Abstract
Acetylcholine (ACh)-induced translocation of RhoA in bronchial smooth muscle of repeatedly antigen-challenged rats that have a marked airway hyperresponsiveness (AHR) was examined.
ACh induced time- and concentration-dependent translocation of RhoA to the plasma membrane, indicating an activation of RhoA in bronchial smooth muscle.
The level of ACh-induced RhoA translocation was further increased markedly in the AHR group as compared to that in the control group.
It is suggested that the augmented activation of RhoA observed in the hyperresponsive bronchial smooth muscle might be responsible for the enhanced ACh-induced Ca2+ sensitization of bronchial smooth muscle contraction associated with AHR.
Keywords: Asthma, airway hyperresponsiveness, bronchial smooth muscle, Ca2+ sensitization, RhoA, acetylcholine
Introduction
Smooth muscle contraction has been thought to be induced by an increase in cytosolic [Ca2+] via the activation of plasma membrane Ca2+ channels and/or Ca2+ release from sarcoplasmic reticulum. Recently, additional mechanisms of agonist-induced smooth muscle contraction have been suggested by studies using the simultaneous measurements for force development and intracellular Ca2+ concentration (Sato et al., 1988) and chemically permeabilized preparations (Fujita et al., 1995) in various types of smooth muscle, including airways (Ozaki et al., 1990). It has been demonstrated that agonist stimulation increases myofilament Ca2+ sensitivity in β-escin-permeabilized smooth muscle of the rat coronary artery (Satoh et al., 1994), guinea-pig vas deferens (Fujita et al., 1995) and canine trachea (Bremerich et al., 1997), among others. Although the detailed mechanism is not fully understood, participation of Rho protein, a monomeric GTP binding protein, in the agonist-induced Ca2+ sensitization has been suggested (Otto et al., 1996; Fujita et al., 1995; Gong et al., 1997).
Bronchial asthma is an airway inflammatory disease characterized by increased airway responsiveness. In most cases, asthmatic patients have an increased contractility of airway smooth muscle (Roberts et al., 1984) which might be a major cause of airway hyperresponsiveness (AHR). Similarly, an increased responsiveness of bronchial smooth muscle has also been demonstrated in a rat model of AHR induced by repeated antigen inhalation (Misawa & Chiba, 1993; Chiba & Misawa, 1995a, 1995b). In this animal model of AHR, the bronchial smooth muscle contraction induced by receptor agonists such as acetylcholine (ACh), but not by high [K+] depolarization, is markedly augmented (Misawa & Chiba, 1993; Chiba & Misawa, 1995a, 1995b). Moreover, it has been found that muscarinic receptor density and antagonist affinity of airway smooth muscle are normal (Chiba & Misawa, 1995a). Thus, it is possible that the mechanisms responsible for AHR exist, at least in part, in the downstream pathway of muscarinic receptor signalling, including the ACh-mediated Ca2+ sensitization. Indeed, by using β-escin-permeabilized bronchial smooth muscle, we recently revealed the existence of ACh-induced, RhoA-mediated Ca2+ sensitization in rat bronchial smooth muscle contraction and a marked augmentation of this Ca2+-sensitizing effect in the increased smooth muscle contractility observed at AHR (Chiba et al., 1999).
To determine whether the augmented Ca2+ sensitization of bronchial smooth muscle in AHR is mediated by an enhancement of activation of RhoA, the ACh-induced translocation of RhoA to the plasma membrane was investigated in bronchial smooth muscle of AHR rats. Although the activation pathway of RhoA via membrane receptors is not yet clear in smooth muscle, it is known that translocation of RhoA from cytosol to plasma membrane occurs when RhoA is activated (Gong et al., 1997; Fujihara et al., 1997; Taggart et al., 1999).
Methods
Sensitization and antigenic challenge
Male Wistar rats (6 weeks of age, specific pathogen-free, 170 – 190 g, Charles River Japan, Inc.) were sensitized and repeatedly challenged with 2,4-dinitrophenylated Ascaris suum antigen (DNP-Asc) by the method described in previously (Misawa & Chiba, 1993; Chiba & Misawa, 1995a, 1995b; Chiba et al., 1999). Age-matched non-sensitized normal animals were used as a control (Chiba & Misawa, 1995a, 1995b; Chiba et al., 1999).
