Abstract
In the present study, for the first time, PDE4 subtypes were identified and semi-quantified in both CD4 and CD8 lymphocytes from healthy and asthmatic individuals.
CD4 and CD8 lymphocytes from healthy and mild asymptomatic asthmatic subjects (receiving β-agonist therapy only) were isolated from peripheral venous blood using appropriate antibody coated paramagnetic beads. PDE4 subtypes and β-actin were identified by digoxigenin (DIG)-labelling reverse transcriptase-polymerase chain reaction and semi-quantified by DIG-detection enzyme-linked immunosorbance assay.
In CD4 and CD8 lymphocytes PDE4A, PDE4B and PDE4D were detected, with no significant differences observed between healthy and asthmatic groups. In CD8 lymphocytes, enzyme subtype expression was lower and showed more intersubject variability.
In functional studies investigating the effects of various PDE inhibitors on PHA-induced proliferation of mononuclear cells from healthy and asthmatic subjects, CDP840 (0.03–10 μM), rolipram (0.1–10 μM) and theophylline (10 μM–1 mM) inhibited PHA-induced proliferation of mononuclear cells from healthy and asthmatic subjects in a concentration-dependent manner, although no significant difference was observed between the groups investigated.
In additional studies, total monocyte cyclic AMP PDE activity was investigated in cells isolated from asthmatic subjects both prior to and 24 h after allergen challenge. Total monocyte cyclic AMP PDE activity remained unaffected following challenge of asthmatic subjects with either house dust mite or cat dander and was inhibited in a concentration-dependent manner by rolipram (0.01–100 μM) both before and after allergen challenge.
Keywords: Lymphocyte, phosphodiesterase, cyclic AMP, asthma
Introduction
Phosphodiesterases (PDEs) are a family of enzymes capable of hydrolysing cyclic adenosine monophosphate (cyclic AMP) to the 5′ mononucleotide adenosine monophosphate (AMP) and are thus key regulators of intracellular levels of this second messenger. In this way PDEs control a wide range of cellular responses. It is now known that PDEs exist in at least 11 heterologous families (Giembycz & Kelly, 1994; Fisher et al., 1998a, 1998b; Fujishige et al., 1999; Torphy & Page, 2000) and that in inflammatory cells, subtypes of the low Km cyclic AMP-specific PDE4 are predominant in terms of expression and function (Giembycz, 1992). In addition, 16 splice variants of PDE4 have now been identified, although little is known of their distribution in inflammatory cells obtained from patients with disease. A large body of evidence exists which suggests PDE activity may be altered in cells obtained from patients with atopic dermatitis (Banner et al., 1995) and it has previously been demonstrated that cyclic AMP PDE activity is elevated in leukocytes from individuals with atopic dermatitis (Grewe et al., 1982), suggested to be secondary to the expression of a novel PDE isoenzyme (Chan et al., 1993). Similarly, lymphocyte PDE activity was augmented in lymphocytes from asthmatic compared to healthy subjects (Tohda et al., 1998) and we have recently extended these findings (Landells et al., 2000). However, some studies have demonstrated that cyclic AMP and cyclic GMP PDE activities, and isoenzyme profiles are similar in CD4+ and CD8+ T lymphocyte homogenates of healthy and atopic subjects (Tenor et al., 1995; Gantner et al., 1997).
To date, most of the published data showing an upregulation of PDE activity and changes in isoenzyme profile, with consequent altered cell function have been with cells obtained from patients with atopic dermatitis or ill-defined atopy (Banner et al., 1995; Hanifin et al., 1996). In one study, semi-quantitative RT–PCR analyses of mRNA from healthy and mild atopic subjects revealed that PDE4A and PDE4B2 were present in both CD4+ and CD8+ cells and that PDE4D was expressed only in CD8+ cells (Gantner et al., 1997). Increased PDE4A and PDE4B2 expression was observed in CD4+ cells from atopic subjects, although this did not appear to result in significantly higher cyclic AMP PDE activity (Gantner et al., 1997). However, it is not yet known which PDE4 subtypes are present in the inflammatory cells of patients with atopic asthma, but it is plausible that differential expression of these isoenzyme subtypes may contribute to the increased PDE activity that has been observed in inflammatory cells isolated from patients with asthma (Tohda et al., 1998; Landells et al., 2000). Currently, much research is being undertaken to develop novel PDE4 inhibitors for the treatment of inflammatory conditions including asthma and a greater understanding of PDE4 subtype expression profiles in inflammatory cells from asthmatic patients may encourage further research and would provide new insight into the causes of the exaggerated immune responses observed in these individuals. It is clearly of interest to know therefore, which PDE4 subtypes are present in inflammatory cells from patients with asthma and whether there is a differential expression of these enzymes relative to the normal population.
The aim of the present study was to investigate whether differences in PDE subtype expression occur in CD4 and CD8 lymphocytes from asthmatic subjects and, if so, whether these have any functional consequence. The protocols applied provided highly purified populations of inflammatory cells and allowed semiquantitative analysis and thus a direct comparison of PDE4 subtype expression.
