Abstract
The effects of n-alcohols (methanol to 1-decanol) on kainate-activated AMPA receptor subunit GluR1 and GluR3 ion currents were studied in Xenopus oocytes using the two-electrode voltage-clamp recording technique.
For short-chain alcohols from methanol to 1-hexanol, potency for inhibition of GluR1 and GluR3 receptor-mediated current increased in proportion to the chain length or hydrophobicity of the alcohol.
The IC50 values of these alcohols for GluR1 were: methanol, 702 mM; ethanol, 170 mM; 1-propanol, 69 mM; 1-butanol, 20 mM; 1-pentanol, 17 mM; and 1-hexanol, 10 mM. For GluR3, IC50 values were: methanol, 712 mM; ethanol, 238 mM; 1-propanol, 50 mM; 1-butanol, 32 mM; 1-pentanol, 13 mM; and 1-hexanol, 7 mM.
For long-chain alcohols, 1-heptanol was less potent than 1-hexanol (estimated IC50: 19 mM for GluR1 and 18 mM for GluR3), 1-octanol had little effect only on GluR3, and 1-nonanol and 1-decanol did not significantly inhibit both GluR1 and GluR3 responses.
The observations indicate that straight-chain n-alcohols exhibit a cutoff in their potency for inhibition of the function of non-NMDA glutamate receptor subunits, GluR1 and GluR3. The cutoff in potency of n-alcohols for inhibition of non-NMDA glutamate receptor function is consistent with the interpretation that alcohols affect the function of these receptor-channels by interacting with an alcohol binding site of specific dimensions on the receptor protein.
Keywords: AMPA receptor subunit, straight-chain alcohol, cutoff effect, GluR1, GluR3, Xenopus oocyte, voltage-clamp, alcohol intoxication, non-NMDA glutamate receptor
Introduction
The molecular mechanism of alcohol action in the nervous system has not been established. Traditionally, alcohols have been thought to act primarily on membrane lipids, perturbing their function, which secondarily affects the function of membrane proteins such as receptors and ion channels (Janoff & Miller, 1981; Goldstein, 1984; Deitrich et al., 1989). Some recent observations, however, have suggested that alcohols may interact directly with certain neuronal membrane proteins. For example, straight-chain and halogenated alcohols with molecular volume ⩽42.2 ml mol−1 were found to inhibit the function of ATP-gated ion channels with potency proportional to their hydrophobicity, whereas alcohols with a molecular volume ⩾46.1 ml mol−1 do not affect the function of these receptors (Li et al., 1994). It has also been reported that straight-chain alcohols from methanol to 1-heptanol inhibit the function of NMDA receptors with increasing potency in proportion to their hydrophobicity; however, 1-nonanol and 1-decanol do not inhibit NMDA receptor-mediated responses (Peoples & Weight, 1995a). The authors suggested that these cutoffs result from an interaction of the alcohols with a hydrophobic pocket of circumscribed dimensions on the receptor protein (Li et al., 1994; Peoples & Weight, 1995a). It has not been established however, whether alcohol effects on the function of other types of neuronal membrane receptors are due to a direct interaction with the receptor protein or are secondary to a perturbation of the membrane lipids.
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system (CNS). The NMDA-type of glutamate receptor-ion channels are thought to be involved in a number of important physiologic and pathophysiologic phenomena, such as synaptic plasticity (Collingridge & Bliss, 1987; 1990), neural development (Collingridge et al., 1991) and neurotoxicity (Choi & Rothman, 1990). The non-NMDA-type of glutamate receptor-ion channels mediates fast excitatory postsynaptic potentials (EPSPs) at the majority of excitatory synapses in the mammalian CNS (Jonas & Sakmann, 1992; Seeburg, 1993). Ethanol has been found to inhibit the function of both NMDA and non-NMDA glutamate receptors (Lovinger et al., 1989; Dildy-Mayfield & Harris, 1992; Lovinger, 1993).
In neurons, NMDA receptors are usually more sensitive to the inhibitory actions of ethanol than are non-NMDA glutamate receptors (Weight et al., 1993). As noted above, it has been found that the inhibition of NMDA receptors by a series of straight-chain alcohols of increasing chain length exhibits a cutoff (Peoples & Weight, 1995a). In order to determine whether non-NMDA receptors exhibit a similar cutoff in potency, we studied the effect of a series of straight-chain alcohols on the function of GluR1 and GluR3 receptor subunits expressed in Xenopus oocytes. The GluR1 and GluR3 homomeric receptor subunits belong to a family of AMPA receptors (including GluR2 and GluR4) that are of similar size, but share a maximum sequence identity of just 68–73% (Boulter et al., 1990; Keinänen et al., 1990). The results from these studies have been reported previously in preliminary form (Akinshola & Weight, 1995; Akinshola et al., 1996).