Functional studies
Approximately 4 mm lengths of the left main bronchus were isolated. The isometric contraction of the circular smooth muscle was measured at a resting tension of 1.0 g as described previously (Chiba & Misawa, 1995a, 1995b).
Preparation of membrane and cytosolic fractions
Bronchial tissue preparations were prepared by the method described previously (Chiba et al., 1999) with minor modifications. In brief, the airway tissues below the main bronchi were removed and immediately soaked in ice-cold, oxygenated Krebs-Henseleit solution (in mM: NaCl 18, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 10; pH 7.4). The airways were carefully cleaned of adhering connective tissues, blood vessels and lung parenchyma under stereomicroscopy. The epithelium was removed as much as possible by gently rubbing with sharp-edged tweezers. Then the bronchial tissue segments (containing main and intrapulmonary bronchi) were equilibrated in oxygenated Krebs-Henseleit solution (37°C) for 60 min with 10-min washout intervals. After the equilibration period, the tissue segments were stimulated by a concentration of ACh for the indicated time. The reaction was stopped by quickly freezing with liquid nitrogen and the tissue was then homogenized with Physcotron (Niti-on, Co. Ltd., Japan: six times for 5 s; level max) in 2 ml ice-cold homogenization buffer with the following composition mM: Tris-HCl (pH 7.5) 10, MgCl2 5, EDTA 2, sucrose 250, DTT 1, 4-(2-aminoethyl) benzenesulphonyl fluoride 1, 20 μg ml−1 leupeptin and 20 μg ml−1 aprotinin. The tissue homogenate was centrifuged (105,000 × g, 4°C for 30 min) and the supernatant was collected as the cytosolic fraction. The pellet was resuspended in 3 ml homogenization buffer and recentrifuged (105,000 × g, 4°C for 30 min). The resultant pellet was resuspended in 2 ml ice-cold homogenization buffer containing 1% (v v−1) Triton X-100 and 1% (w v−1) sodium cholate and used as the membrane fraction. These preparations were stored at −80°C until use. The protein concentrations of these preparations were determined by the method of Lowry et al. (1951) in triplicate with bovine serum albumin as a standard.
Western blots
Immunoblotting was performed in the preparations obtained from the AHR rats (sensitized and repeatedly antigen-challenged) and normal control rats as described above. Briefly, the membrane and cytosolic fractions were dissolved in SDS sample buffer and heated at 100°C for 4 min. The samples (10 μg protein per lane) were subjected to 15% SDS – PAGE. Proteins were then electrophoretically transferred for 4 h onto nitrocellulose membranes (Hybond-ECL, Amersham, Little Chalfont, U.K.) in cold transfer buffer (20% methanol containing Tris 25 mM and glycine 192 mM). After repeated washing with Tris buffer (Tris 20 mM NaCl 500 mM, pH 7.5) containing 0.1% (v v−1) Tween 20 (TTBS), the nitrocellulose membranes were incubated with blocking buffer (3% gelatine in TTBS) for 1.5 h at room temperature. The nitrocellulose membranes were then incubated with polyclonal rabbit anti-RhoA (amino acids 119 – 132; 1 : 3000 dilution; Santa Cruz Biotechnology, Santa Cruz, California, U.S.A.) in antibody buffer (1% gelatine in TTBS) for 12 h at room temperature. The nitrocellulose membranes were then washed five times (each for 15 min) with TTBS. They were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1 : 5000 dilution; Amersham) for 1.5 h at room temperature, and then washed five times with TTBS. The blots were detected with an enhanced chemiluminescent method (ECL System; Amersham) and quantified by densitometry (Atto Densitograph Software ver. 4.0; Atto Co., Japan). To normalize the RhoA contents to an internal control protein, β-actin, immunoblotting was also performed on the same gel by using monoclonal mouse anti-β-actin N-terminal (1 : 5000 dilution; Sigma, St. Louis, Missouri, U.S.A.) and goat anti-mouse IgG (1 : 5000 dilution; Amersham). Under the above conditions, a linear relationship between the band density of RhoA and amounts of loaded proteins was found for protein quantities ranging between 5 and 25 μg (Chiba et al., 1999). Similar results were obtained for β-actin. The ratios of corresponding RhoA/β-actin in each lane were calculated as indexes of RhoA protein levels. The per cent of membrane/total RhoA was calculated according to the formula (membrane RhoA/β-actin)/[(membrane Rho/β-actin)+(cytosolic RhoA/β-actin)].
