Abstract
Fibrosis leads to chronic impairment of cardiac and renal function and thus reversal of existing fibrosis may improve function and survival. This project has determined whether pirfenidone, a new antifibrotic compound, and spironolactone, an aldosterone antagonist, reverse both deposition of the major extracellular matrix proteins, collagen and fibronectin, and functional changes in the streptozotocin(STZ)-diabetic rat.
Streptozotocin (65 mg kg−1 i.v.)-treated rats given pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone; approximately 200 mg kg−1 day−1 as 0.2–2g 1−1 drinking water) or spironolactone (50 mg kg−1 day−1 s.c.) for 4 weeks starting 4 weeks after STZ showed no attenuation of the increased blood glucose concentrations and increased food and water intakes which characterize diabetes in this model.
STZ-treatment increased perivascular and interstitial collagen deposition in the left ventricle and kidney, and surrounding the aorta. Cardiac, renal and plasma fibronectin concentrations increased in STZ-diabetic rats. Passive diastolic stiffness increased in isolated hearts from STZ-diabetic rats. Both pirfenidone and spironolactone treatment attenuated these increases without normalizing the decreased +dP/dtmax of STZ-diabetic hearts.
Left ventricular papillary muscles from STZ-treated rats showed decreased maximal positive inotropic responses to noradrenaline, EMD 57033 (calcium sensitizer) and calcium chloride; this was not reversed by pirfenidone or spironolactone treatment. STZ-treatment transiently decreased GFR and urine flow rates in isolated perfused kidneys; pirfenidone but not spironolactone prevented the return to control values.
Thus, short-term pirfenidone and spironolactone treatment reversed cardiac and renal fibrosis and attenuated the increased diastolic stiffness without normalizing cardiac contractility or renal function in STZ-diabetic rats.
Keywords: Diabetes, fibrosis, pirfenidone, spironolactone
Introduction
Diabetics have a markedly greater incidence of cardiovascular disease and renal failure than the non-diabetic population (Laasko, 1999). Both the heart and kidney show a slowly-developing increase in extracellular matrix deposition, termed fibrosis, in diabetes (Riva et al., 1998; Ziyadeh, 1993). Since fibrosis results in cardiac and renal dysfunction (Brilla & Weber, 1992) including arrhythmias (Assayag et al., 1997), reversal of fibrosis may improve organ function and improve survival. However, current therapeutic approaches to the reversal of progressive renal fibrosis in diabetes have been relatively ineffective (El-Nahas et al., 1997).
Reversal is more clinically relevant than prevention since fibrosis and the accompanying loss of function may first occur many years after the onset of diabetes. Pirfenidone both prevents and reverses lung fibrosis and attenuates pulmonary dysfunction following bleomycin treatment in hamsters (Iyer et al., 1995; Schelegle et al., 1997) by suppressing inflammatory events and down-regulating lung procollagen I over-expression (Iyer et al., 1999a). Pirfenidone also reduces both renal fibrosis and renal damage in the post-obstructed kidney (Shimizu et al., 1997). Hypertension-associated cardiac fibrosis in rats can be prevented (Brilla & Weber, 1992) and partially reversed (Young et al., 1995) by the aldosterone antagonist, spironolactone. Thus, both pirfenidone and spironolactone are candidate drugs for reversal of cardiac and renal fibrosis in diabetic rats.
The aims of this study were firstly to quantify the deposition of collagens and fibronectin in the streptozotocin-diabetic rat heart and kidney, secondly to determine whether administration of pirfenidone or spironolactone can reverse this fibrosis and thirdly to measure the functional changes in isolated hearts, cardiac and vascular tissues and kidneys of these rats.
Methods
Male Wistar rats (8–10 weeks old) were obtained from the Central Animal Breeding House of The University of Queensland. All experimental protocols were approved by the Animal Experimentation Ethics Committee of The University of Queensland. Rats, anaesthetized with tiletamine (25 mg kg−1 i.p.) and zolazepam (25 mg kg−1 i.p.) (Zoletil®) together with xylazine (10 mg kg−1 i.p.), were given a single rapid injection of freshly prepared streptozotocin (STZ, 65 mg kg−1) into the jugular vein. Experiments were performed 4 or 8 weeks after STZ treatment. After 4 weeks, rats were given either pirfenidone (2 g l−1 drinking water for control rats; 200 mg l−1 drinking water for STZ-treated rats) or spironolactone (50 mg kg−1 day−1 s.c.) for a further 4 weeks.
Intakes of food and water as well as body weight were measured daily for all rats. Systolic blood pressure was measured in unanaesthetized rats using a tail-cuff method. Rats were killed with pentobarbitone (200 mg kg−1 i.p.). Blood was taken from the abdominal vena cava, centrifuged and the plasma frozen. Plasma glucose was measured by Precision Plus Blood Glucose Electrodes (Medisense, Abbott Laboratories); plasma Na+ and K+ were measured by flame photometry.