Methods
Blood donors
Non-smoking healthy donors (18–35 years, male and female, n=16) with no history of asthma, allergic rhinitis or atopic dermatitis were selected and compared to a group of mild asymptomatic asthmatics (18–35 years, male and female, n=16) taking β-agonist therapy only and who had refrained from taking all medication including non-steroidal anti-inflammatory drugs for at least 8 h before blood samples were drawn. In a separate study, blood was taken from subjects with allergic asthma (18–35 years, male and female, n=6) immediately before and 24 h after allergen challenge with either cat dander or house dust mite. These studies were approved by the Ethics Committee of King's College Hospital.
Materials
Dynabeads M-450 CD4 and CD8, and DetachaBead (Dynal U.K. Ltd). TriPure, PCR ELISA Dig-labelling and Dig-detection kits (Roche Diagnostics, Meylan, France). SMEM, L-glutamine, non-essential amino acids (10×), sodium pyruvate, penicillin streptomycin (Gibco, Paisely, U.K.), HEPES, dimethylsulphoxide, Histopaque, Trypan blue, chromotrope 2R, Erlichs haematoxylin, leupeptin, trizma preset crystals, magnesium chloride, dithiothreitol, ethylenediaminetetraacetic acid (EDTA), nα-p-tosyl-L-lysine chloromethylketone, Triton X-100, 2-mercaptoethanol, ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid (EGTA), bovine serum albumin, Snake venom (O. hannah), Dowex-1 strongly basic ion exchange (Sigma-Aldrich Company Ltd, Poole, U.K.). Methanol ‘Analar' (Merck Ltd, Lutterworth, U.K.) Ultroser (Jones Chromatography, Mid Glamorgan, U.K.), phytohaemagglutinin (PHA), Leucosep tubes (Poly Labo, Paris, France). [6-3H]-thymidine, [2,3-3H] adenosine 3′5′ cyclic phosphate ammonium salt (Amersham International, Buckinghamshire, U.K.). Lumagel Lumac Universal LSC cocktail (Prolabo, Paris, France). The following drugs were used: Rolipram (a gift of Schering AG, Berlin, Germany), CDP840 (a gift of Celltech Chirosciences, Slough, U.K.), Siguazodan (a gift of Glaxo SmithKline, U.S.A.), theophylline (Sigma Chemicals, Poole, U.K.).
Purification of CD4 and CD8 lymphocytes from peripheral blood
Peripheral venous blood was obtained from healthy and asthmatic volunteers and collected into ethylenediaminetetraacetic acid (EDTA)-coated Vacutainers. CD4 and CD8 lymphocytes were obtained using paramagnetic beads conjugated to anti-CD4 or anti-CD8 monoclonal antibody (Dynabeads M-450 CD14). CD4- and CD8-positive cells were isolated according to the manufacturer's instructions (Dynal U.K. Ltd). The paramagnetic beads were detached from the isolated cells using DetachaBead (Dynal U.K. Ltd). Using this method, the isolated cells were more than 99% pure and 98% viable, and free of antibody bound to the surface. The cells were then frozen (−70°C) until required for RNA extraction.
Extraction of total RNA
Total RNA was purified from the lymphocytes using Tripure™ Isolation Reagent (Roche Diagnostics, Meylan, France), a single-step liquid phase separation for total RNA isolation developed by Chomczynski & Sacchi (1987). Washed and pelleted cells were resuspended at a concentration of 1×106 cells ml−1 Tripure and total RNA extracted following the manufacturer's instructions. RT–PCR was carried out using the commercial PCR enzyme-linked immunosorbance assay (ELISA) Digoxigenin (Dig) Labelling Kit (Roche Diagnostics, Meylan, France). The RNA samples obtained were dried under vacuum and then the RNA was redissolved in water and quantified using a spectrophotometer, the quality of RNA was assessed by running the samples in an agarose gel (1%).
Reverse transcription and specific amplification by PCR
Complementary DNA (cDNA) was generated from 1 μg of total RNA per sample using random hexamers to prime the reverse transcription (First Strand cDNA kit, Roche Diagnostics, Meylan, France), before use, samples for PCR were incubated for 5 min in boiling water to allow enzyme denaturation. The samples were then directly amplified by PCR following the addition of specific PDE4 primer pairs and Taq polymerase or stored (−20°) until further use.
PCR was carried out using the commercial PCR ELISA Dig-labelling kit (Roche Diagnostics). cDNA was added to a mastermix containing PCR buffer, magnesium chloride, DIG-labelled deoxynucleotide mix, upstream and downstream primers and Taq polymerase. The following fragments were amplified: β-actin, PDE4A, PDE4B and PDE4D. PDE4C was not amplified as previous experiments showed that it was not expressed in CD4 and CD8 lymphocytes; moreover this subtype has been shown to not be present in inflammatory cells (Giembycz et al., 1996). β-actin, which is expressed ubiquitously, was amplified to ensure the preparation of cDNA was homogenous and also served as a positive control in later stages of the study. The oligonucleotide primer pairs for each of these fragments were chosen using the PDE4 sequences corresponding to the following GenBank accession numbers: PDE4A (L20965), PDE4B (L20966) and PDE4D (L20970). The sequence of the different primers, and the conditions required for amplification by PCR are documented in Table 1. All the PDE4 subtype PCR fragments correspond to conserved regions among the variants derived from the gene of each PDE4 subtype; thus, the primers were able to detect all the members of the same subtype family. The number of PCR cycles was optimized for each subtype family, in order to be in the exponential phase of the reaction. A negative control (sterile water in the place of cDNA) was performed for each PCR to verify that no cross contamination had taken place. Following thermocycling, the samples were visualized by running through an agarose gel (1%) containing ethidium bromide and viewing under ultraviolet light. The size of each of the amplified fragments was ascertained by running a DNA molecular weight marker (VI or VIII; Roche Diagnostics) through the gel alongside samples.