Statistical analyses
All data are expressed as mean with s.e.mean. Statistical significance of differences was determined by Dunnett's multiple comparisons test or two-way analysis of variance (ANOVA). A value of P<0.05 was considered significant.
Results
Translocation of RhoA in bronchial smooth muscle from normal rats
In normal rats, both the membrane and cytosolic fractions of bronchial smooth muscle contained RhoA proteins (RhoA/β-actin, n=5: 0.19±0.08 and 0.47±0.07, respectively) at resting state (no ACh stimulation). The ratio of membrane to total RhoA at resting state was 25.7±6.9%. ACh (1 mM) stimulation elicited rapid increases in tension and RhoA contents in the membrane fractions, while cytosolic RhoA was decreased (Figure 1). One minute after addition of 1-mM ACh to the intact (non-permeabilized) bronchial smooth muscle, tension reached 53.6±5.5% of the maximal ACh-induced contraction (n=5); this was accompanied by an increase in membrane RhoA, increasing from the resting value of 25.7±6.9% (n=5) to 63.5±7.8% membrane/total RhoA (n=5, P<0.05). Thereafter, both contraction and membrane RhoA gradually increased and reached plateaux at 10 – 20 min after ACh stimulation (Figure 1c).
Figure 1.
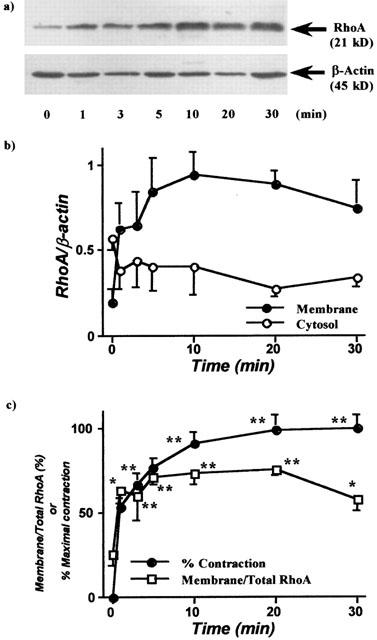
Time course of acetylcholine (ACh)-induced translocation of RhoA in bronchial smooth muscle of non-sensitized normal rats. The isolated main and intrapulmonary bronchi were stimulated by ACh (1 mM), and homogenized to prepare cytosolic and membrane fractions after stopping the reaction by liquid nitrogen at the time indicated. Western blotting was performed by using these fractions both on RhoA and β-actin in the identical transferred membrane. (a) Representative Western blots of membrane RhoA (21 kD) and β-actin. (b) Relevance of the time courses of ACh (1 mM)-induced increase in membrane RhoA and decrease in cytosolic RhoA. (c) Relevance of the time courses of ACh (1 mM)-induced contraction and translocation of RhoA. Values are means±s.e.mean from five experiments in duplicate. *P<0.05 and **P<0.01 vs respective time 0 (no stimulation).
As shown in Figure 2, ACh also induced concentration-dependent increases in tension and membrane RhoA. The ACh (1 mM)-induced increase in membrane RhoA (69.4±8.6% membrane/total RhoA, n=5) was completely blocked by pre-treatment with 1 μM atropine (29.1±4.4% membrane/total RhoA, n=4; P<0.05). On the other hand, depolarizing stimulation induced by isotonic 60 mM K+ (in the presence of 1 μM atropine) had no effect on the localization of RhoA (31.3±3.4 and 29.5±4.5% membrane/total RhoA in resting and 10 min after 60 mM K+ treatment, respectively; n=4), although distinct contraction was observed (data not shown).
Figure 2.
