Abstract
The ability of four endogenous vasodilators, nitric oxide (NO; 0.01 – 30 μM), atrial (ANP), brain (BNP) and C-type (CNP) natriuretic peptide (0.1 – 300 nM), to reverse endothelin-1 (ET-1; 10 nM) constrictions in human resistance and conductance coronary arteries (CA) in vitro was investigated.
ET-1 (0.1 – 300 nM) constricted resistance CA more potently than conductance CA (P<0.05; EC50 values 2.98 nM (95% CI: 1.49 – 5.95 nM and 8.58 (4.72 – 15.6 nM) respectively)).
The NO-donor diethylamine NONOate fully reversed the ET-1 constriction in conductance CA (EMAX 127±9.16%), however only partial reversal was observed in resistance CA (EMAX 78.8±8.13; P<0.05). The soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (100 μM) reduced the maximum response to diethylamine NONOate to 76.9±14.4% in conductance CA (P<0.05), but had no effect on resistance CA (EMAX 77.2±18.4%).
There was no difference between responses to ANP in conductance and resistance CA (EC50 values 4.25 nM (0.84 – 21.4 nM) and 18.4 nM (2.92 – 116 nM), EMAX 53.1±14.7% and 48.6±11.8% respectively).
BNP was a more potent vasodilator of conductance than resistance CA. In conductance CA the mean EC50 value was 2.4 nM (0.74 – 7.75 nM), EMAX 54.5±14.9%. Concentration-response curves to BNP were incomplete in resistance CA.
Concentration-response curves to CNP were incomplete in both conductance and resistance CA.
The greater potency of ET-1 in resistance vessels may exacerbate the effects of increased circulating levels of the peptide in disease. Only NO could fully reverse ET-1 mediated constrictions in conductance CA, and none of the dilators tested could completely counteract constrictions in resistance CA.
Keywords: Human coronary artery, endothelin-1, atrial natriuretic peptide, brain natriuretic peptide, C-type natriuretic peptide, nitric oxide
Introduction
Endothelin-1 (ET-1) is an important constrictor of human blood vessels, with an unusually long duration of action compared to other endogenous vasoactive compounds (Yanagisawa et al., 1988; Davenport et al., 1989; Franco Cereceda, 1989). It is synthesized and released from the endothelium throughout the human vasculature and to date, no vessel has been reported unresponsive to the peptide. ET-1 is unique amongst constrictor peptides in being released both via a regulated pathway in response to external stimuli, as well as being continuously released from a constitutive pathway (Russell et al., 1998). Infusion of the antagonist TAK-44 in normotensive human volunteers results in systemic vasodilatation, indicating a role for ET in the maintenance of basal vascular tone (Haynes et al., 1996).
In pathophysiological conditions, increased levels of the ET peptide have been demonstrated both within atherosclerotic vessels (Bacon et al., 1996) and in the plasma of patients with cardiovascular disease (Lerman et al., 1991; Miyauchi et al., 1989). In addition, ischaemic myocytes have been reported to secrete more ET than controls (Wang et al., 1995). Large epicardial coronary arteries (CA) are particularly prone to atherosclerosis, and small resistance CA, although not susceptible to plaque formation, are exposed to ischaemic conditions, as they are downstream of the occluded vessels, and therefore increased levels of ET-1. In order to restore perfusion of the myocardium it is important to understand what substances are capable of reversing the constrictor effects of ET-1 both in conductance and resistance human CA.
As with ET-1, the vasodilator nitric oxide (NO) is continuously released from the endothelium and contributes to the regulation of vascular tone (Palmer et al., 1987; Vallance et al., 1989). Focally, NO levels are increased in atherosclerotic plaques owing to the induction of the enzyme nitric oxide synthase (Nos; Lafond Walker et al., 1997), however the overall basal release of NO from the endothelium is decreased in atherosclerotic CA (Chester et al., 1990). Hence the importance of other dilators directly acting on the vascular smooth muscle layer, independent of endothelium-derived NO.
The natriuretic peptides are a group of molecules originally identified as stimulators of sodium and water secretion in the kidney, and have also been shown to have vasodilator function (for review see: Levin et al., 1998). All members contain a conserved 17 amino acid ring structure formed by a disulphide bridge. Atrial natriuretic peptide (ANP) is a 28 amino acid peptide and acts as a circulating hormone (Kangawa & Matsuo, 1984). It is secreted by atrial myocytes in response to stimuli such as stretch, ET and low NO levels. Secretion increases in cardiovascular disease and ventricular myocytes also begin to produce ANP (Saito et al., 1989). Brain natriuretic peptide (BNP) comprises 32 amino acids and is also a circulating hormone. The main site of synthesis is the ventricle, with very little BNP produced by atrial myocytes. Plasma levels of BNP rise in cardiovascular disease and are particularly closely correlated to the severity of cardiomyopathy (Troughton et al., 2000). The 22 amino acid residue C-type natriuretic peptide (CNP) is not secreted by myocytes but is synthesized in the endothelium (Stingo et al., 1992). It is thought to act in a paracrine manner on the vascular smooth muscle layer. Low levels of the peptide are detectable in plasma, although plasma CNP is not elevated in cardiovascular disease (Wei et al., 1993).