Table 1.
Primers and conditions used in RT–PCR

Quantification of PCR products
All samples were visualized as described in the previous section, then the density of the bands of the amplified fragments on the gel were analysed using a Multianalyst programme (Biorad). Quantification was also performed spectrophotometrically by ELISA. For this purpose, amplified dig-labelled PCR fragments were analysed using the PCR ELISA Dig Detection kit from Roche Diagnostics (Meylan, France). Briefly, this PCR ELISA allows the specific detection of PCR products in a semi-quantitative microtiter plate format. The PCR products were labelled with digoxigenin during the amplification process (Dig labelling). The labelled PCR products were analysed by solution hybridization to a specific capture probe that is complementary to the inner part of the amplification product (Dig detection). This capture probe is labelled with biotin to allow immobilization of the hybrid to a streptavidin coated microtiter plate surface. Non-specific amplification products will not hybridize to this capture probe and thus will be removed during the subsequent washing steps. The bound hybrid is detected by an anti-digoxigenin peroxidase (anti-Dig-POD) conjugate and use of the colorimetric substrate, ABTS. By comparing with suitable standards, the colour signal can be used for semi-quantitative evaluation of the amplification reaction. In our experiments, the biotinylated capture probes used for the detection of PCR fragments were 5′-AGCGCCAGCGCAGAGCCTAC-3′ for PDE4A (hybridization temperature 55°C), 5′-TAGACCCCTAACATG-3′ for PDE4B (hybridization temperature 40°C), 5′-CAATGTCTCAGATCAGTGCA-3′ for PDE4D (hybridization temperature 40°C) and 5′-CTTCTACAATGAGCTGCGTG-3′ for β-actin (hybridization temperature 50°C).
The samples were incubated with the probe for 1 h 30 min, with the temperature of incubation dependent on the sequence of the probe. After this time, the plate was washed five times with phosphate buffered saline (PBS), then anti-Dig-POD solution (1 μg ml−1) was added (37°C, 30 min). The plate was then washed as before and substrate solution was added (37°C, 30 min). The absorbance of each well was determined at 405 nm. The negative control (containing sterile water in place of cDNA) from each PCR was included, as was a negative control for the ELISA (sterile water was substituted for a PCR product).
Mononuclear cell proliferation studies
Twenty-five ml of blood was collected and mononuclear cells separated and purified according to a previously described method (Banner et al., 1995). Human peripheral mononuclear cells (100,000 per well) were stimulated for 24 h with phytohaemagglutinin (PHA, 2 μg ml−1) in the absence or presence of rolipram (0.1–10 μM), CDP-840 (0.03–10 μM) or theophylline (1–1000 μM) at 37°C in a 95% air, 5% CO2 atmosphere. Twenty-four hours later, [3H]-thymidine (0.1 μCi) was added to each well and cells incubated for a further 24 h period. Cells were then harvested onto glass fibre filters using a cell harvester (ICN Flow, Buckinghamshire, U.K.) and counted in a scintillation counter.
The effect of allergen challenge on monocyte PDE activity
Blood was taken from asthmatic subjects (n=6) who exhibited both an early and a late response, just before and 24 h after allergen challenge. In order to ascertain the effects of the selective PDE4 inhibitor rolipram on total cyclic AMP PDE activity, a two-step method modified from that of Thompson et al. (1979) was employed. Freshly prepared mononuclear cells, separated and purified as for proliferation studies (Banner et al., 1995), were suspended in ice-cold homogenization buffer (leupeptin 10 μg ml−1, Tris HCL 20 mM (pH 7.5), magnesium chloride 2 mM, dithiothreitol 1 mM, EDTA 5 mM, nα-p-tosyl-L-lysine chloromethylketone 0.2 mM and Triton 1%) and homogenized with a Dounce homogenizer (30 strokes). The homogenates were assayed in a mixture containing Tris HCL 20 mM, magnesium chloride 10 mM, 2-mercaptoethanol 4 mM, ethyleneglycol-bis-(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid (EGTA) 0.2 mM, bovine serum albumin (BSA; 0.05 mg ml−1), 1 μM cyclic AMP (approximately 170,000 c.p.m. of tritiated cyclic AMP) in the presence of either drug vehicle, or the PDE4 inhibitor rolipram for 25 min at 30°C. The reaction was terminated (1 min, 100°C) and the assay mixture cooled on ice. O. Hannah snake venom (0.5 mg ml−1) was added to the assay mixture which was then incubated for 10 min at 37°C, following this, methanol (1 ml) was added. The assay mixture was loaded onto Dowex 1 (1×8–400) columns (1 ml) and eluted into Lumagel scintillation fluid before washing the columns with 1 ml methanol and eluting to dryness. The radioactivity in the eluate was counted using a liquid scintillation counter.