Methods
Recombinant receptor: synthesis of cRNAs
cDNAs coding for the FLOP form of the GluR1 and GluR3 receptor subunits subcloned into pBluescript vector were kindly provided by Drs Stephen Heinemann and Jim Boulter, (Salk Institute, San Diego, CA, USA) The GluR1 and GluR3 subunit cRNAs were prepared by in vitro transcription with T3 RNA polymerase in the presence of cap dinucleotide 7meGpppG, using XhoI-linearized pBS SKGluR3 as a template. Concisely, 10 mg of plasmid DNA was linearized by XhoI, in a total volume of 50 μl, for 2 h at 37°C, extracted with phenol/chloroform, precipitated with ethanol, dried, and suspended in 5 μl RNAse-free distilled water. One mg of linearized DNA was used as a template for in vitro capping reactions (cDNA synthesis), using the mCAP capping kit from Stratagene (La Jolla, CA, USA). In vitro synthesized capped cRNA transcripts were analysed on 1.2% formaldehyde-agarose gels for size and length.
Oocyte preparation and microinjection
Mature female Xenopus laevis frogs (Xenopus I, Ann Arbor, MI, USA) were kept in dechlorinated water tanks at 19–21°C, with a 12 h light/dark cycle, and fed beef liver at least twice a week. Ovarian lobes were surgically excised from frogs anaesthetized with 0.2% 3-aminobenzoic acid ethyl ester (Sigma Chemical Co., St. Louis, MO, USA), and manually separated into clusters of 5–10 oocytes, then placed in a calcium free modified Barth's Solution (MBS) containing (in mM): NaCl 88, KCl 1, NaHCO3 2.4, MgSO4 0.8 and HEPES 10 pH 7.5. The follicular cell layer was removed by incubating the oocytes in 2 mg ml−1 collagenase A (Boehringer Mannheim, Indianapolis, IN, USA) with shaking for 2 h. Oocytes were washed, first in the calcium free MBS and then transferred into MBS containing 0.3 mM Ca(NO3)2 and 0.9 mM CaCl2 prior to the selection of healthy stage V and VI denuded oocytes for cRNA injection. The prepared oocytes were injected with 16–20 ng of cRNA/oocyte, using a micropipette with a 10 μm tip diameter, and a PV 800 Pneumatic picopump (World Precision Instruments, Sarasota, FL, USA). The oocytes were incubated at 19–21°C, in MBS supplemented with 2 mM Na pyruvate, 10,000 u l−1 penicillin, 10 mg l−1 streptomycin, 50 mg l−1 gentamycin and 0.5 mM theophylline, and used for electrophysiological recording for 3–7 days after receptor expression.
Electrophysiological recording
Oocytes were placed in a recording chamber (∼100 μl volume) and superfused with MBS constantly at a rate of ∼2.5 ml min−1. The oocytes were impaled with two microelectrodes (1–10 MΩ) filled with a 3 M KCl solution and voltage-clamped at a holding potential of −70 mV using a Gene-Clamp 500 amplifier (Axon Instruments Inc., Foster City, CA, USA). Oocyte membrane ion currents were measured using the standard two-electrode voltage-clamp method and were recorded on a Gould RS 3400 pen recorder (Gould Inc., Cleveland, OH, USA). All experiments were performed at room temperature.
Solutions
Solutions of agonists and alcohols were prepared in MBS, and applied by gravity flow through macropipets (external diameter, ∼1.0 mm) placed ∼1 mm from the oocyte. Agonists were usually applied for 30–50 s, with at least 2 min between applications to allow for full recovery from each treatment. The alcohols were purchased from Aldrich Chemical (Milwaukee, WI, USA). To minimize loss of the higher n-alcohols (heptanol–decanol) from experimental solutions, these solutions were prepared and stored in sealed teflon bags and applied through teflon tubing. Higher alcohols were pre-applied for 30 s to ensure adequate equilibration in the recording chamber. There was no difference in the effect of higher alcohols dissolved in MBS or DMSO.
Statistical analysis
Statistical analysis of concentration-response data was performed using the nonlinear curve-fitting program ALLFIT (DeLean et al., 1978). Values reported for slope factors (n) and alcohol concentrations producing half-maximal inhibition (IC50) are those obtained by fitting the data to the equation
Where x is ethanol concentration, y is response, and Emax is maximal response. In these studies, Emax was constrained to 100% inhibition. Data were also statistically compared using the paired t-test, or InStat program, where appropriate. Average values are expressed as mean±standard error (s.e.).
Results
Effect of n-alcohols on AMPA GluR1 subunit receptor currents
Figure 1 illustrates the inhibition of kainate-activated currents in oocytes expressing GluR1 receptor subunit by a series of straight-chain aliphatic n-alcohols from methanol to 1-decanol. In Figure 1a, the effects of three representative alcohol (ethanol, 1-hexanol and 1-nonanol) are shown, with 100 mM ethanol and 10 mM 1-hexanol inhibiting receptor current by 35.8±1.4% and 46.1±2.0% respectively. However, saturating concentrations (1 mM) of 1-nonanol did not inhibit receptor current. All the alcohols at concentrations used in this study failed to generate currents in oocytes in the absence of agonist. The concentration-response curves for the inhibition of kainate-activated GluR1 receptor currents in oocytes by n-alcohols from methanol to 1-decanol shown in Figure 1b, demonstrate the alcohol cutoff effect. The curves from methanol to 1-hexanol were successively shifted to the left with increasing carbon chain length whereas there was a cutoff in inhibitory potency with 1-heptanol. A reversal of alcohol inhibitory potency on GluR1 currents begins with 1-heptanol, where 10 mM 1-heptanol with 27.6±2.7% inhibition is less potent than 10 mM 1-hexanol with 46.1±2% inhibition. The curves for 1-heptanol could only be fitted by linear regression because maximal concentrations (10 mM) of the alcohol did not inhibit receptor current by 50%. Curves for 1-octanol, 1-nonanol and 1-decanol could not be adequately fitted because saturating concentrations of 1-octanol (3 mM) only inhibited receptor current by 6±2.6% whereas 1 mM 1-nonanol and 0.3 mM 1-decanol did not inhibit GluR1 receptor subunit current.
