Abstract
The transcription factor family CCAAT/enhancer binding proteins (C/EBP) is involved in inflammation via the regulation of the gene expression of various pro-inflammatory cytokines and proteins. PAF and endotoxin (lipopolysaccharide, LPS) are known agents causing intestinal inflammation and injury. In this study, we examined the binding activity of C/EBP isoforms in rat small intestine in response to PAF (1.5 μg kg−1, i.v.) or LPS (5 mg kg−1, i.v.).
We found that C/EBP is constitutively active in normal small intestine, mainly as C/EBP-α and β (C/EBP-β>α). Both C/EBP-α and β are localized in the intestinal epithelial cells: C/EBP-α mainly in the crypts, and C/EBP-β in both villi and crypts, as well as in some lamina propria cells. Only minute amounts of C/EBP-δ were found.
PAF rapidly upregulates the binding activity of C/EBP-α and β within 30 min. The increase in C/EBP-α is prominent in the crypt cells, whereas the change of C/EBP-β is more widespread. LPS also increases the binding activity of C/EBP-α and β, and the response is slower than PAF. PAF synergizes with LPS to markedly activate all three subunits. The increase in C/EBP-α is transient, whereas the other two have a sustained elevation until 120 min.
After challenge with PAF (but not LPS), small amounts of nuclear factor -κB (NF-κB) p50 and p65 subunits are found in the C/EBP-DNA binding complex, indicating cross-dimerization of the two transcription families. Pretreatment of rats with pyrrolidine dithiocarbamate (PDTC) suppresses LPS-, but not PAF-, induced NF-κB and C/EBP binding activity, and significantly increases the C/EBP-δ subunit in LPS- or PAF-induced C/EBP complex.
These results suggest that PAF and LPS activate intestinal C/EBP in vivo, probably via different pathways.
Keywords: Transcription factors, intestines, rat, endotoxin, lipopolysaccharide, platelet activating factor, CCAAT/enhancer binding proteins, NF-kappa B
Introduction
PAF (platelet-activating factor) is a potent inflammatory mediator synthesized endogenously by many cells (Snyder, 1990). PAF may play a pivotal role in the pathogenesis of gastrointestinal inflammatory diseases including gastric ulcer (Rosam et al., 1986), necrotizing enterocolitis (Caplan et al., 1990), and inflammatory bowel disease (Nassif et al., 1996). Administration of endotoxin, or lipopolysaccharide (LPS), a gram-negative bacterial cell wall component, causes shock and intestinal injury; the latter effect probably mediated by endogenous PAF (Hsueh et al., 1987). Systemic administration of either PAF or LPS induces intestinal injury in rats, and the effect of these two agents is synergistic (Gonzalez-Crussi & Hsueh, 1983). At high doses (>2.5 μg kg−1, i.v.), PAF causes severe bowel necrosis and systemic inflammatory response. Similar adverse effects can be achieved at low doses of PAF (<1.5 μg kg−1), if the animal is first ‘primed' with a low dose of LPS (Gonzalez-Crussi & Hsueh, 1983).
The in vivo effect of PAF is long lasting, despite its extremely brief half-life due to its immediate degradation to lyso-PAF by serum and tissue acetylhydrolase (Wang et al., 1997). This prolonged effect may be attributed to, at least in part, by the role of PAF as a regulator of various pro-inflammatory genes. The promoter/enhancer region of many pro-inflammatory cytokines, enzymes and adhesion molecules such as IL-1, IL-6, tumour necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), ICAM-1, containing binding sites for transcription factors NF-κB and C/EBP (Baeuerle, 1994; Poli, 1998; Umek et al., 1991). We have previously shown that PAF up-regulates the gene expression (Tan et al., 1994) and activity (de plaen et al., 1998) of NF-κB in the intestine. Although it has been reported that C/EBP-α, C/EBP-β and C/EBP-δ mRNA are constitutively expressed in the intestine (Alam et al., 1992), the effect of PAF on the C/EBP activation in this organ has not been investigated.
C/EBPs are a family of leucine zipper protein transcription factors involved in the inflammatory response. These proteins consist of three structural components: a C-terminal leucine-zipper dimerization domain, a basic DNA-binding region, and a N-terminal activation domain. C/EBP-β and C/EBP-δ are involved in the regulation of the expression of acute phase plasma proteins during the acute phase response and cellular response to inflammation (Poli, 1998). The gene expression of these two isoforms is induced by stimuli such as LPS, IL-1, and IL-6 (Alam et al., 1992; Hu et al., 1998; Ramji et al., 1993). C/EBP-α, on the other hand, had been traditionally considered mainly as a regulator of cell growth and proliferation (Umek et al., 1991; Yeh et al., 1995), but the recent demonstration that it is indispensable for the neonatal acute inflammatory response, suggests that it also plays a role in inflammation (Burgess-Beusse & Darlington, 1998). Recent in vitro studies indicated that NF-κB and C/EBP synergistically up-regulate the gene expression of inflammatory cytokines IL-6 and IL-8 (Matsusaka et al., 1993), acute phase protein reactant serum amyloid A2 (Xia et al., 1997), pro-inflammatory enzyme iNOS (Eberhardt et al., 1998), adhesion molecule ICAM-1 (Catron et al., 1998) and C-reactive protein (Cha-Molstad et al., 2000).
Both LPS and PAF (Huang et al., 1994) stimulate the intestinal production of TNF-α, a pro-inflammatory cytokine implicated in bowel injury (Beutler et al., 1986). However, despite the fact that PAF is a major endogenous mediator for LPS-induced intestinal injury (Hsueh et al., 1987), PAF and LPS may differ in their effects on the gene regulation of pro-inflammatory cytokines and enzymes in the intestine. For instance, LPS potently induces iNOS (Boughton-Smith et al., 1993), whereas the effect of PAF on intestinal iNOS is minimal (Qu et al., 1999). This may be accounted for, at least in part, by the dissimilar effect of PAF and LPS on NF-κB activation. Although both PAF and LPS (de plaen et al., 1998; 2000) activate NF-κB in the small intestine, the subunit composition of this transcription factor differs in response to PAF and LPS. LPS induces the activation of the classical NF-κB, i.e., p50–p65 heterodimers (Essani et al., 1996), which has been shown to be necessary for the gene expression of iNOS. In contrast, in response to PAF, NF-κB in the intestine consists predominantly of p50 subunits (de plaen et al., 1998). Since previous reports showed functional and physical association between NF-κB p50 or p65 with C/EBP family members (Stein et al., 1993), it is possible that NF-κB may dimerize with C/EBP subunits to activate the expression of pro-inflammatory proteins. The purpose of this study is to examine: (i) the activity of C/EBP-α, C/EBP-β, and C/EBP-δ isoforms in normal small intestine and after stimulation with PAF, LPS, or LPS plus PAF; (ii) the cellular localization of C/EBPs in the intestine and their change following challenge with PAF or LPS; and (iii) the cross-coupling interaction between the two transcription factor families C/EBP and NF-κB in the C/EBP-DNA binding complex after PAF or LPS injection.