Given the potency and long duration of ET-1-mediated constrictions in the human cardiovascular system, we aimed to investigate the extent to which four endogenous vasodilators, NO, ANP, BNP and CNP, could reverse the effects of ET-1 in human endothelium-denuded resistance and conductance CA in vitro. Part of this study has previously been reported to the British Pharmacological Society (Wiley & Davenport, 2000).
Methods
Tissue collection
CA were obtained (with local ethical approval) from the explanted hearts of 29 patients, mean age 46 years (range 12 – 60 years; 12 female, 17 male) undergoing transplant operations. Tissue was also collected from four hearts not required for further transplantation, mean age 37 years (range 17 – 61 years; 3 female, 1 male).
In vitro pharmacology
Resistance vessels
Branch CA were isolated from the apex of the hearts with the aid of a dissecting microscope. Surrounding fat and connective tissue was removed and 1 – 2 mm segments were mounted on 40 μm steel wires in a Mulvany myograph for the measurement of isometric tension. The endothelium was removed using a human hair (verified by the lack of dilator response to bradykinin) and sections were allowed to equilibrate to 37°C in oxygenated Krebs' solution for 1 h before the optimal resting tension was determined using the active wall tension to length relationship. The myograph jaws were pulled apart incrementally and the Laplace equation was used to calculate the internal diameter at which the intramural pressure was equal to 100 mmHg. Each segment was then set to 90% of the internal diameter as optimal force is obtained under these conditions (Mulvany & Halpern, 1977). The maximal force was measured three times with a potassium rich Krebs' solution (100 mM) at 15 min intervals.
Conductance vessels
Right, left circumflex or left anterior descending CA were dissected free from surrounding tissue and cut into 4 mm rings. The rings were denuded of their endothelium using a blunt seeker (verified histologically), mounted in 5 ml organ baths (Linton Instrumentation, Norfolk, U.K.) for the measurement of isometric tension (F30 force transducers; Hugo Sachs, March-Hugstetten, Germany) and bathed in oxygenated Krebs' solution at 37°C. To obtain the optimal resting tension, 100 mM KCl was added at increasing levels of basal tension until no further increase in response was obtained.
Agonist studies
Both conductance and resistance CA were allowed to equilibrate to their own resting tension for at least 1 h before the start of the experiment. Concentration-response curves to ET-1 (0.1 – 300 nM) were constructed and expressed as a percentage of the KCl response obtained prior to the start of the experiment. In the studies on the dilator compounds, a submaximal concentration of ET-1 (10 nM) was added and once the response had reached a plateau concentration-response curves to the natriuretic peptides (0.1 – 300 nM), SNAP (0.1 – 30 μM) and DEA/NO (0.1 – 30 μM), alone and in the presence of 100 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; preincubated with the tissue for 20 min), were constructed. Vessels were left until responses reached a plateau before further addition of agonist, or for a minimum of 5 min if no response was observed. Control rings of artery were contracted with 10 nM ET-1 and the tension measured over the time course of the experiment. The inactive nucleophile diethylamine (DEA; 10 – 30 μM) was used as a negative control. Concentration-response curves to the vasodilators were expressed as percentage ET-1 constrictor response.
Data analysis
Human CA are prone to spontaneous phasic contractions in vitro, but only tonic changes in the basal tension (upon which the spontaneous activity is superimposed) were measured in this study. EC50 values were determined for each curve using the iterative curve-fitting software Fig. P (Biosoft, Cambridgeshire, U.K.). EC50 values were expressed as geometric means with 95% confidence intervals (CI) and were compared using the Mann – Whitney U-test (P<0.05). All other data were expressed as arithmetic means±s.e.mean and were compared using Student's two-tailed t-test (P<0.05).
Materials
ET-1, ANP, BNP and CNP (Peptide Institute Inc., Osaka, Japan) stock solutions (0.1 mM) were prepared in 0.1% acetic acid (ET-1) and distilled water (ANP, BNP, CNP) and stored at −20°C. DEA/NO (Alexis Biochemicals, Nottinghamshire, U.K.) was stored under nitrogen at −70°C and dissolved in 0.01 M NaOH immediately prior to experiments. Stock solutions of ODQ (0.1 M; Tocris Bristol, U.K.) were prepared in dimethylsulphoxide. All other reagents were from Sigma-Aldrich Ltd. (Dorset, U.K.) or BDH Ltd. (Dorset, U.K.). Krebs' solution comprised (mM): NaCl 90, NaHCO3 45, KCl 5, MgSO4.7H2O 0.5, Na2HPO4.2H2O 1, CaCl2 2.25, fumaric acid 5, glutamic acid 5, glucose 10, sodium pyruvate 5 (pH 7.4).
Results
Vasoreactivity of conductance and resistance coronary arteries
Resistance CA had a mean internal diameter of 467±23.5 μm and produced a maximal contractile force of 1.99±0.19 mN mm−1 to 100 mM KCl (62 segments from 26 hearts). Conductance CA produced a maximal contractile force to 100 mM KCl of 14.62±0.86 mN mm−1 (76 segments from 18 hearts).