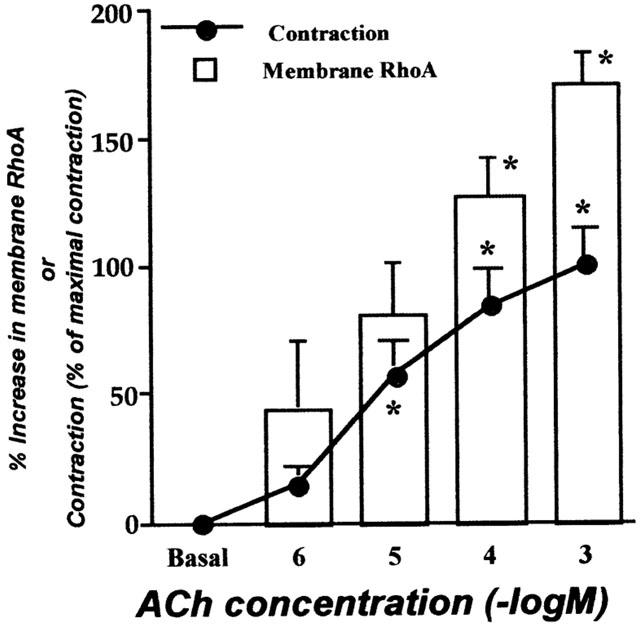
The concentration-dependence of acetylcholine (ACh)-induced translocation of RhoA in bronchial smooth muscle of non-sensitized normal rats. The isolated main and intrapulmonary bronchi were stimulated by ACh (1 μM – 1 mM), and homogenized to prepare cytosolic and membrane fractions after stopping the reaction by liquid nitrogen 10 min after stimulation. Western blotting was performed by using these fractions both on RhoA and β-actin in the identical transferred membrane. The ACh-induced translocation of RhoA seems correlated with contraction induced by the identical concentrations of ACh. Values are means±s.e.mean from five experiments in duplicate. *P<0.05 and **P<0.01 vs respective basal level (no stimulation).
Effects of repeated antigen exposure on the ACh-induced contraction and translocation of RhoA
Figure 3 shows the ACh responsiveness of the bronchial smooth muscle isolated from control and repeatedly antigen-challenged rats. ACh elicited a concentration-dependent contractile response in both groups. However, the concentration-response curve to ACh was significantly shifted upward after repeated antigenic challenge (P<0.01 by ANOVA).
Figure 3.
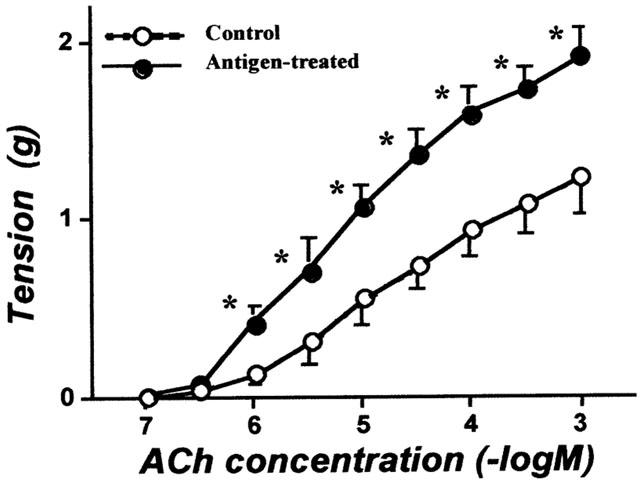
Acetylcholine (ACh) concentration-response curves for contractile responses of the isolated bronchial rings from non-sensitized normal (Control) and repeatedly antigen challenged rats (Antigen-treated). Each value is the mean±s.e.mean from eight experiments. *P<0.05 vs Control. The ACh responsiveness was significantly augmented in antigen-treated group (P<0.01 by ANOVA).
In bronchial smooth muscle of the AHR rats, basal RhoA contents (no ACh stimulation) estimated by RhoA/β-actin were significantly greater (0.68±0.17 in membrane and 1.13±0.23 in cytosolic fractions, n=5) than those of normal animals (0.19±>0.08 and 0.47±0.07, respectively; P<0.05), whereas the ratio of membrane to total RhoA was within normal levels (33.3±7.2%; Figure 4). As shown in Figure 4, ACh elicited a concentration-dependent translocation of RhoA in both groups. Interestingly, the ACh concentration-response curve for translocation of RhoA in the AHR rats was significantly shifted to the left as compared to that in normal animals (P<0.05 by ANOVA; Figure 4).
Figure 4.