Methods
Materials
PAF (1-0-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine) and Poly(dT-dC) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.), PAF was prepared fresh in saline containing 5 mg/ml bovine serum albumin. LPS from Salmonella typhosa was purchased from Difco (Detroit, MI, U.S.A.), [γ-32P]-ATP (3000 Ci mmol−1, 10 mCi ml−1) and ECL kit were purchased from Amersham, Arlington Heights (IL, U.S.A.), C/EBP gel shift oligonucleotide (sc-2525) and mutant oligonucleotide (sc-2526), rabbit polyclonal antibodies against C/EBP-α (#sc-61X), β (#sc-150X), δ (#sc-151X); against NF-κB p50 (#sc-114X), p65 (#sc-109X) and control peptides; and goat polyclonal antibodies against NF-κB p50 (#sc-1191), p65 (#sc-372-G) were obtained from Santa Cruz Biotechnology, Santa Cruz (CA, U.S.A.).
Animal experiment
Young male Sprague-Dawley rats (60–100 g) were fasted overnight, anaesthetized with Nembutal (65 mg kg−1, i.p.) and placed under warm light. After endotracheal incubation, the carotid artery and jugular vein were catheterized for continuous blood pressure recording, drug injection and blood sampling. Blood samples were collected at 0, 30, 60, and 120 min for white blood cell count (WBC) and assessment of haematocrit (Hct). The rats were divided into six groups: (a) PAF (1.5 μg kg−1, i.v.); (b) LPS (5 mg kg−1, i.v.); (c) LPS (0.5 mg kg−1) 30 min before PAF (1.5 μg kg−1, i.v.); (d) PDTC (100 mg kg−1, i.p.) 1 h before PAF (1.5 μg kg−1, i.v.); (e) PDTC (100 mg kg−1, i.p.) 1 h before LPS (5 mg kg−1, i.v.); (f) Sham-operated (saline only). Low doses of PAF and LPS were selected to avoid massive intestinal necrosis, which may hinder the extraction of intact nuclei. Rats were euthanized at different time points after PAF or LPS injection. The small intestine was removed, rinsed with ice-cold saline to remove the intestine content, minced, and immediately frozen in liquid nitrogen. Sections were also taken for immunohistochemical studies. The protocol was approved by the Institutional Animal Care and Use Committee.
Preparation of nuclear extracts
Approximately 0.5 g of frozen tissue was ground into powder with a mortar in liquid nitrogen. The powder was briefly homogenized in 5 ml Buffer A in mM (HEPES 10, NaCl 150, EDTA 1, 0.6% Nonidet P-40, PMSF 0.5) and centrifuged at 2000 r.p.m. for 1 min at 4°C to remove any large debris. The supernatant was placed on ice for 5 min and nuclei were obtained by centrifugation at 5000 r.p.m. for 5 min at 4°C. After staining with Mayer's haematoxylin (Sigma) and checking the integrity of nuclei with a microscope, the nuclei were lysed in 0.5 ml Buffer B (in mM: HEPES 20, NaCl 420, MgCl2 1.2, DTT 0.5, 25% glycerol, EDTA 0.2, benzamidine 2.0, PMSF 0.5, and 5 μg/ml each of leupeptin, aprotinin, trypsin-chymotrypsin inhibitor, pepstatin) and centrifuged at 15,000×g for 1 min at 4°C. The nuclear extract (supernatant) was aliquoted and stored at −80°C. Protein concentrations were determined by Bradford's method using a protein assay kit (Biorad Labs, Hercules, CA, U.S.A.).
Electrophoretic mobility shift assay (EMSA)
The C/EBP consensus double strand oligonucleotide DNA (5′-TGCAGATTGCGCAATCTGCA-3′ annealed with 3′-ACGTCTAACGCGTTAGACGT-5′) was labelled by r-32P-ATP (3000 Ci mmol−1, 10 mCi ml−1, Amersham, Arlington Heights, IL, U.S.A.). (Underlined sequence corresponds to the binding motif of the CCAAT enhancer binding proteins). All binding reactions were performed at 25°C for 30 min in a 20 μl mixture containing 10 μl of nuclear extract (1 μg μl−1 in Buffer B), in mM 4% glycerol, MgCl2 1, EDTA 0.5, DTT 0.5, NaCl 50, Tris-HCl 10, pH 7.5, 2 μg of poly(dI-dC), and 0.5 ng of probe. Some binding reactions included 20 fold and 100 fold excess quantities of unlabelled C/EBP consensus oligonucleotide DNA or mutant oligonucleotide DNA (5′-TGCAGAGACTAGTCTCTGCA-3′ annealed with 3′-ACGTCTCTGATCAGAGACGT-5′). The sample was mixed with loading buffer and electrophoresed through 7.5% polyacrylamide gels in 0.5×TBE buffer, using a gel shift assay system kits (Promega, Madison, WI, U.S.A.). For supershifts, after binding reaction described above, 1 μg of antibody against C/EBP-α, C/EBP-β, C/EBP-δ, NF-κB p50 or NF-κB p65 was added, and the sample was incubated at 4°C overnight. To confirm the specificity of C/EBP-DNA binding activity, a competitive experiment was done by adding unlabelled or mutant oligonucleotide to the reaction mixture.
Immunoprecipitation and Western blot analysis
After precleaning with protein G plus agarose, 1.0 ml nuclear extract (1.0 mg protein) was incubated with rabbit polyclonal antibody against C/EBP-α (5 μg) and against C/EBP-β (5 μg) at 4°C with gentle shaking for 3 h, followed by incubation with 50 μl protein G plus agarose beads overnight at 4°C. The immunoprecipitate was collected and the bound immune complex was eluted by the addition of 50 μl Laemmli buffer and boiling for 5 min. After centrifugation at 10,000×g for 5 min, 50 μl supernatant was loaded on a 10% Tris-HCl Ready gel (Bio-Rad, Hercules, CA, U.S.A.) for electrophoresis resolution. The resolved protein was transferred to a nitrocellulose membrane, and the blot was detected subsequently with goat polyclonal anti- NF-κB p50 or NF-κB p65 antibody, horseradish peroxidase-labelled mouse anti-goat IgG antibody (Pierce, Rockford, IL, U.S.A.), and visualized with an ECL kit.
