Abstract
Using a rat model which allows serial blood sampling and concurrent brain microdialysis sampling, we have investigated the temporal kinetic inter-relationship of levetiracetam in serum and brain extracellular fluid (frontal cortex and hippocampus) following systemic administration of levetiracetam, a new antiepileptic drug. Concurrent extracellular amino acid concentrations were also determined.
After administration (40 or 80 mg kg−1), levetiracetam rapidly appeared in both serum (Tmax, 0.4 – 0.7 h) and extracellular fluid (Tmax, 2.0 – 2.5 h) and concentrations rose linearly and dose-dependently, suggesting that transport across the blood-brain barrier is rapid and not rate-limiting. The serum free fraction (free/total serum concentration ratio; mean±s.e.mean range 0.93 – 1.05) was independent of concentration and confirms that levetiracetam is not bound to blood proteins.
The kinetic profiles for the hippocampus and frontal cortex were indistinguishable suggesting that levetiracetam distribution in the brain is not brain region specific. However, t1/2 values were significantly larger than those for serum (mean range, 3.0 – 3.3 h vs 2.1 – 2.3 h) and concentrations did not attain equilibrium with respect to serum.
Levetiracetam (80 mg kg−1) was associated with a significant reduction in taurine in the hippocampus and frontal cortex. Other amino acids were unaffected by levetiracetam.
Levetiracetam readily and rapidly enters the brain without regional specificity. Its prolonged efflux from and slow equilibration within the brain may explain, in part, its long duration of action. The concurrent changes in taurine may contribute to its mechanism of action.
Keywords: New antiepileptic drug, levetiracetam, microdialysis, extracellular fluid, amino acids, pharmacokinetics, frontal cortex, hippocampus
Introduction
Epilepsy is one of the most common serious neurological disorders, affecting about 1% of the world population at any one time. The life-time chance of developing epilepsy is 3 – 5% (Sander & Shorvon, 1987). Even though globally, since 1989, nine new antiepileptic drugs have been licensed for clinical use (Patsalos, 1999), they have had little impact on the prognosis of refractory epilepsy and therefore there is still a need for new antiepileptic drugs with improved risk/benefit ratios (Marson & Chadwick, 2001) and with simple pharmacokinetics to aid clinical use (Patsalos, 2000).
Levetiracetam (Keppra™, ucb L059, (S)-α-ethyl-2-oxo-pyrrolidine acetamide) is a novel drug which not only possesses potent anticonvulsant properties (Doheny et al., 1997; Gower et al., 1992; 1995; Loscher & Honack, 1993; Margineanu & Wulfert, 1997; Loscher et al., 1998) but also exhibits antiepileptogenic effects (Gower et al., 1992; Loscher et al., 1998). In addition, levetiracetam displays a highly favourable therapeutic index in that adverse effects in rodents are minimal with mild sedation and muscle relaxation occurring at doses 50 to 100 fold higher than those associated with seizure protection (Gower et al., 1992; Klitgaard et al., 1998). In man, levetiracetam has been observed to possess significant efficacy, good tolerability and a desirable therapeutic index (Kasteleijn-Nolst et al., 1996; Sharief et al., 1996; Betts et al., 2000; Cereghino et al., 2000; Ben-Menachem & Falter, 2000; Shorvon et al., 2000; Grant & Shorvon, 2000) and it has recently been licensed for use as adjunctive therapy in patients with intractable partial epilepsy.
Levetiracetam exhibits a broad spectrum of action in that it is effective in a variety of animal seizure models including audiogenic, chemoconvulsive (including systemically administered bicuculline and picrotoxin and centrally administered N-methyl-D-aspartate and tetanus toxin) and kindled (pentylenetetrazole and amygdala) seizures (Birnstiel et al., 1997; Gower et al., 1992; 1995; Loscher et al., 1993; 1996; 1998; Noyer et al., 1995; Margineanu & Wulfert, 1995; 1997; Doheny et al., 1997). These data suggest that levetiracetam may be effective against absence, generalized and partial seizures. However, the exact mechanism of action of levetiracetam, which appears to be mediated by levetiracetam itself and not by one of its metabolites and is stereospecific (only the S-enantiomer has anticonvulsant activity; Gower & Matagne, 1994), is unknown. The drug has no significant affinity for known γ-aminobutyric acid (GABA), benzodiazepine or various excitatory amino acid related receptors (Noyer et al., 1995) and does not interact directly with the benzodiazepine-GABA ionophore (Gower et al., 1992). An action via a specific binding site confined to brain synaptic plasma membranes has been proposed (Noyer et al., 1995). Although levetiracetam has been reported to significantly decrease spontaneous substantia nigra pars reticulata neuronal activity, which is dependent on strong GABAergic input from the striatum (Loscher et al., 1996), a significant anticonvulsant action via an action on GABA is considered to be unlikely (Margineanu & Wulfurt, 1997; Sills et al., 1997).
In man, levetiracetam exhibits simple straightforward linear pharmacokinetic characteristics with rapid absorption following oral ingestion resulting in peak plasma concentrations within 20 min and an elimination half-life of 6 – 8 h (Patsalos, 2000). Such linear dose-dependent pharmacokinetic characteristics have recently been confirmed in a freely behaving rat model which allows the study of the temporal kinetic inter-relationship of drugs in blood and cerebrospinal fluid (CSF; Doheny et al., 1999). Levetiracetam was observed to rapidly and readily enter the CSF compartment, however, its efflux from CSF (mean t1/2 range, 4.4 – 4.9 h) was significantly slower than that suggested by serum concentrations (mean t1/2 range, 1.8 – 2.8 h). As concentrations of numerous antiepileptic drugs in CSF have been shown to be good indices of their concentrations at central sites of action and that the CSF is kinetically indistinguishable from their central sites of action (Danhof & Levy, 1984; Dingemanse et al., 1987; Klockowski & Levy, 1988; Sokomba et al., 1988), we have investigated the pharmacokinetics of levetiracetam in the extracellular fluid of the frontal cortex and hippocampus of the freely behaving rat using the technique of intracerebral microdialysis. The purpose of this study was to determine the brain extracellular pharmacokinetics of levetiracetam and to ascertain whether or not levetiracetam exhibits brain region specificity in its pharmacokinetics. Furthermore, we have systematically determined the concurrent extracellular concentration profile of various amino acid neurotransmitters in the hippocampus and frontal cortex in order to further investigate the possible mechanism of action of levetiracetam. The usefulness of the microdialysis technique in pharmacokinetic studies and monitoring of extracellular events considered to reflect pharmacologically relevant synaptic events, is well established (Abercombie et al., 1988; Delgado et al., 1984; Hutson & Curzon, 1989; Rocha et al., 1999; Patsalos et al., 1995; Stahle, 1993; Stahle et al., 1991; Steele et al., 1991; Stenken et al., 1993; Walker et al., 1996; 2000; Wang & Welty, 1996).