Collagen distribution and fibronectin concentrations
Collagen distribution was determined by image analysis of picrosirius red (0.1% Sirius Red F3BA in picric acid)-stained sections of heart and kidney. Slides were left in 0.2% phosphomolybdic acid for 5 min, washed, left in picrosirius red for 90 min, then in 1 mM HCl for 2 min and 70% ethanol for 45 s. The stained sections were analysed with an Image Pro plus analysis program with results expressed as a percentage of red area in each screen. At least four areas were examined in each heart.
Fibronectin concentrations were determined in hearts stored at −10°C in phenylmethyl-sulphonyl fluoride (0.1 M). Hearts were ground and centrifuged to separate soluble and insoluble fibronectin. A sandwich enzyme-linked immunosorbent assay (ELISA) protocol was followed using a rabbit antihuman fibronectin antibody in PBS-Tween 20 (10 mM) buffer with 2% bovine serum albumin, and sheep anti-rabbit fibronectin conjugated to horse radish peroxidase. Colour formation was measured at 495 nm after addition of o-phenylenediamine and hydrogen peroxide. Concentrations were read against appropriate standards.
Isolated Langendorff heart preparation
Rats were deeply anaesthetized with sodium pentobarbitone (100 mg kg−1 i.p.) and heparin (2000 iu) was administered via the femoral vein. After allowing 2 min for the heparin to fully circulate, the heart was excised and placed in cooled (0°C) crystalloid perfusate (Krebs-Henseleit solution of the following composition in mM: NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.3, NaHCO3 25.0, glucose 11.0). The heart was then attached to the cannula (with the tip of the cannula positioned immediately above the coronary ostia of the aortic stump) and perfused in a non-recirculating Langendorff fashion at 100 cm of hydrostatic pressure. The buffer temperature was maintained at 35°C. The hearts were punctured at the apex to facilitate thebesian drainage and paced at 250 b.p.m. by surface electrodes to the right atrium.
A balloon catheter was inserted in the left ventricle via the mitral orifice for measurement of left ventricular developed pressure. The catheter was connected via a three-way tap to a micrometer syringe and to a Statham P23 pressure transducer. The outer diameter of the catheter was similar to the mitral annulus to prevent ejection of the balloon during the systolic phase. After a 10 min stabilization period, steady-state left ventricular pressure was recorded from isovolumetrically beating hearts. Increments in balloon volume were applied to the heart until left ventricular end-diastolic pressure reached approximately 30 mmHg. At the end of the experiment, the atria and right ventricle were dissected away and the weight of the left ventricle plus septum was recorded. Myocardial diastolic stiffness was calculated as the diastolic stiffness constant (k, dimensionless), the slope of the linear relation between tangent elastic modulus (E, dyne cm−2) and stress (σ, dyne cm−2) (Mirsky & Parmley, 1973; Brown et al., 1999). To assess contractile function, maximal +dP/dt was calculated at a diastolic pressure of 5 mmHg.
Isolated cardiac muscles and thoracic aortic rings
The heart was removed under deep anaesthesia with sodium pentobarbitone (100 mg kg−1) The right atria and papillary muscles from the left ventricle were removed and suspended in organ baths at a resting tension of 5–10 mN adjusted to give the maximal twitch response. Tissues were bathed in a modified Tyrodes solution (in mM): NaCl 136.9, KCl 5.4, MgCl2 1.05, CaCl2 1.8, NaHCO3 22.6, NaH2PO4 0.42, glucose 5.5, ascorbic acid 0.28, sodium edetate 0.05, bubbled with 95% O2/5%CO2 and stimulated at 1 Hz at 35°C as previously described (Brown et al., 1991a). Cumulative concentration-response curves were measured for noradrenaline or EMD 57033 and, following washout and re-equilibration of noradrenaline-treated muscles only, to calcium chloride. At the end of the experiment, papillary muscle dimensions were measured under the loading conditions of the experiment; all tissues were blotted and weighed.
Thoracic aortic rings (approximately 4 mm in length) were suspended with a resting tension of 10 mN (Brown et al., 1991b) and contracted twice with isotonic KCl (100 mM). The presence of endothelium was demonstrated by addition of acetylcholine (10 μM). Cumulative contraction responses to noradrenaline were measured. Separate thoracic aortic rings were perfused with 10% neutral buffered formalin, embedded in wax and stained with haemotoxylin and eosin. Image analysis (Wild-Leitz MD30+ system) was used to calculate wall area of the thoracic aorta.
Isolated perfused right kidney preparation
Kidney function was measured either 4 or 8 weeks after STZ-treatment. Rats were anaesthetized with pentobarbitone (80 mg kg−1). The right ureter was cannulated and a catheter introduced into the renal artery via the superior mesenteric artery to avoid decreases in the oxygenation of the kidney (Nishiitsutsuji et al., 1967). The kidney was perfused from a reservoir containing Krebs-Henseleit buffer with glucose (5 mM), 20 physiological amino acids and 6% bovine serum albumin, maintained at 37°C and gassed with carbogen (95% O2/5% CO2) (Radcliffe et al., 1988).