Immunohistochemistry
Frozen sections of the small intestine were post-fixed in PLP solution (0.02 M NaIO4/PBS (pH 7.4)/2% (w v−1) paraformaldehyde) for 10 min at 4°C and then incubated with 0.3% (v v−1) H2O2 in PBS for 30 min to quench the endogenous peroxidase activity. After blocking with 1.5% (v v−1) normal serum in PBS, the slides were incubated with specific anti-C/EBP antibody in 1% BSA in PBS for 1 h, and immunostained by the avidin-biotin peroxidase complex method using a Vectastain Kit. The peroxidase label was visualized by exposing the sections to diaminobenzidine. The positive signals were confirmed by comparing with negative controls (antibody pre-incubated with a 10 fold excess of control peptide before reacting with the tissue sections).
Statistical analysis
All values in the figures and text are expressed as mean±s.e.mean. Data sets were examined by one-way analysis of variance, and individual group means were compared with the Student's unpaired t-test. A P value of <0.05 was considered significant.
Results
C/EBP is constitutively active in normal small intestinal epithelial cells and lamina propria cells, which includes mainly C/EBP-α and C/EBP-β subunits
C/EBP activity is constitutively expressed in the nuclei of normal small intestinal cells, as shown by EMSA (Figure 1). A competitive experiment with excess 20 fold or 100 fold unlabelled or mutant oligonucleotide probes confirms the specificity of the C/EBP-DNA binding activity. One hundred fold excess of unlabelled probe, but not mutant probe, completely abolishes the C/EBP-DNA binding band. Supershift experiments using specific antibody against C/EBP-α, β, and δ show that the DNA-binding activity of C/EBP is composed of all three subunits, with C/EBP-β being the predominant component, followed by C/EBP-α. C/EBP-δ is present only in a minute amount (Figure 1). Immunohistochemical studies reveal that both C/EBP-α and β are localized in the intestinal epithelial cells: C/EBP-α especially intense in the crypts, and C/EBP-β in both villi and crypts, as well as in some lamina propria cells (Figure 2).
Figure 1.
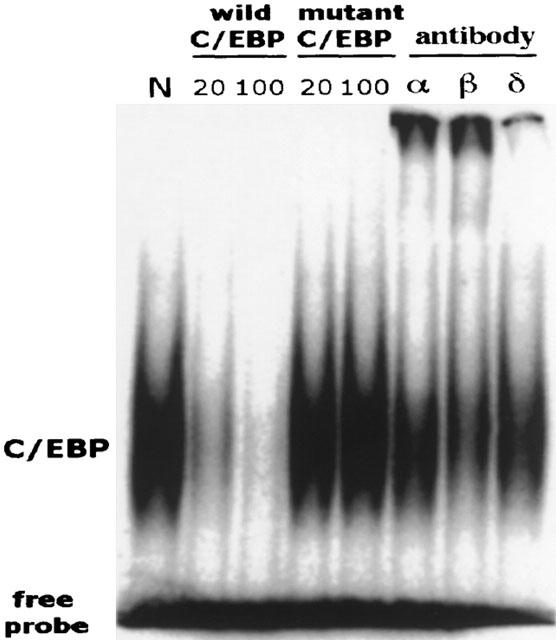
EMSA and supershift assay showing constitutive DNA-binding activity of C/EBP α, C/EBP β, and C/EBP δ in normal rat intestine and its blocking by unlabelled C/EBP probe. Nuclear extract was prepared from normal small intestine, and EMSA was performed using a 32P-labelled C/EBP probe. Supershift assays were performed using normal rabbit serum (N), anti-C/EBP-α, anti-C/EBP-β, or anti-C/EBP-δ Ab. Competitive experiments were done with 20 fold or 100 fold excess of unlabelled C/EBP or mutant C/EBP oligonucleotide probe that was added to the sample during the binding reaction. Since the normal, constitutive C/EBP activity is very weak, the gel was over-exposed to enhance the density of the bands to facilitate visualization.
Figure 2.
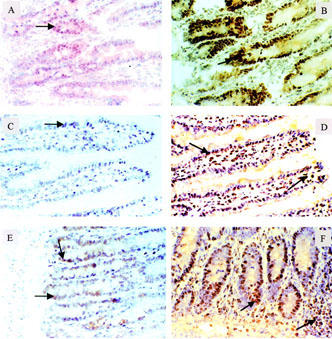
Immunohistochemical localization of C/EBP's in rat small intestine. Frozen sections of the ileum were prepared and reacted with anti-C/EBP-α or anti-C/EBP-β antibody (1 : 2000 dilution), followed by staining with a Vectastain ABC kit. (A): C/EBP-α, untreated. (B): C/EBP-α, PAF (1.5 μg kg−1), 60 min. (C and E): C/EBP β, untreated. (D and F): C/EBP-β, PAF 60 min. Note arrows showing the markedly increased C/EBP-α and β in PAF-treated groups (B, D and F). The increase in C/EBP-α is especially marked in crypt cells (B), whereas the increase in C/EBP-β appears to be widespread, in both villus and crypt epithelial cells (D and F).
PAF or LPS increases the activity of C/EBP, mostly α and β subunits, in the intestinal epithelium
Synergy of PAF and LPS
PAF (1.5 μg kg−1) rapidly activates C/EBP as early as 30 min, peaking at 60 min (Figure 3). The increase of C/EBP binding activity in response to LPS injection is slower than that of PAF. The increase becomes apparent at 60 min and reaches a peak at 120 min (Figure 3). LPS at a low dose (0.5 mg kg−1) synergizes with PAF to induce C/EBP activity quickly, peaking at 30–60 min (Figure 3).
Figure 3.
