Abstract
The effects of agonists with endothelin (ET) ETA-receptor activity have been analysed in relation to their interaction with ETB receptors in rat mesenteric arteries.
ET-1, sarafotoxin 6b (S6b) and ET-3 induced large, slow-onset and sustained contractions whereas S6c induced weak transient contractions. However, following pre-contraction with U46619 and subsequent relaxation with forskolin, the effect of S6c was amplified, indicating a potential for powerful ETB-receptor mediated contraction.
The selective ETA-receptor antagonist, FR139317, produced parallel rightward shifts of ET-1, S6b and ET-3 concentration-effect curves indicating that the contractions were mediated by ETA receptors. However, the corresponding FR139317 pKB values were significantly different between the agonists. As expected FR139317 had no effect on S6c responses.
Pre-treatment with S6c to desensitize ETB receptors, increased ET-1 potency and the pKB value for FR139317. In contrast, neither the potency of S6b and ET-3 nor the pKB values for FR139317 estimated using these agonists were affected by ETB-receptor desensitization.
Segments pre-contracted with submaximal concentrations of S6b and ET-3, but not ET-1, rapidly relaxed following wash-out or FR139317 administration.
The results indicate that the small contractile response to selective ETB receptor activation, barely detectable under standard bioassay conditions, is greatly amplified when adenylate cyclase activity is elevated. Moreover, the response to ETA receptor activation by ET-1, but not ET-3 and S6b, is significantly modified by co-activation of ETB receptors. This interaction has a significant effect on the apparent affinity of ETA-receptor selective antagonists when ET-1 is used as agonist and decreases the potency of ET-1.
Keywords: Rat mesenteric artery, myograph, endothelin receptors, FR139317, IRL2500, sarafotoxin 6b, sarafotoxin 6c, cross-talk, adenylate cyclase
Introduction
The two dominant endothelin (ET) peptides in mammals are ET-1 and ET-3 (Inoue et al., 1989). ET-1, produced by the endothelial cells, induces vasoconstriction by activating ETA and ETB receptors and relaxation via endothelial ETB receptors coupled to NO generation (Bax & Saxena, 1994). Radioligand binding studies have shown that ET-1 expresses higher affinity than ET-3 for the ETA receptor, whereas both ligands express the same affinity for the ETB receptor (Panek et al., 1992; Williams et al., 1991). In vascular tissue, the ETA receptor is usually considered to be the most abundant as judged by the observation that activation of it, but not the ETB receptor, induces contraction in all vascular regions irrespective of species (for references, see Gray & Webb, 1996). The distribution of ETB receptors is more variable and it has been suggested that ETB receptor mediated vasoconstriction is preferentially localized to veins and the micro-circulation (Ekelund et al., 1994; Moreland et al., 1994). In addition to the differences in affinity for the endogenous ligands, the two receptor subtypes also differ markedly with respect to the kinetic profile of the contractile responses associated with their activation. Thus, the responses mediated by ETB receptors are transient in contrast to the relatively sustained responses obtained following ETA receptor activation (Sudjarwo et al., 1994). The sustained effect of ETA receptor activation has been attributed to the localization of the receptors in caveolae with a low rate of internalization (Chun et al., 1994). In contrast, the ETB receptor is desensitized shortly after activation due to phosphorylation and subsequent sequestration of the receptors (Cramer et al., 1997).
It has been reported that ETB receptor activation does not make an important contribution to arterial contraction in response to the endothelins. However, significant quantities of both ETB receptor binding sites and mRNA have been shown to be present in arterial smooth muscle cells (Davenport et al., 1995a, 1995b; Nilsson et al., 1997; Opgaard et al., 1996). Moreover, there have been several reports of so-called ‘cross-talk' between ETA and ETB receptors in situations where both receptors can separately induce strong responses (Clozel & Gray, 1995; Fukuroda et al., 1996). This raises the possibility that the functional significance of the ETB receptor may only be apparent during concomitant ETA receptor activation. Attempts have been made to analyse the nature of the interaction between the two receptors (Adner et al., 2000) but so far no explanatory model has been proposed to explain this ‘cross-talk'.
The aim of this study was to analyse the effect of manipulating the contribution of ETB receptors on the agonist activity of the ET agonists, ET-1, sarafotoxin 6b (S6b), ET-3 and S6c, in an isolated vascular tissue bioassay with functionally-coupled ETA and ETB receptors. Previously, the interaction between ET receptors, also with variable small ETB receptor mediated responses, has been described in the rat mesenteric third arterial branch (Mickley et al., 1997). Here, the rat mesenteric first arterial branch was chosen as the test assay as not only has it been shown that ETA receptor activation produces powerful vasoconstrictor responses but also that selective ETB receptor activation produces small although reproducible responses. Thus, in principle at least, it should have been possible to characterize agonist responses mediated by simultaneous as well as individual activation of each receptor type. This was attempted by use of selective antagonists and manipulation of the functional ETB receptor density using a selective receptor desensitization procedure (Lodge et al., 1995).
Methods
Rat small mesenteric artery preparation
The mesentery was removed from male, Wistar – Kyoto rats (250 – 350 g; M&B, Denmark) and placed in cold (4°C) buffer solution (see below). The first branches from the superior mesenteric artery were dissected free from surrounding adipose tissue and the endothelium was removed by a 10 s infusion of 0.1% Triton-X 100. Thereafter, the arteries were cut into ∼1 mm long segments which were mounted in a small vessel myograph (J.P. Trading, Aarhus, Denmark). Tissue responses were measured as changes in isometric tension in thermostatically controlled (37.0±0.5°C) 4 ml baths continuously gassed with 95% O2 and 5% CO2 (pH 7.40±0.05).