Renal function was evaluated at perfusion pressures in about 15 mmHg increments over the range of 77 to 135 mmHg. Kidneys were equilibrated for 3–5 min before urine collection at each pressure. Renal arterial perfusion pressure, corrected for the Venturi effect, was continuously monitored by a catheter located within the renal artery catheter. Flow was recorded from an ultrasonic device positioned on the renal line. Urine flow rate was determined at each pressure. The glomerular filtration rate (GFR) was estimated from the 14C-inulin clearance (Uin volume−1 Pin−1 min−1).
Data analysis
All results are given as mean±s.e.mean of at least six experiments. The negative log EC50 of the increase in either force of contraction in mN or rate of contraction in beats/min was determined from the concentration giving half-maximal responses in individual concentration-response curves. Renal function results were corrected for kidney wet weight measured at the end of the experiment. These results were analysed by two-way analysis of variance followed by the Duncan test to determine differences between treatment groups and by paired or unpaired t-tests as appropriate; P<0.05 was considered significant.
Drugs
Streptozotocin, spironolactone, noradrenaline, PBS-Tween 20 and bovine serum albumin were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.). Noradrenaline was dissolved in distilled water; streptozotocin was dissolved in citrate buffer (pH 4.5) shortly before injection; spironolactone was dissolved in dimethylformamide. Pirfenidone was provided by Marnac, Inc. (Dallas, TX, U.S.A.).
Results
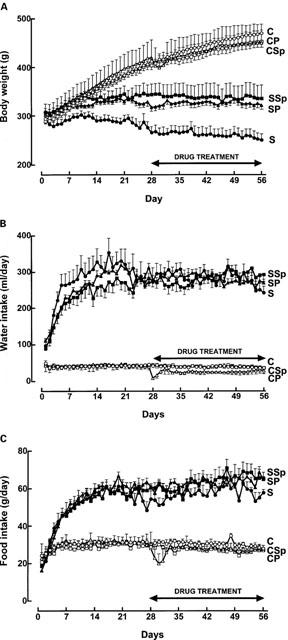
STZ treatment increased blood glucose which was unchanged by 4 weeks of treatment with pirfenidone or spironolactone starting 4 weeks after STZ administration (Table 1). Body weight increased in control rats but not in STZ rats during the 8 weeks of measurement; both water and food intake increased in STZ rats (Figure 1). Neither pirfenidone nor spironolactone administration altered water intake body weight or food intake (Figure 1). Pirfenidone intake was similar in control and STZ-treated rats (Figure 2). Plasma sodium and potassium concentrations in control rats were unchanged by STZ, pirfenidone or spironolactone treatment (Table 1). Plasma aldosterone concentrations were markedly increased in spironolactone-treated rats. Systolic blood pressure was lower in all STZ-treated rats but this was significant only in STZ-rats with no further treatment; resting heart rate was significantly decreased in STZ-treated rats.
Table 1.
Pathophysiological parameters in control and STZ-rats and rats treated with pirfenidone or spironolactone
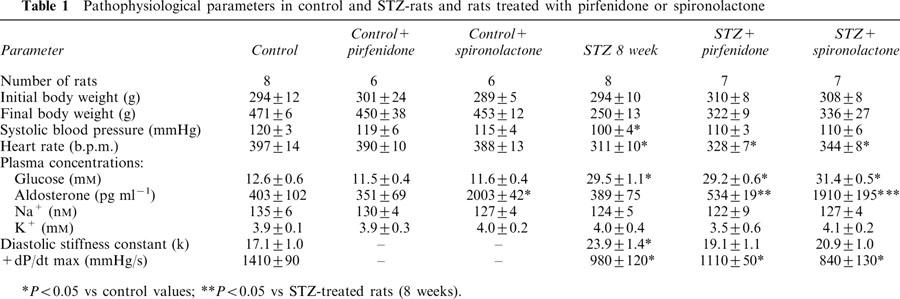
Figure 1.
Body weight (A), daily water intake (B) and food intake (C) for control (open circle), control+pirfenidone (open triangle), control+spironolactone (open square), STZ (filled circle), STZ+pirfenidone (filled triangle) and STZ+spironolactone (filled square) rats.
Figure 2.
Daily pirfenidone dose in control+pirfenidone (open triangles) and STZ+pirfenidone (filled triangles) rats.