Constrictions to ET-1
ET-1 contracted all vessels tested and produced a sustained constriction in control segments. ET-1 contracted resistance CA significantly more potently than conductance CA (P<0.05, Mann – Whitney U-test; Figure 1) with EC50 values of 2.98 nM (95% CI: 1.49 – 5.95 nM; n=6) and 8.58 nM (95% CI: 4.72 – 15.6 nM; n=9) respectively. The maximal response (EMAX) to ET-1 was 140±8.22% KCl response in resistance CA and 129±25.9% KCl response in conductance CA.
Figure 1.
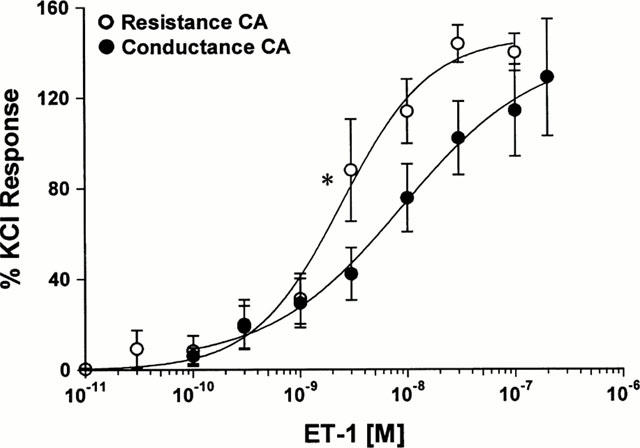
Cumulative concentration-response curves for ET-1 in human conductance and resistance coronary arteries (0.01–100 and 0.1–200 nM; n=6 and 9 respectively). EC50 values for ET-1 are significantly different in resistance compared to conductance CA (*P<0.05, Mann–Whitney U-test).
Reversal of ET-1 induced constrictions by NO
In resistance CA, the NO-donor SNAP (0.1 – 30 μM) only partially reversed (EMAX 60.5±11.7%; n=9) a constriction induced by a sub-maximal concentration of 10 nM ET-1 (Figure 2; Table 1). In contrast, constrictions induced by the same concentration of ET-1 in conductance CA were completely reversed by SNAP, and there was also an additional relaxation to below the original baseline (EMAX 125±11.5%; P<0.05, Student's t-test; n=6). The potency of SNAP was more variable in resistance CA, yielding an EC50 value of 1.13 μM (95% CI: 0.25 – 5 μM) compared to 0.1 μM (95% C: 0.01 – 0.89 μM) in conductance CA (Table 1).
Figure 2.
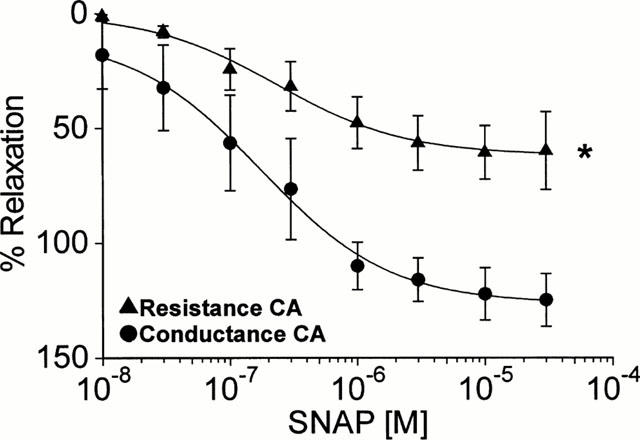
Cumulative concentration-response curves to the NO-donor SNAP in human conductance and resistance coronary arteries (n=6 and 9 respectively; *P<0.05, Student's t-test).
Table 1.
Effect of ANP, BNP and CNP and the NO-donors SNAP and DEA/NO on constrictions induced by ET-1 in human conductance and resistance coronary arteries
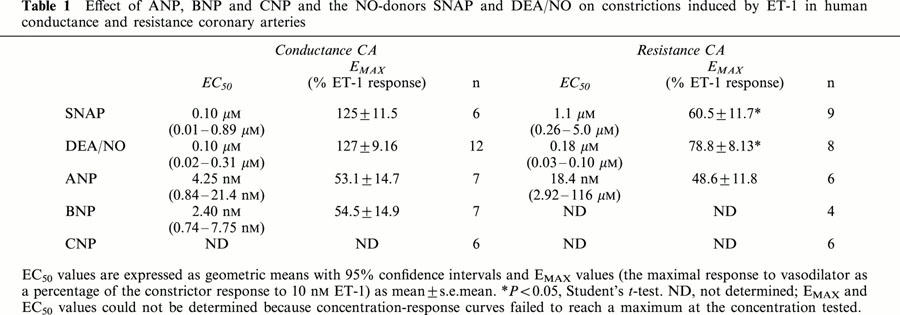
The NO-donor DEA/NO was also used to study the effects of NO in human CA as the breakdown product DEA can be used as an additional negative control. Single, submaximal doses of DEA/NO produced rapid, transient relaxations to ET-1 in conductance CA (Figure 3). Cumulative concentration-response curves to DEA/NO gave similar response to SNAP in conductance CA (P>0.15, Mann – Whitney U-test; Figures 4 and 5A, Table 1; n=12). DEA/NO was more potent in resistance CA, although, as with SNAP the EC50 values were variable (n= 8; Table 1).