Experimental protocol
Following a 30 min stabilization period, the internal diameter of each segment was set to a tension equivalent to 0.9 times the estimated diameter at 100 mmHg effective transmural pressure (l100=329±4 μm, 186 segments) according to the standard protocol of Mulvany & Halpern (1977). The contractile capacity of each tissue segment was determined after a further 15 min stabilization period by a double exposure to a KCl-rich buffer solution (60 mM; 8.40±0.42 mN mm−1). All segments were then exposed to 10 μM noradrenaline (NA) and, when a stable contraction was achieved, 1 μM acetylcholine (ACh) was administered to verify, by the lack of a relaxant response, the absence of endothelium. Antagonists were incubated for 60 min before the cumulative application of agonists. Eight segments were used from each animal and experimental treatments were allocated according to a randomized block design so that data from one control for each treatment group were obtained from one animal. Therefore, n refers to the number of replicate experiments and is identical to the number of animals used. In a preliminary experiment, a high concentration (10 μM) of the selective ETB receptor antagonist, IRL2500 (183 fold selective for the ETB receptor in rat heart; Russell & Davenport, 1996), decreased the upper asymptote of the ET-1 concentration-effect curve (data not shown). However, at this concentration IRL 2500 was dissolved in 100% methanol (i.e. 1% in the bath) and the vehicle control revealed that this effect could be attributed to the methanol. Therefore, a lower concentration was selected (1 μM) that had no significant effect on the ET-1 concentration-effect curve (α=115±5 and 118±10; p[A]50=9.73±0.15 and 9.86±0.15; nH=1.00±0.14 and 0.98±0.08, for control segments and segments incubated with IRL 2500, respectively; n=5; see below for the definition of the concentration-effect curve fitting parameters α, nH and [A]50). In some experiments, ETB receptors were first desensitized by a 60 min incubation with a supramaximal concentration of sarafotoxin 6c (S6c; 0.3 μM) during which time the S6c response faded to baseline levels (Lodge et al., 1995).
Analysis
All data are expressed as mean values±s.e.mean. Contractile responses in each segment are expressed as a percentage of the contraction induced by KCl. Individual agonist concentration-effect curve data were fitted to the Hill equation [1] using an iterative, least squares method (GraphPad Prism, San Diego, CA, U.S.A), to provide estimates of the Hill coefficient (nH; the midpoint slope index), [A]50 (the agonist concentration, [A], for half-maximum effect, E) and upper asymptote (α; maximum effect).
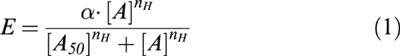 |
pKB values were estimated by fitting the individual log[A]50 values obtained in the absence (log[A]50) and presence (log[A]50B) of antagonist concentrations ([B]) to the following derivation of the Schild equation as described previously (Black et al., 1985),
In the first fit, the Schild slope parameter (b) was allowed to vary. If the value of b was found not to be significantly different from unity a second fit was performed with b constrained to unity. When only one antagonist concentration was investigated, a pKB' value was estimated using equation [2] with the value of b constrained to unity. Thus, the term pKB' is used to denote that those criteria for competitive antagonism that can be tested with a single antagonist concentration (i.e. surmountable, parallel shift of the agonist curve) have been satisfied although the linearity and slope of the Schild plot were not investigated.
The effect of drug treatments on the computed Hill equation parameters was assessed by one-way analysis of variance (ANOVA) and the Bonferroni modified t-test for multiple comparisons. P values of less than 0.05 were considered to be significant.
Solutions and drugs
Buffer solution was of the following composition in (mM): 119 NaCl, 15 NaHCO3, 4.6 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 1.5 CaCl2 and 5.5 glucose. In the KCl-rich buffer an equivalent of NaCl was replaced by 60 mM KCl. The sources of the agonists and antagonists were: endothelin-1, endothelin-3, sarafotoxin 6b, sarafotoxin 6c (Auspep, Parkville, Australia), FR139317; (R)2-[(R)-2-[(S)-2-[[1-(hexahydro-1H-azepinyl)]carbonyl]amino-4-methylpentanoyl]amino-3-[3-(1-methyl-1H-indoyl)]propionyl]amino-3-(2-pyridyl)propionic acid (Fujisawa Pharmaceuticals Co., Osaka, Japan), IRL2500, N-(3,5-dimethylbenzoyl)-N-methyl-(D)-(4-phenylphenyl)-alanyl-L-tryptophan, (Ciba-Geigy, Takarazuka, Japan), noradrenaline and acethylcholine (Sigma, St. Louis, U.S.A.).
Results
Contractile responses to ET-1, S6b and ET-3
ET-1, S6b and ET-3 all produced concentration-dependent contractile responses in the rat small mesenteric artery segments (Table 1). Interestingly, there was a significant difference between the kinetic profile of the responses to ET-1 and those to S6b or ET-3. Thus, the time for individual responses to attain a plateau was longer for ET-1 than for ET-3 or S6b, especially for low concentrations of ET-1 (Figure 1a). The individual replicate curve data for each agonist, measured at steady-state response levels, could be fitted to the Hill equation and the results indicated that the curve maxima for each agonist were indistinguishable (∼140% of 60 mM KCl). ET-1 was ∼2 fold more potent (p[A]50=9.59±0.11; n=10) than S6b (p[A]50=9.25±0.14; n=5) and ∼100 fold more potent than ET-3 (p[A]50=7.58±0.03; n=5). There were significant differences in the midpoint slopes of the curves as judged by the estimated Hill coefficients of the agonist curves (nH=1.39±0.23 for ET-1, 1.81±0.17 for S6b and 3.13±0.38 for ET-3).
Table 1.