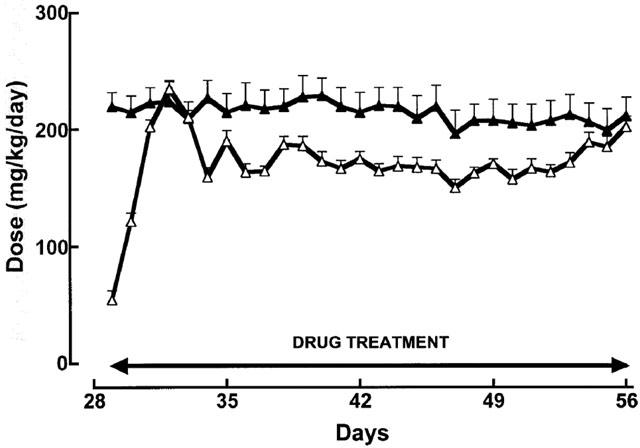
The deposition of collagen was increased in the heart, thoracic aorta and kidneys of STZ-rats (Figure 3). This increase was measured in interstitial and perivascular areas of the left ventricle, in vascular smooth muscle layers of the aorta and in the tubulointerstitium and glomerulus of the kidney although no scarring (reparative fibrosis) was observed in the heart or kidney of STZ-treated rats. Treatment with pirfenidone and spironolactone for 4 weeks did not change collagen deposition in control rats but attenuated or reversed deposition in all tissues from STZ-treated rats. STZ-treatment increased ventricular, renal and plasma fibronectin concentrations; this was attenuated both by pirfenidone and spironolactone (Figure 4).
Figure 3.
Collagen distribution in the left ventricle (A), thoracic aorta (B) and kidney (C) of control (C), control+pirfenidone (CP), control+spironolactone (CSp), STZ (S), STZ+pirfenidone (SP) and STZ+spironolactone (SSp) rats; n=6 [* P<0.05 vs C; ** P<0.05 vs S].
Figure 4.
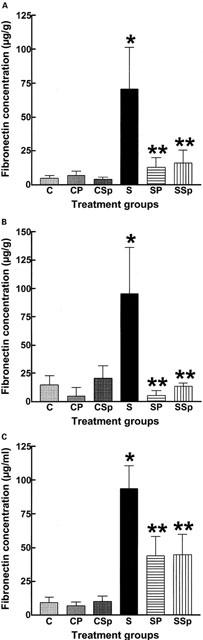
Fibronectin concentration in the left ventricle (A), kidney (B) and plasma (C) of control (C), control+pirfenidone (CP), control+spironolactone (CSp), STZ (S), STZ+pirfenidone (SP) and STZ+spironolactone (SSp) rats; n=6 [* P<0.05 vs C; ** P<0.05 vs S].
Cardiac function was determined in the isolated Langendorff heart as well as in isolated cardiac muscles and thoracic aortic rings. STZ treatment increased passive diastolic stiffness and decreased ventricular contractility defined as +dP/dt; pirfenidone and spironolactone reversed only the increases in passive stiffness (Table 1).
STZ treatment significantly decreased maximal positive inotropic responses to noradrenaline (control 4.2±0.7 mN; STZ 2.0±0.5 mN, n=8), the calcium sensitizer EMD 57033 (control 5.4±0.8 mN; STZ 2.6±0.4 mN, n=8) and calcium chloride (control 5.1±0.4 mN; STZ 2.0±0.3 mN, n=8 in the left ventricular papillary muscle; neither pirfenidone nor spironolactone returned responses to normal (Figure 5). Chronotropic responses to noradrenaline were unaltered (Figure 6; control, −log EC50 6.5±0.1; maximal increase 174±6 beats min−1, n=8). Noradrenaline potency was significantly increased in isolated thoracic aortic rings following STZ treatment (control −log EC50 6.4±0.1; STZ 7.1±0.1); pirfenidone and spironolactone treatment further increased noradrenaline potency (−log EC50 7.4±0.1) without changing maximal vasoconstrictor responses in these isolated thoracic aortic rings (Figure 7).
Figure 5.
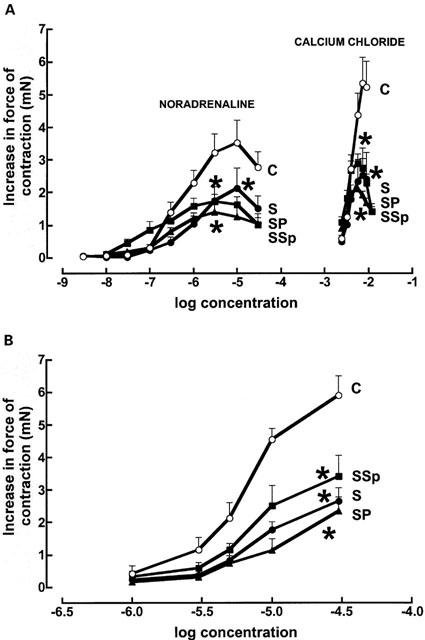
Cumulative concentration-response curves for noradrenaline and calcium chloride (A) and EMD 57033 (B) in left ventricular papillary muscles from control (C, open circles) (n=8), STZ (S, filled circles) (n=8), STZ+pirfenidone (SP, filled triangles) (n=8) and STZ+spironolactone (SSp, filled squares) (n=8) rats. [*p<0.05 vs C].
Figure 6.