Statistical analyses
For PDE4 subtype identification and quantification studies, results are presented as mean±s.e.mean of three independent readings. Data are expressed in arbitrary units; (subtype absorbance/β actin absorbance) for each subject, where absorbance is expressed as units of optical density (OD) for the ELISA experiments. These calculations were performed to allow direct comparison of results from studies in which PCR products were processed and quantified at different times. Readings for the MultiAnalyst analysis of density of the bands of amplified fragments on the gel were taken as density mm−2 or OD mm−2. Statistical evaluation was carried out on all results in order to compare healthy and asthmatic groups. Data were analysed parametrically using a Student's unpaired t-test. In each case the null hypothesis would have been rejected if P<0.05, and any differences considered to be significant.
For mononuclear cell proliferation studies, each drug was examined on blood samples from six to eight volunteers and each concentration of PDE inhibitor was examined in triplicate. Geometric means were calculated together with 95% confidence limits. Data are expressed as mean±s.e.mean. Drug treated cells were compared with vehicle-treated cells by Dunnett's test.
In studies investigating the effect of allergen challenge on monocyte PDE activity in cells from asthmatic subjects, cyclic AMP PDE activity is expressed as pmol cyclic AMP hydrolyzed per min per mg of protein. The PDE profiles of different subjects were analysed using a one-way ANOVA followed by a Tukey-Kramer post-hoc test. Differences were considered to be significant if P<0.05.
Results
In preliminary experiments it was necessary to determine the optimal number of cycles for the PCR, in order to be able to suitably compare the PDE4 subtypes profiles in CD4 and CD8 cells from healthy and asthmatic subjects. For β-actin and all PDE4 subtypes, it was found that 30 cycles gave a visible signal on a gel without reaching saturation.
The expression of β-actin in CD4 (n=10) and CD8 (n=6) lymphocytes was chosen as an internal standard to assess PCR efficacy in each tested sample. It was then necessary to show that the expression of this PCR product was stable among the different subjects in the same group and also in both groups of control and asthmatic patients. Expression of β-actin was initially visualized using MultiAnalyst software, computer analysis of the density of bands observed on the agarose gel allowed comparison of samples with others on the same gel. As shown in Figures 3a and 4a, the expression of this PCR product was stable and identical in all the subjects used in the experiment, allowing us to use it as an internal standard to measure the variation, if any, of PDE4 subtype expression between the two groups of patients. In a second step, β-actin expression was also quantified by an ELISA, using 0.5 μl of sample.
Figure 3.
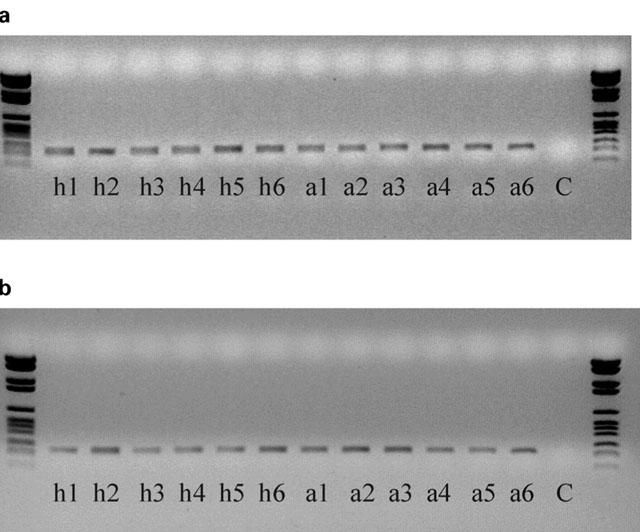
Amplified fragments of (a) β-actin (237 base pairs) and (b) PDE4D (339 base pairs) from CD4 lymphocytes from healthy and asthmatic subjects. ‘h' denotes healthy, ‘a' denotes asthmatic, ‘C' denotes negative control (sterile water substituted for cDNA). Numbers correspond to subject numbers.
Figure 4.
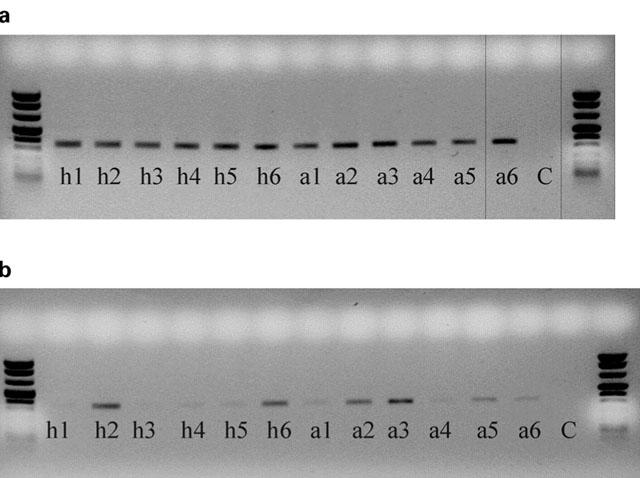
Amplified fragments of (a) β-actin (237 base pairs) and (b) PDE4D (339 base pairs) from CD8 lymphocytes from healthy and asthmatic subjects. ‘h' denotes healthy, ‘a' denotes asthmatic, ‘C' denotes negative control (sterile water substituted for cDNA). Numbers correspond to subject numbers.