Analysis of ET-1, S6b and ET-3 contractions in rat mesenteric arterial branch
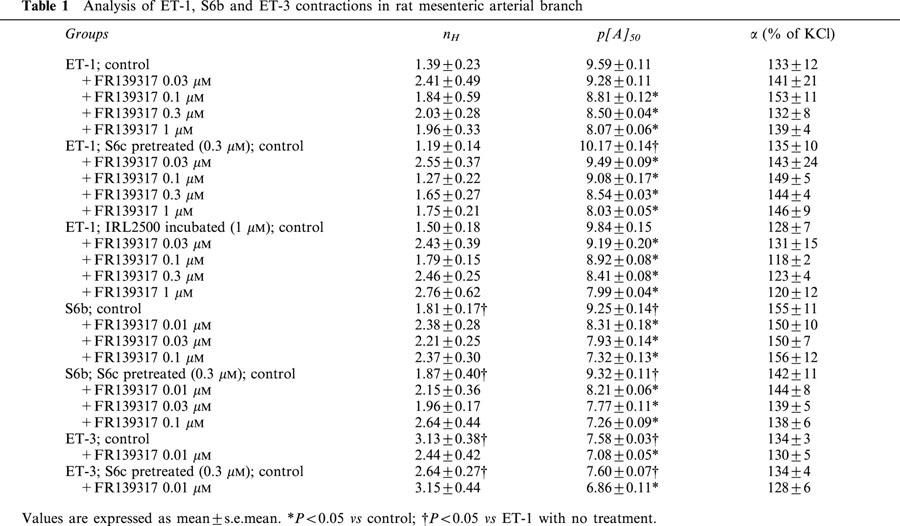
Figure 1.
Representative experimental traces showing the contraction developed in rat mesenteric artery branch segments during cumulative administration of ET-1 (a), ET-1 pre-treated for 1 h with FR139317 (b), S6b (c), ET-3 (d), S6c (e; insert – 20×amplitude magnification) and S6c following pre-contraction with 0.1 μM U46619 and subsequent dilatation with 0.1 μM forskolin (f). Agonist concentrations are expressed as log10 values.
Interaction of ET-1, S6b and ET-3 with FR139317
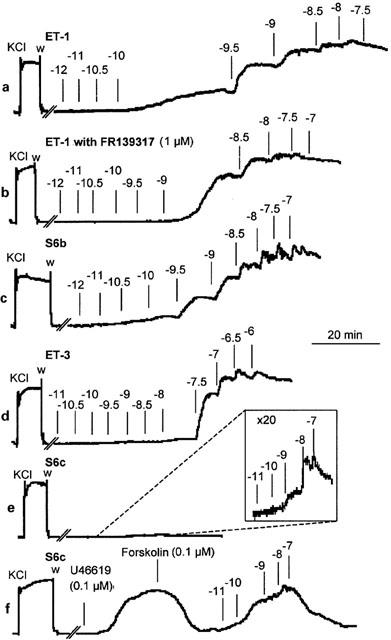
The competitive antagonist FR139317 (∼10,000 fold ETA-receptor selective; Sogabe et al., 1993) produced a concentration-dependent, rightward shift of the ET-1 concentration-effect curve (Figure 2a and Table 1). The Hill coefficients were not significant different as tested by ANOVA even though, by inspection, the control curve appeared to be shallower than those obtained in the presence of FR139317. The Schild slope parameter was not different from unity (b=0.91±0.10) and when the slope was constrained to unity a pKB value of 7.57±0.08 (d.f.=28) was obtained.
Figure 2.
Concentration-effect curves obtained on rat small mesenteric artery segments to ET-1 in the absence and presence of 0.03, 0.1, 0.3 and 1 μM FR 139317 – control (a), pre-treated with 0.3 μM S6c (b) and pre-treated with 1 μM IRL 2500 (c). Contractions are expressed as a percentage of the response to 60 mM KCl. Corresponding Schild plots are shown in panel (d). Each point represents the mean of all segments tested with error bars representing s.e.mean from 5 – 10 animals.
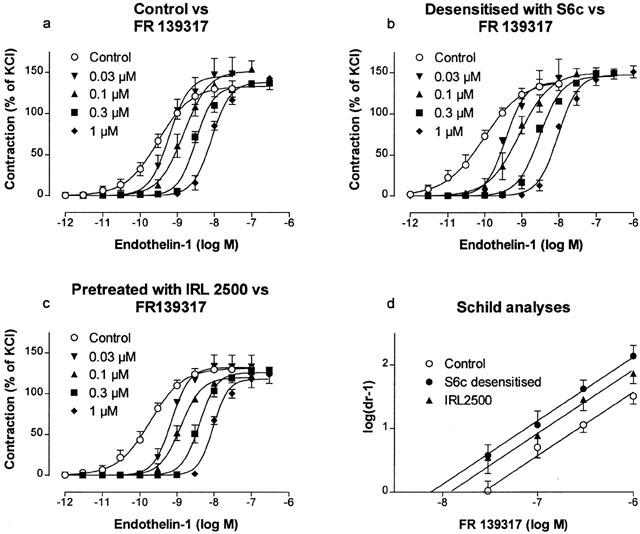
FR139317 produced concentration-dependent, parallel rightward shifts of the S6b concentration-effect curves and the corresponding Schild plot slope (1.04±0.16) was not significantly different from unity (Figure 3a and Table 1). The pKB value estimated (8.87±0.12; d.f.=18) was significantly higher than when ET-1 was used as agonist.
Figure 3.