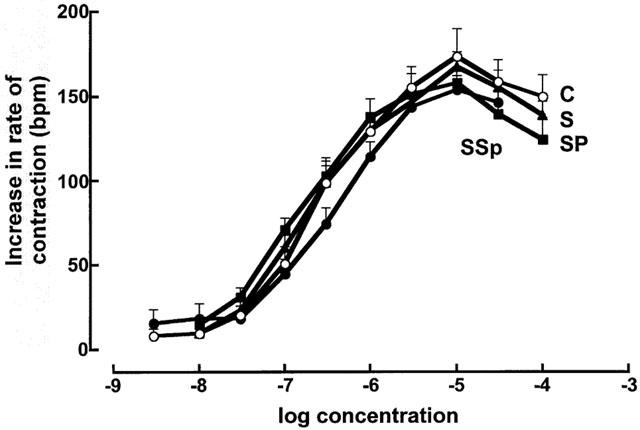
Concentration-response curves for noradrenaline in right atria (n=8) from control (C, open circles), STZ (S, filled circles), STZ+pirfenidone (SP, filled triangles) and STZ+spironolactone (SSp, filled squares) rats.
Figure 7.
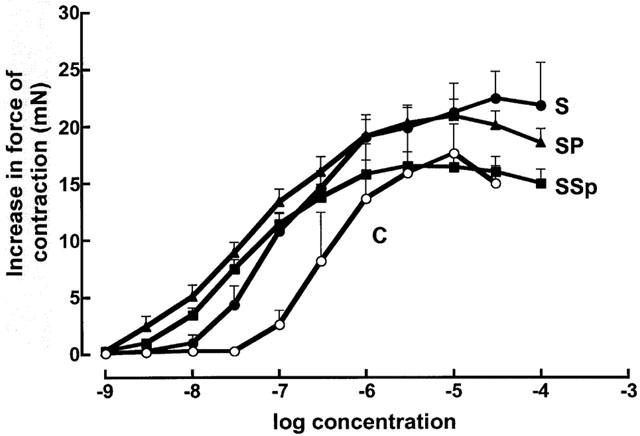
Concentration-response curves for noradrenaline in thoracic aortic rings (n=8) from control (C, open circles), STZ (S, filled circles), STZ+pirfenidone (SP, filled triangles) and STZ+spironolactone (SSp, filled squares) rats.
Four weeks after STZ treatment, GFR and urine flow rates were decreased in the isolated perfused kidney (respectively: P<0.005 at all pressure levels, and P<0.01 at 120 and 135 mmHg); values returned to control 8 weeks after induction of diabetes, but tended to flatten out at the higher pressures. Administration of pirfenidone, but not spironolactone, prevented the return to control values; in addition, renal arterial flow was decreased by pirfenidone treatment (P<0.05 at 105, 120 and 135 mmHg; Figure 8). Fractional sodium excretion was increased at 4 weeks (P<0.05 at all pressure levels except 80 and 120 mmHg), and not improved at 8 weeks (P<0.05 at all pressure levels except 135 mmHg), reflecting impaired kidney function: both spironolactone and pirfenidone partially returned this parameter towards control values, so that no statistically significant differences with control values were present (except for pirfenidone at 90 mmHg, Figure 8).
Figure 8.
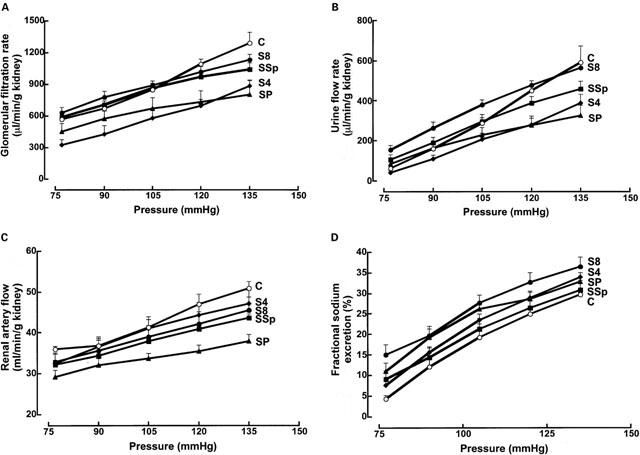
Glomerular filtration rates (A), urine flow rates (B), renal arterial flow (C) and fractional excretion of sodium (D) (n=6) in isolated perfused kidneys from control (C, open circles), STZ (4 weeks after STZ, S4, filled diamonds; 8 weeks after STZ, S8, filled circles), STZ+pirfenidone (SP, filled triangles) and STZ+spironolactone (SSp, filled squares) rats.