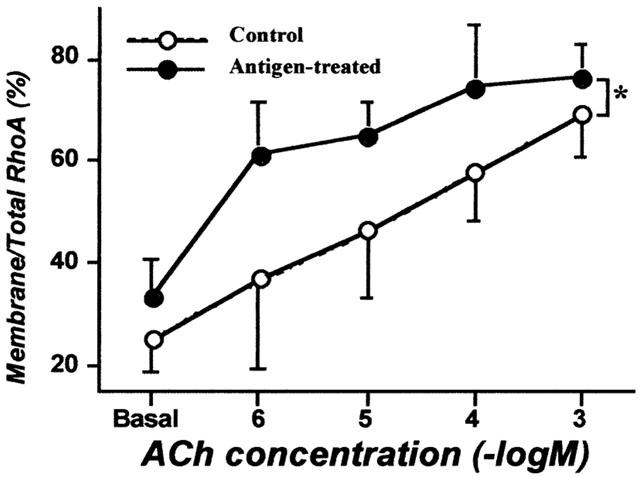
Acetylcholine (ACh) concentration-response curves for translocation of RhoA in bronchial smooth muscles from non-sensitized normal (Control) and repeatedly antigen challenged rats (Antigen-treated). The isolated main and intrapulmonary bronchi were stimulated by ACh (1 μM – 1 mM), and homogenized to prepare cytosolic and membrane fractions after stopping the reaction by liquid nitrogen 10 min after stimulation. Western blotting was performed by using these fractions both on RhoA and β-actin in the identical transferred membrane and the per cent of membrane to total RhoA was calculated. Values are means±s.e.mean from five experiments in duplicate. The ACh-induced translocation of RhoA was significantly augmented in antigen-treated group (*P<0.05 by ANOVA).
Effects of papaverine and Y-27632 on the resting tone of bronchial smooth muscle
As shown in Figure 5, papaverine elicited a concentration-dependent decrease in resting muscle tone in both groups. No significant difference in the papaverine responsiveness was observed between groups (Figure 5). On the other hand, Y-27632 (1 μM – 1 mM), an inhibitor of Rho-associated protein kinase (Uehata et al., 1997), had no effect on the resting muscle tone in either group (data not shown).
Figure 5.
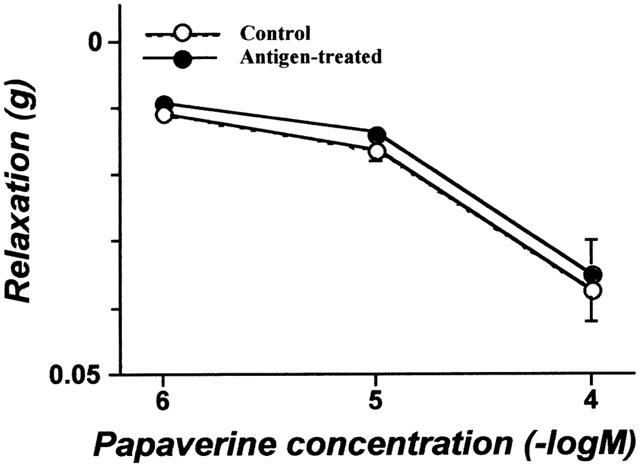
The effect of papaverine on the resting tone of the isolated bronchial rings from non-sensitized normal (Control) and repeatedly antigen challenged rats (Antigen-treated). Each value is the mean±s.e.mean from four experiments. No significant difference in the papaverine responsiveness was observed between groups.
Discussion
We previously reported that repeated challenge of actively sensitized rats with aerosolized antigen causes distinct airway inflammation and marked AHR to inhaled ACh in vivo (Misawa & Chiba, 1993). The isolated bronchial smooth muscle from these animals also displays hyperresponsiveness to ACh (Misawa & Chiba, 1993; Chiba & Misawa, 1995a, 1995b; Chiba et al., 1999). More recently, a marked augmentation of ACh-induced, RhoA-mediated Ca2+ sensitization has been demonstrated in bronchial smooth muscle of this animal model of AHR (Chiba et al., 1999). In the present study, ACh-induced translocation of RhoA in intact bronchial smooth muscle of AHR rats was compared with that of normal animals to demonstrate an augmented activation of RhoA at antigen-induced AHR.
In the present study, the ratio of membrane to total RhoA of normal rats at resting state (25.7±6.9%) is approximately equal to that reported in the portal vein and ileum (Fujihara et al., 1997), indicating a successful separation of the membrane RhoA proteins. ACh stimulation elicited time- and concentration-dependent increases in tension and RhoA content in the membrane fraction, while cytosolic RhoA was decreased (Figures 1 and 2). The ACh-induced increase in membrane RhoA was completely blocked by pre-treatment with atropine. On the other hand, high [K+] stimulation had no effect on the localization of RhoA. These findings indicate that ACh induces translocation of RhoA from the cytosol to the membrane (that is an activation of RhoA (Gong et al., 1997; Fujihara et al., 1997; Taggart et al., 1999)) in rat bronchial smooth muscle via an activation of muscarinic receptors. Furthermore, the translocation of RhoA induced by ACh might be a parallel event to the induction of contraction in the intact (non-permeabilized) bronchial smooth muscle of the rat (Figures 1 and 2).