Methods
Animals
Male Sprague-Dawley rats (B&K Universal Ltd., Hull, U.K.) weighing 300 – 350 g (age range 5 – 6 months) were used. They were housed in groups of four for 7 – 14 days prior to surgery under a 12-h light/dark cycle (light on 0600 h) with free access to water and a normal laboratory diet (SDS R and M number 1 expanded. Scientific Dietary Services, Witham, Essex, U.K.).
Surgical procedures, microdialysate and blood sampling
Experimental procedures were licensed under the Animals (Scientific Procedures) Act 1986, and performed in accordance with Home Office guidelines (U.K.). Rats were anaesthetized with halothane and microdialysis probes stereotaxically implanted in the hippocampus (from bregma 5.3 mm posterior, 4.5 mm lateral, 7.5 mm ventral) and frontal cortex (from bregma 3.0 mm anterior, 2.5 mm lateral, 5.0 mm ventral) as previously described (Walker et al., 1996). The co-ordinates used were according to the atlas of Paxinos & Watson (1986). In addition, a catheter was implanted in the right internal jugular vein for blood samplings as previously described (Patsalos et al., 1992). Post-surgery, animals were housed individually in contiguous perspex cages.
Two days later when the animals were fully recovered from the surgical procedure, the microdialysis probes and the jugular vein catheter were checked for patency. Artificial CSF, composition (mmol): NaCl 125, KCl 2.5, MgCl2 1.18, CaCl2 1.26 was perfused through the probes at a rate of 2 μl min−1. Two baseline samples of blood (100 μl) at 30 min intervals and three baseline dialysate samples (40 μl) at 20 min intervals were collected during a 1 h period. The rats were then randomly assigned to three groups and administered, by intraperitoneal injection at 0900 – 1000 h, with 40 or 80 mg kg−1 levetiracetam (UCB Pharmaceutical Sector, Chemin du Foriest, Belgium) or isotonic saline. Levetiracetam was constituted in isotonic saline at a concentration of 20 mg ml−1. Blood samples (100 μl) were withdrawn at 20 min intervals for the first hour and then at 30 min intervals for a further 7 h. The catheter was flushed with 100 μl 100 u ml−1 heparinized saline after each sampling so as to maintain patency and prevent hypovolaemia. For the levetiracetam treated animals, microdialysate samples were collected at 15 min intervals (30 μl) for the first 2 h and then at 30 min intervals (60 μl) for a further 6 h. For the isotonic saline treated animals (controls) microdialysate samples were collected at 20 min intervals for the first 4 h and then at 30 min intervals for a further 4 h. Blood and microdialysate samples were collected in 0.5 ml polyethylene tubes (Treff Labs, Degershein, Switzerland). Sera were separated from whole blood by centrifugation (model 2K15, Sigma, U.K.) at 10,000×g for 5 min. After transferring sera into new tubes, sera and microdialysate samples were stored at −70°C until analysed for levetiracetam or amino acid content.
Levetiracetam analysis
The concentration of levetiracetam in sera (total and free non-protein-bound) and microdialysates was determined by high performance liquid chromatography (HPLC) as previously described (Ratnaraj et al., 1996). All solvents were of HPLC grade (Paterson Scientific, Luton, U.K.) and reagents were of ‘analar' grade (BDH-Merck, Poole, U.K.). The HPLC system comprised a Spectra-Physics spectra system pump P4000, an autosampler (AS3000), a UV2000 detector and a chromjet integrator (Spectra-Physics, Maidenhead, U.K.). Chromatograms were run at 35°C on a steel cartridge column (250×4 mm I.D.) with precolumn (4×4 mm I.D.) packed with LiChrospher 60 RP-select B, 5 μm (Merck, Poole, U.K.). A mobile phase of acetonitrile/50 mmol phosphate buffer (pH 5.6, 10/90) with a flow rate of 0.7 ml min−1 and at 1000 p.s.i. was used. The mobile phase was filtered through a 0.45 μm Millipore filter (Amicon, Stonehouse, U.K.) prior to use. The column eluent was monitored at 220 nm with a sensitivity range of 1.0 a.u.f.s and a chart speed of 0.25 cm min−1.
Sera and microdialysates were prepared for analysis of levetiracetam concentrations as follows: Into a 1.5 ml microcentrifuge tube (Sarstedt Ltd, Leicester, U.K.), 25 μl of sera, 10 μl microdialysate or standard, 10 μl 5 M sodium hydroxide solution, 500 μl dichloromethane and 25 μl of the working internal standard (50 mmol; α,2,2-dimethyl-5-oxo-1-pyrrolidine acetamide; UCB SA Pharma Sector, Chemin du Foriest, Belgium) were added. After mixing the contents for 1 min using a Vibrax electronic shaker (Sartorius-IKA, Epsom, U.K.), the mixture was centrifuged for 5 min at 11,000×g and the upper aqueous layer discarded. The solvent extract was then transferred to a clean microcentrifuge tube and evaporated to dryness at 50°C using a Gyro Vap centrifugal evaporator (Howe, Banbury, U.K.). The residue was then reconstituted in 50 μl of mobile phase and 10 μL were injected into the HPLC system.
Free non-protein bound serum levetiracetam concentration
The procedure used for the determination of the free non-protein-bound serum levetiracetam concentrations was essentially the same as for total concentrations except that samples were first filtered through an Amicon Centrifree Micropartition System (Amicon, Stonehouse, U.K.) using a Sorvall RC-5B refrigerated centrifuge (Du Pont, Stevenage, U.K.) set at 25°C. Fifteen μl samples of resultant ultrafiltrate were then processed as described above.
Microdialysis probes
Concentric microdialysis probes, constructed from Filtral 12 dialysis membrane (4 mm long, 200 μm diameter; Hospal, Rugby, U.K.) as previously described, were used (Walker et al., 1996). Prior to use the in vitro recovery of each probe was determined. This was achieved by placing the probe into a 40 μmol solution of levetiracetam constituted in artificial CSF, composition (mmol): NaCl 125, KCl 2.5, MgCl2 1.18, and CaCl2 1.26 and then perfusing the probes with artificial CSF at 2 μl min−1. Forty μl samples were collected every 20 min for 80 min and these samples, along with a sample of the 40 μmol levetiracetam solution, were stored at −70°C until analysis for levetiracetam content.
