Abstract
Phagocytosis of unopsonized zymosan by RAW 264.7 macrophages upregulated protein expression of haem oxygenase-1 (HO-1), inducible nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2) in a time- and concentration-dependent manner.
In the presence of zymosan, exogenous prostaglandin E2 (PGE2) did not exert significant effects on the expression of these three enzymes. In contrast, exogenous leukotriene B4 (LTB4) and LTC4 in the nanomolar range inhibited HO-1 and iNOS expression, as well as nitrite accumulation.
The COX inhibitors indomethacin and NS398 weakly inhibited HO-1 expression but had no effect on iNOS and COX-2 expression or nitrite. In contrast, the 5-lipoxygenase (5-LO) inhibitor ZM 230,487 significantly decreased HO-1, iNOS and nitrite, which were not affected by zileuton. Dexamethasone showed an inhibitory effect on HO-1 expression induced by zymosan.
ZM 230,487 but not zileuton, inhibited the shift due to nuclear factor-κB (NF-κB), whereas they did not modify activator protein-1 (AP-1) binding. Our results suggest that inhibition of NF-κB binding could mediate the effects of ZM 230,487 on the modulation of HO-1 and iNOS protein expression.
NOS inhibition by L-NG-nitroarginine methyl ester (L-NAME) or 1400 W abolished nitrite production and strongly reduced HO-1 expression. These results show an induction of HO-1 protein expression by zymosan phagocytosis in macrophages, with a positive modulatory role for endogenous NO and a negative regulation by exogenous LTs, likely dependent on the reduction of iNOS expression and NO production.
Keywords: Haem oxygenase-1, nitric oxide, inducible nitric oxide synthase, RAW 264.7 macrophages, cyclo-oxygenase-2, leukotrienes, 5-lipoxygenase
Introduction
Macrophages play a key role in the immune defence system against microorganisms or tumour cells since they participate in the processing of antigens and presentation to lymphocytes, as well as in the phagocytosis and killing of microbes. In this process, high amounts of nitric oxide (NO) and reactive oxygen intermediates are generated contributing to intracellular destructive mechanisms. In addition, activated macrophages secrete degradative enzymes and produce cytokines and eicosanoids (Macmicking et al., 1997).
Zymosan, a yeast cell wall derivative composed of alfa-mannan and beta-glucan, is a particulate ligand for different receptors leading to cellular activation and production of inflammatory mediators. Phagocytosis of unopsonized zymosan by macrophages is mediated at least in part through the mannose receptor (Linehan et al., 1999), which is involved in the recognition and phagocytosis of unopsonized microorganisms, such as bacteria, fungi, and protozoa through interactions with polysaccharide components of cell walls (Ofek et al., 1995).
Excessive mediator generation can lead to the spread of cytotoxicity to the host tissues, resulting in detrimental effects. Nevertheless, macrophages possess regulatory pathways where protective mechanisms can operate to control proinflammatory responses and thus limit the destructive potential. Haem oxygenase (HO) is a rate-limiting enzyme in haem catabolism, leading to the formation of biliverdin, which is reduced to bilirubin, carbon monoxide (CO) and iron. Inducible (HO-1) and constitutive (HO-2, HO-3) isozymes have been identified in many cell types (Abraham et al., 1988; McCoubrey et al., 1997).
There is a large body of evidence suggesting that induction of HO-1 is a protective cellular response against environmental and oxidative stress, although the precise mechanisms remain unclear. Overexpression of HO-1 prevents tumour necrosis factor-α-induced apoptosis in murine fibroblasts, an effect possibly mediated via CO (Petrache et al., 2000). In addition, cells from HO-1 knockout mice are less resistant to the cytotoxicity induced by oxidative stress (Poss & Tonegawa, 1997b) and these findings have been confirmed in vivo in mice (Poss & Tonegawa, 1997a) as well as in human HO-1 deficiency (Yachie et al., 1999). Nevertheless, exaggerated HO-1 expression may not be beneficial due to iron accumulation (Suttner & Dennery, 1999) and reduction in haem availability for haem-dependent proteins (Deramaudt et al., 1999).