As shown in Figure 3, ACh-induced bronchial contraction was significantly augmented in the repeatedly antigen-challenged rats, indicating that reproducible AHR occurs at the level of bronchial smooth muscle after repeated antigen inhalation (Misawa & Chiba, 1993; Chiba & Misawa, 1995a, 1995b; Chiba et al., 1999). In this animal model of AHR, basal RhoA content (no ACh stimulation) was significantly greater than that of normal animals (see Results). The increased content of RhoA in bronchial smooth muscle from AHR rats is consistent with our previous study (Chiba et al., 1999). Although the basal content of bronchial membrane RhoA was increased in AHR, the functional role of this membrane-associated RhoA is unclear. If this membrane-associated RhoA were involved in the Ca2+ sensitization of contractile apparatus, an increase in intracellular Ca2+ concentration of smooth muscle (by non-receptor stimulation) would induce further contraction. However, we previously demonstrated that the K+-depolarization-induced contraction of intact (non-permeabilized) bronchial smooth muscle and the Ca2+-induced contraction of β-escin permeabilized bronchial smooth muscle were within normal levels even in AHR (Chiba & Misawa, 1995b; Chiba et al., 1999). Moreover, no significant difference in the relaxant effect of papaverine on the resting muscle tone was observed between control and AHR groups (Figure 5), indicating that the inherent tone was not elevated in the hyperresponsive bronchial smooth muscle. Additionally, Y-27632, an inhibitor of Rho-associated protein kinase (Uehata et al., 1997), had no effect on the resting tone of bronchial smooth muscle in either group (see Results). Gong et al. (1997) suggested that membrane-associated RhoA exists in at least two states, resting and activated states, in the rabbit portal vein. In the absence of ACh stimulation, a large proportion of the membrane-associated RhoA observed in the hyperresponsive bronchial smooth muscle may be in the resting state.
As in the normal group, ACh stimulation induced concentration-dependent translocation of RhoA to the membrane in bronchial smooth muscle from AHR rats (Figure 4). Interestingly, the ACh-induced translocation of RhoA was significantly enhanced in bronchial smooth muscle of AHR rats (Figure 4). Thus, in addition to the up-regulation of RhoA proteins (Chiba et al., 1999), it is suggestive that RhoA activation pathway(s) via muscarinic receptors of bronchial smooth muscle might be functionally augmented in AHR in rats. Although the detailed mechanism of activation pathway of RhoA in airway smooth muscle is not clear at present, an involvement of heterotrimeric G protein(s) has been suggested (Kai et al., 1998; Croxton et al., 1998). We also demonstrated an up-regulation of heterotrimeric G proteins (Chiba et al., 2000), but not muscarinic receptors (Chiba & Misawa, 1995a), in bronchial smooth muscle in the AHR model. Therefore, it is possible that enhanced signalling by the increased heterotrimeric G proteins may be involved in the augmented activation of RhoA in AHR in rats.
In conclusion, ACh induced a RhoA translocation to plasma membrane – i.e. an activation of RhoA – in rat bronchial smooth muscle. The level of ACh-induced RhoA translocation was markedly increased in AHR as compared to that in the control group. Our data suggest that the augmented activation of RhoA observed in the hyperresponsive bronchial smooth muscle might be responsible for the enhanced ACh-induced Ca2+ sensitization of bronchial smooth muscle contraction at the AHR in rats.