Amino acid analysis
Amino acid concentrations in microdialysates of frontal cortex and hippocampus after levetiracetam administration (80 mg kg−1, i.p.) were determined by HPLC using pre-column derivatization and fluorescence detection after binary gradient liquid chromatographic separation. The method is based on that previously described by Godel et al. (1992). The highest purity amino acids and O-phthalaldehyde and 3-mercaptopropionic acid were used (Sigma, U.K.). All other chemicals were of analytical grade. Five μl of dialysate were used for pre-column derivatization of amino acids by means of an O-phthaldialdehyde/3-mercaptopropionic acid reaction. Derivatization was conducted using an autoinjector, and the dialysate/reagent mixture was injected onto the HPLC system via a valve fitted with a 2 μl sample loop. The HPLC system consisted of two high pressure pumps with a gradient controller and mixing chamber (Micro-Tech Scientific, Sunnyvale, U.S.A.). Separation of amino acids was achieved using an AminoQuant column and guard cartridge (Hewlett Packard, Cheshire, U.K.) placed inside a column oven operating at 40°C, using a linear gradient (5 to 100% elution mobile phase B in 18 min). Mobile phases A and B were prepared exactly as described in the AminoQuant method (Godel et al., 1992). The detector was a Gilson model 122 fluorometer (Gilson, Middleton, U.S.A.) fitted with a 5 μl flow cell, and was operated with excitation and emission wavelengths of 340 and 450 nm respectively. Calibration of the system was achieved using aqueous working standards prepared by dilution of an amino acid standard mixture (Hewlett Packard, Waldbronn, Germany) following the additions of L-asparagine, L-glutamine, citrulline, L-taurine, GABA, norvaline (internal standard) and L-tryptophan. Calibration curves were constructed to cover the range 0.005 to 200 μmol of amino acid. The within-batch and between-batch coefficient of variation varied between 3.9 and 5.8% and 4.6 and 7.9%, respectively, for the various amino acids measured.
Microdialysate amino acids measured include: alanine, arginine, aspartic acid, asparagine, citrulline, GABA, glutamate, glutamine, glycine, isoleucine, leucine, lycine, methionine, phenylanine, serine, taurine, threonine, tryptophan, tyrosine and valine.
Pharmacokinetic analysis
Levetiracetam concentration versus time profiles for both sera and microdialysate were analysed according to a one compartment model. The parameters computed (using a Dell 486D/16 computer) were: area under the concentration versus time curve (AUC) and apparent elimination half-life (t1/2). The AUC from 0 to 8 h (AUC0 – 8) was calculated by the trapezoid rule. Time to maximum concentration (Tmax) and maximum concentration (Cmax) were obtained by visual inspection of the levetiracetam versus time profiles.
Statistical analysis
Microdialysate amino acid concentrations were compared (levetiracetam versus saline control) by two-way analysis of variance over time.
Results
Blood pharmacokinetics
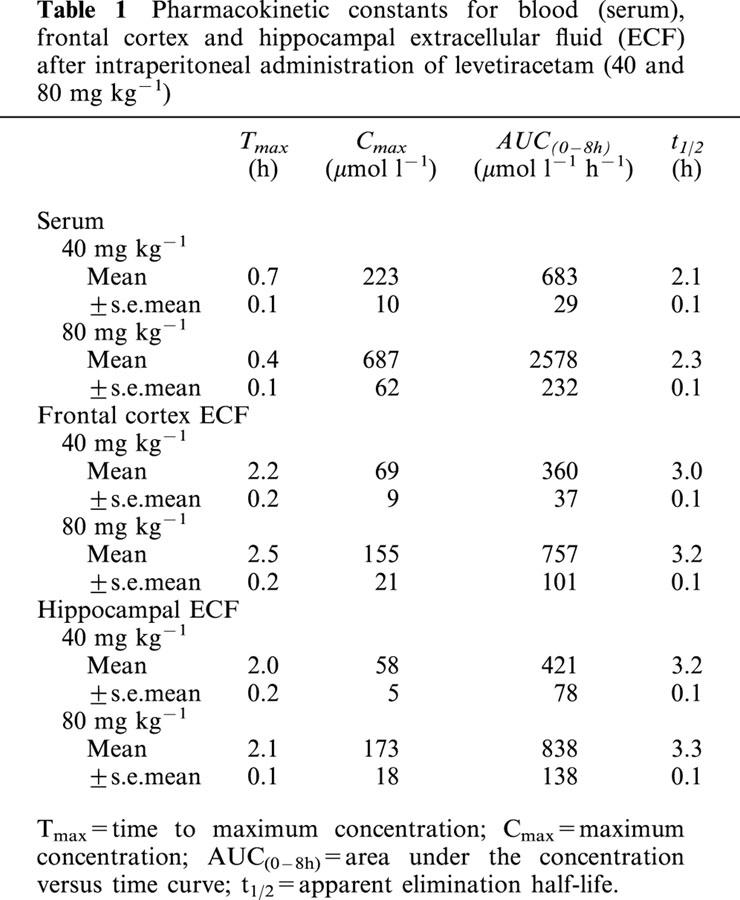
The serum concentration versus time profiles of levetiracetam following intraperitoneal administration of 40 and 80 mg kg−1 levetiracetam are shown in Figures 1 and 2 respectively. Levetiracetam demonstrated rapid absorption after intraperitoneal injection with mean peak concentrations being achieved 0.4 – 0.7 h later. Levetiracetam concentrations rose dose-dependently and declined exponentially. The mean±s.e.mean pharmacokinetic constants, as calculated from the log concentration versus time plots of individual rats, is shown in Table 1. The pharmacokinetic constants for individual rats showed moderate variability within the two dose groups. Mean Cmax and AUC values for 80 mg kg−1 levetiracetam were slightly higher than twice those achieved for 40 mg kg−1. Mean t1/2 values were indistinguishable for the two dose groups.
Figure 1.
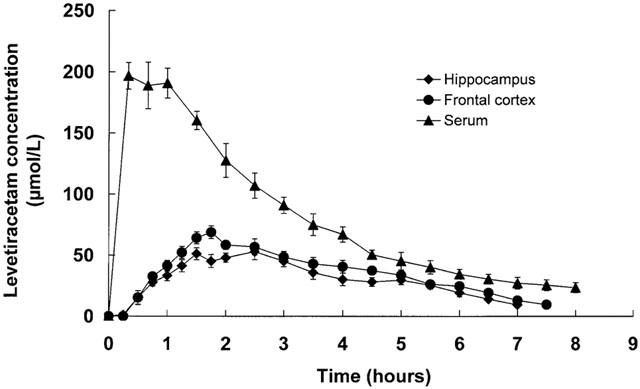
Serum and frontal cortex and hippocampal microdialysate concentration versus time profiles for levetiracetam following intraperitoneal administration of 40 mg kg−1 levetiracetam. Data are mean±s.e.mean of six rats.
Figure 2.
