Abstract
We have examined the effect of an inhibitor of Rho-kinase, (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride monohydrate (Y-27632), on the contractions elicited by noradrenergic nerve stimulation and by phenylephrine in the human and rabbit penile corpus cavernosum. In both tissues, after treatment with scopolamine (10 μM) and NG-nitro-L-arginine methyl ester (L-NAME; 300 μM), electrical field stimulation (EFS) elicited noradrenergic contractions. These contractions were inhibited by Y-27632 in a concentration-dependent manner. The compound caused concentration-dependent relaxation of phenylephrine-contracted tissues, which were treated with scopolamine (10 μM), guanethidine (10 μM) and L-NAME (300 μM). These results suggest that Rho-kinase is involved in noradrenergic contractile pathway in the cavernosal smooth muscle of the penis.
Keywords: Contraction, Rho-kinase, corpus cavernosum, human, rabbit, Y-27632
Introduction
In the human penile cavernous tissue, sympathetic stimulation causes contraction of the smooth muscle. This action is elicited by noradrenaline released from sympathetic nerves acting on the postjunctional α1-adrenoceptors (for reviews see Andersson & Wagner, 1995; Cellek, 2000). The mechanism of noradrenergic contraction in the smooth muscle is based on an increase in intracellular concentrations of calcium ([Ca2+]i). This is achieved by release of Ca2+ from internal stores via the action of inositol 1,4,5 trisphosphate (IP3), which is produced by G-protein-coupled phospholipase C (Berridge, 1993). It has also been suggested that noradrenaline can induce a change in the membrane potential which elicits the opening of voltage-gated channels in the plasma membrane and thereby allows the entry of Ca2+ into the cell (Himpens et al., 1995).
Recently, an additional mechanism has been proposed for smooth muscle contractility. Activation of excitatory receptors coupled to G-proteins can also cause contraction of smooth muscle by increasing Ca2+-sensitivity and without changing [Ca2+]i. This pathway involves RhoA, a small, monomeric G-protein that activates Rho-kinase. Activated Rho-kinase phosphorylates the regulatory subunit of smooth muscle myosin phosphatase (SMPP-1M). Inhibitory phosphorylation of SMPP-1M leads to sensitization of myofilaments to Ca2+ (for review see Somlyo & Somlyo, 2000). A specific inhibitor of Rho-kinase, (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride monohydrate (Y-27632; Uehata et al., 1997; Ishizaki et al., 2000), has been shown to relax vascular and non-vascular smooth muscle in different preparations (Fu et al., 1998; Iizuka et al., 2000; Robertson et al., 2000; Sward et al., 2000). Y-27632 has been demonstrated to lower blood pressure of hypertensive but not of normotensive rats (Uehata et al., 1997) suggesting that it has relaxant activity preferentially on the vascular beds with high basal tone. Since cavernosal smooth muscle is kept contracted by basal noradrenergic activity in the detumescent state, we were interested in investigating the effect of Y-27632 on this tissue.
Methods
Tissue specimens were prepared as described previously (Cellek & Moncada, 1997). Male New Zealand rabbits (3.5 – 5.0 kg; n=3) were sacrificed by an overdose of pentobarbitone and the penis was excised. The isolated strips of corpus cavernosum (four from each animal) were placed horizontally between two ring electrodes in tissue chambers at 37°C. The tissues were perfused at a constant flow of 1 ml min−1 by means of peristaltic pumps (Miniplus 2, Gilson) with a medium of the following composition (mM): NaCl 136.9, KCl 2.7, CaCl2 1.8, MgSO4 0.6, NaHCO3 11.9, KH2PO4 0.5, glucose 11.5, indomethacin 0.01, dexamethasone 0.01 and gassed with 5% CO2 in O2 (pH 7.4 – 7.6). One end of the preparation was tied to a Grass FT 03C force-displacement transducer connected to a Linearcorder WR 3101 (Graphtec) for registration of isometric changes in tension. The preparations were stretched (2 to 5 mN) until they reached approximately the in situ length and allowed to equilibrate for 90 min. The preparations were stimulated electrically (electrical field stimulation; EFS) with 5 s trains of rectangular pulses of 50 V, 0.3 ms pulse duration and frequencies ranging from 1 to 50 Hz, delivered by Grass S88 stimulators every 120 s throughout the experiment. Drugs were applied into the medium reservoir.
Whole corpus cavernosum was obtained from patients (ages 28, 32, 43; n=3) undergoing penectomy for gender reassignment in Sussex Nuffield Hospital (Brighton, U.K.). None of the patients had erectile dysfunction or any systemic disease. All patients gave written informed consent to the study, which was approved by the East Unit Research Ethics Committee of Mid Sussex National Health Service Hospital, U.K. The tissues were transported from the hospital to the laboratories in ice-cold medium (composition as above) in less than 3 h. On arrival, they were transferred into the medium at room temperature and cleaned of adherent tissue and blood. Four to eight strips were cut from the middle part of each corpus cavernosum. Each strip was mounted into the tissue chambers as described above.