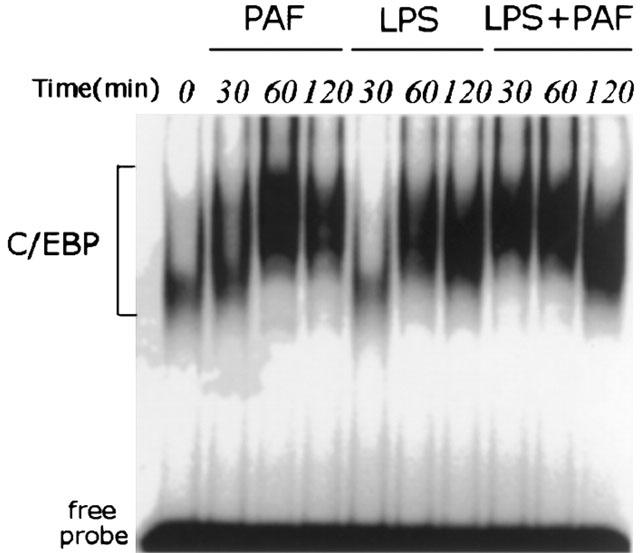
EMSA showing the time course of C/EBP activation in rat small intestine after challenge with PAF, LPS, or LPS plus PAF. Animals were killed at different time points after receiving PAF (1.5 μg kg−1), LPS (5 mg kg−1), or PAF (1.5 μg kg−1) following pretreatment with low dose LPS (0.5 mg kg−1). Nuclear extract was prepared from small intestine, and EMSA was performed using a 32P-labelled C/EBP probe.
Supershift experiments show that the kinetics of PAF-induced C/EBP activation for each subunit is different. C/EBP-α and C/EBP-β, which are the predominant subunits activated by PAF, peak at 60 min. In contrast, no significant change of C/EBP-δ is noted (Figure 4).
Figure 4.
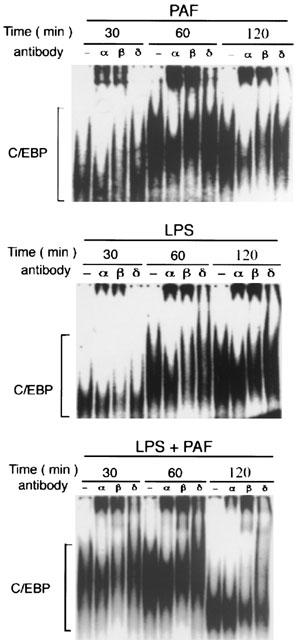
EMSA and supershift assays showing the activation of various subunits of C/EBP in rat small intestine after challenge with PAF, LPS, or LPS plus PAF. Animals were killed at different time points after receiving PAF (1.5 μg kg−1), LPS (5 mg kg−1), or PAF (1.5 μg kg−1) following pretreatment with LPS (0.5 mg kg−1). Nuclear extracts were prepared from the intestine, and supershift assays were performed by adding normal serum (‘–' sign) or specific antibody against different subunits of C/EBP's.
Immunohistochemical studies confirm the results of EMSA and supershift experiments. There is a marked increase of C/EBP-α and C/EBP-β within 60 min. The increase in C/EBP-α is especially prominent in crypt cells, whereas the increase in C/EBP-β appears to be widespread, in villus epithelial cells, crypt epithelial cells, and lymphocytes (Figure 2).
LPS also induces intestinal C/EBP activity, which slowly increases up to 120 min. Activated C/EBP consists mainly of α and β subunits. Supershift experiments show that the kinetics of activation of the subunits are different, with C/EBP-α peaking at 60 min and C/EBP-β peaking at 120 min after LPS challenge (Figure 4). Immunohistochemical studies show a pattern of cellular localization of C/EBP-α and C/EBP-β similar to that of PAF-stimulated intestine (not shown), except that the rate of enhancement in response to LPS is slower than that of PAF.
The effect of combined LPS and PAF on C/EBP induction is synergistic and rapid. Unlike PAF alone, combined LPS/PAF induces all three subunits (Figure 4), as indicated by the slowing down of the migration of the C/EBP-oligonucleotide-DNA complex by antibodies against C/EBP-α, β and δ. Both C/EBP α and β are markedly activated at 30 and 60 min. However, C/EBP-α declines sharply at 120 min, leaving C/EBP-β as the predominant binding species at this time point, followed by C/EBP-δ.
NF-κB p50 and p65 are both components of C/EBP-DNA binding complex in response to the stimulation by PAF (but not LPS)
Figure 5 shows the presence of small amounts of NF-κB p50 and p65 in the C/EBP-DNA binding complex. In the PAF-treated group, NF-κB p50 and p65 subunits become detectable at 60 min, and remain elevated throughout the 2 h period. In contrast, no apparent NF-κB p50 or p65 subunit is detected in the LPS treated group. In rats receiving LPS plus PAF, the appearance of NF-κB p50 or p65 is more rapid than that of rats receiving PAF alone. NF-κB p50 or p65 appears at 30 min and 60 min, but declines rapidly and becomes undetectable at 2 h.
Figure 5.
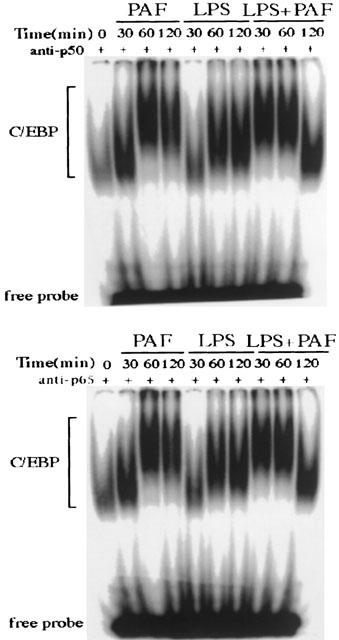
EMSA and supershift assays showing that NF-κB p50 and p65 are components of intestinal C/EBP-DNA binding complex after PAF adminstration. Animals were killed at different time points after challenged with PAF, LPS, or LPS plus PAF. Nuclear extracts were prepared from the small intestine, and supershift assays were performed using anti-NF-κB p50, or anti-NF-κB p65 Ab.
In order to confirm that NF-κB p50 and p65 are components of C/EBP-DNA binding complex after PAF-stimulation, we performed Western blotting of NF-κB p50 or p65 using goat anti-NF-κB p50 or p65 antibody, after immunoprecipitation with the specific antibodies (anti-C/EBP-α and anti-C/EBP-β). As shown in Figure 6, there were faint bands of NF-κB p50 or p65 in PAF- or LPS plus PAF- challenged rats, indicating small amounts of these subunits in the C/EBP-DNA binding complex. These bands were not observed in rats receiving LPS alone.
Figure 6.