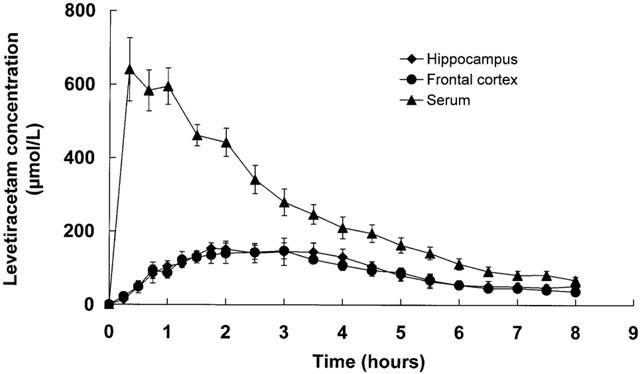
Serum and frontal cortex and hippocampal microdialysate concentration versus time profiles for levetiracetam following intraperitoneal administration of 80 mg kg−1 levetiracetam. Data are mean±s.e.mean of six rats.
Table 1.
Pharmacokinetic constants for blood (serum), frontal cortex and hippocampal extracellular fluid (ECF) after intraperitoneal administration of levetiracetam (40 and 80 mg kg−1)
The free non-protein-bound levetiracetam concentration was determined in nine sera which were chosen so as to reflect the spectrum of levetiracetam concentration and sampling time (Figure 3). The free fraction (free/total serum levetiracetam concentration ratio) varied between 0.93 and 1.05 (mean±s.e.mean; 0.99±0.01).
Figure 3.
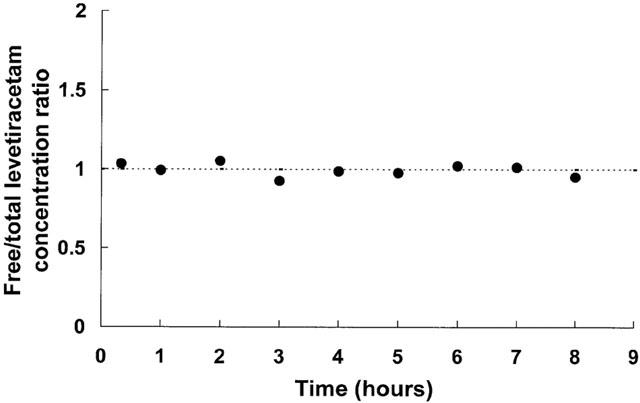
Serum free fraction (free/total concentration ratio) versus time profile for levetiracetam. Dotted line indicates mean value of ratio.
Brain extracellular fluid pharmacokinetics
The corresponding concentration versus time profiles of levetiracetam in frontal cortex and hippocampal extracellular fluid are shown in Figures 1 and 2 and it can be seen that the profiles are essentially superimposable for a given levetiracetam dose. The calculated mean±s.e.mean pharmacokinetic constants as calculated from the log concentration versus time plots of individual rats can be seen in Table 1. Both AUC and Cmax mean values increased dose-dependently and linearly. Levetiracetam concentrations were detectable at time of first microdialysate sampling (15 min) with concentrations peaking somewhat later (mean Tmax, 2.0 – 2.5 h) than serum (0.4 – 0.7 h). Mean Cmax values for extracellular levetiracetam was 3.2 – 4.0 fold lower to those in serum and this difference, which was similar for both the frontal cortex and the hippocampus, may, however, be attributed to the use of probe in vitro relative recovery characteristics to calculate brain extracellular fluid concentrations. The extracellular fluid/serum Cmax ratio was independent of dose (3.2 and 3.8; 4.0 and 4.0 at 40 and 80 mg kg−1 respectively for frontal cortex and hippocampus). Extracellular fluid t1/2 values were identical for the frontal cortex and hippocampus and, as observed for serum, values were dose-independent. However, compared to serum, extracellular fluid t1/2 values were significantly larger.
Figure 4 shows the levetiracetam concentration ratio of hippocampal extracellular fluid/serum over time for 40 and 80 mg kg−1 levetiracetam. It can be seen that at 5.0 to 7.5 h post levetiracetam administration, there was a tendency towards equilibration (as measured by a constant extracellular fluid/serum levetiracetam concentration ratio) between the blood and extracellular fluid compartments. A similar profile was observed for the frontal cortex (data not shown).
Figure 4.
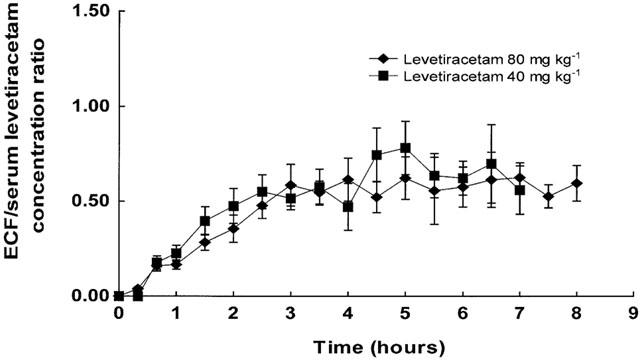
Levetiracetam hippocampal extracellular fluid/serum concentration versus time profiles after 40 mg kg−1 and 80 mg kg−1 levetiracetam. Data are mean±s.e.mean of six rats.
Mean±s.e.mean levetiracetam relative recovery in vitro (37°C) for the 24 microdialysate probes used in the present study was 13±0.6% at a dialysate flow rate of 2 μ1 min−1. The extracellular fluid levetiracetam concentration data shown in this study have been adjusted on the basis of in vitro relative recovery.
Brain extracellular fluid amino acid profiles
Of the 20 amino acids investigated only taurine was associated with significant changes. Figure 5 shows dialysate taurine in the frontal cortex and the hippocampus after giving isotonic saline or 80 mg kg−1 levetiracetam. Results are expressed as percentage changes with respect to baseline. Three baseline samples were collected in each experiment and mean sample values are shown. Mean±s.e.mean basal dialysate values for taurine in frontal cortex and hippocampus were 5.91±0.69 μM and 10.61±0.59 μM respectively. These values are similar to those reported in other microdialysis studies (Baldwin et al., 1994; Butcher et al., 1990; Tossman et al., 1987; Taylor et al., 1995).
Figure 5.