Discussion
Fibrosis is a slowly-evolving process which eventually leads to chronic impairment of cardiac and renal function (Brilla & Weber, 1992; Ziyadeh, 1993). Since fibrosis is a dynamic process (Sun & Weber, 2000), reversal of collagen deposition may be feasible; this process could return disease-induced cardiac and renal dysfunction towards normal. In contrast, prevention of collagen deposition has mechanistic relevance only since patients are not treated before the onset of symptoms. This study has clearly shown that cardiac, renal and aortic fibrosis in STZ-diabetic rats can be attenuated or reversed by administration of pirfenidone or spironolactone. Further, the increased cardiac stiffness in the isolated diabetic heart can be reversed by treatment with pirfenidone or spironolactone. However, the myocardial changes in diabetes, such as a decreased contractility and positive inotropic response to noradrenaline, were not altered. The changes in GFR and urine flow rates were paralleled by changes in arterial flow; the lack of normalization with pirfenidone probably reflects effects on the vasculature, possibly similar to the myocardium. The excretion of sodium, exaggerated during STZ treatment, tended to normalize with both treatments, possibly due to attenuation of fibrosis improving renal function. Thus, although these compounds have considerable potential in reversing cardiac fibrosis and functional impairment due to the increased collagen deposition, they did not normalize all aspects of myocardial or renal dysfunction in STZ-diabetic rats.
Long-term diabetes induced by streptozotocin leads to an increased collagen deposition (Riva et al., 1998) resulting in defects in contractility and relaxation such as a decrease in left ventricular compliance as a result of increased myocardial stiffness (Litwin et al., 1990; Norton et al., 1996). Diabetic nephropathy is characterized by tubulointerstitial fibrosis; high glucose concentrations increased TGFβ-expression and collagen I mRNA and protein production by renal cortical fibroblasts in culture (Han et al., 1999). An activated renin-angiotensin system, as in streptozotocin-diabetic rats (Brown et al., 1996), may lead to cardiac fibrosis (Weber et al., 1994) although oral captopril treatment did not reduce stiffness in diabetic rat hearts (Norton et al., 1996).
Several studies have shown that initiating treatment 2–4 weeks after induction of streptozotocin-diabetes can prevent or reverse the cardiac changes due to collagen accumulation. The enhanced collagen fluorescence and myocardial stiffness in streptozotocin-diabetic rats was prevented by treatment with aminoguanidine, probably by preventing the formation of advanced glycosylation end products (Norton et al., 1996). Treatment with the Ginkgo biloba extract EGb 761 partly diminished interstitial fibrosis in the streptozotocin-diabetic myocardium (Welt et al., 1999). Antioxidative treatment with tocopherol acetate significantly reduced collagen I and III deposition in the streptozotocin-diabetic heart and prevented the impairment of endothelium-dependent vasodilatation (Rosen et al., 1995).
The antifibrotic actions of pirfenidone have been shown in bleomycin-induced pulmonary fibrosis in hamsters (Iyer et al., 1995; 1999a, 1999b) and in rats with sclerosing peritonitis (Suga et al., 1995) or with renal fibrosis following partial nephrectomy (Shimizu et al., 1997). This last study used a reversal protocol, starting pirfenidone treatment 2 weeks after renal ablation; the other studies used prevention protocols. Pirfenidone doses ranged from 0.5% in food (Iyer et al., 1995; 1999a, 1999b), 350 mg kg−1 day−1 (Suga et al., 1995) or 500 mg kg−1 day−1 (Shimizu et al., 1997); the current study used 0.2–2 g l−1 drinking water which approximated 200 mg kg−1 day−1. Despite these differences in protocol, the results have shown that pirfenidone consistently prevents, and probably reverses, collagen accumulation. Iyer et al. (1999a) have shown that pirfenidone suppressed bleomycin-induced inflammatory events and down-regulated bleomycin-induced overexpression of lung procollagen I and III genes. This is consistent with a suppression of an increased TGFβ mRNA expression by pirfenidone (Shimizu et al., 1997; Iyer et al., 1999b) which may increase collagen breakdown by reducing TGFβ-induced inhibition of the degrading enzymes, the matrix metalloproteinases. Since diabetes is associated with an increased TGFβ mRNA expression, at least in the kidneys (Hoffman et al., 1998; Han et al., 1999), suppression of the expression of this cytokine may be the mechanism for the antifibrotic actions of pirfenidone in diabetic rat heart and kidneys. Further, pirfenidone attenuated pulmonary functional deficits in bleomycin-treated hamsters (Schelegle et al., 1997) and improved renal function in the remnant kidney (Shimizu et al., 1997). These results are consistent with the actions of pirfenidone to attenuate diabetes-induced increases in cardiac stiffness in this study.
Spironolactone prevented collagen synthesis and fibrosis in the DOCA-salt hypertensive rat heart (Brown et al., 1999) and aortic fibrosis in the spontaneously hypertensive rat (Benetos et al., 1997). Although aldosterone receptors are clearly implicated in collagen deposition, this does not explain the reversal of existing fibrosis by spironolactone as in the current study. The renin-angiotensin system is clearly involved in regulation of TGFβ bioactivity; for example, chronic angiotensin receptor blockade decreased expression of Smad proteins, the downstream effector molecules of TGFβ, as well as fibrosis (Dixon et al., 2000). Further, increased levels of plasminogen activator inhibitor-1 (PAI-1) have been associated with local accumulation of extracellular matrix; inhibition of angiotensin converting enzyme prevented PAI-1 expression and perivascular fibrosis (Katoh et al., 2000). It is not known whether spironolactone can also act by these mechanisms. The mechanisms of action of spironolactone are important given the RALES (Randomized Aldactone Evaluation Study) results where patients with an ejection fraction of 35% or less showed a 35% reduction in risk of mortality from progressive heart failure after taking the sub-haemodynamic 25 mg dose of spironolactone (Pitt et al., 1999). This project suggests that the reversal of cardiac fibrosis and the resulting decrease in cardiac stiffness could be partly responsible for the clinical improvement of these patients during chronic treatment with spironolactone.