The expression of PDE4A, PDE4B and PDE4D was demonstrated in CD4 and CD8 lymphocytes obtained from healthy and asthmatic subjects (Figures 1 and 2). The expression of these isoforms from CD4 and CD8 lymphocytes were compared by density analysis of the bands on gels and quantified by an ELISA. In CD4 cells, there was no significant difference (P>0.05) in the expression of PDE4A (healthy 1.118±0.261 vs asthmatic 0.836±0.130), PDE4B (healthy 0.806±0.115 vs asthmatic 1.033±0.109) and PDE4D (healthy 0.984±0.103 vs asthmatic 1.186±0.179). An example of an agarose gel showing PDE4D expression in CD4 lymphocytes of healthy and asthmatic subjects is shown in Figure 3b. As with CD4, in CD8 lymphocytes there was no significant difference (P>0.05) in the expression of PDE4A (healthy 0.884±0.455 vs asthmatic 0.431±0.217), PDE4B (healthy 1.238±0.544 vs asthmatic 1.062±0319) or PDE4D (healthy 1.045±0.268 vs asthmatic 0.903±0.232). The expression of PDE4 subtypes was less in CD8 lymphocytes and more variable among individuals. An example of an agarose gel showing PDE4D expression in CD8 lymphocytes of healthy and asthmatic subjects is shown in Figure 4b. Moreover, PDE4D subtype expression was reduced in CD8 when compared to CD4 lymphocytes, this can be seen by comparing Figures 3b and 4b.
Figure 1.
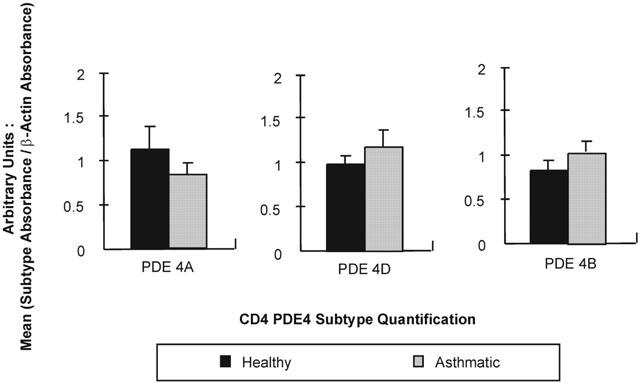
Quantification of PDE4A, PDE4B and PDE4D in CD4 lymphocytes from both healthy and asthmatic subjects. An ELISA technique was used on fragments amplified by RT–PCR (sample sizes vary: PDE4A=5 μl sample, PDE4B=1 μl sample, PDE4D=0.5 μl sample). Readings were taken as absorbance at 402 nm after 30 min incubation with the substrate and data are expressed in arbitrary units (subtype absorbance/β actin absorbance). In each case, values are expressed as mean±s.e.mean (n=10). None of these results were found to be statistically significant (P>0.05).
Figure 2.
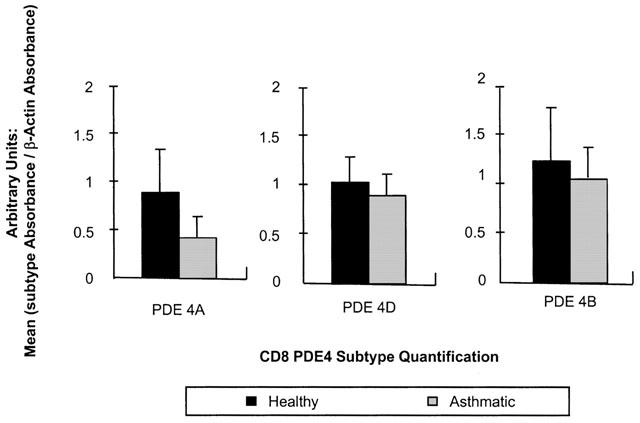
Quantification of PDE4A, PDE4B and PDE4D in CD8 lymphocytes from both healthy and asthmatic subjects. An ELISA technique was used on fragments amplified by RT–PCR (sample sizes vary: PDE4A=10 μl sample, PDE4B=10 μl sample, PDE4D=1 μl sample). Readings were taken as absorbance at 402 nm after 30 min incubation with the substrate and data are expressed in arbitrary units (subtype absorbance/β actin absorbance). In each case, values are expressed as mean±s.e.mean (n=6). None of these results were found to be statistically significant (P>0.05).
Mononuclear cell proliferation
PHA induced proliferation of mononuclear cells, obtained from both healthy and asthmatic subjects, to the same extent (counts per minute (c.p.m.): healthy 7812±766.5 vs asthmatic 8890±369.2, P>0.05). The PDE4 inhibitors CDP480 (Figure 5a) and rolipram (Figure 5b) and the non-selective PDE inhibitor, theophylline (Figure 5c) inhibited PHA-induced proliferation of mononuclear cells from healthy and asthmatic subjects in a concentration-dependent manner, although there was no significant difference in the inhibitory activity of any of these drugs between the two groups (P>0.05).
Figure 5.