Figure 1.
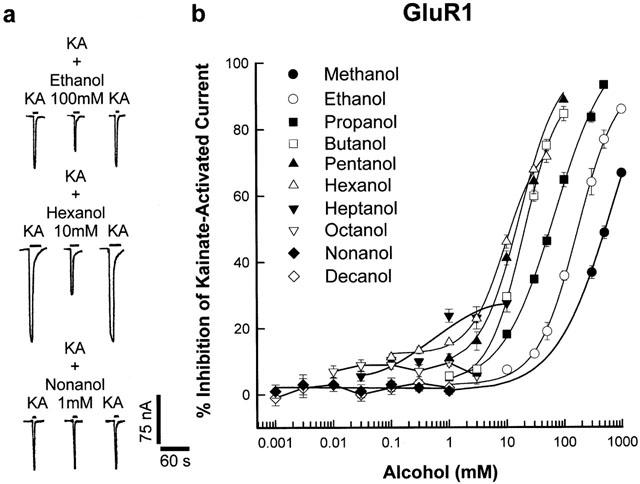
Inhibition of kainate-activated ion-currents in oocytes expressing recombinant AMPA GluR1 receptor subunit by aliphatic n-alcohols. (a) Record of currents activated by 200 μM KA in oocytes voltage-clamped at −70 mV and the inhibition of these currents by 100 mM ethanol and 10 mM 1-hexanol. 1 mM 1-decanol did not inhibit receptor current. Current and time calibration applies to all records. (b) Concentration-response curves for inhibition of kainate-activated current by aliphatic n-alcohols from methanol to 1-decanol in oocytes expressing recombinant AMPA GluR1 receptor subunit. Values are mean (±s.e.mean) percentage inhibition of 5–8 oocytes; error bars not visible are smaller than the symbol size. Each curve was plotted by fitting the values to the logistic equation in the methods section, except for the 1-octanol, 1-nonanol and 1-decanol curves, which were fitted by linear regression.
Effect of n-alcohols on AMPA GluR3 subunit receptor currents
Figure 2 similarly shows the inhibition of kainate-activated currents in oocytes expressing GluR3 receptor subunit by a series of straight-chain aliphatic n-alcohols from methanol to 1-decanol. In Figure 2a, 100 mM ethanol and 10 mM 1-hexanol likewise inhibited receptor current by 29±1.3% and 60.8±2.5% respectively whereas 1 mM 1-nonanol did not inhibit receptor current. From the concentration-response graph of alcohol inhibition of GluR3 subunit current in Figure 2b, the curves depicting methanol to 1-hexanol inhibitions were successively shifted to the left as alcohol carbon chains increase. A cutoff in alcohol inhibitory potency for GluR3 occurred with 1-heptanol, similar to the effect seen with the GluR1 subunit. Consequently, 10 mM 1-heptanol with 41.8±2.8% inhibition is less potent than the same concentration of 1-hexanol with 60.8±2.5% inhibition and the 1-heptanol curve is shifted to the right of the 1-hexanol curve. The 1-heptanol curve was also fitted by linear regression due to the inability to achieve 50% current inhibition with a saturating concentration of 1-heptanol. The curves for 1-octanol, 1-nonanol and 1-decanol could not be adequately fitted because saturating concentrations of the alcohols did not inhibit GluR3 receptor subunit current.
Figure 2.
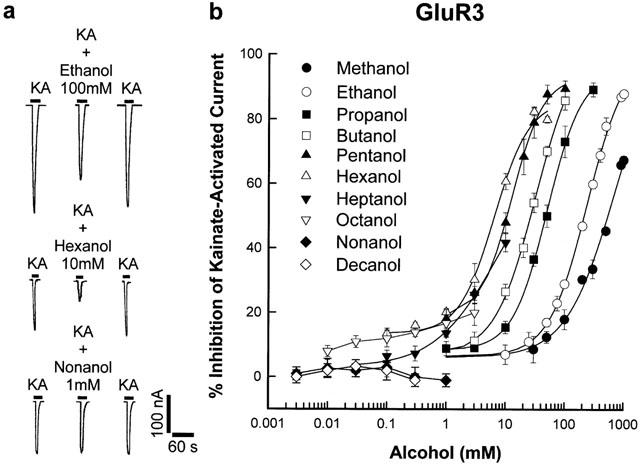
Inhibition of kainate-activated ion-currents in oocytes expressing recombinant AMPA GluR3 receptor subunit by aliphatic n-alcohols. (a) Record of currents activated by 200 μM KA in oocytes voltage-clamped at −70 mV and the inhibition of these currents by 100 mM ethanol and 10 mM 1-hexanol. One mM 1-decanol did not inhibit receptor current. Current and time calibration applies to all records. (b) Concentration-response curves for inhibition of kainate-activated current by aliphatic n-alcohols from methanol to 1-decanol in oocytes expressing recombinant AMPA GluR3 receptor subunit. Values are mean (±s.e.mean) percentage inhibition of 5–8 oocytes; error bars not visible are smaller than the symbol size. Each curve was plotted by fitting the values to the logistic equation in the Methods section, except for the 1-octanol, 1-nonanol and 1-decanol curves.