This study clearly shows that reversal, rather than prevention, of cardiac and renal fibrosis is feasible which emphasises the dynamic nature of the extracellular matrix. Since extracellular matrix protein synthesis and degradation is complex, there are many possible points of attack for pharmacological reversal of fibrosis; however, the most relevant may be suppression of TGFβ production or bioactivity. Our results suggest that treatment with either pirfenidone or spironolactone is a viable mechanism to reverse fibrosis and, more importantly, to reverse the functional impairment associated with the increased collagen deposition in the diabetic heart and kidney.
Abbreviations
- DOCA
deoxycorticosterone acetate
- ELISA
enzyme-linked immunosorbent assay
- GFR
glomerular filtration rate
- PAI-1
plasminogen activator inhibitor-1
- RALES
Randomized Aldactone Evaluation Study
- STZ
streptozotocin
- TGFβ
transforming growth factor β
References
- ASSAYAG P., CARRÉ F., CHEVALIER B., DELCAYRE C., MANSIER P., SWYNGHEDAUW B. Compensated cardiac hypertrophy: arrhythmogenicity and the new myocardial phenotype. I. Fibrosis. Cardiovasc. Res. 1997;34:439–444. doi: 10.1016/s0008-6363(97)00073-4. [DOI] [PubMed] [Google Scholar]
- BENETOS A., LACOLLEY P., SAFAR M.E. Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 1997;17:1152–1156. doi: 10.1161/01.atv.17.6.1152. [DOI] [PubMed] [Google Scholar]
- BRILLA C.G., WEBER K.T. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc. Res. 1992;26:671–677. doi: 10.1093/cvr/26.7.671. [DOI] [PubMed] [Google Scholar]
- BROWN L., CRAGOE E.J., JR, ABEL K.C., MANLEY S.W., BOURKE J.R. Amiloride analogues induce responses in isolated rat cardiovascular tissues by inhibition of Na+/Ca2+ exchange. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991b;344:220–224. doi: 10.1007/BF00167222. [DOI] [PubMed] [Google Scholar]
- BROWN L., DUCE B., MIRIC G., SERNIA C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J. Am. Soc. Nephrol. 1999;10:S143–S148. [PubMed] [Google Scholar]
- BROWN L., SERNIA C., NEWLING R., FLETCHER P. Comparison of inotropic and chronotropic responses in rat isolated atria and ventricles. Clin. Exp. Pharmacol. Physiol. 1991a;18:753–760. doi: 10.1111/j.1440-1681.1991.tb01393.x. [DOI] [PubMed] [Google Scholar]
- BROWN L., WALL D., MARCHANT C., SERNIA C. Tissue-specific changes in angiotensin II receptors in streptozotocin-diabetic rats. J. Endocrin. 1996;154:355–362. doi: 10.1677/joe.0.1540355. [DOI] [PubMed] [Google Scholar]
- DIXON I.M.C., HAO J., REID N.L., ROTH J.C. Effect of chronic AT1 receptor blockade on cardiac Smad overexpression in hereditary cardiomyopathic hamsters. Cardiovasc. Res. 2000;46:286–297. doi: 10.1016/s0008-6363(00)00035-3. [DOI] [PubMed] [Google Scholar]
- EL-NAHAS A.M., MUCHANETA-KUBARA E.C., ESSAWY M., SOYLEMEZOGLU O. Renal fibrosis: Insights into pathogenesis and treatment. Int. J. Biochem. Cell. Biol. 1997;29:55–62. doi: 10.1016/s1357-2725(96)00119-7. [DOI] [PubMed] [Google Scholar]
- HAN D.C., ISONO M., HOFFMAN B.B., ZIYADEH F.N. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J. Am. Soc. Nephrol. 1999;10:1891–1899. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B.B., SHARMA K., ZHU Y., ZIYADEH F.N. Transcriptional activation of transforming growth factor-betal in mesangial cell culture by high glucose concentration. Kidney Int. 1998;54:1107–1116. doi: 10.1046/j.1523-1755.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- IYER S.N., GURUJEYALAKSHMI G., GIRI S.N. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J. Pharmacol. Exp. Ther. 1999a;289:211–218. [PubMed] [Google Scholar]
- IYER S.N., GURUJEYALAKSHMI G., GIRI S.N. Effects of pirfenidone on transforming growth factor-β gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J. Pharmacol. Exp. Ther. 1999b;291:367–373. [PubMed] [Google Scholar]
- IYER S.N., WILD J.S., SCHIEDT M.J., HYDE D.M., MARGOLIN S.B., GIRI S. Dietary intake of pirfenidone ameliorates bleomycin induced lung fibrosis in hamsters. J. Lab. Clin. Med. 1995;125:779–785. [PubMed] [Google Scholar]