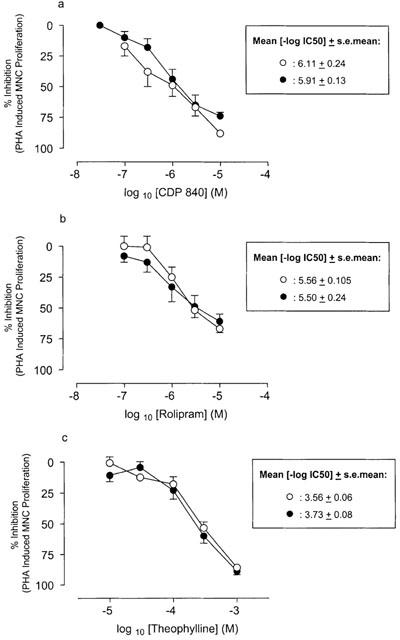
Effect of (a) CDP-840, (b) rolipram and (c) theophylline on PHA-induced proliferation of mononuclear cells from healthy (open circles) and asthmatic (closed circles) subjects. Data are expressed as per cent of the values obtained for PHA stimulated cells without drug. Results represent mean±s.e.mean (n=6–8 per group).
The effect of allergen challenge on monocyte PDE activity
The effect of allergen challenge (house dust mite or cat dander) on monocyte cyclic AMP PDE activity (pmol min−1 mg protein−1) and the response to rolipram was investigated using cells isolated from asthmatic subjects exhibiting both an early (27.8±4.6% fall in FEV1) and late (30.7±5.3% fall in FEV1) asthmatic response. Allergen challenge did not alter monocyte total cyclic AMP PDE activity (pmol min−1 mg−1) of these subjects (28.0±5.8 vs 23.2±5.0; n=6).
Monocyte cyclic AMP PDE activity was inhibited in a concentration-dependent manner by rolipram before and after allergen challenge in all subjects (P<0.05, n=6) (Figure 6) and the degree of inhibition by rolipram was not altered in any way by allergen challenge (P>0.05; n=6).
Figure 6.
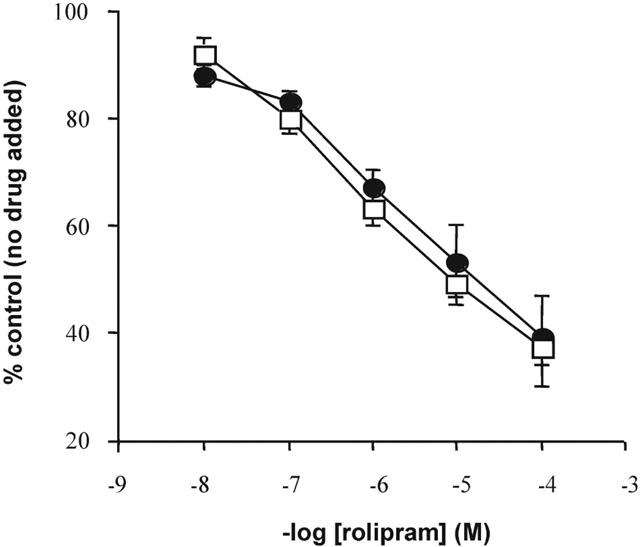
Effect of rolipram on total monocyte cyclic AMP PDE activity in cells from asthmatic subjects exhibiting both an early and late asthmatic response, before (open symbols) and 24 h after (closed symbols) allergen challenge. Data are expressed as per cent control, i.e. no drug added. Values are presented as mean±s.e.mean (n=6).
Discussion
The results of the present study demonstrate for the first time the profile of PDE4 subtypes in lymphocytes obtained from mild asthmatic individuals in comparison with lymphocytes obtained from healthy subjects. The PCRs for the PDE4 subtypes showed that PDE4A, PDE4B and PDE4D were present in high quantities and to a similar extent in CD4 lymphocytes obtained from both healthy and asthmatic subjects as verified by analysis of the density of the bands on the gel and by ELISA. A PCR for β-actin was performed as a positive control for the CD4 and CD8 cells. The level of β-actin expression was similar in healthy and asthmatic subjects, indicating that the measurement of RNA was accurate and that the cDNA synthesis had been completed successfully. It appears that there was no significant difference in the expression of any of the PDE4 subtypes in CD4 and CD8 lymphocytes between the healthy and mild asymptomatic subjects using quantification by an ELISA. Our data are at variance with a previous study (Gantner et al., 1997) which showed that the PDE4D subtype was not expressed in the CD4 lymphocytes obtained from normal or atopic donors, although, CD8 lymphocytes exhibited a strong signal corresponding to this subtype. In the present experiment, PDE4D was highly expressed in both CD4 and CD8 lymphocytes with even a stronger signal in CD4 cells. Our finding that the three subtype families were expressed in both CD4 and CD8 cells is in good agreement with the data obtained by others using cells obtained from atopic donors (Giembycz et al., 1996). The difference between our results and those of Gantner et al. (1997) concerning PDE4D expression could be due to the choice of the specific primers used for the PCR and/or to different PCR conditions, i.e. the number of amplification cycles and the choice of the hybridization temperature. This distribution of the three subtypes in the CD4 and CD8 lymphocyte subsets appears to be identical to the one found in human eosinophils and monocytes but is rather different from that found in Jurkat cells commonly used as a lymphocyte representative cell line (Engels et al., 1994). This finding highlights the potential limitations of using some cell lines as models of human leukocytes when studying inflammation and especially if PDE4 is the protein of interest.