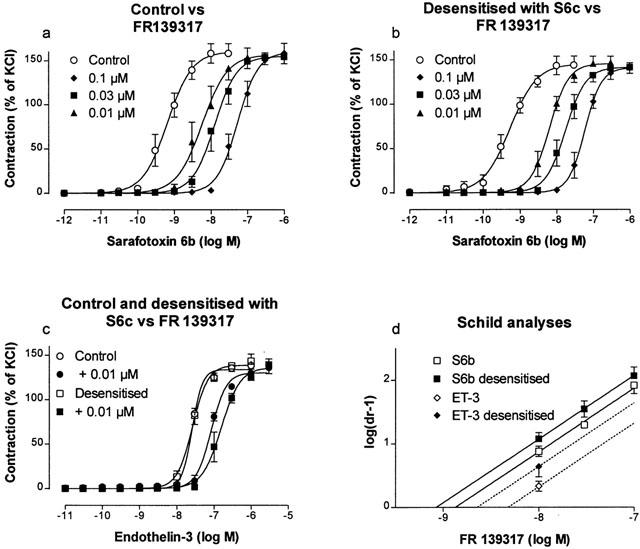
Concentration-effect curves obtained on rat small mesenteric artery segments to S6b in the absence and presence of 0.01, 0.03 and 0.1 μM FR 139317 – control (a), pre-treated with 0.3 μM S6c (b). Concentration-effect curves to ET-3 (c) in the absence and presence of 0.01 μM FR 139317 for controls and in the absence and presence of 0.01 μM FR 139317 in arteries pre-treated with 0.3 μM S6c. Contractions are expressed as a percentage of the response to 60 mM KCl. Corresponding Schild plots are shown in panel (d). The hatched line shows the extrapolated Schild plot for the interaction between ET-3 and the single concentration of FR139317 investigated. Each point represents the mean of all segments tested with error bars representing s.e.mean from five animals.
Due to the relatively low potency of ET-3 (Figure 3c and Table 1), that limited the experimental window available for measuring antagonist effects, a pKB value for FR139317 could only be estimated from a concentration-effect curve obtained with a single antagonist concentration (10 nM). At this concentration, FR139317 produced a parallel, surmountable, rightward shift and the corresponding pKB value (8.32±0.07; d.f.=8) was significantly different from the values obtained using ET-1 or S6b as agonist.
Contractile responses to S6c
Given that the rat small mesenteric artery expresses ETB in addition to ETA receptors, we investigated the possibility that the complexity in the agonist responses and the antagonism expressed by FR139317 was due to concomitant stimulation of ETB receptors. First, an attempt to characterize ETB receptor-mediated agonism was made using the selective ETB receptor agonist, S6c. Under the standard assay conditions, a significant, although small, concentration-dependent, contractile response was obtained to S6c in all segments tested (p[A]50=9.12±0.20; α=3.3±0.7% of KCl; nH=1.15±0.14; n=8; Figure 1e). Subsequently, the effect of a single, maximal, concentration (0.3 μM) of S6c was measured in the tissues during the ETB receptor desensitization studies described below. From these data the contraction was measured to be 4.4±0.4% of KCl, ranging from 0.6 – 16.5% (n=19). The contractions were fast, relative to those to ET-1, and transient returning to basal levels within 60 min. The S6c response was not modified by the presence of a high concentration of FR139317 (1 μM; data not shown). In addition, we did not detect ETB-receptor mediated responses to ET-1, S6b or ET-3 in the presence of high concentrations of FR139317. However, the cumulative dosing protocol with relatively long periods between dosing might have allowed significant desensitization to occur with these agonists in contrast to the rapid cumulative dosing used when S6c was investigated.
Contractile responses to ET-1, S6b and ET-3 and interaction with FR139317 following ETB receptor desensitization
In order to study agonist-induced ETA receptor activation in the absence of any ETB receptor stimulation, ETB receptors were desensitized using S6c (see Methods). Following desensitization, there were no obvious changes in any of the agonist time-response profiles and no significant changes in the maxima or Hill coefficients of the corresponding concentration-effect curves. However, although the potency of S6b and ET-3 was unchanged, there was a significant 3.8 fold increase in the potency of ET-1 (p[A]50=10.17±0.14; n=10).
Following ETB receptor desensitization, the profile of interaction between ET-1 and FR139317 was indistinguishable (Figure 4a) from that observed in control arteries (Figure 2a). However, subsequent competitive analysis revealed a significant, 3.5 fold, increase in the apparent affinity value of FR139317 (pKB=8.12±0.09; d.f.=28). In contrast, desensitization of ETB receptors had no significant effect on the interaction between FR139317 and either S6b (pKB=9.07±0.08; d.f.=18) or ET-3 (pKB′=8.64±0.14; d.f.=8).
Figure 4.
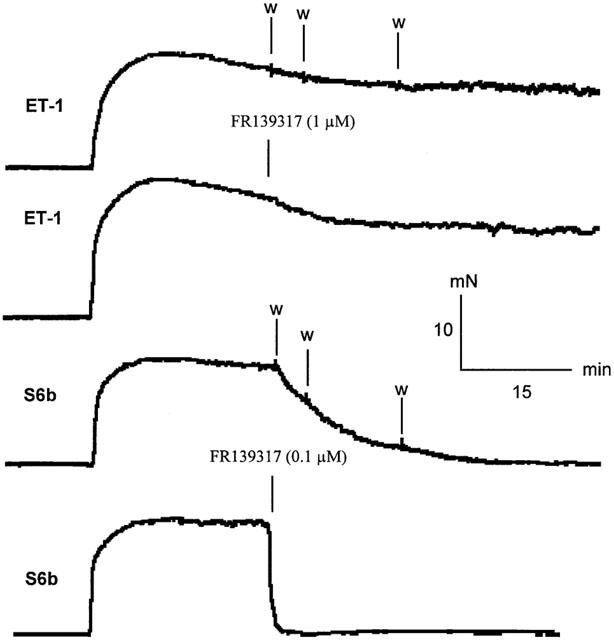
Representative experimental traces showing the contractions developed in rat mesenteric arterial branch segments to a single concentration (10 nM) of ET-1 and S6b followed by washout (three successive exchanges of the organ bath solution) or administration of FR139317 (1 and 0.1 μM for ET-1 and S6b, respectively).