Y-27632 was provided by Welfide Inc (Osaka, Japan). All other compounds were from Sigma, U.K. Y-27632 was dissolved in phosphate-buffered saline at 4°C and added to the medium reservoir cumulatively in incremental concentrations once the effect of the compound on EFS- or phenylephrine-induced tone was stabilised. At the end of the experiment the tissues were exposed to medium without Y-27632 in order to assess the reversibility of its effect.
Results
Effect of Y-27632 on EFS-induced contractions
In the presence of scopolamine (10 μM) and NG-nitro-L-arginine methyl ester (L-NAME; 300 μM) to inhibit cholinergic and nitrergic neurotransmission respectively, the tissue was stimulated with EFS. Under these conditions EFS of the human and rabbit corpus cavernosum has been shown to elicit noradrenergic contractions (Cellek & Moncada, 1997). Since a frequency of 5 Hz was found to give optimum contractile responses for both tissues under these conditions (not shown), this frequency was used in all of the experiments. Contractions elicited with EFS were inhibited with Y-27632 (1 nM – 300 μM). The inhibition was partial (97.0±1.5% and 85.6±3.6% inhibition at 300 μM) with an IC50 of 3.3±0.25 μM and 2.8±0.2 μM in human and rabbit tissues respectively (n=3, Figures 1A and 2). The inhibition was reversible; the contractions returned to control values following removal of Y-27632 from the medium (Figure 2).
Figure 1.
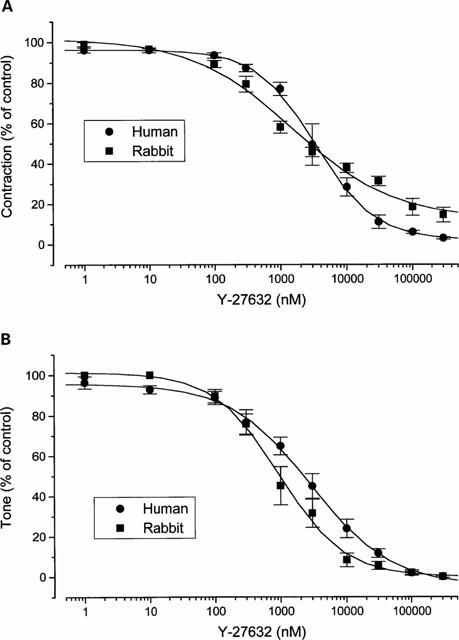
Effect of Y-27632 on EFS-induced noradrenergic contractions (A) and phenylephrine-induced tone (B) in the human (circles) and rabbit (squares) corpus cavernosum (n=3 for both).
Figure 2.
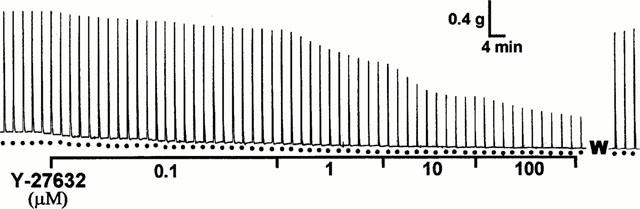
Effect of Y-27632 on EFS-induced contractions of rabbit corpus cavernosum. ‘W' denotes the washing period for 140 min in the absence of Y-27632. Each train of EFS (50 V, 5 Hz, 0.3 ms pulse duration, for 5 s, every 120 s) is shown with a closed circle. The mechanogram is an original recording of the responses of one preparation and is representative of all experiments in this series (n=3).
Effect of Y-27632 on elevated tone with phenylephrine
The tone of the tissue was elevated with 10 and 1 μM phenylephrine (EC80 for human and rabbit tissues respectively) in the presence of scopolamine (10 μM), guanethidine (10 μM) and L-NAME (300 μM). Y-27632 (1 nM – 100 μM) caused relaxation of the tissue in a concentration-dependent manner (EC50: 2.2±0.25 μM and 0.99±0.3 μM for human and rabbit tissues respectively) (n=3, Figure 1B). The tone returned to control level after Y-27632 was removed from the medium (not shown).
Discussion
Noradrenergic neurotransmission plays an important role in the regulation of cavernosal tone. In the detumescent state, penile corpus cavernosum is kept contracted by release of noradrenaline from sympathetic nerves acting on postjunctional α-adrenoceptors (Andersson & Wagner, 1995). During erection the noradrenergic pathway seems to be under control of the nitrergic pathway (Cellek & Moncada, 1997). In diabetic erectile dysfunction, noradrenergic neurotransmission takes over, resulting in enhanced noradrenergic and diminished nitrergic responses (Cellek et al., 1999).