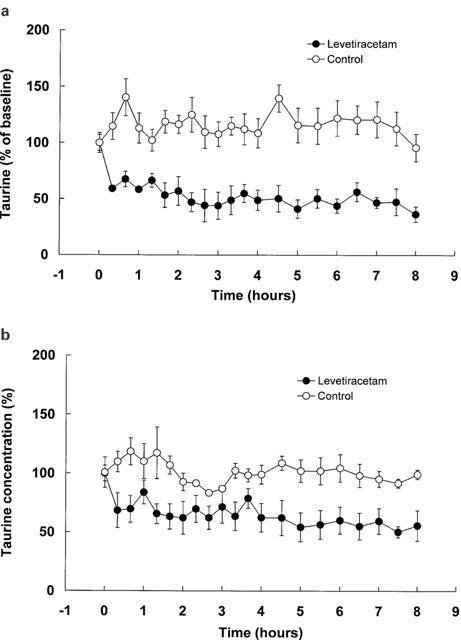
Dialysate taurine in the frontal cortex (a) and hippocampus (b) before and after the administration of levetiracetam (80 mg kg−1) or 0.9% saline (control). Values are mean of four rats and are expressed as percentage of baseline; vertical bars show s.e.mean.
Discussion
The present study was undertaken to investigate the pharmacokinetic inter-relationship of levetiracetam in blood and the brain and to investigate any concurrent brain extracellular amino acid neurotransmitter changes which may further enhance our understanding of the mechanism of action of levetiracetam. The freely behaving rat model used in this study, which allows concurrent blood and microdialysate sampling of brain extracellular fluid, has been extensively validated and used in numerous studies of antiepileptic drugs (Patsalos et al., 1995; Walker et al., 1996; 1998; 2000).
Microdialysis has several advantages over classical tissue excision methods in examining drug distribution in the brain. Firstly, brain drug concentrations in brain tissue homogenates can be associated with large errors because of uncertainty as to tissue blood content (Gallo et al., 1992). In contrast, microdialysis allows sampling without any vascular contamination, provided the blood-brain barrier is intact after probe insertion. Indeed it has been demonstrated that approximately 30 min after implantation, the blood-brain barrier becomes intact again and that the optimal time for undertaking microdialysis experiments is 8 – 48 h after probe implantation (Benveniste, 1989; Westerink & de Vries, 1988). In the present study microdialysate sampling occurred 2 days after probe implantation which can be considered more than sufficient time for restructuring of the blood – brain barrier. Secondly, brain extracellular drug concentrations measured by microdialysis are considered to reflect the amount of drug that is available for receptor occupancy and therefore is of considerable pharmacological relevance. Finally, microdialysate monitoring of neurochemical events in the brain extracellular fluid are considered to reflect pharmacodynamic events and the technique has contributed substantially to our understanding of drug action (Biggs & Starr, 1999; Gur et al., 1999; Ipponi et al., 1999; Rocha et al., 1999). However, it is important to understand the inherent characteristics of microdialysis so that data collection can be optimal and interpretation appropriate. Thus, because microdialysis sampling represents period sampling over a period of time (in contrast to direct sampling of, for example, blood which represents a single sampling time), sampling frequency, particularly during monitoring of periods of rapid change, is very important (Patsalos et al., 1995). Consequently, in pharmacokinetic studies, Cmax and Tmax will be significantly underestimated and overestimated respectively, if sampling time is longer rather than shorter and is indeed the rationale for using the microdialysate sampling frequency described in the present study.
We previously reported on the temporal pharmacokinetic inter-relationship of levetiracetam in rat blood and CSF (Doheny et al., 1999). In the present study we have extended our studies to the brain by monitoring levetiracetam extracellular fluid concentrations in the hippocampus and frontal cortex by microdialysis. The pharmacokinetic profile of levetiracetam in serum, as observed in the present study, is exactly what we previously reported and these data not only serve to further validate the animal model used, but also further confirm the predictable pharmacokinetic nature of levetiracetam. Also, since the serum free/total serum concentration ratio (free fraction) was 0.99±0.01 (mean±s.e.mean; range 0.93 – 1.05), it confirms that levetiracetam is not bound to blood proteins (Doheny et al., 1999; Patsalos, 2000).
The temporal pharmacokinetic profile of levetiracetam in frontal cortex and hippocampal extracellular fluid reveals some interesting characteristics. After intraperitoneal administration, levetiracetam rapidly appeared in the brain as evidenced by the fact that levetiracetam was detected in the extracellular fluid of both the frontal cortex and hippocampal at time of first sample (15 min). These data suggest ready penetration of the blood – brain barrier by levetiracetam. However, as might be expected, Tmax values for extracellular fluid were somewhat larger (mean Tmax 2.0 – 2.5 h) than that for serum (mean Tmax 0.4 – 0.7 h) but, surprisingly, Tmax values were also larger than that previously reported for the CSF compartment (mean Tmax 1.58 – 1.92 h; Doheny et al., 1999). The observed difference in Tmax values between the extracellular and CSF compartments may be attributed, in part, to methodology in that CSF was directly sampled whereas extracellular fluid was not. The dose-dependent increase in extracellular fluid concentrations after levetiracetam administration (40 and 80 mg kg−1), suggests that transport is not rate limiting over the concentration range observed in the present study. Furthermore, as the pharmacokinetic profiles of levetiracetam in the frontal cortex and hippocampus are essentially identical and superimposable, it can be concluded that levetiracetam distribution in the brain is not brain region specific. This is in contrast to some other antiepileptic drugs (e.g. vigabatrin and phenytoin) which exhibit substantial regional specificity in their brain pharmacokinetics (Walker et al., 1996; Tong et al., 1999).
Efflux of levetiracetam from the brain extracellular fluid compartment appears to be restricted, as demonstrated by the half-life of levetiracetam, which is approximately 50% longer than that observed in the blood compartment. These data, combined with similar data for CSF (where efflux of levetiracetam from the CSF compartment was 170% slower; Doheny et al., 1999), may in part explain the substantial clinical efficacy of levetiracetam during twice a day dosing (Ben-Menachem & Falter, 2000; Cereghino et al., 2000; Shorvon et al., 2000) despite the fact that its blood pharmacokinetics (t1/2 6 – 8 h) would suggest that three times a day dosing is more appropriate.
Previously, we showed that at equilibrium, the CSF/serum concentration ratio was similar to that of the blood free fraction (0.96 versus 1.01), suggesting the blood levetiracetam concentration reflected central brain concentrations (Doheny et al., 1999). However, in the present study, although there was a tendency towards equilibration at 5.0 to 7.5 h post levetiracetam administration, the extracellular fluid/serum concentration ratio for both the frontal cortex and hippocampus was only approximately 0.5 (Figure 4), 50% less than the blood free fraction. This would suggest that, at least in the acute situation, blood levetiracetam concentrations do not reflect concentrations at its site of action in the brain or that equilibration had not been achieved over the time period of the study. However, it should be noted that since the extracellular fluid levetiracetam concentration data have been adjusted on the basis of in vitro relative recovery and since in vitro recovery can be expected to be greater than in vivo recovery, the extracellular fluid/serum concentration ratio may be underestimated.