Interaction of ET-1 with FR139317 in presence of ETB receptor blockade
As an alternative method of studying the interaction between ET-1 and FR139317, in the absence of an involvement of ETB receptors, the Schild analysis was repeated in the presence of a high but selective concentration of the ETB receptor antagonist, IRL2500 (KD=55 and 0.3 μM for the ETA and ETB receptors, respectively; Russell & Davenport, 1996). In the presence of 1 μM of IRL2500, parallel shifts of the ET-1 concentration-effect curve were obtained in the presence of FR139317. The corresponding pKB value (7.92±0.09; d.f.=28) lay midway between the values estimated in the control segments (7.57) and in the vessels desensitized with S6c treatment (8.12) although not significantly different from either.
ETB receptor mediated agonism following stimulation of adenylate cyclase activity
The above results indicate that although the contractile response observed following stimulation of the ETB receptor is small, its influence on the expression of agonism by ET-1, and its blockade by FR139317, was significant. The possibility that the impact of ETB receptor stimulation was being underestimated under the current isolated tissue assay conditions was investigated. The method established by Thomas & Ehlert (1994) for the exposure of muscarinic M2-receptor mediated agonism in smooth muscle assays was adopted on the basis that, like M2 receptors, vascular ETB receptors have been reported to couple through Gi proteins (Eguchi et al., 1993). Accordingly, we investigated whether the effect of ETB-receptor activation would be enhanced following stimulation of adenylate cyclase. Segments were pre-contracted with the selective thromboxane A2 receptor agonist U46619 (0.1 μM; 58±7% of KCl) and then relaxed with the adenylate cyclase activator forskolin (0.1 μM). Under these conditions, a fully-defined concentration-effect curve was obtained to S6c (α=42±10% of KCl; p[A]50=9.61±0.32; nH=1.10±0.10; n=8; Figure 1f). Thus, it appeared that under basal conditions, the contractile effect of S6c is limited by the extent of basal adenylate cyclase activity rather than by the number and coupling efficiency of the ETB receptor (an amplification of the S6c contraction could be seen when forskolin was omitted but with a smaller magnitude; not shown). Interestingly, even when adenylate cyclase activity was elevated, the S6c contractile responses faded to baseline within 60 min.
Effect of washout and FR139317 on plateau responses to ET-1, S6b and ET-3
The reversibility of the agonist responses was assessed by measuring the effect of washout (three successive exchanges of the organ bath solution) and addition of FR139317 (at concentrations that produced dose ratios of ⩾20) on the steady-state response to a single, sub-maximal concentration of each agonist (10 nM S6b and 10 nM ET-1, Figure 4; 100 nM ET-3, data not shown). The responses to ET-3 and S6b were wholly reversed within 20 min by both washout and administration of the antagonist. In contrast, the response to ET-1 was relatively refractory to both treatments (50% reduction after 70±40 min and 160±50 min for washout and addition FR139317, respectively; n=3).
Discussion
The aim of this study was to determine the nature of any interaction between ETA and ETB receptors using three ETA receptor agonists, ET-1, S6b and ET-3, in the rat mesenteric artery assay. Under the standard bioassay conditions, ETA receptor activation produced a large, sustained contraction whereas the responses following ETB receptor activation were significantly smaller and faded rapidly. However, when the tissue was pre-contracted with the thromboxane A2 agonist U46619 and then relaxed with forskolin, it was found that selective ETB receptor activation could produce a large contraction indicating the presence of a significant population of ETB receptors coupled to the inhibition of adenylate cyclase. A significant effect of ETB receptor activation, even under standard assay conditions, was also revealed by the finding that both the potency for ET-1 and the apparent affinity for FR139317, estimated using ET-1 as agonist, were increased in the arterial segments following ETB receptor desensitization. The apparent affinity of FR139317 was higher when S6c and ET-3 were used as agonists, but interestingly, desensitization of ETB receptors did not affect potency of these agonists or the apparent affinity of FR139317. The difference in apparent affinity of FR139139 may be attributed in part to the much slower dissociation rate of ET-1, which was illustrated when both the wash-out procedure and administration of FR139317, although producing rapid relaxation of S6b and ET-3 pre-contracted arteries, had little effect on ET-1 pre-contracted arteries. However, the situation was further complicated by the findings that the slopes (i.e. Hill coefficients) of the S6b and ET-3 concentration effect curves were different. Moreover, although both agonist responses were blocked by FR139317, the block was associated with different apparent pKB values.