The aim of this work was to assess if HO-1 participates in the response to zymosan in the macrophage and characterize the mediators involved. We have shown that zymosan induces HO-1 protein expression in a time- and concentration-dependent manner in RAW 264.7 macrophages. This induction appears to be mediated by NO and regulated by exogenous products of the 5-lipoxygenase (5-LO) pathway.
Methods
Cell culture
RAW 264.7 murine macrophage cell line (American Type Culture Collection, Manassas, VA, U.S.A.) were maintained in DMEM medium supplemented with 10% foetal bovine serum, 2 mM L-glutamine and penicillin/streptomycin. Cells were plated onto 24-well tissue culture dishes (7.5×105 cells/well) and allow to grow until confluence. Suspensions of zymosan A from Saccharomyces cerevisiae in saline were autoclaved prior to use. After incubation with zymosan and/or drugs at the indicated times, cell supernatants were collected to measure prostaglandin E2 (PGE2) by radioimmunoassay (Moroney et al., 1988) and nitrite levels by the fluorimetric assay of Misko et al. (1993). Degranulation was assessed by the method of Barrett & Heath (1979) using 4-methylumbelliferyl-β-D-glucuronide as substrate and results were expressed as fluorescence units (F.U.). In parallel, cell pellets were scraped and leukotriene C4 (LTC4) was measured by radioimmunoassay (Moroney et al., 1988). Cell viability was assessed by the mitochondrial-dependent reduction of 3-(4,5-dimethyltiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) to formazan. After appropriate stimulation times, cells were incubated with MTT (200 μg ml−1) for 60 min. The medium was then removed and cells were solubilized in dimethylsulphoxide (100 μl) to quantitate formazan at 550 nm (Gross et al., 1991).
Western blot analysis
After incubation, RAW 264.7 macrophages were lysed in 100 μl of buffer (1% Triton X-100, 1% deoxicholic acid, 20 mM NaCl and 25 mM Tris, pH 7.4) and centrifuged at 4°C for 5 min at 10,000×g. The protein content was determined by the Bradford method using bovine serum albumin as standard. Cell lysate (40 μg of protein) was mixed with Laemmli sample buffer under reducing conditions. Samples were size-separated in 12.5% SDS – PAGE and transferred onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech Europe GmbH, Barcelona, Spain), which were blocked in phosphate buffer saline (0.02 M, pH 7.0)-Tween 20 (0.1%) containing 3% fat-free dry milk. Membranes were incubated with specific antibodies: polyclonal antibody against cyclo-oxygenase-2 (COX-2) (1/1000, Cayman Chemical, Ann Arbor, MI, U.S.A.), polyclonal antibody against inducible NO synthase (iNOS) (1/1000, Cayman Chemical, Ann Arbor, MI, U.S.A.), and anti-HO-1 monoclonal antibody (1/2000, Stressgen, Victoria, Canada). Blots were washed and incubated with peroxidase-conjugated goat anti-rabbit IgG (1/20,000, Dako, Gloustrup, Denmark) or peroxidase-conjugated goat anti-mouse IgG (1/20,000, Sigma Chemical Co., St Louis, MO, U.S.A.). The immunoreactive bands were visualized by an enhanced chemiluminescence system (ECL, Amersham Pharmacia Biotech Europe GmbH, Barcelona, Spain). Band intensity was quantitated using computer-assisted densitometry.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared as described (López-Collazo et al., 1998). Protein was determined by the DC Bio-Rad protein reagent (Richmond, CA, U.S.A.). Double-stranded oligonucleotides containing either the consensus nuclear factor-κB (NF-κB) or activator protein-1 (AP-1) sequence (Promega Corp., Madison, WI, U.S.A.) were end-labelled using T4 polynucleotide kinase (Amersham Pharmacia Biotech Europe GmbH, Barcelona, Spain) and [γ-32P]-ATP, followed by purification using G-25 microcolumns (Amersham Pharmacia Biotech Europe GmbH, Barcelona, Spain). Incubations were performed on ice with 6 μg of nuclear extract, 100,000 c.p.m. of labelled probe, 2 μg poly(dI-dC), 5% v/v glycerol, 1 mM EDTA, 5 mM MgCl2, 1 mM dithiothreitol, 100 mM NaCl and 10 mM Tris-HCl buffer (pH 8.0) for 15 min. Complexes were analysed by nondenaturating 6% polyacrylamide gel electrophoresis in 0.5×Tris-borate buffer followed by autoradiography of the dried gel.