Levetiracetam is unique amongst the new antiepileptic drugs in that it was not identified by screening in the traditional models (maximal electroshock and pentylenetetrazole) used to identify potential antiepileptic drugs. Consequently, it is not surprising that its profile of action is somewhat different to other drugs and that its mechanism of action is not that typically associated with antiepileptic drugs. Since in animal models of epilepsy, the effective dose at which 50% of animals respond (ED50) ranged from 5 to 30 mg kg−1 (Gower et al., 1992) and the ED50 for suppression of secondarily generalized seizures in the amygdala-kindled model of epilepsy is 40 mg kg−1 (Loscher & Honack, 1993), the levetiracetam dose (80 mg kg−1) used in the present study is appropriate. Levetiracetam administration (80 mg kg−1) was associated with a significant reduction in taurine concentrations in both the frontal cortex and hippocampus (Figure 5). However, the taurine concentrations measured in the present study reflect total extracellular fluid concentrations and it is well established that concentrations of neurotransmitter molecules measured by microdialysis reflect concentrations that are both neuronal and metabolic in origin (Reith et al., 1997; Rowley et al., 1995; 1998; Osborne et al., 1990; Westerink et al., 1987). Therefore, as to what proportion of the changes seen in taurine concentration is neuronal in origin, and therefore relevant in relation to the pharmacological action of levetiracetam, is not know. Indeed, the role of taurine, if any, in the mechanism of action of levetiracetam remains to be determined.
Acknowledgments
This work was supported in part by the National Society for Epilepsy. We are grateful to Dr H.C. Doheny, Miss A. Hay and Dr N. Ratnaraj for some technical assistance, Dr M.C. Walker for the help with the pharmacokinetic analysis and Dr M.T. O'Connell for help with the amino acid analysis. We thank UCB SA Pharma Sector (Chemin du Foriest, Belgium) for the supply of levetiracetam and α,2,2,-dimethyl-5-oxo-1-pyrrolidine acetamide.
Abbreviations
- AUC
area under the concentration versus time curve
- Cmax
maximum concentration
- CSF
cerebrospinal fluid
- HPLC
high performance liquid chromatography
- t1/2
apparent elimination half-life
- Tmax
time to maximum concentration
References
- ABERCOMBIE E.D., KELLER R.W., ZIGMOND M.J. Characterisation of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioural studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- BALDWIN H.A., WILLIAMS J.L., SNARES M., FERREIRA T., CROSS A.J., GREEN A.R. Attenuation by chlormethiozole administration of the rise in extracellular amino acids following focal ischaemia in the cerebral cortex of the rat. Br. J. Pharmacol. 1994;112:188–194. doi: 10.1111/j.1476-5381.1994.tb13050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEN-MENACHEM E., FALTER U. Efficacy and tolerability of levetiracetam 3000 mg/day in patients with refractory partial seizures: a multicentre, double-blind, responder-selected study evaluating monotherapy. Epilepsia. 2000;41:1276–1283. doi: 10.1111/j.1528-1157.2000.tb04605.x. [DOI] [PubMed] [Google Scholar]
- BENVENISTE H. Brain microdialysis. J. Neurochem. 1989;52:1667–1679. doi: 10.1111/j.1471-4159.1989.tb07243.x. [DOI] [PubMed] [Google Scholar]
- BETTS T., WAEGEMANS T., CRAWFORD P. A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure. 2000;9:80–87. doi: 10.1053/seiz.2000.0380. [DOI] [PubMed] [Google Scholar]
- BIGGS C.S., STARR M.S. Microdialysis study of the effects of the antiparkinsonian drug budipine on L-DOPA-induced release of dopamine and 5-hydroxytryptamine by rat substantia nigra and corpus striatum. Synapse. 1999;34:36–46. doi: 10.1002/(SICI)1098-2396(199910)34:1<36::AID-SYN5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- BIRNSTIEL S., WULFERT E., BECK S.G. Levetiracetam (ucb L059) affects in vitro models of epilepsy in CA3 pyramidal neurons without altering normal synaptic transmission. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:611–618. doi: 10.1007/pl00005097. [DOI] [PubMed] [Google Scholar]
- BUTCHER S.P., BULLOCK R., GRAHAM D.L., MCCULLOCK J. Correlation between amino acids release and neuropathological outcome in rat brain following middle cerebral artery occlusion. Stroke. 1990;21:1727–1733. doi: 10.1161/01.str.21.12.1727. [DOI] [PubMed] [Google Scholar]
- CEREGHINO J.J., BITON V., ABOU-KHALIL B., GREIFUSS F., GAUER L.J., LEPPIK I., THE UNITED STATES LEVETIRACETAM STUDY GROUP Levetiracetam for partial seizures. Results of a double-blind, randomized clinical trial. Neurology. 2000;55:236–242. doi: 10.1212/wnl.55.2.236. [DOI] [PubMed] [Google Scholar]
- DANHOF M., LEVY G. Kinetics of drug action in disease states. I. Effect of infusion rate on phenobarbital concentrations in serum, brain and cerebrospinal fluid of normal rats at onset of loss of righting reflex. J. Pharmacol. Exp. Ther. 1984;229:44–50. [PubMed] [Google Scholar]
- DELGADO J.M.R., LERMA J., MARTIN DEL RIO R., SOLIS S.M. Dialytrode technology and local profiles of amino acids in the awake rat brain. J. Neurochem. 1984;42:1218–1228. doi: 10.1111/j.1471-4159.1984.tb02775.x. [DOI] [PubMed] [Google Scholar]
- DINGEMANSE J., VAN BREE J.B.M.M., DANHOF M. Pharmacokinetic modelling of the anticonvulsant action of phenobarbital in rats. J. Pharmacol. Exp. Ther. 1987;249:601–608. [PubMed] [Google Scholar]
- DOHENY H.C., RATNARAJ N., WHITTINGTON M.A., JEFFERYS J.G.R., PATSALOS P.N. Blood and cerebrospinal fluid pharmacokinetics of the novel anticonvulsant levetiracetam (ucb L059) in the rat. Epilepsy Res. 1999;34:161–168. doi: 10.1016/s0920-1211(98)00104-1. [DOI] [PubMed] [Google Scholar]
- DOHENY H.C., WHITTINGTON M.A., JEFFERYS J.G.R., PATSALOS P.N. Levetiracetam in a chronic model of epilepsy. Epilepsia. 1997;38 Suppl. 8:30. [Google Scholar]