All the longer-chain alcohols were preapplied for 30 s before agonist application to allow for maximum equilibration in solutions bathing the oocytes. See Methods for detailed explanation. In order to ensure that the effects of the higher alcohols were not limited by their sparing solubility, we compared MBS solutions of alcohols to DMSO (0.05%) solutions of alcohols on kainate current inhibition. There were no differences in MBS or DMSO (0.05%) solubilized alcohol effect. However, DMSO (0.05%) in the absence of alcohol, inhibited kainate-activated current in oocytes by 6%.
Influence of the partition coefficient of alcohols on their relative inhibitory potency of GluR1 and GluR3 receptor subunit
Figure 3 is a plot of the relative potency of n-alcohols (ethanol IC50/alcohol IC50) for inhibiting GluR1 (a) and GluR3 (b) subunit currents as a function of their membrane/buffer partition coefficient P(m:b). The P(m:b) was accomplished by using the partition coefficient of 1-octanol in water (P(octanol/water)) to determine the value for each primary alcohol and then dividing by five (according to the method of Roth & Seeman, 1972) to obtain each P(m:b). There was an exponential increase in the relative potency of GluR1 and GluR3 receptor inhibition up to 1-pentanol and maximum receptor inhibition at 1-hexanol. A least squares plot of maximum receptor inhibitory potency data yields a slope value of 0.9437 (r=0.9952) for both receptor subunits. Similarly, a least squares plot of the membrane disordering potency data (depicted by the dashed lines) yields a slope value of 0.9609 (r=0.9982). A significant correlation (with a correlation coefficient approximating 1.0) therefore exists between membrane disordering potency and inhibitory potency of GluR1 and GluR3 receptor subunits by the n-alcohols. In contrast, inhibitory potency for the subunits declined with 1-heptanol, and 1-octanol, 1-nonanol and 1-decanol were not effective in inhibiting GluR1 and GluR3 receptor currents, despite increased lipid solubility effects.
Figure 3.
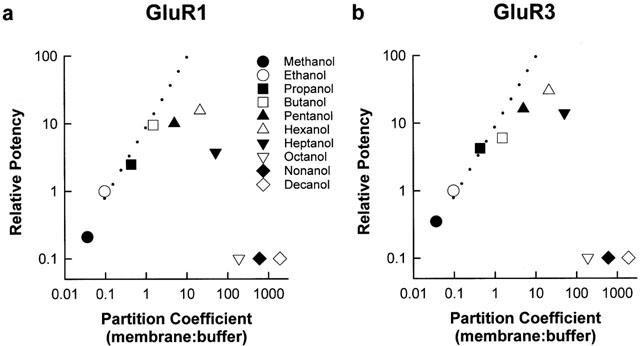
Cutoff in the potency of aliphatic n-alcohols for inhibition of GluR1 (a) and GluR3 (b) receptor subunits. Relative potency of aliphatic n-alcohols for inhibiting receptor-mediated current in oocytes (ethanol IC50/alcohol IC50), expressed as a function of their membrane : buffer partition coefficients. Dashed line represents membrane disordering potency data for aliphatic n-alcohols from ethanol to 1-octanol, (Lyon et al., 1981). The symbols for 1-octanol, 1-nonanol, and 1-decanol are shown on the x-axis because IC50 values for these alcohols could not be determined.