Materials
Z-Leu-Leu-Leu-CHO (MG-132) was purchased from Biomol Res. Labs. Inc. (Plymouth Meeting, PA, U.S.A.). Culture reagents were from Life Technologies Inc. (Barcelona, Spain) and [γ-32P]-ATP (3000 Ci mmol−1) from NEN Life Science Products Inc. (Boston, MA, U.S.A.). [5,6,8,11,12,14,15(n)-3H] PGE2 and [5,6,8,9,11,12,14,15(n)-3H] LTC4 were from Amersham Iberica (Madrid, Spain). 6-[[3-fluoro-5-(4-methoxy-3,4,5,6-tetrahydro-2H-pyran-4-yl)phenoxy]methyl]-1-ethylquinol-2-one (ZM 230,487) and zileuton were kind gifts from Zeneca Pharmaceuticals (Macclesfield, Cheshire, U.K.) and Abbott Laboratories (Abbott Park, Illinois, U.S.A.), respectively. N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS398) and N-[[3-(aminoethyl)phenyl]methyl]-ethanimidamide, dihydrochloride (1400 W) were purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.) and the rest of reagents were from Sigma Chemical Co. (St Louis, MO, U.S.A.).
Statistical analysis
Results are presented as mean±s.e.mean of n experiments. Data were analysed by two-way ANOVA followed by Dunnett's t-test for multiple comparisons.
Results
Zymosan induces HO-1 expression in RAW 264.7 macrophages
Induction of HO-1 by zymosan treatment of RAW 264.7 macrophages was accompanied by induction of enzymes relevant to the inflammatory response, such as COX-2 and iNOS. Figure 1a depicts the increase in HO-1, iNOS and COX-2 protein expression when RAW 264.7 macrophages were stimulated with zymosan (0.3 mg ml−1) for different time periods. The time course indicated that HO-1 protein expression was apparent by 6 h and reached a maximum at 18 h, whereas iNOS was induced later and increased with time. In contrast, high levels of COX-2 protein were already observed at 6 h and also increased with time. These changes in protein expression paralleled the production of PGE2 and nitrite (Figure 1b). Cell activation was also followed by measuring degranulation as β-D-glucuronidase release. As shown in Figure 2, zymosan stimulation of RAW 264.7 macrophages was concentration-dependent in the range tested (0.1 – 0.6 mg ml−1). Maximal levels of PGE2 and nitrite were reached by stimulation with 0.3 mg ml−1 zymosan, although for degranulation the highest effect was already observed at 0.2 mg ml−1.
Figure 1.

Time-course of zymosan stimulation in RAW 264.7 macrophages. Cells were incubated for 6, 18 and 32 h in the presence of zymosan (Zy, 0.3 mg ml−1). (a) Effect on HO-1, iNOS and COX-2 protein expression. Band intensity is represented as arbitrary units. (b) Effect on nitrite, PGE2 and β-D-glucuronidase accumulation. Percentages were calculated with respect to the maximum level measured (21.0±1.2 ng ml−1 PGE2 2529.7±75.3 ng ml−1 nitrite and 20.5±1.0×106 F.U. ml−1 of β-D-glucuronidase). Open symbols and broken lines represent unstimulated cells. Data are the mean±s.e.mean of three experiments.
Figure 2.

Concentration-dependence of zymosan stimulation. Cells were incubated with zymosan (0.1, 0.2, 0.3 and 0.6 mg ml−1) for 18 h. (a) Effect on HO-1, iNOS and COX-2 protein expression. Results are representative of three experiments. (b) Effect on nitrite, PGE2 and β-D-glucuronidase accumulation. Percentages were calculated with respect to the maximum level measured (67.7±2.1 ng ml−1 PGE2, 1585.8±20.6 ng ml−1 nitrite and 19.9±0.9×106 F.U. ml−1 of β-D-glucuronidase). Data are the mean±s.e.mean of three experiments.