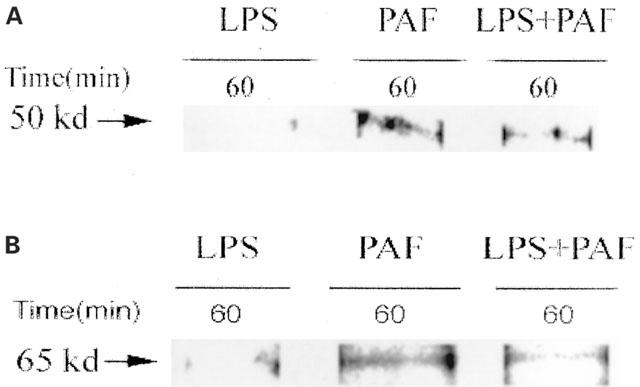
Immuno-blotting of NF-κB p50 or p65 in PAF or LPS-induced nuclear C/EBP complex. Animals were killed at 60 min after challenge with PAF, LPS, or LPS plus PAF. Nuclear extracts were prepared from the small intestine. After immunoprecipitation with rabbit polyclonal antibody against C/EBP-α and against C/EBP-β, Western blotting was done with goat anti- NF-κB p50 polyclonal antibody (A), or with goat anti- NF-κB p65 polyclonal antibody (B).
PDTC suppresses LPS- (but not PAF-) induced NF-κB and C/EBP binding activity, and significantly increases the C/EBP-δ subunit in LPS- or PAF-induced C/EBP complex
Previous studies reported that PDTC specifically inhibits NF-κB activation, but not AP-1, CREB, and Sp-1 in vitro (Schreck et al., 1992) or in vivo (Liu et al., 1999). The data presented here demonstrate that PDTC inhibits intestinal NF-κB and C/EBP binding activity in LPS-challenged rats, but not in rats receiving PAF (Figure 7A,B). Supershift experiments show that the subunit composition of activated C/EBP activation in PDTC pretreated rats changes, compared to rats receiving PAF or LPS alone: As shown above, C/EBP-α and C/EBP-β are the predominant subunits activated by PAF or LPS (Figure 4). In contrast, PDTC pretreatment induces C/EBP-δ activation, which becomes the predominant subunit (Figure 7C).
Figure 7.
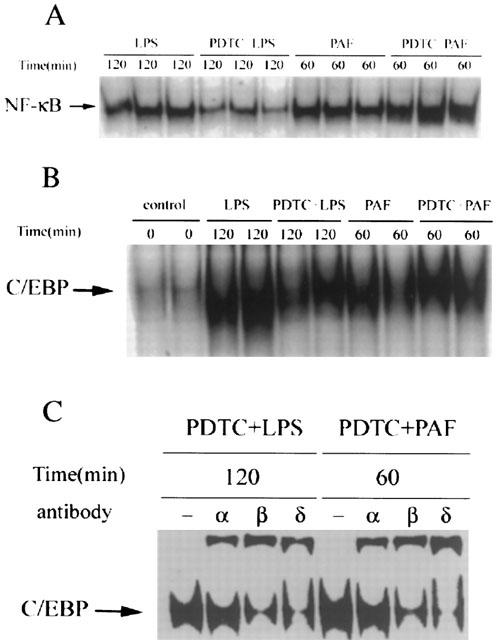
EMSA and supershift assay showing PDTC selectively inhibits the LPS- (but not PAF-) induced NF-κB and C/EBP binding activity, and significantly increases the C/EBP-δ subunit in LPS- or PAF-induced C/EBP complex. Animals were pretreated with PDTC (100 mg kg−1, i.p.) 1 h before PAF or LPS, and were killed at the time point at which PAF or LPS-induced C/EBP activation reached peak levels. Nuclear extracts were prepared from the small intestine, and subjected to EMSA with 32P-labelled oligonucleotide probes for NF-κB (A) and C/EBP (B). Supershift assays were also performed by adding normal serum (‘–') or specific antibody against different subunits of C/EBP's (C).
Systemic pathophysiological changes
PAF at a dose of 1.5 μg kg−1 induces only a transient hypotension and the blood pressure returns to near normal after 60 min. LPS (5 mg kg−1) causes mild hypotension after injection, which persists to the end of the experiment. Priming with low dose (0.5 mg kg−1) LPS aggravates and prolongs PAF- induced hypotension. PAF also causes peripheral leukocytosis and haemoconcentration (increased haematocrit), and LPS causes haemoconcentration but peripheral leukopenia. LPS priming before PAF injection results in marked leukopenia and haemoconcentration. No gross intestinal injury is found in rats receiving PAF or LPS alone. Combined LPS and PAF induces intestinal injury in all rats treated.
Discussion
C/EBP-binding motifs have been identified in the functional regulatory regions of many genes involved in the acute inflammatory response, such as acute phase proteins (Ramji et al., 1993), TNF-α (Pope et al., 1994), IL-6 (Bretz et al., 1994; Matsusaka et al., 1993), IL-1 beta (Godambe et al., 1994), IL-8 (Matsusaka et al., 1993; Stein & Baldwin, 1993), iNOS (Lowenstein et al., 1993), lysozyme (Ness et al., 1993), myeloperoxidase (Ford et al., 1996), elastase (Oelgeschlager et al., 1996;) and adhesion molecule ICAM-1 (Hou et al., 1994). Most of the early studies on C/EBP were done in the liver or liver cell lines because of the well-established relationship between this transcription factor, IL-6, and the acute phase response. In the liver both C/EBP-β and δ are strongly up-regulated by LPS and by cytokines such as IL-6, IL-1 and TNF (Alam et al., 1992; Akira & Kishimoto, 1997). More recent studies demonstrated similar induction of C/EBP β and δ in other tissues and organs during the acute phase response (Alam et al., 1992), suggesting that the role of these two isoforms in inflammation may be a more generalized phenomenon. Moreover, C/EBP β has also been found to be important in the activation and differentiation of myelomonocytic lineage, and in immune regulation, as suggested by the observation that C/EBP β-deficient mice had distorted humoral, innate and cellular immunity (Screpanti et al., 1995). There have been some in vivo studies on the role of C/EBPs in the maturation and differentiation of the intestine (Chandrasekaran & Gordon, 1993; Montgomery et al., 1997; Oesterreicher et al., 1998) and on the regulation of haptoglobin in intestinal epithelial cells (Pelletier, et al., 1998). However, intestinal C/EBP in inflammatory states has only rarely been examined (Alam et al., 1992). Given the facts that intestinal epithelial cells produce IL-6, IL-1, TNF, IL-8, and iNOS (Jobin et al., 1998; 1999; Hoffman et al., 1997; McGee et al., 1995; Tan et al., 1993; Waterhouse & Stadnyk, 1999), and that the lamina propria contains many immune cells, it is very likely that C/EBP β and δ are involved in intestinal inflammation and injury. The present study confirms the previous observation (Alam et al., 1992) that three isoforms C/EBP-α, C/EBP-β, and C/EBP-δ, are constitutively present at low levels in normal small intestine. In addition, we demonstrated that this transcription factor is activated in vivo in response to inflammatory stimuli. PAF and LPS elicit similar responses on the activation of C/EBP isoforms, except that PAF action is much more rapid than LPS. With respect to C/EBP-β, the activation of this transcription factor by inflammatory stimuli such as PAF and LPS is not unexpected, due to its well established role in acute phase response (Poli, 1998). Contrary to a previous report (Chandrasekaran & Gordon, 1993), we found that C/EBP-β is constitutively expressed in the epithelium and some lamina propria cells. After PAF or LPS injection, the increase of C/EBP β seems widespread, in lamina propria cells, lymphocytes and epithelial cells, in both villi and crypts. It is possible that C/EBP β, as transactivator, upregulates the gene expression of pro-inflammatory cytokines and enzymes in both epithelial cells and lamina propria cells, in response to inflammatory stimuli PAF and LPS.