Figure 3.
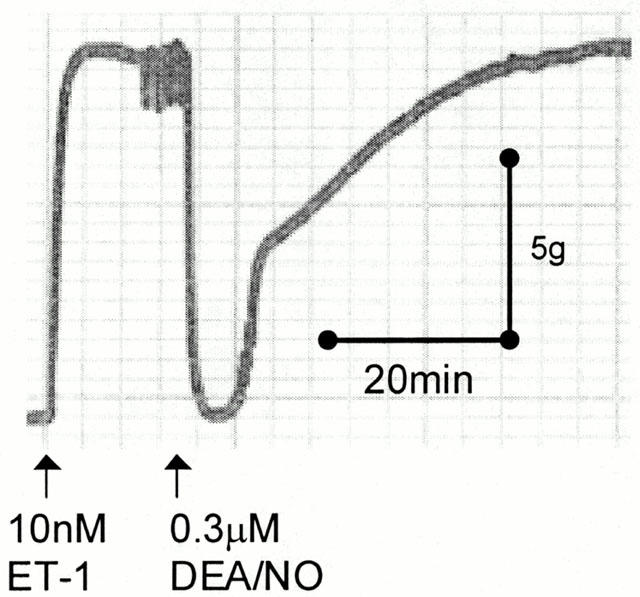
An original trace recording showing a constriction to 10 nM ET-1 and subsequent transient reversal with 0.3 μM DEA/NO in human conductance coronary artery.
Figure 4.
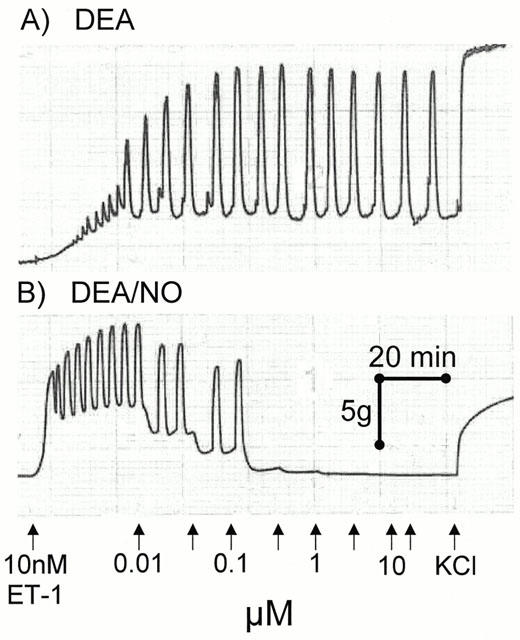
Original trace recording showing concentration-response curves to DEA/NO and the inactive breakdown product DEA in sections of human conductance coronary artery. The phasic contractions seen are spontaneous activity of the tissue.
Figure 5.
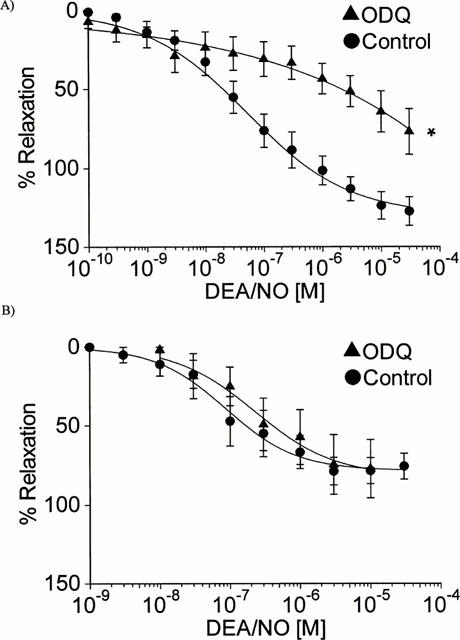
Cumulative concentration-response curves to DEA/NO alone and in the presence of 100 μM ODQ (A) Human conductance coronary artery (n=12 and 7; *P<0.05, Student's t-test, maximum control response compared to maximum response in the presence of ODQ) (B) Human resistance coronary artery (n=8 and 3).
Inhibition of soluble guanylate cyclase (sGC)
Cumulative concentration-response curves to DEA/NO were repeated in segments of CA that had been pre-incubated with the sGC inhibitor ODQ (Figure 5A). In conductance CA the maximum response to DEA/NO was significantly reduced in the presence of ODQ (EMAX 76.9±14.4%; P<0.05, Student's t-test; n=7), however ODQ had no effect on relaxations to the NO-donor in resistance CA (Figure 5B; EMAX 77.2±18.4%; n=3).