Effects of eicosanoids
Exogenous PGE2 did not exert significant effects on HO-1, COX-2 or iNOS expression induced by zymosan (Figure 3 and Table 1). We next determined the influence of exogenous LTs at the time of maximal HO-1 expression and observed that challenging macrophages during zymosan stimulation with either LTB4 or LTC4 in the nanomolar range, had inhibitory effects on HO-1 and iNOS expression and nitrite levels. None of these treatments alone significantly modified the expression of these enzymes or metabolite levels (data not shown).
Figure 3.

Effect of LTB4, LTC4 and PGE2 on zymosan-induced HO-1, iNOS and COX-2 protein expression (a), and nitrite production (b). (**P<0.01). Cells were incubated with LTB4 (30 nM), LTC4 (30 nM) or PGE2 (30 nM) in the presence of zymosan (0.3 mg ml−1) for 18 h. Nitrite production was calculated as percentage with respect to control (zymosan). Data are the mean±s.e.mean of four experiments.
Table 1.
Effect of LTB4, LTC4 and PGE2 on zymosan-induced HO-1, iNOS and COX-2 protein expression

Effects of inhibitors of eicosanoid synthesis
To determine if products of COX activity could mediate HO-1 induction by zymosan, macrophages were exposed to this stimulus in the presence of the dual COX-1/COX-2 inhibitor indomethacin or the selective COX-2 inhibitor NS398, at concentrations able to abolish PGE2 production. Both compounds failed to significantly modify HO-1, iNOS or COX-2 protein expression as well as nitrite accumulation (Figure 4 and Table 2). The influence of the 5-LO pathway was assessed by using selective inhibitors with different mechanisms of action, the non-redox agent ZM 230,487 and the iron-ligand inhibitor zileuton. Incubation of RAW 264.7 macrophages with zymosan in the presence of either ZM 230,487 or zileuton, gave divergent results. While HO-1 and iNOS expression was inhibited in cells treated with ZM 230,487, zileuton failed to exert significant modifications. Changes in iNOS were paralleled by changes in nitrite levels. The inhibitory effect of ZM 230,487 was higher on HO-1 expression. In contrast, COX-2 expression was not modified by these compounds. It is known that 5-LO activity is weak in RAW 264.7 macrophages. In our experimental conditions, LTC4 levels detected by radioimmunoassay did not change significantly after zymosan stimulation of cells for 1 – 4 h (data not shown).
Figure 4.
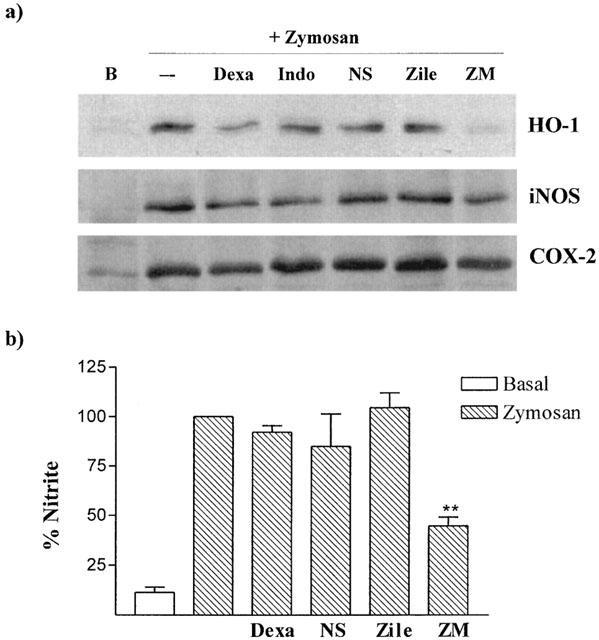
Effect of dexamethasone, indomethacin, NS398, zileuton and ZM 230,487 on zymosan-induced HO-1, iNOS and COX-2 protein expression (a), and nitrite production (b) (**P<0.01). Cells were incubated with dexamethasone (Dexa, 1 μM), indomethacin (Indo, 1 μM), NS398 (NS, 10 μM), zileuton (Zile, 10 μM) or ZM 230,487 (ZM, 5 μM) in the presence of zymosan (0.3 mg ml−1) for 18 h. Dexamethasone was added 1 h before zymosan. Nitrite production was calculated as percentage with respect to control (zymosan). Data are the mean±s.e.mean of 3 – 9 experiments.