The expression of the PDE4 subtypes was lower and more variable among individuals in CD8 lymphocytes obtained from both normal and asthmatic subjects, as greater sample volumes were required for the ELISA and the resultant bands were fainter upon visualization. Since β-actin expression was similar for each healthy and asthmatic subject, this would indicate that the cDNA preparations were completed accurately. Furthermore, since the fragments for CD4 and CD8 samples were concurrently amplified, this would indicate that the low expression of PDE4 subtypes in CD8 lymphocytes was genuine and not as a result of experimental error. As with CD4 lymphocytes, there was no significant difference in the expression of any of the PDE4 subtypes between healthy and asthmatic subjects.
The fact that no significant difference in expression of any of the three PDE4 subtypes identified and quantified was found when comparing cell samples from healthy and asthmatic subjects was also reflected in the functional studies that we conducted. The selective PDE4 inhibitors CDP840 or rolipram and the non-selective PDE inhibitor theophylline, inhibited PHA-induced proliferation of mononuclear cells from both healthy and asthmatic subjects to similar levels. No significant difference was found when comparing the two groups. These data extend our previous observations that despite an increased overall PDE activity in inflammatory cells obtained from patients with asthma compared to cells obtained from healthy subjects, there was no altered PDE4 activity or altered sensitivity to PDE4 inhibitors with respect to proliferation or TNFα release (Landells et al., 2000). These results differ markedly from studies with cells isolated from the peripheral blood of patients with atopic dermatitis (Banner et al., 1995; Hanifin et al., 1996).
We were unable to demonstrate any evidence of altered PDE4 expression in mild asymptomatic subjects and our study does not support the hypothesis that asthma is characterized by a molecular difference in PDE4 subtype expression. Moreover, it suggests that work with atopic dermatitis cannot be extrapolated to atopic asthma and indicates that PDE4 subtype dysregulation is unlikely to be a major molecular cause of atopy. Nonetheless, the selection of the patients (severity of the disease, medication) may be critical in this kind of study and it remains plausible that any possible molecular differences in PDE4 activity in asthma may only be detected when studying the splice variants or may be due to short-term regulation of the enzyme, i.e. phosphorylation (Conti et al., 1995), binding with phosphatidic acid (Marcoz et al., 1993) and thus not detected by the techniques used in the present study. It would be of interest, therefore, to conduct similar studies using cells obtained from patients with more severe asthma. In an attempt to address the question of whether or not monocyte PDE activity and therefore, PDE isoenzyme expression, may be different in more severe asthmatics, we measured monocyte cyclic AMP PDE activity in cells from allergic individuals following allergen-induced airways obstruction and thus exhibiting symptoms of asthma. However, allergen challenge did not alter monocyte total cyclic AMP PDE activity in cells isolated from asthmatic subjects exhibiting both an early and late asthmatic response. Furthermore, monocyte cyclic AMP PDE activity was inhibited in a concentration-dependent manner by rolipram in all asthmatic subjects.
PDE3 and PDE7 are also expressed in CD4 and CD8 lymphocytes (Giembycz et al., 1996) and it is possible that these isoenzymes may account for the previously reported changes in PDE activity in leukocytes from asthmatic subjects. We have recently shown that siguazodan (a PDE3 inhibitor) produces significantly greater inhibition of cyclic AMP PDE activity in monocyte homogenates from asthmatic subjects compared to healthy subjects (Landells et al., 2000). Furthermore, it has recently been shown that lymphocytes which are activated by co-stimulation of CD3 and CD28 have increased expression of PDE7 and that the increased expression correlates with decreased cyclic AMP, augmented IL-2 release and proliferation (Li et al., 1999). When PDE7 expression was reduced by the addition of a PDE7 antisense oligonucleotide, proliferation was reduced also (Li et al., 1999). These results indicate that PDE7 may also have an important role in activated lymphocytes.
In summary, these results demonstrate that there is expression of PDE4A, PDE4B and PDE4D in CD4 lymphocytes and to a lesser extent in CD8 lymphocytes of patients with mild atopic asthma. However, there was no difference in expression of any of the PDE4 subtypes observed between healthy and mild asthmatic subjects and no differences were noted between these subjects with respect to the effect of selective or non-selective PDE inhibition on lymphocyte proliferation.
Acknowledgments
We wish to thank Pfizer Global Research and Development, Fresnes, France for the funding of these studies.