We found that C/EBP-δ is also constitutively active in the intestine, although at a very low level. LPS and PAF only mildly increases C/EBP-δ binding activity. These results suggest that in the intestine, C/EBP-δ may not play an important role in the early phase of inflammation in response to PAF or LPS. However, when a low dose of LPS was given together with PAF to induce severe bowel necrosis and systemic inflammation, there was a strong and rapid increase of the activities of intestinal C/EBP-α, β, as well as δ. The activity of C/EBP-α was relatively transient, unlike the other two isoforms. It is possible that the prolonged activation of C/EBP-β and δ may contribute to the exaggerated, sustained inflammatory response induced by PAF plus LPS. Interestingly, after pretreating rats with PDTC, C/EBP-δ became the predominant subunit in both PAF or LPS-induced C/EBP complex. The physiological implication of this isoform ‘switch' is unclear.
The present study also demonstrates a widespread, constitutive expression of C/EBP-α in ileal epithelial cells. After challenge with PAF or LPS, we found a marked increase of C/EBP-α in the epithelium, especially pronounced in the crypt. This is discordant from a previous study (Chandrasekaran & Gordon, 1993) which showed no C/EBP-α in adult mouse ileal and colonic epithelium, but abundant C/EBP-α in the duodenum and jejunum, predominantly in the differentiated epithelium of the villus, and not in the epithelial cells in the crypt. Previous studies using liver and cultured macrophages have shown that, contrary to C/EBP-β and δ, C/EBP-α is usually down-regulated in the acute phase response (Alam et al., 1992; Hu et al., 1998), and the amount of complexes containing C/EBP α is dramatically reduced, replaced by C/EBP-β and δ. This change of C/EBP-α may be organ-specific, since C/EBP-α mRNA in the intestine has been reported to either remain unchanged or slightly increase after LPS challenge (Alam et al., 1992). We found that PAF or LPS administration markedly enhances the activity of this isoform in the intestine. The exact role of C/EBP-α in the small intestine is unclear. Although it is generally assumed that C/EBP-α is important for cell growth, differentiation (Yeh et al., 1995), and the maintenance of energy homeostasis (Wang et al., 1995), a recent study revealed a critical role of C/EBP-α for the neonatal acute phase response (Burgess-Beusse & Darlington, 1998). Interestingly, we found C/EBP-α localized in the intestinal crypt epithelium, where two potent pro-inflammatory proteins, TNF (Tan et al., 1993) and group II phospholipase A2 (PLA2-II) (Nevalainen et al., 1995) are also localized. Further, since binding sites for C/EBP have been found in the promoter region of TNF (Pope et al., 1994) and PLA2-II (Crowl et al., 1991), and the gene expression of both of these proteins is up-regulated after administration of PAF or LPS (Huang et al., 1994; Tan et al., 1996), it is possible that C/EBP-α plays a role in the induction of TNF and PLA2-II by these agents. It is interesting to note that the induction of the genes of TNF and PLA2-II by PAF is also rapid, within 30 min after injection (Huang et al., 1994; Tan et al., 1996), which coincides with the activation of C/EBP-α. In contrast, the induction of IL-6 in the intestine is much slower (unpublished observation), in parallel with the elevation of C/EBP-β.
One observation in this study is that both p50 and p65 subunits of NF-κB are components of C/EBP-DNA binding complexes in the small intestine, only after PAF (but not LPS) administration. Functional and physical associations between C/EBP and NF-κB p50 and p65 have been previously reported (Stein et al., 1993). This cross coupling was hypothesized to suppress promoters with κB enhancer motifs but synergistically stimulate promoters with C/EBP binding sites. The physiological implication of the association of these two transcription factor families in the intestine after PAF stimulation is unclear. We have demonstrated that PAF induces the gene expression of TNF in the intestine. As mentioned above, the TNF gene contains, in its promoter, binding sites for NF-κB (p65–p50) as well as C/EBP-α and β (Pope et al., 1994). Since PAF activates NF-κB p50 homodimers in the intestine (de plaen et al., 1998), which lack transactivation domain (Baeuerle & Henkel, 1994; Kopp & Ghosh, 1995), it is possible the PAF-induced TNF gene expression is via transcription factors other than NF-κB, such as C/EBP-α and β homo- or heterodimers or C/EBP-NF-κB heterodimers. Previous studies indicated that PDTC is a relatively selective NF-κB inhibitor in vitro (Schreck et al., 1992) and in vivo (Liu et al., 1999). We demonstrated here that PDTC inhibits LPS-, but not PAF-induced NF-κB and C/EBP binding activity in rat small intestine, suggesting that the activation mechanisms of these transcription factors are different for PAF and LPS.
In conclusion, PAF and LPS activate C/EBP-α and β in the intestine probably via different pathways. Unlike LPS, PAF acts more rapidly, and induces cross-dimerization of C/EBP and NF-κB. Combining PAF and LPS synergistically activates all three subunits of C/EBP.
Acknowledgments
This work was supported by NIH grant DK34574. The authors thank Ms Qianping Liu, Hong Chong, Jing Chen, and Liya Wang for their excellent technical help.