Table 2.
Effect of dexamethasone, indomethacin, NS398, zileuton and ZM 230,487 on zymosan-induced HO-1, iNOS and COX-2 protein expression

In addition, the effect of the glucocorticoid dexamethasone was also studied. This anti-inflammatory compound inhibited HO-1 expression, whereas iNOS or COX-2 were reduced to a lower extent (Figure 4 and Table 2).
Effects of 5-LO inhibitors on NF-κB and AP-1 binding
To establish if the behaviour of 5-LO inhibitors could be related to differences in binding of relevant nuclear factors, such as NF-κB and AP-1, we performed EMSA experiments. As shown in Figure 5, nuclear protein extracts from zymosan-stimulated RAW 264.7 macrophages slowed the migration of an oligonucleotide containing the NF-κB-binding site. This shift was inhibited in cells incubated with either the reference inhibitor MG-132 or ZM 230,438, but not in those treated with zileuton. None of these compounds affected the shift due to AP-1 binding.
Figure 5.
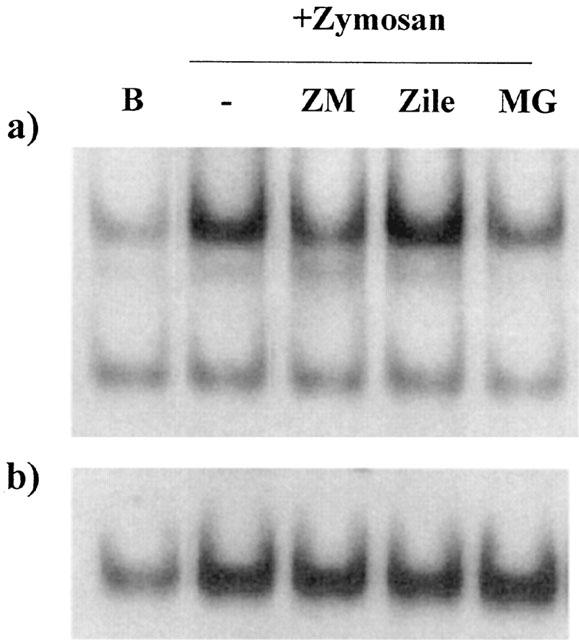
EMSA experiments. (a) NF-κB, (b) AP-1. Cells were preincubated with ZM 230,487 (ZM, 5 μM), zileuton (Zile, 10 μM) or MG-132 (MG, 1 μM) for 15 min before zymosan. (Zy, 0.3 mg ml−1) addition and then incubation proceeded for 2 h. B=unstimulated cells. Results are representative of three independent experiments.
Effect of NOS inhibitors
Addition of L-NG-nitroarginine methyl ester (L-NAME) or the selective iNOS inhibitor, 1400 W significantly reduced HO-1 protein expression and abolished nitrite levels in RAW 264.7 macrophages stimulated with zymosan (Figure 6 and Table 3). In contrast, these agents did not modify COX-2 and L-NAME slightly increased iNOS expression. Other parameters such as degranulation or PGE2 levels were not affected by NOS inhibitors (data not shown).
Figure 6.

Effect of NOS inhibitors on zymosan-induced HO-1, iNOS and COX-2 protein expression (a), and nitrite production (b) (**P<0.01). Cells were incubated with L-NAME (1 mM) or 1400 W (10 μM) in the presence of zymosan (0.3 mg ml−1) for 18 h. Nitrite production was calculated as percentage with respect to control (zymosan). Data are the mean±s.e.mean of four experiments.
Table 3.