Noradrenaline elicits its contractile effect in the cavernosal smooth muscle via α1-adrenoceptors. Indeed, contractile responses elicited either by EFS or by exogenous noradrenaline in the human corpus cavernosum are completely inhibited by prazosin (Andersson & Wagner, 1995). Binding of noradrenaline to the α1-receptor activates G-protein-coupled phospholipase-C, which initiates IP3-dependent Ca2+ release from internal stores to start the contractile process (Berridge, 1993; Himpens et al., 1995).
Other mechanisms have been proposed for the contractile action of noradrenaline in smooth muscle. In vascular smooth muscle, noradrenaline can induce a change in the membrane potential, which elicits the opening of voltage-gated channels in the plasma membrane and thereby allows the entry of extracellular Ca2+ into the cell (Himpens et al., 1995). The effect of Ca2+-channel blockers to reduce [Ca2+]i and to relax the vascular smooth muscle is consistent with this mechanism. In the urogenital smooth muscle, however, Ca2+-channel blockers do not affect noradrenaline-induced contractions (Vila et al., 1985). Moreover, these contractions have been shown not to involve a change in the membrane potential (Large, 1984) and are not affected by reducing extracellular Ca2+ concentrations (Elemide & Oriowo, 1986). It appears that IP3-dependent Ca2+ release from internal stores is responsible for smooth muscle contraction in the urogenital smooth muscle (Vila et al., 1985).
Ancillary regulatory mechanisms, which could modify the sensitivity of the contractile and regulatory proteins to [Ca2+]i, are suggested to be operating in the smooth muscle (Somlyo & Somlyo, 2000). This ‘sensitizing mechanism' can cause contraction of smooth muscle by increasing Ca2+-sensitivity without necessarily changing [Ca2+]i. One such mechanism is the Rho-kinase pathway, which has been suggested to be the major Ca2+-sensitizing mechanism in the smooth muscle (Somlyo & Somlyo, 2000). Rho-kinase is activated by RhoA, a small, monomeric G-protein believed to be coupled to excitatory receptors (e.g. α1-adrenoceptor). Activated Rho-kinase phosphorylates the regulatory subunit of smooth muscle myosin phosphatase (SMPP-1M). Inhibitory phosphorylation of SMPP-1M leads to sensitization of myofilaments to Ca2+ (Somlyo & Somlyo, 2000).
The role of Rho-kinase pathway in the regulation of vascular and non-vascular smooth muscle tone has been elucidated in vitro and in vivo using a specific inhibitor of Rho-kinase, Y-27632. Oral administration of the compound to spontaneously hypertensive, renal hypertensive and DOCA-salt hypertensive rats caused a persistent fall in blood pressure while the same oral dose did not affect the blood pressure of normotensive rats (Uehata et al., 1997). Inhalation of Y-27632 inhibited acetylcholine- or ovalbumin-induced increase in lung resistance of guinea-pig (Iizuka et al., 2000). Application of Y-27632 into perfused rat lungs caused no change in basal perfusion pressure but was found to inhibit hypoxic pulmonary vasoconstriction (Robertson et al., 2000). Thus, Rho-kinase inhibitor, Y-27632, has been shown to antagonise contractile mechanisms in vascular and non-vascular smooth muscle preparations, particularly in conditions where an elevated tone exists.
In order to elucidate the role of Rho-kinase pathway in the cavernosal smooth muscle contraction, we applied Y-27632 to isolated penile corpus cavernosum strips from human and rabbit contracted either with EFS or phenylephrine. Both type of contractions were inhibited by Y-27632 in a concentration-dependent manner, suggesting that the Rho-kinase pathway is involved in the noradrenergic contractile response of human and rabbit penile cavernosal smooth muscle. These results support the findings of Chitaley et al. (2001) who showed the relaxant effect of Y-27632 on rat cavernous tissue in vitro. To our knowledge, our results are the first to describe the effect of a Rho-kinase inhibitor in human cavernous tissue.
Phasic (transient) contractile responses elicited by EFS were more resistant to inhibition by Y-27632 than the tonic (sustained) contraction by phenylephrine (IC50 for Y-27632 was 3.3±0.25 μM for EFS-induced contractions and 2.2±0.25 μM for phenylphrine-induced contraction in human cavernous tissue; P<0.05). This is in accordance with the previous reports in the guinea-pig ileum and human trachea in which Y-27632 has been found to be effective against the tonic but not the transient phase of contraction (Yoshii et al., 1999; Sward et al., 2000). Our results further support the role of Rho-kinase pathway in regulating the smooth muscle tone in conditions where a tonic contraction or a high basal tone are involved. Indeed, recently intracavernous application of Y-27632 has been reported to elicit penile erection in rats (Chitaley et al., 2001), suggesting that Rho-kinase is critically involved in maintaining basal noradrenergic tone in a detumescent state.