Under standard assay conditions, the agonist potency order was consistent with previously defined expectations for the activation of ETA receptors. Thus, the finding that ET-1 was more potent than S6b is in agreement with other functional studies (Panek et al., 1992; Maguire & Davenport, 1995) and reflects its relatively high ETA receptor affinity found in radioligand binding studies. Similarly, the potency of ET-3 was about 100 fold lower than ET-1 which is similar to reports of previous functional studies (Panek et al., 1992; Adner et al., 1996). That ETA receptors were involved was further supported by the almost negligible contractile response to the selective ETB receptor agonist, S6c, and the antagonist behaviour of FR139317. Thus, when ET-1, S6b and ET-3 were used as agonist, FR139317 produced concentration-dependent, surmountable antagonism. Overall, these data viewed at a superficial level suggest that ETB receptors did not play a significant role in the contractile activity of ET-1, S6b and ET-3. However, closer examination of the behaviour of FR139317 revealed significant agonist-dependent differences. Thus, when S6b and ET-3 were used as agonist, the apparent affinity of FR139317 was 20 and 6 fold higher, respectively, than when ET-1 was used. Such an increase in the apparent affinity of ETA receptor antagonists when agonists other than ET-1 are used has been reported previously (Bax et al., 1993; Devadason & Henry, 1997; Maguire et al., 1996). Previous explanations for this phenomenon include both the existence of an additional ETA receptor subtype that preferentially binds ET-1 (Maguire et al., 1996) and the irreversible nature of ET-1 binding at ETA receptors (Devadason & Henry, 1997). In addition, not surprisingly, it has been reported that the apparent affinity of ETA receptor antagonists is altered in tissues with a significant component of ETB receptor mediated contraction (Clozel & Gray, 1995; Fukuroda et al., 1996; Mickley et al., 1997). However, how the very small ETB receptor mediated contraction, as obtained in this study, could affect the estimate of affinity for ETA receptor antagonists does not appear to have been considered before.
These findings prompted the investigation into whether concomitant ETB receptor activation influenced the response profiles for the ET agonists and the variation in the apparent affinity of FR139317. Since ET-1, S6b and ET-3 have been reported to express similar affinity for the ETB receptor as S6c in radioligand binding studies (Williams et al., 1991), any interaction between ETA and ETB receptors might have been expected to occur with all three agonists under the standard assay conditions. As an alternative method of exposing such an influence, the effect of ETB receptor desensitization was determined on the interaction between FR139317 and each of ET-1, S6b and ET-3.
The ETB receptor desensitization procedure had no significant effect on S6b and ET-3 concentration-effect curve shape or their interactions with FR139317, indicating that the responses obtained to these agonists was not influenced by any concomitant ETB receptor activation. In contrast, following ETB receptor desensitization, ET-1 was significantly more potent than under control conditions. Furthermore, the apparent affinity of FR139317 was increased towards those values obtained when S6b or ET-3 was used as agonist and literature values obtained in ETA receptor radioligand binding studies (Doherty et al., 1993; Sogabe et al., 1993). These data suggest that under standard assay conditions ET-1 activates both ETA and ETB receptors. The effect of concomitant ETB stimulation was to decrease the effectiveness of the ETA receptor stimulation as though the nature of the ‘cross-talk' between the receptors is one of functional antagonism (Clozel & Gray, 1995; Fukuroda et al., 1996; Mickley et al., 1997; Adner et al., 2000). This interaction could be due to activation of different intracellular pathways since, although both have been shown to couple to Gq-proteins, ETA and ETB receptors have also been shown be coupled to Gs- and Gi-proteins, respectively (Eguchi et al., 1993). In addition, pre-treatment with the competitive ETB-receptor antagonist IRL2500 produced a small increase in the apparent affinity of FR139317 and the potency of ET-1 in agreement with the ETB receptor desensitization studies. However, in this case both the ET-1 potency and the FR139317 affinity values were midway between those obtained from control and S6c-desensitized arteries, which raise the question if only interaction between the of intracellular pathways are involved in the complex cross-talk between the ET receptors. Interestingly, these results may explain the wide variation in reported pKB/pA2 values for FR139317 in functional assays when ET-1 was used as agonist (range 6.0 to 8.2, Doherty et al., 1993; Sogabe et al., 1993; Lodge et al., 1995, Devadason & Henry, 1997). Thus, even when no overt contractile effects of ETB-receptor activation are observed under standard assay conditions, extrapolation of the current results suggests that the variation could be attributed to assay differences in ETB receptor density and degree of activation.
The presence of a significant functional population of ETB receptors in the rat small mesenteric artery was confirmed by the results obtained when the arteries were pre-contracted with a TXA2 analogue and relaxed with forskolin (Figure 1f). Thus, it was evident that the ETB receptors can provide a powerful contractile pathway when there is tonic vascular relaxation driven by adenylate cyclase activity. As with Thomas & Ehlerts' (1994) work which revealed the potential importance of the Gi-coupled ACh M2 receptor in gastrointestinal smooth muscle contractility, the present results demonstrate the potential for error when using isolated tissue assays to obtain information on physiological control mechanisms. Thus, in vivo, where adenylate cyclase activity might be expected to be elevated, effective inhibition of ET-1 stimulated vasoconstriction would require both ETA and ETB receptor blockade, which could explain the incomplete inhibition by a high dose of the ETA receptor antagonist of the ET-1 induced pressor effect in anaesthetized rat (McMurdo et al., 1993).
Previously, it has been suggested that the ‘cross-talk' between the ETA and the ETB receptors is in the form of a mutual inhibition so that blockade of one receptor subtype will free the other receptor subtype from the inhibition (Fukuroda et al., 1996; Adner et al., 2000). The present results support this hypothesis since desensitization of the ETB receptors caused an increase in the potency of ET-1 and apparently rectified the pKB estimate for the ETA receptor antagonist. As far as we are aware, ‘cross-talk' has not been described when any ET receptor agonists other than ET-1 have been used and no evidence of such interactions could be found in this study as the responses to S6b and ET-3 were unaffected by ETB receptor desensitization. A possible explanation for this phenomenon can be that the effect induced by ET-1 is due to induction of another conformational state of the ETA receptor (Sokolovsky, 1993) that activates a different intracellular signal. The idea of such ET-receptor coupling promiscuity was raised independently by Eguchi et al. (1993) who found that ET-3 could generate cyclic AMP through Gs-coupling in addition to standard Gq-mediated contractile effects (Panek et al., 1992; Adner et al., 1998).