Effect of L-NAME and 1400 W on zymosan-induced HO-1, iNOS and COX-2 protein expression

Discussion
Results in this study show for the first time that activation of RAW 264.7 cells by unopsonized zymosan upregulates HO-1 protein expression, suggesting that HO-1 induction represents a regulatory mechanism to limit the cytotoxic effects of this inflammatory response. In fact, a protective role of HO-1 has been reported in vitro for a variety of stress inducers such as haemoglobin, hypoxia, glutathione depletion, cytokines . . . (Terry et al., 1998; Dennery et al., 1997; Shibahara et al., 1987). In vivo, exogenous administration of HO-1 has been useful in hyperoxia-induced lung injury in rats (Otterbein et al., 1999), whereas expression of HO-1 induced by different agents protects renal cells from ischaemia reperfusion injury (Shimizu et al., 2000) and has been associated to inhibitory effects in different inflammatory states (Willis et al., 1996; Laniado-Schwartzman et al., 1997; Mosley et al., 1998). Recently, Otterbein et al. (2000) have described the participation of CO generated by HO-1 activity in the anti-inflammatory effects exhibited by this enzyme (Otterbein et al., 2000).
In the response to zymosan, iNOS and COX-2 were also induced, leading to significant increases in NO and PGE2 production. However, according to its role as a stress-inducible protein, HO-1 expression during zymosan stimulation was more rapid in reaching maximal levels than iNOS and COX-2. Our experimental evidence suggests a relationship between HO-1 and endogenous NO which may play a positive modulatory role in HO-1 expression induced by zymosan, likely as a protective mechanism in the macrophage against the biological consequences of high levels of NO generated during phagocytosis. It has been reported previously using stimuli other than zymosan or NO donors, that NO induces the expression of HO-1 in different cellular systems such as vascular smooth muscle (Durante et al., 1997), endothelial cells (Foresti et al., 1997), HeLa (Bouton & Demple, 2000) and fibroblasts (Alcaraz et al., 2000). We have demonstrated in the present work that inhibition of NO synthesis during prolonged stimulation with zymosan (18 h) decreases HO-1 protein expression. Nevertheless other mediators may play a role in HO-1 induction at early times, when there is no significant NO production. To this respect, it is known that reactive oxygen species are quickly generated during zymosan phagocytosis by mouse peritoneal macrophages or RAW 264.7 cells (Herencia et al., 2001).
We have also found an interesting negative modulation of HO-1 and iNOS expression by exogenous LTs. The ability of these eicosanoids to modify HO-1 expression may depend on the reduction of iNOS and consequently of NO, observed in our experiments performed in the presence of zymosan for 18 h. On the other hand, our data do not support a role for endogenous 5-LO or COX metabolites in the response to zymosan in RAW 264.7 macrophages. It is interesting to note that inhibition of 5-LO by ZM 230,487 resulted in strong HO-1 reduction, whereas zileuton had no effect. This difference in behaviour is likely due to inhibition of NF-κB binding and independent of 5-LO inhibition. To this respect, Bowie & O'neill (2000) have recently speculated that ZM 230,487 may have other targets distinct from 5-LO. It is known that upregulation of iNOS and HO-1 gene expression can depend on activation of NF-κB and AP-1 (Alam & Den, 1992; Nunokawa et al., 1996; Oshiro et al., 1999; Lee et al., 2000). Thus, inhibition of NF-κB binding by ZM 230,487 could explain the decrease in HO-1 and iNOS protein expression produced by this drug. This last effect would lead to reduced NO production that may also contribute to the strong inhibitory effect of ZM 230,487 on HO-1 expression.
In this study, we have shown that dexamethasone inhibits HO-1 expression induced by zymosan, which is in line with reports indicating that this glucocorticoid blocks the induction of HO-1 mRNA in response to interleukin-6 in endothelial cells, as part of its anti-inflammatory mechanisms (Deramaudt et al., 1999). Long-term expression of HO-1 can result in deleterious effects on cells since it can decrease the availability of haem, with negative effects on haem proteins (Albakri & Stuehr, 1996; Deramaudt et al., 1999). Thus, inhibition of HO-1 may be beneficial for the control of chronic inflammation.