Sensitivity of GluR1 and GluR3 receptor subunits to n-alcohol inhibition
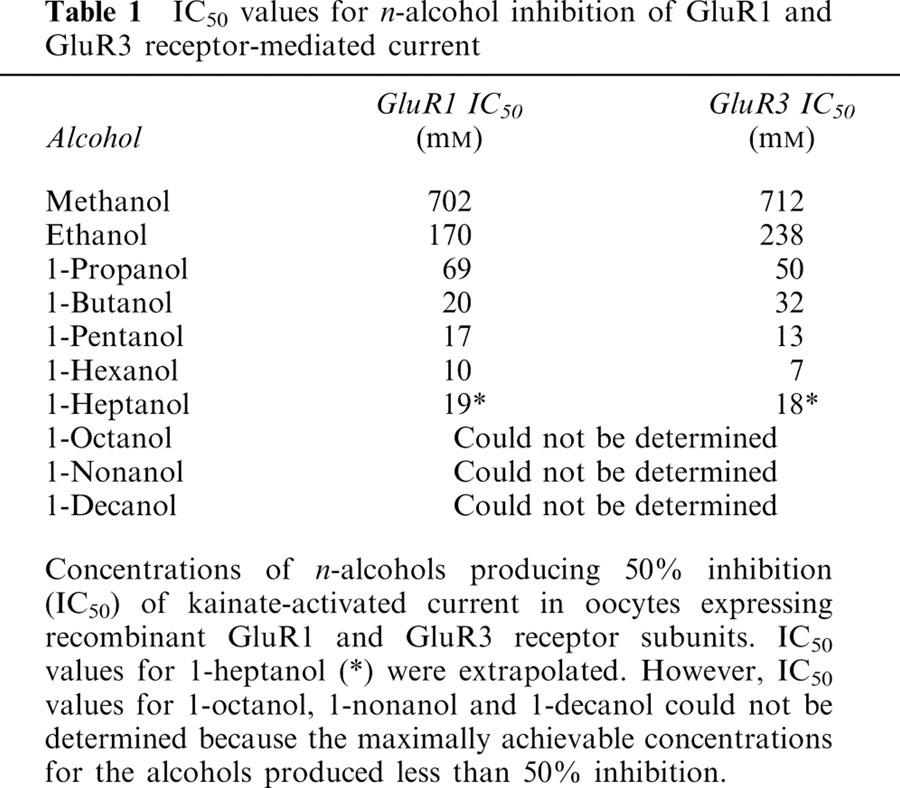
Table 1 shows the values for concentrations of n-alcohols (methanol–1-heptanol) producing 50% inhibition (IC50) of GluR1 and GluR3 receptor-mediated currents. The IC50 values were determined by fitting the data to the logistic equation described in the Methods. From methanol through 1-hexanol, IC50 values decreased correspondingly with increasing carbon number. At seven carbons (1-heptanol), IC50 values increased when compared with 1-hexanol, and could only be obtained by extrapolation. The IC50 values for 1-octanol, 1-nonanol and 1-decanol could not be determined, because the maximally achievable concentrations of the alcohols produced little or no inhibition. With the exception of methanol, ethanol and 1-butanol, the IC50 values for GluR3 are lower than the IC50 values for GluR1. This is an indication of increased potency of n-alcohol inhibition of GluR3 subunit current in comparison to GluR1 current inhibition.
Table 1.
IC50 values for n-alcohol inhibition of GluR1 and GluR3 receptor-mediated current
Discussion
Our observations show that the increase in n-alcohol potency for inhibition of AMPA receptor current in oocytes cuts off at 1-heptanol for GluR1 and GluR3 subunits. Inhibitory potency of kainate-activated receptor current increased up to 1-hexanol and decreased with 1-heptanol for both GluR1 and GluR3 subunits. 1-octanol, 1-nonanol and 1-decanol did not inhibit the GluR1 subunit current. For the GluR3 subunit current however, inhibition by 1-octanol was less than the inhibition by 1-heptanol, whereas 1-nonanol and 1-decanol did not inhibit receptor current.
The cutoff in inhibitory effect of alcohol on GluR1 and GluR3 subunits in this study is consistent with a direct interaction of these alcohols with a hydrophobic binding site (or pocket) of fixed dimensions on the ion-channel receptor protein. The alcohols failing to inhibit the receptor subunits therefore are unable to fit the binding pockets within the receptor because their molecular size prevented binding. The estimated volume of the alcohol-binding pocket on the receptor subunits will be less than (91.24 cm3, Bondi, 1964) the molecular volume for 1-octanol on GluR1 and (103.5 cm3, Bondi, 1964), the molecular volume for 1-nonanol on GluR3. The differences in molecular volume of the hydrophobic pockets for the GluR1 and GluR3 subunits may be attributed to the differences in the sequence of amino acids constituting the hydrophobic pocket. Ethanol is known to bind non-competitively in its inhibition of GluR receptors (Dildy-Mayfield & Harris, 1992; 1995), suggesting a site of interaction, different from the receptor agonist site. The two-agonist binding domains reported for the GluR receptor are both located extracellularly (Stern-Bach et al., 1994).
The cutoff effect from this study is well illustrated in Figure 3a, b where the linear relationship between relative potency of alcohols and their partition coefficient for the receptor subunits change with 1-heptanol. In order to compare and contrast between a protein effect and a lipid effect in the binding of alcohol to the GluR receptor, the data in Figure 3a, b were plotted against the dashed line, representing alcohol effect on the disordering of mouse brain membranes (Lyon et al., 1981). In the study by Lyon et al. (1981), the increase in potency of the alcohols (methanol to 1-octanol) in effecting mouse brain synaptic membrane perturbation was found to increase with increasing hydrophobicity as shown by the dashed lines. This result was used to interpret the lipid theory of alcohol effect on ion-channel proteins, as being dependent on membrane lipid perturbation (McCreery & Hunt, 1978; Lyon et al., 1981). However, the intoxicating potency of the alcohols could not be explained by this theory, because its measurement by the loss of righting reflex in mice, decreased with 1-heptanol, despite increasing membrane lipid disordering potency and increasing lipid solubility. In contrast, our result showing maximum relative potency of alcohol inhibition of receptor current at 1-hexanol correlates well with potency of alcohol intoxication in mice, as measured by their loss of righting reflex (Lyon et al., 1981).