- GALLO J.M., SANZGIRI Y., HOWERTH E.W., FINCO T.S., WILSON J., JOHNSON J., TACKETT R., BUDSBERG S.C. Serum, cerebrospinal fluid, and brain concentrations of a new zidovudine formulation following chronic administration via an implantable pump in dogs. J. Pharm. Sci. 1992;81:11–15. doi: 10.1002/jps.2600810103. [DOI] [PubMed] [Google Scholar]
- GODEL H., SEITZ P., VERHOEF M. Automated amino acid analysis using combined FMOC-CL precolumn derivatization. LC/GC Internat. 1992;5:44–49. [Google Scholar]
- GOWER A.J., HIRSCH E., BOEHRER A., NOYER M., MARESCAUX C. Effects of levetiracetam, a novel antiepileptic drug, on convulsant activity in two genetic rat models of epilepsy. Epilepsy Res. 1995;22:207–213. doi: 10.1016/0920-1211(95)00077-1. [DOI] [PubMed] [Google Scholar]
- GOWER A.J., MATAGNE A. Levetiracetam (ucb LO59): anticonvulsant effects are mediated by the parent compound. Epilepsia. 1994;35 Suppl. 7:75. [Google Scholar]
- GOWER A.J., NOYER M., VERLOES R., GOBERT J., WULFERT E. ucb L059, a novel anti-convulsant drug: pharmacological profile in animals. Eur. J. Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-x. [DOI] [PubMed] [Google Scholar]
- GRANT R., SHORVON S.D. Efficacy and tolerability of 1000–4000 mg per day of levetiracetam as add-on therapy in patients with refractory epilepsy. Epilepsy Res. 2000;42:89–95. doi: 10.1016/s0920-1211(00)00158-3. [DOI] [PubMed] [Google Scholar]
- GUR E., DREMENCOV E., LERER B., NEWMAN M.E. Venlafaxine: acute and chronic effects on 5-hydroxytryptamine levels in rat brain in vivo. Eur. J. Pharmacol. 1999;372:17–24. doi: 10.1016/s0014-2999(99)00164-8. [DOI] [PubMed] [Google Scholar]
- HUTSON P.H., CURZON G. Concurrent determination of effects of p-chloramphetamine on central extracellular 5-hydroxytraptamine concentration and behaviour. Br. J. Pharmacol. 1989;96:801–806. doi: 10.1111/j.1476-5381.1989.tb11887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPPONI A., LAMBERTI C., MEDICA A., BARTOLINI A., MALMBERG-AIELLO P. Tiagabine antinociception in rodents depends on GABA(B) receptor activation: parallel antinociception testing and medial thalamus GABA microdialysis. Eur. J. Pharmacol. 1999;368:205–211. doi: 10.1016/s0014-2999(99)00034-5. [DOI] [PubMed] [Google Scholar]
- KASTELEIJN-NOLST TRENITE D.G.A., MARESCAUX C., STODIECK S., EDELBROEK P.M., OOSTING J. Photosensitive epilepsy: a model to study the effect of antiepileptic drugs. Evaluation of the piracetam analogue, levetiracetam. Epilepsy Res. 1996;25:225–230. doi: 10.1016/s0920-1211(96)00031-9. [DOI] [PubMed] [Google Scholar]
- KLITGAARD H., MATAGNE A., GOBERT J., WULFERT E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- KLOCKOWSKI P.M., LEVY G. Kinetics of drug action in disease states. XXIV. Pharmacodynamics of diazepam and its active metabolites in rats. J. Pharmacol. Exp. Ther. 1988;244:912–918. [PubMed] [Google Scholar]
- LOSCHER W., HONACK D. Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats. Eur. J. Pharmacol. 1993;232:147–158. doi: 10.1016/0014-2999(93)90768-d. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., HONACK D., BLOMS-FUNKE P. The novel antiepileptic drug levetiracetam (ucbL059) induces alterations in GABA metabolism and turnover in discrete areas of rat brain and reduces neuronal activity in substantia nigra pars reticulata. Brain Res. 1996;735:208–216. doi: 10.1016/0006-8993(96)00587-2. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., HONACK D., RUNFELDT C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- MARGINEANU D.G., WULFERT E. ucb L059, a novel anticonvulsant, reduces bicuculline-induced hyperexcitability in rat hippocampal CA3 in vivo. Eur. J. Pharmacol. 1995;286:321–326. doi: 10.1016/0014-2999(95)00597-8. [DOI] [PubMed] [Google Scholar]
- MARGINEANU D.G., WULFERT E. Inhibition by levetiracetam of a non-GABAA receptor-associated epileptiform effect of bicuculline in rat hippocampus. Br. J. Pharmacol. 1997;122:1146–1150. doi: 10.1038/sj.bjp.0701476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSON A.G., CHADWICK D.W. New drug treatments for epilepsy. J. Neurol. Neurosurg. Psychiat. 2001;70:143–148. doi: 10.1136/jnnp.70.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOYER M., GILLARD M., MATAGNE A., HENICHART J.P., WULFERT E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur. J. Pharmacol. 1995;286:137–146. doi: 10.1016/0014-2999(95)00436-o. [DOI] [PubMed] [Google Scholar]
- OSBORNE P.G., O'CONNOR W.T., DREW K.L., UNGERSTEDT U. An in vivo microdialysis characterization of extracellular dopamine and GABA in dorsolateral striatum of awake freely moving and halothane anaesthetised rats. J. Neurosci. Meth. 1990;34:99–105. doi: 10.1016/0165-0270(90)90047-j. [DOI] [PubMed] [Google Scholar]
- PATSALOS P.N.The new generation of anti-epileptic drugs Emerging Drugs: The Prospect for Improved Medicines. Volume 4 1999London: Ashley Publications Ltd; 87–106.ed. Bowman, W.C., Fitzgerald, J.D. & Taylor, J.B. pp [Google Scholar]
- PATSALOS P.N. Pharmacokinetic profile of levetiracetam: Toward ideal characteristics. Pharmacol. Ther.. 2000;85:77–85. doi: 10.1016/s0163-7258(99)00052-2. [DOI] [PubMed] [Google Scholar]
- PATSALOS P.N., ABED W.T., ALAVIJEH M.S., O'CONNELL M.T. The use of microdialysis for the study of drug kinetics: some methodological consideration illustrated with antipyrine in rat frontal cortex. Br. J. Pharmacol. 1995;115:503–509. doi: 10.1111/j.1476-5381.1995.tb16362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATSALOS P.N., ALAVIJEH M.S., SEMBA J., LOLIN Y.I. A freely moving and behaving rat model for the chronic and simultaneous study of drug pharmacokinetics (blood) and neuropharmacokinetics (cerebrospinal fluid): hematological and biochemical characterization and kinetic evaluation using carbamazepine. J. Pharmacol. Toxicol. Methods. 1992;28:21–28. doi: 10.1016/1056-8719(92)90061-5. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1986San Diego: Academic Press; Second Edition [Google Scholar]