Reversal of ET-1 induced constrictions by natriuretic peptides
Cumulative concentration-response curves to three natriuretic peptides, ANP, BNP and CNP were constructed in conductance and resistance CA after constrictions were elicited with 10 nM ET-1 (Figure 6). Concentration-response curves to CNP did not always reach a maximum in the concentration range tested and neither did the response curves to BNP in some resistance CA. Consequently mean EC50 and EMAX values could not be calculated in these cases. ANP dilated both conductance and resistance CA with nanomolar potency (Table 1). EMAX values for ANP were 53.1±14.7% (n=7) and 48.6±11.8% (n=6), in conductance and resistance CA respectively.
Figure 6.
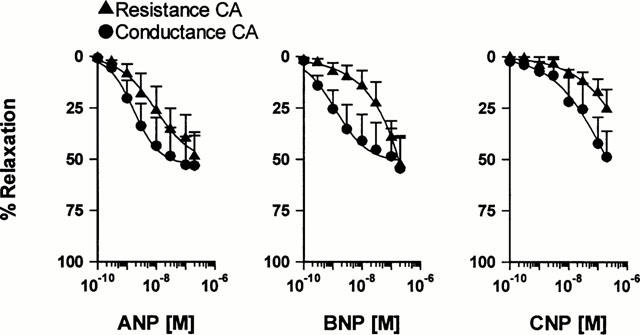
Cumulative concentration-response curves to natriuretic peptides in human conductance and resistance coronary arteries. ANP (n=7 and 6) BNP (n=7 and 4) CNP (n=4/6 and 6).
Responses to BNP only reached a plateau in conductance CA, yielding an EC50 value of 2.4 nM (95% CI: 0.74 – 7.75; n=7) and an EMAX of 54.5±14.9%. Concentration-response curves to CNP were not completed in either conductance or resistance CA.
Discussion
The spontaneous activity of human coronary arteries has been well characterized in normal, diseased and cadaveric tissue in vitro (Ross et al., 1980; Godfraind et al., 1984; Stork & Cocks, 1994). Phasic contractions can develop at any stage of the experiment and are not caused by the addition of constrictor agonists. Activation of voltage operated calcium channels has been shown to mediate the phasic contractions as they can be removed by the addition of calcium channel blockers such as nifedipine (Godfraind et al., 1984; Stork & Cocks, 1994). However, as a component of the response to ET-1 is dependent on the influx of calcium through these channels, the addition of nifedipine in the present study would have blunted the response of the arteries to ET-1 (Pierre & Davenport, 1999). The phasic contractions are superimposed on tonic changes in tension and in the present study, only tonic changes in tension have been measured.
ET-1 produced a prolonged contraction in all vessels tested, with contractions in control segments typically lasting over 3 h. In contrast single, submaximal concentrations of NO caused a rapid but transient relaxation, and only cumulative concentration curves caused a sustained dilatation. ET-1 was significantly more potent in resistance compared to conductance CA, and the EC50 values derived were similar to those previously published in CA of similar internal diameters (Maguire et al., 1997; Pierre & Davenport, 1998). ETA receptors predominate in the coronary vasculature and mediate constriction (Maguire & Davenport, 1995; Pierre & Davenport, 1998). In vivo, infusions of the ETA antagonist BQ123 in humans caused greater vasodilatation in distal epicardial CA and resistance arterioles than proximal CA (Kyriakides et al., 2000), providing further evidence to support the hypothesis that ET receptors are more sensitive in the distal coronary artery tree.
The NO donor SNAP was found to cause significantly greater vasodilatation against ET-1 constrictions in conductance compared to resistance arteries. The vasodilator action of DEA/NO is closely correlated to the spontaneous generation of NO (Morley & Keefer, 1993), thus, DEA/NO was used to more accurately mimic the continuous release of NO from the endothelium in vivo. Similar responses to those obtained with SNAP were seen in both vessel types; there was complete reversal of ET-1-mediated constrictions in conductance CA, with an additional relaxation of the basal tone, but only partial reversal in resistance CA.
In vivo infusions of NOS inhibitors in healthy human volunteers show that NO contributes to resting tone in both conductance and resistance CA, but that inhibition of NOS has a greater effect in the larger CA (Quyyumi et al., 1995).
In order to assess the extent to which NO-mediated vasodilatation was dependent on cyclic GMP in conductance CA, concentration-response curves to DEA/NO were repeated in the presence of the sGC inhibitor ODQ. Surprisingly, the maximum response to DEA/NO was only reduced by approximately half, even in the presence of 100 μM ODQ, a concentration which we have previously shown to completely block cyclic GMP production in human tissue (Wiley & Davenport, 2001). Intriguingly, sGC inhibition had no effect on relaxations to DEA/NO in resistance CA. The lack of effect of ODQ on resistance CA, coupled with the fact that in conductance CA, ODQ reduced the maximum response to NO to a similar level to that seen in resistance vessels, suggests that the difference between the two vessel types could be that small vessels do not express sGC. An alternative explanation is that resistance CA are much less sensitive to cyclic GMP. Further studies would be required to test these hypotheses, however the measurement of cyclic GMP in resistance CA is not possible at present as the levels generated would be below the level of detection.