During inflammatory responses macrophages are exposed to high concentrations of eicosanoids derived from the LO or COX pathways. We have shown that LTB4 and LTC4 potently decreased the expression of HO-1 and iNOS induced by zymosan and these results are consistent with LT products of inflammatory cells having paracrine effects to control macrophage activation after a prolonged exposure to this phagocytic stimulus. Although the role of LTs in modulating innate host defense mechanisms related to NO is poorly understood, it is known that LTB4 and cysteinyl-LTs contribute to antimicrobial defence and quickly activate NO release and secretory events in human neutrophils (Larfars et al., 1999) and mediate the phagocytic response after stimulation of rat alveolar macrophages by K. pneumoniae (Mancuso et al., 1998). Evidence has also been provided that inhibition of 5-LO during cell activation decreases NO production and cytolytic capacity in thyoglycollate-elicited macrophages (Hubbard & Erickson, 1995), whereas other authors have reported a lack of interaction between LT and NO pathways in RAW 264.7 macrophages stimulated with lipopolysaccharide+interferon-γ (Hulkower et al., 1996).
Further studies would be necessary to elucidate the mechanism of action of LTs in this setting although previous work has suggested an anti-inflammatory effect of LTB4 mediated by peroxisome proliferator-activated receptor-α activation (Devchand et al., 1996). We have provided in vitro evidence for a regulatory role of LTs working to limit the inflammatory response in RAW 264.7 macrophages.
Acknowledgments
A.M. Vicente thanks Generalitat Valenciana for a fellowship. The authors are grateful to Zeneca Pharmaceuticals (Macclesfield, Cheshire, U.K.) for providing ZM 230,487 and Abbott Laboratories (Abbott Park, Illinois, U.S.A.) for the gift of zileuton.
Abbreviations
- AP-1
Activator protein-1
- CO
carbon monoxide
- COX
cyclo-oxygenase
- ECL
enhanced chemiluminescence
- EMSA
electrophoretic mobility shift assay
- F.U.
fluorescence units
- HO
haem oxygenase
- iNOS
inducible nitric oxide synthase
- LT
leukotriene
- L-NAME
L-NG-nitroarginine methyl ester
- 5-LO
5-lipoxygenase
- MTT
3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- PG
prostaglandin
References
- ABRAHAM N.G., LIN J.H., SCHWARTZMAN M.L., LEVERE R.D., SHIBAHARA S. The physiological significance of heme oxygenase. Int. J. Biochem. 1988;20:543–558. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- ALAM J., DEN Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J. Biol. Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- ALBAKRI Q.A., STUEHR D.J. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996;271:5414–5421. doi: 10.1074/jbc.271.10.5414. [DOI] [PubMed] [Google Scholar]
- ALCARAZ M.J., HABIB A., LEBRET M., CREMINON C., LEVY-TOLEDANO S., MACLOUF J. Enhanced expression of haem oxygenase-1 by nitric oxide and antiinflammatory drugs in NIH 3T3 fibroblasts. Br. J. Pharmacol. 2000;130:57–64. doi: 10.1038/sj.bjp.0703281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRETT A.J., HEATH M.F.Lysosomal enzymes Lysosomes: a Laboratory Handbook 1979Amsterdam: North Holland; 118–120.ed. Dingle, A.T. pp [Google Scholar]
- BOUTON C., DEMPLE B. Nitric oxide-inducible expression of heme oxygenase-1 in human cells: Translation-independent stabilization of the mRNA and evidence for direct action of NO. J. Biol. Chem. 2000;275:32688–32693. doi: 10.1074/jbc.275.42.32688. [DOI] [PubMed] [Google Scholar]
- BOWIE A., O'NEILL L.A. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- DENNERY P.A., SRIDHAR K.J., LEE C.S., WONG H.E., SHOKOOHI V., RODGERS P.A., SPITZ D.R. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J. Biol. Chem. 1997;272:14937–14942. doi: 10.1074/jbc.272.23.14937. [DOI] [PubMed] [Google Scholar]
- DERAMAUDT T.B., DA SILVA J.L., REMY P., KAPPAS A., ABRAHAM N.G. Negative regulation of human heme oxygenase in microvessel endothelial cells by dexamethasone. Proc. Soc. Exp. Biol. Med. 1999;222:185–193. doi: 10.1046/j.1525-1373.1999.d01-130.x. [DOI] [PubMed] [Google Scholar]