- KATOH M., EGASHIRA K., MITSUI T., CHISHIMA S., TAKESHITA A., NARITA H. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J. Mol. Cell. Cardiol. 2000;32:73–83. doi: 10.1006/jmcc.1999.1053. [DOI] [PubMed] [Google Scholar]
- LAASKO M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- LITWIN S.E., RAYA T.E., ANDERSON P.G., DAUGHERTY S., GOLDMAN S. Abnormal cardiac function in the streptozotocin-diabetic rat. Changes in active and passive properties of the left ventricle. J. Clin. Invest. 1990;86:481–488. doi: 10.1172/JCI114734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRSKY I., PARMLEY W.W. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ. Res. 1973;33:233–243. doi: 10.1161/01.res.33.2.233. [DOI] [PubMed] [Google Scholar]
- NISHIITSUTSUJI J.M., ROSS B.D., KREBS H.A. Metabolic activities of the isolated perfused rat kidney. Biochem. J. 1967;103:852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON G.R., CANDY G., WOODIWISS A.J. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- PITT B., ZANNAD F., REMME W.J., CODY R., CASTAIGNE A., PEREZ A., PALENSKY J., WITTES J. , for the Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- RADCLIFFE P.J., ENDRE Z.H., SHEINMAN S.J., TANGE J.D., LEDINGHAM J.G.G., RADDA G.K. 31P nuclear magnetic resonance study of steady-state adenosine 5′-triphosphate levels during graded hypoxia in the isolated perfused rat kidney. Clin. Sci. 1988;74:437–448. doi: 10.1042/cs0740437. [DOI] [PubMed] [Google Scholar]
- RIVA E., ANDREONI G., BIANCHI R., LATINI R., LUVARÀ G., JEREMIC G., TRAQUANDI C., TUCCINARDI L. Changes in diastolic function and collagen content in normotensive and hypertensive rats with long-term streptozotocin-induced diabetes. Pharmacol. Res. 1998;37:233–240. doi: 10.1006/phrs.1998.0290. [DOI] [PubMed] [Google Scholar]
- ROSEN P., BALHAUSEN T., BLOCH W., ADDICKS K. Endothelial relaxation is disturbed by oxidative stress in the diabetic rat heart: influence of tocopherol as antioxidant. Diabetologia. 1995;38:1157–1168. doi: 10.1007/BF00422364. [DOI] [PubMed] [Google Scholar]
- SCHELEGLE E.S., MANSOOR J.K., GIRI S. Pirfenidone attenuates bleomycin-induced changes in pulmonary functions in hamsters. PSEBM. 1997;218:392–397. doi: 10.3181/00379727-216-44187. [DOI] [PubMed] [Google Scholar]
- SHIMIZU T., FUKAGAWA M., KURODA T., HATA S., IWASAKI Y., NEMOTO M., SHIRAI K., YAMAUCHI S., MARGOLIN S.B., SHIMIZU F., KUROKAWA K. Pirfenidone prevents collagen accumulation in the remnant kidney in rats with partial nephrectomy. Kidney Int. 1997;52 suppl 63:S239–S243. [PubMed] [Google Scholar]
- SUGA H., TERAOKA S., OTA K., KOMEMUSHI S., FURUTANI S., YAMAUCHI S., MARGOLIN S.B. Preventive effect of pirfenidone against sclerosing peritonitis in rats. Exp. Toxic. Pathol. 1995;47:287–291. doi: 10.1016/s0940-2993(11)80261-7. [DOI] [PubMed] [Google Scholar]
- SUN Y., WEBER K.T. Infarct scar: a dynamic tissue. Cardiovasc. Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- WEBER K.T., SUN Y., TYAGI S.C., CLEUTJENS J.P.M. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J. Mol. Cell. Cardiol. 1994;26:279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- WELT K., WEISS J., KOCH S., FITZL G. Protective effects of Ginkgo biloba extract EGb 761 on the myocardium of experimentally diabetic rats. II. Ultrastructural and immunohistochemical investigation on microvessels and interstitium. Exp. Toxic. Pathol. 1999;51:213–222. doi: 10.1016/S0940-2993(99)80099-2. [DOI] [PubMed] [Google Scholar]
- YOUNG M., HEAD G., FUNDER J. Determinants of cardiac fibrosis in experimental hyper-mineralocorticoid states. Am. J. Physiol. 1995;269:E657–E662. doi: 10.1152/ajpendo.1995.269.4.E657. [DOI] [PubMed] [Google Scholar]
- ZIYADEH F.N. The extracellular matrix in diabetic nephropathy. Am. J. Kidney Dis. 1993;22:736–744. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]