In addition to the altered sensitivity to concomitant ETB receptor activity, several other differences between the responses to the agonists were observed. Thus, the time for responses to reach plateau at lower concentrations was longer for ET-1 than for S6b and ET-3, the responses to ET-1, but not S6b and ET-3, were unaffected by subsequent wash-out or administration of FR139317, the pKB values differed between all the agonists used and the Hill coefficients associated with each agonist curve were significantly different (ET-1<S6b<ET-3). These differences in the profile of agonist action also suggest heterogeneity in the formation of receptor activation states. It is known that ET-1 has a very slow rate of dissociation compared to S6b and ET-3 (Devadason & Henry, 1997, Hilal Dandan et al. 1997). Theoretically, this could explain why ET-1 responses were relatively slow to reach a plateau and the resistance to wash-out and administration of FR139317. As previously suggested, this phenomenon alone could also explain the low apparent affinity for ETA receptor antagonists (Devadason & Henry, 1997) although the increase in apparent affinity following ETB receptor desensitization indicates that ‘cross-talk' is also a contributing factor. However, the differences in Hill coefficients for S6b and ET-3 and the associated pKB values for FR139317 suggest the existence of yet more complicating factors in the activation of the ETA receptor.
In conclusion, the results obtained indicate that the small contractile response to selective ETB receptor activation in rat mesenteric arteries, barely detectable under standard bioassay conditions, is greatly amplified when adenylate cyclase activity is elevated. Furthermore, the response to ETA receptor activation by ET-1, but not ET-3 and S6b, is significantly modified by co-activation of ETB receptors. However, even when the ETB receptors were inhibited, there were still significant differences in the profile of agonist responses to ET-1, ET-3 and S6b. Therefore, there are at least two sources of complexity to be considered in the analysis of ET receptor responses in vascular tissue. First, the consequences of simultaneous activation of ETA and ETB receptors and second, an intrinsic feature of agonism at ETA receptors that may be related to differences in agonist receptor binding kinetics and the formation of multiple activated receptor states.
Acknowledgments
This work was supported by Wellcome Travelling Research Fellowship, Swedish Medical Research Council (grant nos 0598, 12904 and 12905 and the Royal Physiografic Society. The authors would like to thank Dr Nicola Welsh for valuable discussion of the data obtained in this study.
Abbreviations
- ET
Endothelin
- S6b
sarafotoxin 6b
- S6c
sarafotoxin 6c
References
- ADNER M., CANTERA L., EHLERT F., NILSSON L., EDVINSSON L. Plasticity of contractile endothelin-B receptors in human arteries after organ culture. Br. J. Pharmacol. 1996;119:1159–1166. doi: 10.1111/j.1476-5381.1996.tb16018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADNER M., EDVINSSON L., SHANKLEY N.P.Analysis of the interaction between contractile endothelin ETA and ETB receptors following organ culture of human omental artery segments Proceedings of the IUPHAR 1998 Receptor Satellite Meeting 2000New York: Annals of the New York Academy of Sciences; eds: Humphrey, P.P.A., Leff, P. & Shankley, N.P [Google Scholar]
- ADNER M., GREG G.G., EDVINSSON L. Appearance of contractile endothelin-B receptors in rat mesenteric arterial segments following organ culture. Acta Physiol. Scand. 1998;163:121–129. doi: 10.1046/j.1365-201X.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- BAX W.A., BOS E., SAXENA P.R. Heterogeneity of endothelin/sarafotoxin receptors mediating contraction of the human isolated saphenous vein. Eur. J. Pharmacol. 1993;239:267–268. doi: 10.1016/0014-2999(93)91010-k. [DOI] [PubMed] [Google Scholar]
- BAX W.A., SAXENA P.R. The current endothelin receptor classification: time for reconsideration. Trends Pharmacol. Sci. 1994;15:379–386. doi: 10.1016/0165-6147(94)90159-7. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse stomach assay. Br. J. Pharmacol. 1985;86:1581–1587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUN M., LIYANAGE U.K., LISANTI M.P., LODISH H.F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOZEL M., GRAY G.A. Are there different ETB receptors mediating constriction and relaxation. J. Cardiovasc. Pharmacol. 1995;26:S262–S264. [PubMed] [Google Scholar]
- CRAMER H., MULLER ESTERL W., SCHROEDER C. Subtype-specific desensitization of human endothelin ETA and ETB receptors reflects differential receptor phosphorylation. Biochemistry. 1997;36:13325–13332. doi: 10.1021/bi9708848. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., KUC R.E., MAGUIRE J.J., HARLAND S.P. ETA receptors predominate in the human vasculature and mediate constriction. J. Cardiovasc. Pharmacol. 1995a;26:S265–S267. [PubMed] [Google Scholar]
- DAVENPORT A.P., O'REILLY G., KUC R.E. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br. J. Pharmacol. 1995b;114:1110–1116. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVADASON P.S., HENRY P.J. Comparison of the contractile effects and binding kinetics of endothelin-1 and sarafotoxin S6b in rat isolated renal artery. Br. J. Pharmacol. 1997;121:253–263. doi: 10.1038/sj.bjp.0701126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHERTY A.M., CODY W.L., HE J.X., DEPUE P.L., CHENG X.M., WELCH K.M., FLYNN M.A., REYNOLDS E.E., LADOUCEUR D.M., DAVIS L.S., KEISER J.A., HALEEN S.J. In vitro and in vivo studies with a series of hexapeptide endothelin antagonists. J. Cardiovasc Pharmacol. 1993;22:S98–S102. doi: 10.1097/00005344-199322008-00027. [DOI] [PubMed] [Google Scholar]