The large non-cyclic GMP-dependent component of the NO induced vasodilatation could be mediated via several pathways. For example NO could be directly activating potassium channels such as calcium-dependent potassium channels (Bychkov et al., 1998) or ATP-sensitive potassium channels (Miyoshi et al., 1994). Alternatively, NO could be directly modifying the ET receptor to reduce the level of ET-1 binding (Wiley & Davenport, 2001).
The present study also compared the ability of the natriuretic peptides to reverse ET-1 mediated constrictions by directly acting on the vascular smooth muscle layer. ANP partially reversed constrictions to ET-1, with similar potency and maximal response in both vessel groups. Previous studies have presented mixed findings regarding the relative potency of ANP in the coronary vasculature. Single concentrations of ANP partially reversed potassium-induced constrictions in human conductance CA in vitro (Rapoport et al., 1986). In vivo infusions in normotensive individuals and patients coronary artery disease have found ANP to cause vasodilatation in conductance but not resistance CA (Herrmann et al., 1990; Valsson et al., 1996). In contrast Egashira et al. (1991) reported that in addition to dilating epicardial CA, there was an increase in coronary blood flow in healthy volunteers, indicating dilatation of the resistance vasculature. Another study in healthy humans measured sustained dilations in proximal CA and transient increases in coronary flow which lasted less than 5 min after a bolus dose of ANP (Chu et al., 1989). Taken together these results suggest that ANP causes prolonged dilatation in conductance CA, and a transient dilatation in resistance CA where the effects are rapidly reversed in vivo, perhaps by the counter-regulatory action of baroreceptors.
BNP reversed ET-1-induced constrictions in conductance vessels with similar magnitude and potency to ANP. BNP may have a greater effect on resistance arteries in vivo because part of the vasodilatation seen is endothelium-dependent activation of NOS and cyclo-oxygenase (Zellner et al., 1999). Indeed, the response to BNP in resistance CA was nearly abolished in the presence of L-NAME, an effect not observed in the epicardial CA, and as the present study was conducted on endothelium denuded arteries, this may account for the 6 fold greater potency in conductance CA compared to resistance CA.
CNP caused very little vasodilatation in either vessel type at the concentration tested. This is surprising as CNP, unlike ANP and BNP, is only synthesized in the endothelium and is not thought to have any natriuretic properties. Studies have shown that CNP stimulates cyclic GMP production in cultured human vascular smooth muscle cells from internal mammary arteries (Ikeda et al., 1996; Zhang et al., 1996) and 1 μM CNP caused greater than 60% reversal of human internal mammary artery pre-constricted with ET-1 (Protter et al., 1996). However systemic infusion of CNP in humans causes a reduction in diastolic and systolic blood pressure only at a dose approximately 50 times greater than the physiological plasma level (Igaki et al., 1996). Previously, the lack of an up-regulation in plasma CNP in patients with chronic heart failure has been attributed to the paracrine, rather than endocrine nature of CNP (Wei et al., 1993), but the lack of vasodilator potency seen in this study indicates that the main role of CNP in the coronary vasculature is yet to be determined.
In conclusion, the greater potency of ET-1 in resistance vessels may exacerbate the effects of increased circulating levels of the peptide in disease. Whilst NO will reverse ET-1 mediated constrictions in conductance CA, neither of the NO-donors tested could completely reverse constrictions in resistance CA. None of the natriuretic peptides could fully reverse ET-1 in the coronary vasculature and BNP was found to have heterogeneous effects on the vascular smooth muscle of conductance and resistance CA. Other mediators such as endothelium-derived hyperpolarizing factors or prostanoids may also contribute to the maintenance of dilator tone, however these results would suggest that in vivo the constrictor tone generated by ET-1 is opposed by a combination of dilator molecules. An effective therapy to counteract ET-1 in resistance CA in pathophysiological conditions may be an ETA receptor antagonist, particularly as the endothelium is dysfunctional in cardiovascular disease.
Acknowledgments
This work was funded by the British Heart Foundation and the Medical Research Council. We thank Jean Chadderton and the theatre and consultant staff at Papworth Hospital for help with tissue collection. We are also grateful to Dr Janet Maguire for discussion and constructive criticism of the manuscript.