Abbreviations
- C/EBP
CCAAT/enhancer binding proteins
- EMSA
electrophoretic mobility shift assay
- Hct
haematocrit
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-kappa B
- PAF
platelet activating factor
- PDTC
pyrrolidine dithiocarbamate
- PLA2-II
group II phospholipase A2
- TNF
tumour necrosis factor
- WBC
white blood cells
References
- AKIRA S., KISHIMOTO T. NF-IL6 and NF-kappa B in cytokine gene regulation. Adv. Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- ALAM T., AN M.R., PAPACONSTANTINOU J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J. Biol. Chem. 1992;267:5021–5024. [PubMed] [Google Scholar]
- BAEUERLE P.A., HENKEL T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- BEUTLER B., KROCHIN N., MILSARK I.W., LUEDKE C., CERAMI A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., LASZLO F., WHITTLE B.J., MONCADA S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Brit. J. Pharmacol. 1993;110:1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRETZ J.D., WILLIAMS S.C., BAER M., JOHNSON P.F., SCHWARTZ R.C. C/EBP-related protein 2 confers lipopolysaccharide-inducible expression of interleukin 6 and monocyte chemoattractant protein 1 to a lymphoblastic cell line. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7306–7310. doi: 10.1073/pnas.91.15.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGESS-BEUSSE B.L., DARLINGTON G.J. C/EBPalpha is critical for the neonatal acute-phase response to inflammation. Mol. Cell. Biol. 1998;18:7269–7277. doi: 10.1128/mcb.18.12.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPLAN M.S., SUN X.M., HSEUH W., HAGEMAN J.R. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J. Pediatr. 1990;116:960–964. doi: 10.1016/s0022-3476(05)80661-4. [DOI] [PubMed] [Google Scholar]
- CATRON K.M., BRICKWOOD J.R., SHANG C., LI Y., SHANNON M.F., PARKS T.P. Cooperative binding and synergistic activation by RelA and C/EBPbeta on the intercellular adhesion molecule-1 promoter. Cell. Growth Differ. 1998;9:949–959. [PubMed] [Google Scholar]
- CHA-MOLSTAD H., AGRAWAL A., ZHANG D., KUSHNER I. The rel family member P50 mediates cytokine-induced C-reactive protein expression by a novel mechanism. J. Immunol. 2000;165:4592–4597. doi: 10.4049/jimmunol.165.8.4592. [DOI] [PubMed] [Google Scholar]
- CHANDRASEKARAN C., GORDON J.I. Cell lineage-specific and differentiation-dependent patterns of CCAAT/enhancer binding protein alpha expression in the gut epithelium of normal and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8871–8875. doi: 10.1073/pnas.90.19.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWL R.M., STOLLER T.J., CONROY R.R., STONER C.R. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J. Biol. Chem. 1991;266:2647–2651. [PubMed] [Google Scholar]
- DE PLAEN I., TAN X.D., CHANG H., QU X.W., LIU Q.P., HSUEH W. Intestinal NF-kappaB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim. Biophy. Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- DE PLAEN I., TAN X.D., CHANG H., WANG L., REMICK D.G., HSUEH W. Lipopolysaccharide activates nuclear factor κB in rat intestine: role of endogenous platelet-activating factor and tumour necrosis factor. Br. J. Pharmacol. 2000;129:307–314. doi: 10.1038/sj.bjp.0703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBERHARDT W., PLUSS C., HUMMEL R., PFEILSCHIFTER J. Molecular mechanisms of inducible nitric oxide synthase gene expression by IL-1beta and cAMP in rat mesangial cells. J. Immunol. 1998;160:4691–4699. [PubMed] [Google Scholar]
- ESSANI N.A., MCGUIRE G.M., MANNING A.M., JAESCHKE H. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J. Immunol. 1996;156:2956–2963. [PubMed] [Google Scholar]
- FORD A.M., BENNETT C.A., HEALY L.E., TOWATARI M., GREAVES M.F., ENVER T. Regulation of the myeloperoxidase enhancer binding proteins Pu1, C-EBP alpha, -beta, and -delta during granulocyte-lineage specification. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10838–10843. doi: 10.1073/pnas.93.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODAMBE S.A., CHAPLIN D.D., TAKOVA T., BELLONE C.J. Upstream NFIL-6-like site located within a DNase I hypersensitivity region mediates LPS-induced transcription of the murine interleukin-1 beta gene. J. Immunol. 1994;153:143–152. [PubMed] [Google Scholar]
- GONZALEZ-CRUSSI F., HSUEH W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am. J. Pathol. 1983;112:127–135. [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN R.A., ZHANG G., NUSSLER N.C., GLEIXNER S.L., FORD H.R., SIMMONS R.L., WATKINS S.C. Constitutive expression of inducible nitric oxide synthase in the mouse ileal mucosa. Am. J. Physiol. 1997;272:G383–G392. doi: 10.1152/ajpgi.1997.272.2.G383. [DOI] [PubMed] [Google Scholar]
- HOU J., BAICHWAL V., CAO Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11641–11645. doi: 10.1073/pnas.91.24.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSUEH W., GONZALEZ-CRUSSI F., ARROYAVE J.L. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- HU H.M., BAER M., WILLIAMS S.C., JOHNSON P.F., SCHWARTZ R.C. Redundancy of C/EBP alpha, -beta, and -delta in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J. Immunol. 1998;160:2334–2342. [PubMed] [Google Scholar]
- HUANG L., TAN X., CRAWFORD S.E., HSUEH W. Platelet-activating factor and endotoxin induce tumour necrosis factor gene expression in rat intestine and liver. Immunology. 1994;83:65–69. [PMC free article] [PubMed] [Google Scholar]
- JOBIN C., HOLT L., BRADHAM C.A., STREETZ K., BRENNER D.A., SARTOR R.B. TNF receptor-associated factor-2 is involved in both IL-1 beta and TNF-alpha signaling cascades leading to NF-kappa B activation and IL-8 expression in human intestinal epithelial cells. J. Immunol. 1999;162:4447–4454. [PubMed] [Google Scholar]