The n-alcohol potency decrease at 1-heptanol for AMPA GluR1, GluR3 receptor subunits in this study is very comparable to the decrease in intoxicating potencies of these alcohols as measured by the effective doses (ED3) producing ataxia in rats (McCreery & Hunt, 1978). A decrease in alcohol intoxicating potency from 1.7 mmol kg−1 for 1-hexanol to 2.2 mmol kg−1 for 1-heptanol in rats (McCreery & Hunt, 1978) also relate well with a decrease in alcohol inhibitory potency (measured as IC50) from 10 mM for 1-hexanol, to 19 mM for 1-heptanol in GluR1 (Table 1). A similar decrease in alcohol potency from 7 mM for 1-hexanol to 18 mM for 1-heptanol is also seen in the GluR3 receptor inhibition (Table 1).
The cutoff in alcohol effect from our studies is difficult to explain by the lipid hypothesis. This is because the membrane lipid disordering effect of alcohol continue to increase with potency, whereas, receptor current inhibition by alcohol drops off with 1-heptanol as shown in Figure 3. Alcohol cutoff effect from this study, could however be explained by the binding to a hydrophobic pocket of fixed dimensions, as proposed for the anaesthetic inhibition of firefly luciferase protein (Franks & Lieb, 1985).
Cutoffs in alcohol potentiation and inhibition of membrane receptor function have been reported for ligand-gated ion-channels. Alcohol inhibition of ATP-gated ion channel cuts off at 1-propanol, thereby excluding alcohols with a molecular volume ⩾42.2 ml mol−1 (e.g. 1-butanol) from binding or inhibiting the receptor (Li et al., 1994). Cutoff in alcohol inhibition of NMDA ion-channel occurred at 1-octanol, with a size exclusion of 1-nonanol binding (Peoples & Weight, 1995a). A putative hydrophobic binding pocket with a molecular volume less than that of 1-nonanol, which is 103.5 cm3 mol−1 was proposed. A cutoff in alcohol inhibition of neuronal nicotinic acetylcholine ion-channel receptor (AChR) after dodecanol (C12) was reported in Lymnaea stagnalis neurones (McKenzie et al., 1995). The AChR alcohol cutoff occured at the same position (just after dodecanol) as found for the induction of general anaesthesia in tadpoles (Alifimoff et al., 1989).
The observation of an alcohol cutoff effect after dodecanol in tadpoles (Alifimoff et al., 1989) and Lymnaea stagnalis neurones (McKenzie et al., 1995) deals with the anaesthetic effects of alcohol, in contrast to the alcohol potency cutoff effect observed at 1-heptanol in rats (McCreery & Hunt, 1978), mice (Lyon et al., 1981) and AMPA receptors in this study, which is a measurement of alcohol intoxication.
Cutoffs in alcohol potentiation of ion-channel receptor currents have been studied. Alcohols exhibit a cutoff above five carbon atoms (1-pentanol) for the potentiation of serotonin (5-HT3) receptor current in rat nodose ganglion neurons (Fan & Weight, 1994). Potentiation of GABAA receptor currents by alcohols produced cutoff effects after C12 (Peoples & Weight, 1995b), showing a resemblance to alcohol cutoff effect in anaesthesia. Studies have also been performed using the N-terminal domain of a recombinant nAChα7 receptor (known to be inhibited by ethanol), to form a chimera with the transmembrane and C-terminal domains of a recombinant 5-HT3 receptor (known to be potentiated by ethanol). The resulting chimeric receptor was inhibited by ethanol, just like the nAChα7 receptor, suggesting an n-terminal mediation of alcohol's action (Yu et al., 1996).
Furthermore, cutoff in alcohol inhibition of kainate-activated AMPA currents in cultured hippocampal neurons has also been observed at 1-nonanol (unpublished observation). Studies by Wick et al. (1998) provide confirmatory evidence that the size of the alcohol binding pocket responsible for the cutoff effect can be changed. They showed that the glycine receptor (Gly-R-α1) alcohol cutoff can be changed from 1-decanol to 1-propanol and the GABAA-R-ρ1 cutoff alcohol can be also be changed from 1-heptanol to 1-decanol by amino acid mutations on the wild-type receptor. We believe that alcohols interact directly with specific protein sites (that vary in size with the constituent amino acids) on glutamate receptors and other membrane-bound channel receptors to produce their effects.
Contrasting arguments explaining a combined lipid and protein interaction for alcohol cutoff in synaptic membrane perturbation, after dodecanol, has been proposed (Miller et al., 1989). However, this evidence is difficult to reconcile with the difference in chain length and pharmacologic concentrations of alcohols resulting in ion-channel protein cutoffs versus lipid cutoffs. Furthermore, a potentiating, or inert effect may be suggested for 1-nonanol and 1-decanol since they failed to inhibit GluR1 and GluR3 receptor in our study. However, it should be noted that these two alcohols have been shown to potentiate GABAA receptor-ion channel proteins (Nakahiro et al., 1991).