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- CA
coronary artery
- cGMP
guanosine 3′5′-cyclic monophosphate
- CNP
C-type natriuretic peptide
- DEA
diethylamine
- DEA/NO
diethylamine NONOate
- ET-1
endothelin-1
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluble guanylate cyclase
- SNAP
S-nitroso-N-acetyl penicillamine
References
- BACON C.R., CARY N.R., DAVENPORT A.P. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ. Res. 1996;79:794–801. doi: 10.1161/01.res.79.4.794. [DOI] [PubMed] [Google Scholar]
- BYCHKOV R., GOLLASCH M., STEINKE T., RIED C., LUFT F.C., HALLER H. Calcium-activated potassium channels and nitrate-induced vasodilation in human coronary arteries. J. Pharmacol. Exp. Ther. 1998;285:293–298. [PubMed] [Google Scholar]
- CHESTER A., O'NEIL G., MONCADA S., TADJKARIMI S., YACOUB M. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990;336:897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- CHU A., MORRIS K.G., KUEHL W.D., CUSMA J., NAVETTA F., COBB F.R. Effects of atrial natriuretic peptide on the coronary arterial vasculature in humans. Circulation. 1989;80:1627–1635. doi: 10.1161/01.cir.80.6.1627. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., NUNEZ D.J., HALL J.A., KAUMANN A.J., BROWN M.J. Autoradiographical localization of binding sites for porcine [125I]endothelin-1 in humans, pigs, and rats: functional relevance in humans. J. Cardiovasc. Pharmacol. 1989;13 Suppl 5:S166–S170. doi: 10.1097/00005344-198900135-00045. [DOI] [PubMed] [Google Scholar]
- EGASHIRA K., INOU T., IMAIZUMI T., TOMOIKE H., TAKESHITA A. Effects of synthetic human atrial natriuretic peptide on the human coronary circulation in subjects with normal coronary arteries. Jpn. Circ. J. 1991;55:1050–1056. doi: 10.1253/jcj.55.1050. [DOI] [PubMed] [Google Scholar]
- FRANCO CERECEDA A. Endothelin- and neuropeptide Y-induced vasoconstriction of human epicardial coronary arteries in vitro. Br. J. Pharmacol. 1989;97:968–972. doi: 10.1111/j.1476-5381.1989.tb12038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODFRAIND T., FINET M., LIMA J.S., MILLER R.C. Contractile activity of human coronary arteries and human myocardium in vitro and their sensitivity to calcium entry blockade by nifedipine. J. Pharmacol. Exp. Ther. 1984;230:514–518. [PubMed] [Google Scholar]
- HAYNES W.G., FERRO C.J., O'KANE K.P., SOMERVILLE D., LOMAX C.C., WEBB D.J. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- HERRMANN H.C., ROSENTHAL A.D., DAVIS C.A. Cardiovascular effects of intracoronary atrial natriuretic peptide administration in man. Am. Heart. J. 1990;120:308–315. doi: 10.1016/0002-8703(90)90074-8. [DOI] [PubMed] [Google Scholar]
- IGAKI T., ITOH H., SUGA S. C-type peptide in chronic renal failure and its actions in humans. Kidney Int. 1996;49:S144–S147. [PubMed] [Google Scholar]
- IKEDA T., ITOH H., KOMATSU Y., HANYU M., YOSHIMASA T., MATSUDA K., NAKAO K., BAN T. Natriuretic peptide receptors in human artery and vein and rabbit vein graft. Hypertension. 1996;27:833–837. doi: 10.1161/01.hyp.27.3.833. [DOI] [PubMed] [Google Scholar]
- KANGAWA K., MATSUO H. Purification and complete amino acid sequence of α-human atrial natriuretic polypeptide (α-hANP) Biochem. Biophys. Res. Comm. 1984;118:131–139. doi: 10.1016/0006-291x(84)91077-5. [DOI] [PubMed] [Google Scholar]
- KYRIAKIDES Z.S., KREMASTINOS D.T., BOFILIS E., TOUSOULIS D., ANTONIADIS A., WEBB D.J. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart. 2000;84:176–182. doi: 10.1136/heart.84.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFOND WALKER A., CHEN C.L., AUGUSTINE S., WU T.C., HRUBAN R.H., LOWENSTEIN C.J. Inducible nitric oxide synthase expression in coronary arteries of transplanted human hearts with accelerated graft arteriosclerosis. Am. J. Pathol. 1997;151:919–925. [PMC free article] [PubMed] [Google Scholar]
- LERMAN A., EDWARDS B.S., HALLETT J.W., HEUBLEIN D.M., SANDBERG S.M., BURNETT J.C. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N. Engl. J. Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- LEVIN E.R., GARDNER D.G., SAMSON W.K. Natriuretic peptides. New Eng. J. Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br. J. Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Affinity and selectivity of PD156707, a novel nonpeptide endothelin antagonist, for human ETA and ETB receptors. J. Pharmacol. Exp. Ther. 1997;280:1102–1108. [PubMed] [Google Scholar]
- MIYAUCHI T., YANAGISAWA M., TOMIZAWA T., SUGISHITA Y., SUZUKI N. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet. 1989. pp. 53–54. [DOI] [PubMed]
- MIYOSHI H., NAKAYA Y., MORITOKI H. Nonendothelial-derived nitric oxide activates the ATP-sensitive K+ channel of vascular smooth muscle cells. FEBS Lett. 1994;345:47–49. doi: 10.1016/0014-5793(94)00417-x. [DOI] [PubMed] [Google Scholar]