- EGUCHI S., HIRATA Y., IMAI T., MARUMO F. Endothelin receptor subtypes are coupled to adenylate cyclase via different guanyl nucleotide-binding proteins in vasculature. Endocrinology. 1993;132:524–529. doi: 10.1210/endo.132.2.7678793. [DOI] [PubMed] [Google Scholar]
- EKELUND U., ADNER M., EDVINSSON L., MELLANDER S. Effects of selective ETB-receptor stimulation on arterial, venous and capillary functions in cat skeletal muscle. Br. J. Pharmacol. 1994;112:887–894. doi: 10.1111/j.1476-5381.1994.tb13163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKURODA T., OZAKI S., IHARA M., ISHIKAWA K., YANO M., MIYAUCHI T., ISHIKAWA S., ONIZUKA M., GOTO K., NISHIKIBE M. Necessity of dual blockade of endothelin ETA & ETB receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. Br. J. Pharmacol. 1996;117:995–999. doi: 10.1111/j.1476-5381.1996.tb16688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY G.A., WEBB D.J. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol. Ther. 1996;72:109–148. doi: 10.1016/s0163-7258(96)00101-5. [DOI] [PubMed] [Google Scholar]
- HILAL DANDAN R., VILLEGAS S., GONZALEZ A., BRUNTON L.L. The quasi-irreversible nature of endothelin binding and G protein-linked signaling in cardiac myocytes. J. Pharmacol. Exp. Ther. 1997;281:267–273. [PubMed] [Google Scholar]
- INOUE A., YANAGISAWA M., KIMURA S., KASUYA Y., MIYAUCHI T., GOTO K., MASAKI T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LODGE N.J., ZHANG R., HALAKA N.N., MORELAND S. Functional role of endothelin ETA and ETB receptors in venous and arterial smooth muscle. Eur. J. Pharmacol. 1995;287:279–285. doi: 10.1016/0014-2999(95)00494-7. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br. J. Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., ROUS B.A., DAVENPORT A.P. Failure of BQ123, a more potent antagonist of sarafotoxin 6b than of endothelin-1, to distinguish between these agonists in binding experiments. Br. J. Pharmacol. 1996;118:335–342. doi: 10.1111/j.1476-5381.1996.tb15407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCMURDO L., CORDER R., THIEMERMANN C., VANE J.R. Incomplete inhibition of the pressor effects of endothelin-1 and related peptides in the anaesthetized rat with BQ-123 provides evidence for more than one vasoconstrictor receptor. Br. J. Pharmacol. 1993;108:557–561. doi: 10.1111/j.1476-5381.1993.tb12840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICKLEY E.J., GRAY G.A., WEBB D.J. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br. J. Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELAND S., MCMULLEN D., ABBOA OFFEI B., SEYMOUR A. Evidence for a differential location of vasoconstrictor endothelin receptors in the vasculature. Br. J. Pharmacol. 1994;112:704–708. doi: 10.1111/j.1476-5381.1994.tb13133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NILSSON T., CANTERA L., ADNER M., EDVINSSON L. Presence of contractile endothelin-A and dilatory endothelin-B receptors in human cerebral arteries. Neurosurgery. 1997;40:346–351. doi: 10.1097/00006123-199702000-00023. [DOI] [PubMed] [Google Scholar]
- OPGAARD O.S., CANTERA L., ADNER M., EDVINSSON L. Endothelin-A and -B receptors in human coronary arteries and veins. Regul. Pept. 1996;63:149–156. doi: 10.1016/0167-0115(96)00036-5. [DOI] [PubMed] [Google Scholar]
- PANEK R.L., MAJOR T.C., HINGORANI G.P., DOHERTY A.M., TAYLOR D.G., RAPUNDALO S.T. Endothelin and structurally related analogs distinguish between endothelin receptor subtypes. Biochem. Biophys. Res. Commun. 1992;183:566–571. doi: 10.1016/0006-291x(92)90519-q. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., DAVENPORT A.P. Characterization of the binding of endothelin ETB selective ligands in human and rat heart. Br. J. Pharmacol. 1996;119:631–636. doi: 10.1111/j.1476-5381.1996.tb15720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOGABE K., NIREI H., SHOUBO M., NOMOTO A., AO S., NOTSU Y., ONO T. Pharmacological profile of FR139317, a novel, potent endothelin ETA receptor antagonist. J. Pharmacol. Exp. Ther. 1993;264:1040–1046. [PubMed] [Google Scholar]
- SOKOLOVSKY M. BQ-123 identifies heterogeneity and allosteric interactions at the rat heart endothelin receptor. Biochem. Biophys. Res. Commun. 1993;196:32–38. doi: 10.1006/bbrc.1993.2212. [DOI] [PubMed] [Google Scholar]
- SUDJARWO S.A., HORI M., TANAKA T., MATSUDA Y., OKADA T., KARAKI H. Subtypes of endothelin ETA and ETB receptors mediating venous smooth muscle contraction. Biochem. Biophys. Res. Commun. 1994;200:627–633. doi: 10.1006/bbrc.1994.1494. [DOI] [PubMed] [Google Scholar]
- THOMAS E.A., EHLERT F.J. Pertussis toxin blocks M2 muscarinic receptor-mediated effects on contraction and cyclic AMP in the guinea pig ileum, but not M3-mediated contractions and phosphoinositide hydrolysis. J. Pharamacol. Exp. Ther. 1994;271:1042–1050. [PubMed] [Google Scholar]
- WILLIAMS D.J., JR, JONES K.L., PETTIBONE D.J., LIS E.V., CLINESCHMIDT B.V. Sarafotoxin S6c: an agonist which distinguishes between endothelin receptor subtypes. Biochem. Biophys. Res. Commun. 1991;175:556–561. doi: 10.1016/0006-291x(91)91601-8. [DOI] [PubMed] [Google Scholar]