Abbreviations
- cyclic AMP
cyclic adenosine monophosphate
- cDNA
complementary deoxyribonucleic acid
- c.p.m.
counts per minute
- Dig
Digoxigenin
- EDTA
ethylenediaminetetraacetic acid
- EGTA
Ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetra-acetic acid
- ELISA
enzyme-linked immunosorbance assay
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PDE
phosphodiesterase
- POD
peroxidase
- RNA
ribonucleic acid
References
- BANNER K.H., ROBERTS N.M., PAGE C.P. Differential effect of phosphodiesterase 4 inhibitors on the proliferation of human peripheral blood mononuclear cells from normals and subjects with atopic dermatitis. Br. J. Pharmacol. 1995;116:3169–3174. doi: 10.1111/j.1476-5381.1995.tb15120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN S.C., REIFSNYDER D., BEAVO J.A., HANIFIN J.M. Immunochemical characterization of the distinct monocyte cyclic AMP-phosphodiesterase from patients with atopic dermatitis. J. Allergy Clin. Immunol. 1993;91:1179–1188. doi: 10.1016/0091-6749(93)90321-6. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CONTI M., NEMOZ G., SETTE C., VICINI E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr. Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- ENGELS P., FICHTEL K., LUBBERT H. Expression and regulation of human and rat phosphodiesterase type IV isogenes. FEBS Lett. 1994;350:291–295. doi: 10.1016/0014-5793(94)00788-8. [DOI] [PubMed] [Google Scholar]
- FISHER D.A., SMITH J.F., PILLAR J.S., ST DENIS S.H., CHENG J.B. Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem. Biophys. Res. Commun. 1998a;246:570–577. doi: 10.1006/bbrc.1998.8684. [DOI] [PubMed] [Google Scholar]
- FISHER D.A., SMITH J.F., PILLAR J.S., ST DENIS S.H., CHENG J.B. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J. Biol. Chem. 1998b;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- FUJISHIGE K., KOTERA J., OMORI K. Striatum- and testis-specific phosphodiesterase PDE10A isolation and characterization of a rat PDE10A. Eur. J. Biochem. 1999;266:1118–1127. doi: 10.1046/j.1432-1327.1999.00963.x. [DOI] [PubMed] [Google Scholar]
- GANTNER F., TENOR H., GEKELER V., SCHUDT C., WENDEL A., HATZELMANN A. Phosphodiesterase profiles of highly purified human peripheral blood leukocyte populations from normal and atopic individuals: a comparative study. J. Allergy Clin. Immunol. 1997;100:527–535. doi: 10.1016/s0091-6749(97)70146-5. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of bronchial asthma. Biochem. Pharmacol. 1992;43:2041–2051. doi: 10.1016/0006-2952(92)90160-k. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., CORRIGAN C.J., SEYBOLD J., NEWTON R., BARNES P.J. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br. J. Pharmacol. 1996;118:1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., KELLY J.J.Current status of cyclic nucleotide phosphodiesterase isozymes Methylxanthines and Phosphodiesterase Inhibitors in the Treatment of Airways Disease 1994Parthenum Press; 27–80.ed. Costello, J.F. & Piper, P.S. pp [Google Scholar]
- GREWE S.R., CHAN S.C., HANIFIN J.M. Elevated leukocyte cyclic AMP-phosphodiesterase in atopic disease: a possible mechanism for cyclic AMP-agonist hyporesponsiveness. J. Allergy Clin. Immunol. 1982;70:452–457. doi: 10.1016/0091-6749(82)90008-2. [DOI] [PubMed] [Google Scholar]
- HANIFIN J.M., CHAN S.C., CHENG J.B., TOFTE S.J., HENDERSON W.R., , JR, KIRBY D.S., WEINER E.S. Type 4 phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J. Invest. Dermatol. 1996;107:51–56. doi: 10.1111/1523-1747.ep12297888. [DOI] [PubMed] [Google Scholar]
- LANDELLS L.J., SPINA D., SOUNESS J.E., O'CONNOR B.J., PAGE C.P. A biochemical and functional assessment of monocyte phosphodiesterase activity in healthy and asthmatic subjects. Pulm. Pharmacol. Ther. 2000;13:231–239. doi: 10.1006/pupt.2000.0248. [DOI] [PubMed] [Google Scholar]
- LI L., YEE C., BEAVO J.A. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- MARCOZ P., NEMOZ G., PRIGENT A.F., LAGARDE M. Phosphatidic acid stimulates the rolipram-sensitive cyclic nucleotide phosphodiesterase from rat thymocytes. Biochim. Biophys. Acta. 1993;1176:129–136. doi: 10.1016/0167-4889(93)90187-t. [DOI] [PubMed] [Google Scholar]
- TENOR H., STANICIU L., SCHUDT C., HATZELMANN A., WENDEL A., DUKANOVIC R., CHURCH M.K., SHUTE J.K. Cyclic nucleotide phosphodiesterases from purified human CD4+ and CD8+ T lymphocytes. Clin. Exp. Allergy. 1995;25:616–624. doi: 10.1111/j.1365-2222.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- THOMPSON W.J., TERASAKI W.L., EPSTEIN P.M., STRADA S.J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv. Cyclic. Nucleotide. Res. 1979;10:69–92. [PubMed] [Google Scholar]
- TOHDA Y., NAKAHARA H., KUBO H., OHKAWA K., FUKUOKA M., NAKAJIMA S. Effects of theophylline on lymphocyte phosphodiesterase activity. Gen. Pharmacol. 1998;31:409–413. doi: 10.1016/s0306-3623(98)00027-5. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., PAGE C. Phosphodiesterases: the journey towards therapeutics. Trends Pharmacol. Sci. 2000;21:157–159. doi: 10.1016/s0165-6147(00)01478-4. [DOI] [PubMed] [Google Scholar]