Rho-kinase antagonism represents a potential therapeutic use for the treatment of erectile dysfunction where elevated noradrenergic tone has been reported (Taub et al., 1993; for review see Cellek, 2000). Moreover, Rho-kinase inhibitors would not require a functional nitrergic system and could possibly be more effective in patients with full loss of nitrergic function who are unable to benefit from phosphodiesterase-5 inhibitors (Rendell et al., 1999).
Abbreviations
- EFS
electrical field stimulation
- IP3
inositol 1,4,5 trisphosphate
- [Ca2+]i
intracellular concentration of calcium
- L-NAME
NG-nitro-L-arginine methyl ester
- SMPP-1M
smooth muscle myosin phosphatase
References
- ANDERSSON K.E., WAGNER G. Physiology of penile erection. Physiol. Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- CELLEK S. Nitrergic-noradrenergic interaction in penile erection: A new insight into erectile dysfunction. Drugs Today. 2000;36:135–146. doi: 10.1358/dot.2000.36.2-3.568787. [DOI] [PubMed] [Google Scholar]
- CELLEK S., MONCADA S. Nitrergic control of peripheral sympathetic responses in the human corpus cavernosum: a comparison with other species. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8226–8231. doi: 10.1073/pnas.94.15.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELLEK S., RODRIGO J., LOBOS E., FERNANDEZ P., SERRANO J., MONCADA S. Selective nitrergic neurodegeneration in diabetes mellitus – a nitric oxide-dependent phenomenon. Br. J. Pharmacol. 1999;128:1804–1812. doi: 10.1038/sj.bjp.0702981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHITALEY K., WINGARD C.J., WEBB R.C., BRANAM H., STOPPER V.S., LEWIS R.W., MILLS T.M. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nature Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- ELEMIDE J., ORIOWO M.A. The effect of altering the external Ca2+ concentration and TMB-8 on noradrenaline-induced contractions of the rat anococcygeus muscle. Arch. Int. Pharmacodyn. 1986;280:45–52. [PubMed] [Google Scholar]
- FU X., GONG M.C., JIA T., SOMLYO A.V., SOMLYO A.P. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammaS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- HIMPENS B., MISSIAEN L., CASTEELS R. Ca2+ homeostasis in vascular smooth muscle. J. Vasc. Res. 1995;32:207–219. doi: 10.1159/000159095. [DOI] [PubMed] [Google Scholar]
- IIZUKA K., SHIMIZU Y., TSUKAGOSHI H., YOSHII A., HARADA T., DOBASHI K., MUROZONO T., NAKAZAWA T., MORI M. Evaluation of Y-27632, a rho-kinase inhibitor, as a bronchodilator in guinea pigs. Eur. J. Pharmacol. 2000;406:273–279. doi: 10.1016/s0014-2999(00)00504-5. [DOI] [PubMed] [Google Scholar]
- ISHIZAKI T., UEHATA M., TAMECHIKA I., KEEL J., NONOMURA K., MAEKAWA M., NARUMIYA S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- LARGE W.A. The effect of chloride removal on the responses of the isolated rat anococcygeus muscle to α1-adrenoceptor stimulation. J. Physiol. (Lond) 1984;352:17–29. doi: 10.1113/jphysiol.1984.sp015275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENDELL M.S., RAJFER J., WICKER P.A., SMITH D. Sildenafil for the treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–426. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- ROBERTSON T.P., DIPP M., WARD J.P., AARONSON P.I., EVANS A.M. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br. J. Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. (Lond) 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARD K., DREJA K., SUSNJAR M., HELLSTRAND P., HARTSHORNE D.J., WALSH M.P. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J. Physiol. (Lond) 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUB H.C., LERNER S.E., MELMAN A., CHRIST G.J. Relationship between contraction and relaxation in human and rabbit corpus cavernosum. Urology. 1993;42:698–704. doi: 10.1016/0090-4295(93)90538-l. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- VILA E., THOOLEN M.J., BECKERINGH J.J., TIMMERMANS P.B., VAN ZWIETEN P.A. Lack of effect of D600 on α1-adrenoceptor-mediated contractions of rat isolated anococcygeus muscle. Eur. J. Pharmacol. 1985;106:97–105. doi: 10.1016/0014-2999(84)90682-4. [DOI] [PubMed] [Google Scholar]
- YOSHII A., IIZUKA K., DOBASHI K., HORIE T., HARADA T., NAKAZAWA T., MORI M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization. Am. J. Respir. Cell Mol. Biol. 1999;20:1190–1200. doi: 10.1165/ajrcmb.20.6.3441. [DOI] [PubMed] [Google Scholar]