- JOBIN C., PANJA A., HELLERBRAND C., IIMURO Y., DIDONATO J., BRENNER D.A., SARTOR R.B. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J. Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- KOPP E.B., GHOSH S. NF-kappa B and rel proteins in innate immunity. Adv. Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- LIU S.F., YE X., MALIK A.B. Inhibition of NF-kappa B activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN C.J., ALLEY E.W., RAVAL P., SNOWMAN A.M., SNYDER S.H., RUSSELL S.W., MURPHY W.J. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUSAKA T., FUJIKAWA K., NISHIO Y., MUKAIDA N., MATSUSHIMA K., KISHIMOTO T., AKIRA S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGEE D.W., BAMBERG T., VITKUS S.J., MCGHEE J.R. A synergistic relationship between TNF-alpha, IL-1 beta, and TGF-beta 1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995;86:6–11. [PMC free article] [PubMed] [Google Scholar]
- MONTGOMERY R.K., RINGS E.H., THOMPSON J.F., SCHUIJT C.C., ARAS K.M., WIELENGA V.J., KOTHE M.J.C., BULLER H.A., GRAND R.J. Increased C/EBP in fetal rat small intestine precedes initiation of differentiation marker mRNA synthesis. Am. J. Physiol. 1997;272 35:G534, G544. doi: 10.1152/ajpgi.1997.272.3.G534. [DOI] [PubMed] [Google Scholar]
- NASSIF A., LONGO W.E., MAZUSKI J.E., VERNAVA A.M., KAMINSKI D.L. Role of cytokines and platelet-activating factor in inflammatory bowel disease. Implications for therapy. Dis. Colon Rectum. 1996;39:217–223. doi: 10.1007/BF02068079. [DOI] [PubMed] [Google Scholar]
- NESS S.A., KOWENZ-LEUTZ E., CASINI T., GRAF T., LEUTZ A. Myb and NF-M: combinatorial activators of myeloid genes in heterologous cell types. Genes Dev. 1993;7:749–759. doi: 10.1101/gad.7.5.749. [DOI] [PubMed] [Google Scholar]
- NEVALAINEN T.J., GRONROOS J.M., KALLAJOKI M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab. Invest. 1995;72:201–208. [PubMed] [Google Scholar]
- OELGESCHLAGER M., NUCHPRAYOON I., LUSCHER B., FRIEDMAN A.D. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol. Cell. Biol. 1996;16:4717–4725. doi: 10.1128/mcb.16.9.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OESTERREICHER T.J., LEEPER L.L., FINEGOLD M.J., DARLINGTON G.J., HENNING S.J. Intestinal maturation in mice lacking CCAAT/enhancer-binding protein α (C/EBPα) Biochem. J. 1998;330:1165–1171. doi: 10.1042/bj3301165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLETIER N., BOUDREAU F., YU S., ZANNONI S., BOULANGER V., ASSELIN C. Activation of haptoglobin gene expression by cAMP involve CCAAT/enhancer-binding protein isoforms in intestinal epithelial cells. FEBS lett. 1998;439:274–280. doi: 10.1016/s0014-5793(98)01388-x. [DOI] [PubMed] [Google Scholar]
- POLI V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- POPE R.M., LEUTZ A., NESS S.A. C/EBP beta regulation of the tumor necrosis factor alpha gene. J. Clin. Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QU X.W., ROZENFELD R.A., HUANG W., SUN X., TAN X.D., HSUEH W. Roles of nitric oxide synthases in platelet-activating factor-induced intestinal necrosis in rats. Crit. Care Med. 1999;27:356–364. doi: 10.1097/00003246-199902000-00043. [DOI] [PubMed] [Google Scholar]
- RAMJI D.P., VITELLI A., TRONCHE F., CORTESE R., CILIBERTO G. The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBP delta/NF-IL6 beta, are induced by IL-6 to promote acute phase gene transcription via different mechanisms. Nucleic Acid Res. 1993;21:289–294. doi: 10.1093/nar/21.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSAM A.C., WALLACE J.L., WHITTLE B.J. Potent ulcerogenic actions of platelet-activating factor on the stomach. Nature. 1986;319:54–56. doi: 10.1038/319054a0. [DOI] [PubMed] [Google Scholar]
- SCHRECK R., MEIER B., MANNEL D.N., DROGE W., BAEUERLE P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. MED. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCREPANTI I., ROMANI L., MUSIANI P., MODESTI A., FATTORI E., LAZZARO D., SELLITTO C., SCARPA S., BELLAVIA D., LATTANZIO G. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am. J. Physiol. 1990;259:C697–C708. doi: 10.1152/ajpcell.1990.259.5.C697. [DOI] [PubMed] [Google Scholar]
- STEIN B., BALDWIN A.S.J. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol. Cell. Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN B., COGSWELL P.C., BALDWIN A.S.J. Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol. Cell. Biol. 1993;13:3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN X., HSUEH W., GONZALEZ-CRUSSI F. Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells, intestinal eosinophils, and macrophages. Am. J. Pathol. 1993;142:1858–1865. [PMC free article] [PubMed] [Google Scholar]
- TAN X., SUN X., GONZALEZ-CRUSSI F.X., GONZALEZ-CRUSSI F., HSUEH W. PAF and TNF increase the precursor of NF-kappa B p50 mRNA in mouse intestine: quantitative analysis by competitive PCR. Biochim. Biophys. Acta. 1994;1215:157–162. doi: 10.1016/0005-2760(94)90105-8. [DOI] [PubMed] [Google Scholar]
- TAN X.D., WANG H., GONZALEZ-CRUSSI F.X., CHANG H., GONZALEZ-CRUSSI F., HSUEH W. Platelet activating factor and endotoxin increase the enzyme activity and gene expression of type II phospholipase A2 in the rat intestine. Role of polymorphonuclear leukocytes. J. Immunol. 1996;156:2985–2990. [PubMed] [Google Scholar]
- UMEK R.M., FRIEDMAN A.D., MCKNIGHT S.L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- WANG H., TAN X.D., QU X.W., CHANG H., REMICK D.G., GONZALEZ-CRUSSI F., HSUEH W. Platelet-activating factor (PAF) up-regulates plasma and tissue PAF-acetylhydrolase activity in the rat: effect of cycloheximide. Ped. Res. 1997;42:597–603. doi: 10.1203/00006450-199711000-00008. [DOI] [PubMed] [Google Scholar]
- WANG N.D., FINEGOLD M.J., BRADLEY A., OU C.N., ABDELSAYED S.V., WILDE M.D., TAYLOR L.R., WILSON D.R., DARLINGTON G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- WATERHOUSE C.C., STADNYK A.W. Rapid expression of IL-1beta by intestinal epithelial cells in vitro. Cell. Immunol. 1999;10:1–8. doi: 10.1006/cimm.1999.1468. [DOI] [PubMed] [Google Scholar]
- XIA C., CHESHIRE J.K., PATEL H., WOO P. Cross-talk between transcription factors NF-kappa B and C/EBP in the transcriptional regulation of genes. International. Intern. J. Biochem. Cell. Biol. 1997;29:1525–1539. doi: 10.1016/s1357-2725(97)00083-6. [DOI] [PubMed] [Google Scholar]
- YEH W.C., CAO Z., CLASSON M., MCKNIGHT S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]