- RATNARAJ N., DOHENY H.C., PATSALOS P.N. A micromethod for the determination of the new antiepileptic drug levetiracetam (ucb L059) in serum or plasma by high performance liquid chromatography. Ther. Drug Monit. 1996;18:154–157. doi: 10.1097/00007691-199604000-00008. [DOI] [PubMed] [Google Scholar]
- REITH M.E.A., LI M.Y., YAN Q.S. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus acumbens of freely-moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- ROCHA L., ONDARZA-ROVIRA R., MAIDMENT N.T. Gabapentin modifies extracellular opioid peptide content in amygdala: a microdialysis study. Epilepsy Res. 1999;35:13–29. doi: 10.1016/s0920-1211(98)00121-1. [DOI] [PubMed] [Google Scholar]
- ROWLEY H.L., KILPATRICK I.C., NEEDHAM P.L., HEAL D.J. Elevation of extracellular cortical noradrenaline may contribute to the antidepressant activity of zotepine: an in vivo microdialysis study in freely moving rats. Neuropharmacology. 1998;37:937–944. doi: 10.1016/s0028-3908(98)00094-x. [DOI] [PubMed] [Google Scholar]
- ROWLEY H.L., MARTIN K.F., MARSDEN C.A. Decreased GABA release following toni-clonic seizures is associated with an increase in extracellular glutamate in rat hippocampus in vivo. Neuroscience. 1995;68:415–422. doi: 10.1016/0306-4522(95)00159-g. [DOI] [PubMed] [Google Scholar]
- SANDER J.W.A.S., SHORVON S.D. Incidence and prevalence studies in epilepsy and their methodological problems: a review. J. Neurol. Neurosurg. Psychiat. 1987;50:829–839. doi: 10.1136/jnnp.50.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARIEF M.K., SINGH P., SANDER J.W.A.S., PATSALOS P.N., SHORVON S.D. Efficacy and tolerability study of ucb L059 in patients with refractory epilepsy. J. Epilepsy. 1996;9:106–112. [Google Scholar]
- SHORVON S.D., LOWENTHAL A., JANZ D., BIELEN E., LOIUSEAU P. Multicentre double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory seizures. Epilepsia. 2000;41:1179–1186. doi: 10.1111/j.1528-1157.2000.tb00323.x. [DOI] [PubMed] [Google Scholar]
- SILLS G.J., LEACH J.P., FRASER C.M., FORREST G., PATSALOS P.N., BRODIE M.J. Neurochemical studies with the novel anticonvulsant levetiracetam in mouse brain. Eur. J. Pharmacol. 1997;325:35–40. doi: 10.1016/s0014-2999(97)00105-2. [DOI] [PubMed] [Google Scholar]
- SOKOMBA E.N., PATSALOS P.N., LOLIN Y.I., CURZON G. Concurrent monitoring of central carbamazepine and transmitter amine metabolism and motor activity in individual unrestrained rats using repetitive withdrawal of cerebrospinal fluid. Neuropharmacology. 1988;27:409–415. doi: 10.1016/0028-3908(88)90150-5. [DOI] [PubMed] [Google Scholar]
- STAHLE L. Microdialysis in pharmacokinetics. Eur. J. Drug Metab. Pharmacokin. 1993;18:89–96. doi: 10.1007/BF03220011. [DOI] [PubMed] [Google Scholar]
- STAHLE L., SEGERSVARD S., UNGERSTEDT U. Drug distribution studies with microdialysis. II. Caffeine and theophylline in blood, brain and other tissues in rats. Life Sci. 1991;49:1843–1852. doi: 10.1016/0024-3205(91)90487-v. [DOI] [PubMed] [Google Scholar]
- STEELE K.L., SCOTT D.O., LUNTE C.E. Pharmacokinetic studies of aspirin in rats using in vivo microdialysis sampling. Anal. Chim. Acta. 1991;65:2324–2328. [Google Scholar]
- STENKEN J.A., TOPP E.M., SOUTHARD M.X., LUNTE C.E. Examination of microdialysis sampling in a well-characterized hydrodynamic system. Anal Chem. 1993;65:2324–2328. doi: 10.1021/ac00065a026. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.L., DAVIES S.E.C., OBRENOVITCH T.P., DOHENY M.H., PATSALOS P.N., CLARK J.B., SYMON L. Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J. Neurochem. 1995;65:275–281. doi: 10.1046/j.1471-4159.1995.65010275.x. [DOI] [PubMed] [Google Scholar]
- TONG X., RATNARAJ N., PATSALOS P.N. Extracellular fluid (ECF) kinetics of vigabatrin (VGT) in rat frontal cortex and hippocampus. Epilepsia. 1999;40 Suppl. 2:128. doi: 10.1111/j.1528-1167.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- TOSSMAN U., DELIN A., ERIKSSON S., UNGERSTEDT U. Brain cortical amino acids measured by intracerebral dialysis in portocaval shunted rats. Neurochem. Res. 1987;12:265–269. doi: 10.1007/BF00972136. [DOI] [PubMed] [Google Scholar]
- WALKER C.M., ALAVIJEH M.S., SHORVON S.D., PATSALOS P.N. Microdialysis study of the neuropharmacokinetics of phenytoin in rat hippocampus and frontal cortex. Epilepsia. 1996;37:421–427. doi: 10.1111/j.1528-1157.1996.tb00586.x. [DOI] [PubMed] [Google Scholar]
- WALKER C.M., TONG X., BROWN S., SHORVON S.D., PATSALOS P.N. Comparison of single- and repeated-dose pharmacokinetics of diazepam. Epilepsia. 1998;39:283–289. doi: 10.1111/j.1528-1157.1998.tb01374.x. [DOI] [PubMed] [Google Scholar]
- WALKER C.M., TONG X., PERRY H., ALAVIJEH M.S., PATSALOS P.N. Comparison of serum, cerebrospinal fluid and brain extracellular fluid pharmacokinetics of lamotrigine. Br. J. Pharmacol. 2000;130:242–248. doi: 10.1038/sj.bjp.0703337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., WELTY D.F. The simultaneous estimation of the influx and efflux blood–brain barrier permeabilities of gabapentin using a microdialysis – pharmacokinetic approach. Pharm. Res. 1996;13:398–403. doi: 10.1023/a:1016092525901. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., DAMSMA G., ROLLEMA H., DE VRIES J.B., HORN A.S. Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 1987;41:1763–1776. doi: 10.1016/0024-3205(87)90695-3. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., DE VRIES J.B. Characterisatio of in vivo dopamine release as determined by brain microdialysis after acute and subchronic implantation: methodological aspects. J. Neurosci. 1988;51:863–867. doi: 10.1111/j.1471-4159.1988.tb01798.x. [DOI] [PubMed] [Google Scholar]