- MORLEY D., KEEFER L.K. Nitric oxide/nucleophile complexes: A unique class of nitric oxide-based vasodilators. J. Cardiovasc. Pharmacol. 1993;22:S3–S9. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small resistance vessels in spontaneously hypertensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- PALMER R., FERRIDGE A., MONCADA S. Nitric oxide release accounts for biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PIERRE L.N., DAVENPORT A.P. Endothelin receptor subtypes and their functional relevance in human small coronary arteries. Br. J. Pharmacol. 1998;124:499–506. doi: 10.1038/sj.bjp.0701865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERRE L.N., DAVENPORT A.P. Blockade and reversal of endothlin-induced constrictions in pial arteries from human brain. Stroke. 1999;30:638–643. doi: 10.1161/01.str.30.3.638. [DOI] [PubMed] [Google Scholar]
- PROTTER A.A., WALLACE A.M., FERRARIS V.A., WEISHAAR R.E. Relaxant effect of human brain natriuretic peptide on human artery and vein tissue. Am. J. Hypertens. 1996;9:432–436. doi: 10.1016/0895-7061(95)00435-1. [DOI] [PubMed] [Google Scholar]
- QUYYUMI A.A., DAKAK N., ANDREWS N.P., GILLIGAN D.M., PANZA J.A., CANNON R.O., III Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- RAPOPORT R.M., GINSBURG R., WALDMAN S.A., MURAD F. Effects of arteriopeptins on relaxation and cGMP levels in human coronary artery in vitro. Eur. J. Pharmacol. 1986;124:193–196. doi: 10.1016/0014-2999(86)90144-5. [DOI] [PubMed] [Google Scholar]
- ROSS G., STINSIN E., SHROEDER J., GINSBURG R. Spontaneous phasic activity of isolated human coronary arteries. Cardiovasc. Res. 1980;14:613–618. doi: 10.1093/cvr/14.10.613. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., SKEPPER J.N., DAVENPORT A.P. Evidence using immunoelectron microscopy for regulated and constitutive pathways in the transport and release of endothelin. J. Cardiovasc. Pharmacol. 1998;31:424–430. doi: 10.1097/00005344-199803000-00014. [DOI] [PubMed] [Google Scholar]
- SAITO Y., NAKAO K., ARAI H. Augmented expression of atrial natriuretic polypeptide gene in ventricle of human failing heart. J. Clin. Invest. 1989;83:298–305. doi: 10.1172/JCI113872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORK A.P., COCKS T.M. Pharmacological reactivity of human epicardial coronary arteries: phasic and tonic responses to vasoconstrictor agents differentiated by nifedipine. Br. J. Pharmacol. 1994;113:1093–1098. doi: 10.1111/j.1476-5381.1994.tb17108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STINGO A.J., CLAVELLI A.L., HEUBLEIN D.M., WEI C.-M., PITTELKOW M.R., BURNETT J.C. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am. J. Physiol. 1992;263:H1318–H1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- TROUGHTON R.W., FRAMPTON C.M., YANDLE T.G., ESPINER E.A., NICHOLLS M.G., RICHARDS A.M. Treatment of heart failure guided by plasma amino terminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- VALLANCE P., COLLIER J., MONCADA S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- VALSSON F., LUNDIN S., KIRNO K., HEDNER T., SAITO Y., RICKSTEN S.E. Myocardial circulatory and metabolic effects of atrial natriuretic peptide after coronary artery bypass grafting. Anesth. Analg. 1996;83:928–934. doi: 10.1097/00000539-199611000-00007. [DOI] [PubMed] [Google Scholar]
- WANG Q.-D., HEMSEN A., LUNDBERG J.M., URIUDA Y., PERNOW J. Local overflow and enhanced tissue content of endothelin following myocardial ischaemia and reperfusion in the pig: modulation by L-arginine. Cardiovasc. Res. 1995;29:44–49. [PubMed] [Google Scholar]
- WEI C.-M., HEUBLEIN D.M., PARELLA M.A., LERMAN A., RODEHEFFER R.J., MCGREGOR C.G.A., EDWARDS W.D., SCHAFF H.V., BURNETT J.C. Natriuretic peptide system in human heart failure. Circulation. 1993;88:1004–1009. doi: 10.1161/01.cir.88.3.1004. [DOI] [PubMed] [Google Scholar]
- WILEY K.E., DAVENPORT A.P. Nitric oxide is an effective physiological antagonist of endothelin-1 and U46619-mediated constrictions in human coronary arteries. Br. J. Pharmacol. 2000;131:7P. [Google Scholar]
- WILEY K.E., DAVENPORT A.P. Nitric oxide-mediated modulation of the endothelin-1 signalling pathway in the human cardiovascular system. Br. J. Pharmacol. 2001;132:213–220. doi: 10.1038/sj.bjp.0703834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- ZELLNER C., PROTTER A.A., KO E., POTHIREDDY M.R., DEMARCO T., HUTCHISON S.J., CHOU T.M., CHATTERJEE K., SUDHIR K. Coronary vasodilator effects of BNP: Mechanisms of action in coronary conductance and resistance arteries. Am. J. Physiol. 1999;276:H1049–H1057. doi: 10.1152/ajpheart.1999.276.3.H1049. [DOI] [PubMed] [Google Scholar]
- ZHANG L.M., CASTRESANA M.R., MCDONALD M.H., JOHNSON J.H., NEWMAN W.H. Response of human artery, vein, and cultured smooth muscle cells to atrial and C-type natriuretic peptides. Crit. Care Med. 1996;24:306–310. doi: 10.1097/00003246-199602000-00021. [DOI] [PubMed] [Google Scholar]