Acknowledgments
We thank Yuri Ogamino, Takashi Kubota and Masashi Sugisawa for their technical assistance. This work was supported by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- ACh
acetylcholine
- AHR
airway hyperresponsiveness
- DNP-Asc
2,4-dinitrophenylated Ascaris suum extract
- TTBS
Tris-buffered saline containing 0.1% (v v−1) Tween 20
References
- BREMERICH D.H., WARNER D.O., LORENZ R.R., SHUMWAY R., JONES K.A. Role of protein kinase C in calcium sensitization during muscarinic stimulation in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;27317:L775–L781. doi: 10.1152/ajplung.1997.273.4.L775. [DOI] [PubMed] [Google Scholar]
- CHIBA Y., MISAWA M. Characteristics of muscarinic cholinoceptors in airways of antigen-induced airway hyperresponsive rats. Comp. Biochem. Physiol. 1995b;111C:351–357. doi: 10.1016/0742-8413(95)00061-5. [DOI] [PubMed] [Google Scholar]
- CHIBA Y., MISAWA M. Alteration in Ca2+ availability involved in antigen-induced airway hyperresponsiveness in rats. Eur. J. Pharmacol. 1995a;278:79–82. doi: 10.1016/0014-2999(95)00132-5. [DOI] [PubMed] [Google Scholar]
- CHIBA Y., SAKAI H., ARIMOTO T., YOSHIKAWA T., MISAWA M. Gq protein level increases concurrently with antigen-induced airway hyperresponsiveness in rats. Respir. Physiol. 2000;121:75–83. doi: 10.1016/s0034-5687(00)00114-6. [DOI] [PubMed] [Google Scholar]
- CHIBA Y., TAKADA Y., MIYAMOTO S., MITSUI-SAITO M., KARAKI H., MISAWA M. Augmented acetylcholine-induced, Rho-mediated Ca2+ sensitization of bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Br. J. Pharmacol. 1999;127:597–600. doi: 10.1038/sj.bjp.0702585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROXTON T.L., LANDE B., HIRSHMAN C.A.Role of G proteins in agonist-induced Ca2+ sensitization of tracheal smooth muscle Am. J. Physiol. 1998275L748–L755.(Lung Cell. Mol. Physiol.19) [DOI] [PubMed] [Google Scholar]
- FUJIHARA H., WALKER L.A., GONG M.C., LEMICHEZ E., BOQUET P., SOMLYO A.V., SOMLYO A.P. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin D3B. Mol. Biol. Cell. 1997;8:2437–2447. doi: 10.1091/mbc.8.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA A., TAKEUCHI T., NAKAJIMA H., NISHIO H., HATA F. Involvement of heterotrimeric GTP-binding protein and rho protein, but not protein kinase C, in agonist-induced Ca2+ sensitization of skinned muscle of guinea pig vas deferens. J. Pharmacol. Exp. Ther. 1995;274:555–561. [PubMed] [Google Scholar]
- GONG M.C., FUJIHARA H., SOMLYO A.V., SOMLYO A.P. Translocation of rhoA associated with Ca2+ sensitization of smooth muscle. J. Biol. Chem. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- KAI T., JONES K.A., WARNER D.O. Halothane attenuates calcium sensitization in airway smooth muscle by inhibiting G-proteins. Anesthesiology. 1998;89:1543–1552. doi: 10.1097/00000542-199812000-00034. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MISAWA M., CHIBA Y. Repeated antigenic challenge-induced airway hyperresponsiveness and airway inflammation in actively sensitized rats. Jpn. J. Pharmacol. 1993;61:41–50. doi: 10.1254/jjp.61.41. [DOI] [PubMed] [Google Scholar]
- OTTO B., STEUSLOFF A., JUST I., AKTORIES K., PFITZER G. Role of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. J. Physiol. 1996;496:317–329. doi: 10.1113/jphysiol.1996.sp021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI H., KWON S.-C., TAJIMI M., KARAKI H. Changes in cytosolic Ca2+ and contraction induced by various stimulants and relaxants in canine tracheal smooth muscle. Pflügers Arch. 1990;416:351–359. doi: 10.1007/BF00370740. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.A., RAEBURN D., RODGER I.W., THOMSON N.C. Comparison of in-vivo responsiveness and in-vitro smooth muscle sensitivity to methacholine. Thorax. 1984;39:837–843. doi: 10.1136/thx.39.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO K., OZAKI H., KARAKI H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator Fura 2. J. Pharmacol. Exp. Ther. 1988;246:294–300. [PubMed] [Google Scholar]
- SATOH S., KREUTZ R., WILM C., GANTEN D., PFITZER G. Augmented agonist-induced Ca2+-sensitization of coronary artery contraction in genetically hypertensive rats. Evidence for altered signal transduction in the coronary smooth muscle cells. J. Clin. Invest. 1994;94:1397–1403. doi: 10.1172/JCI117475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGGART M.J., LEE Y.-H., MORGAN K.G. Cellular redistribution of PKCα, RhoA, and ROKα following smooth muscle agonist stimulation. Exp. Cell Res. 1999;251:92–101. doi: 10.1006/excr.1999.4565. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]