The intoxicating and anaesthetic effects of alcohol have been partly attributed to its stimulatory and inhibitory actions on ligand-gated ion-channels respectively. To this effect, ethanol concentrations that affect AMPA type glutamate receptors range from that found in moderate-severe intoxication (Lovinger, 1993), to that found during general anaesthesia (Weight et al., 1993). The concentrations of alcohols inhibiting, coupled with the alcohol cutoff for the AMPA receptor inhibition in this study, is consistent with severe intoxicating and early anaesthetic concentrations of alcohol. A recent study (Dildy-Mayfield et al., 1996) linked the relative insensitivity of AMPA glutamate receptors to longer chain alcohols, but missed the alcohol cutoff effect. On closer examination of their results, a kainate agonist concentration of 400 μM (a high dose, near the plateau phase of a binding/displacement curve for the receptor) may have interfered with the non-competitive binding effect of the alcohol for the receptor. In contrast, our kainate concentrations of 200 μM for kainate-activated currents in oocytes, are closer to the EC50 for GluR3 receptor (Egebjerg et al., 1991). A plot of agonist concentration-response curve in this study and other reports (Akinshola & Weight, 1995; Akinshola et al., 1999) show that AMPA receptor desensitization occurs at kainate concentration of 400 μM and higher. An NMDA concentration of 500 μM and higher has also been previously reported (Masood et al., 1994) to produce a desensitizing effect in the NMDA receptor. Studies of the cutoff effect of alcohol in receptor mutants will be helpful in determining the mechanism of alcohol inhibition of AMPA receptors.
Acknowledgments
We thank Drs Jim Boulter and Steve Heinemann for providing the GluR1 and GluR3 cDNA clones, Denise Gates and Dr Randy Stewart for help with graphics and Dr Bob Peoples for helpful comments on the manuscript. This work was done in part in Dr Forrest F. Weight's laboratory at the National Institute on Alcohol Abuse and Alcoholism where the author was a senior staff fellow. These experiments were performed with the approval of Howard University Animal Care and Use Committee (Protocol Approval #A-3742-01) and thereby comply with the current laws of the United States of America.
Abbreviations
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- cDNA
complimentary DNA
- cRNA
complimentary RNA
- GluR
glutamate receptor
- NMDA
N-methyl-D-aspartate
- 1°
primary alcohol
References
- AKINSHOLA B.E., PEOPLES R.W., STEWART R., WEIGHT F.F. Sensitivity of native and recombinant glutamate receptors to aliphatic n-alcohol inhibition. Soc. Neurosci. Abstr. 1996;22:338. [Google Scholar]
- AKINSHOLA B.E., TAYLOR R.E., OGUNSEITAN A.E., ONAIVI E.S. Anandamide inhibition of recombinant AMPA receptor subunits in Xenopus oocytes is increased by forskolin and 8-bromo-cyclic AMP. N. Schmied. Arch. Pharmacol. 1999;360:242–248. doi: 10.1007/s002109900078. [DOI] [PubMed] [Google Scholar]
- AKINSHOLA B.E., WEIGHT F.F. Aliphatic n-alcohols exhibit a cutoff in potency for the inhibition of recombinant GluR3 receptor subunit current in Xenopus oocytes. Soc. Neurosci. Abstr. 1995;21:1815. [Google Scholar]
- ALIFIMOFF J.K., FIRESTONE L.L., MILLER K.W. Anaesthetic potencies of primary alkanols: Implications for the molecular dimensions of the anaesthetic site. Br. J. Pharmacol. 1989;96:9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONDI A. Van der Waals volumes and Radii. J. Phys. Chem. 1964;68:441–451. [Google Scholar]
- BOULTER J., HOLLMANN M., O-SHEA-GREENFIELD A., HARTLEY M., DENERIS E., MARON C., HEINEMANN S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990;249:1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- CHOI D.W., ROTHMAN S.M. The role of glutamate neurotoxicity in hypoxicischemic neuronal death. Annu. Rev. Neurol. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- COLLINGRIDGE G.L., BLISS T.V.P. NMDA receptors – their role in long-term potentiation. TINS. 1987;10:288–293. [Google Scholar]
- COLLINGRIDGE G.L., BLAKE J.F., BROWN M.W. Involvement of excitatory amino acid receptors in long-term potentiation in the schaffer collateral commissural pathway of rat hippocampal slices. Can. J. Physiol. Pharmacol. 1991;69:1084–1090. doi: 10.1139/y91-160. [DOI] [PubMed] [Google Scholar]
- COLLINGRIDGE G.L., SINGER W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol. Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- DIETRICH R.A., DUNWIDDIE T.V., HARRIS R.A., ERWIN V.G. Mechanism of action of ethanol: initial central nervous sytem actions. Pharmacol. Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- DELEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay and physiological dose-response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., HARRIS R.A. Comparison of ethanol sensitivity of rat brain kainate, DL-alpha-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Therap. 1992;262:487–494. [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., HARRIS R.A. Ethanol inhibits kainate responses of glutamate receptors expressed in xenopus oocytes: Role of calcium and protein kinase. C. J. Neurosci. 1995;15:3162–3171. doi: 10.1523/JNEUROSCI.15-04-03162.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., MIHIC S.J., LIU Y., DEITRICH R.A., HARRIS R.A. Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br. J. Pharmacol. 1996;118:378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGEBJERG J., BETTLER B., HERMANS-BORGMEYER I., HEINEMANN S. Cloning of a cDNA for a glutamate receptor subunit activated by a kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- FAN P., WEIGHT F.F. Alcohols exhibit a cutoff effect for the potentiation of 5-HT3 receptor-activated current. Soc. Neurosci. Abst. 1994;20:1127. [Google Scholar]
