARIO G., WANG Y.X., SHARE L., FITZGERALD M.E., REINER A. Evidence from its cardiovascular effects that 7-nitroindazole may inhibit endothelial nitric oxide synthase in vivo. Eur. J. Pharmacol. 1996;303:61–69. doi: 10.1016/0014-2999(96)00106-9. [DOI] [PubMed] [Google Scholar]
- ZANZINGER J. Role of nitric oxide in the neural control of cardiovascular function. Cardiovasc. Res. 1999;43:639–649. doi: 10.1016/s0008-6363(99)00085-1. [DOI] [PubMed] [Google Scholar]
- ZANZINGER J., CZACHURSKI J., SELLER H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am. J. Physiol. 1995;268:R958–R962. doi: 10.1152/ajpregu.1995.268.4.R958. [DOI] [PubMed] [Google Scholar]