- FRANKS N.P., LIEB W.R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985;316:349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN D.B. The effects of drugs on membrane fluidity. Ann. Rev. Pharmacol. Toxicol. 1984;24:43–64. doi: 10.1146/annurev.pa.24.040184.000355. [DOI] [PubMed] [Google Scholar]
- JANOFF A.S., MILLER K.W. Biological Membranes 1981New York: Academic Press; 417–476.ed Chapman, D. pp [Google Scholar]
- JONAS P., SAKMANN B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J. Physiol. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEINÄNEN K., WISDEN W., SOMMER B., WERNER P., HERB A., VERDOORN T.A., SAKMANN B., SEEBURG P.H. A family of AMPA selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Alcohol action on a neuronal membrane receptor: Evidence for a direct interaction with the receptor protein. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVINGER D.M. High ethanol sensitivity of recombinant AMPA-type glutamate receptors expressed in mammalian cells. Neurosci. Lett. 1993;159:83–87. doi: 10.1016/0304-3940(93)90804-t. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., WHITE G., WEIGHT F.F. Ethanol inhibits NMDA-activated ion-current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- LYON R.C., MCCOMB J.A., SCHREURS J., GOLDSTEIN D.B. A relationship between alcohol intoxication and the disordering of brain membranes by a series of short-chain alcohols. J. Pharmacol. Exp. Ther. 1981;218:669–675. [PubMed] [Google Scholar]
- MASOOD K., WU C., BRAUNEIS U., WEIGHT F.F. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol. Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- MCCREERY M.J., HUNT W.A. Physico-chemical correlates of alcohol intoxication. Neuropharmacol. 1978;17:451–461. doi: 10.1016/0028-3908(78)90050-3. [DOI] [PubMed] [Google Scholar]
- MCKENZIE D., FRANKS N.P., LIEB W.R. Actions of general anaesthetics on a neuronal nicotinic acetylcholine receptor in isolated identified neurones of Lymnae stagnalis. Br. J. Pharmacol. 1995;115:275–282. doi: 10.1111/j.1476-5381.1995.tb15874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER K.W., FIRESTONE L.L., ALIFIMOFF J.K., STREICHER P. Nonanesthetic alcohols dissolve in synaptic membranes without perturbing their lipids. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1084–1087. doi: 10.1073/pnas.86.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAHIRO M., ARAKAWA O., NARAHASHI T. Modulation of GABA receptor-channel complex by alcohols. J. Pharmacol. Exp. Ther. 1991;259:235–240. [PubMed] [Google Scholar]
- PEOPLES R.W., WEIGHT F.F. Cutoff in potency implicates alcohol inhibition of N-methyl-D-aspartate receptors in alcohol intoxication. Proc. Natl. Acad. Sci. U.S.A. 1995a;92:2825–2829. doi: 10.1073/pnas.92.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEOPLES R.W., WEIGHT F.F. Aliphatic alcohols exhibit a cutoff in potency for enhancement of GABA-activated ion current. Soc. Neurosci. Abstr. 1995b;21:1814. [Google Scholar]
- ROTH S., SEEMAN P. The membrane concentrations of neutral and positive anesthetics (alcohols, chlorpromazine, morphine) fit the Meyer-Overton rule of anesthesia; negative narcotic do not. Biochim. Biophys. Acta. 1972;255:207–219. doi: 10.1016/0005-2736(72)90023-5. [DOI] [PubMed] [Google Scholar]
- SEEBURG P.H. The TIPS/TINS lecture: The molecular biology of mammalian glutamate receptor channels. Trends Pharmacol. Sci. 1993;14:297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- STERN-BACH Y., BETTLER B., HARTLEY M., SHEPPARD P.O., OÕHARA P.J., HEINEMANN S.F. Agonist selectivity of glutamate receptors is specified by two structurally related bacterial amino acid binding proteins. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- WEIGHT F.F., PEOPLES R.W., WRIGHT J.M., LI C., AGUAYO L.G., LOVINGER D.M., WHITE G.Neurotransmitter-gated ion channels as molecular sites of alcohol action Alcohol, Cell Membranes, and Signal Transduction in Brain 1993New York: Plenum; 107–122.ed. Alling, C. et al., pp [Google Scholar]
- WICK M.J., MIHIC S.J., UENO S., MASCIA M.P., TRUDELL J.R., BROZOWSKI S.J., YE Q., HARRISON N.L., HARRIS R.A. Mutations of γ-aminobutyric and glycine receptors change alcohol cutoff: Evidence for an alcohol receptor. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU D., ZHANG L., JEAN-LUC E., BERTRAND D., CHANGEUX J., WEIGHT F.F. Ethanol inhibition of nicotinic acetylcholine type 7 receptors involves the amino-terminal domain of the receptor. Mol. Pharmacol. 1996;50:1010–1016. [PubMed] [Google Scholar]