- DEVCHAND P.R., KELLER H., PETERS J.M., VAZQUEZ M., GONZALEZ F.J., WAHLI W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- DURANTE W., KROLL M.H., CHRISTODOULIDES N., PEYTON K.J., SCHAFER A.I. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ. Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- FORESTI R., CLARK J.E., GREEN C.J., MOTTERLINI R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J. Biol. Chem. 1997;272:18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- GROSS S.S., JAFFE E.A., LEVI R., KILBOURN R.G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem. Biophys. Res. Commun. 1991;178:823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- HERENCIA F., FERRANDIZ M.L., UBEDA A., GUILLEN I., DOMINGUEZ J.N., CHARRIS J.E., LOBO G.M., ALCARAZ M.J. 4-Dimethylamino-3′,4′-dimethoxychalcone downregulates iNOS expression and exerts anti-inflammatory effects. Free Radic. Biol. Med. 2001;30:43–50. doi: 10.1016/s0891-5849(00)00443-3. [DOI] [PubMed] [Google Scholar]
- HUBBARD N.E., ERICKSON K.L. Role of 5-lipoxygenase metabolites in the activation of peritoneal macrophages for tumoricidal function. Cell. Immunol. 1995;160:115–122. doi: 10.1016/0008-8749(95)80016-c. [DOI] [PubMed] [Google Scholar]
- HULKOWER K.I., POLLOCK J.S., WALSH R.E., HUANG R., OTIS E.R., BROOKS C.D.W., BELL R.L. Leukotrienes do not regulate nitric oxide production in RAW 264.7 macrophages. Prostag. Leukotr. Ess. Fatty Acids. 1996;55:145–149. doi: 10.1016/s0952-3278(96)90089-7. [DOI] [PubMed] [Google Scholar]
- LANIADO-SCHWARTZMAN M., ABRAHAM N.G., CONNERS M., DUNN M.W., LEVERE R.D., KAPPAS A. Heme oxygenase induction with attenuation of experimentally induced corneal inflammation. Biochem. Pharmacol. 1997;53:1069–1075. doi: 10.1016/s0006-2952(97)00080-4. [DOI] [PubMed] [Google Scholar]
- LARFARS G., LANTOINE F., DEVYNCK M.A., PALMBLAD J., GYLLENHAMMAR H. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood. 1999;93:1399–1405. [PubMed] [Google Scholar]
- LEE P.J., CAMHI S.L., CHIN B.Y., ALAM J., CHOI A.M. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L175–L182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- LINEHAN S.A., MARTINEZ-POMARES L., STAHL P.D., GORDON S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÓPEZ-COLLAZO E., HORTELANO S., ROJAS A., BOSCÁ L. Triggering of peritoneal macrophages with IFN-α/ β attenuates the expression of inducible nitric oxide synthase through a decrease in NF-κB activation. J. Immunol. 1998;160:2889–2895. [PubMed] [Google Scholar]
- MACMICKING J., XIE Q.W., NATHAN C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- MANCUSO P., STANDIFORD T.J., MARSHALL T., PETERS-GOLDEN M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect. Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCOUBREY W.K., HUAG T.J., MAINES M.D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., SCHILLING R.J., SALVEMINI D., MOORE W.M., CURRIE M.G. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- MORONEY M.A., ALCARAZ M.J., FORDER R.A., CAREY F., HOULT J.R.S. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J. Pharm. Pharmacol. 1988;40:787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- MOSLEY K., WEMBRIDGE D.E., CATTELL V., COOK H.T. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998;53:672–678. doi: 10.1046/j.1523-1755.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- NUNOKAWA Y., OIKAWA S., TANAKA S. Human inducible nitric oxide synthase gene is transcriptionally regulated by nuclear factor-kappaB dependent mechanism. Biochem. Biophys. Res. Commun. 1996;223:347–352. doi: 10.1006/bbrc.1996.0897. [DOI] [PubMed] [Google Scholar]
- OFEK I., GOLDHAR J., KEISARI Y., SHARON N. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- OSHIRO S., TAKEUCHI H., MATSUMOTO M., KURATA S. Transcriptional activation of heme oxygenase-1 gene in mouse spleen, liver and kidney cells after treatment with lipopolysaccharide or hemoglobin. Cell Biol. Int. 1999;23:465–474. doi: 10.1006/cbir.1999.0375. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., BACH F.H., ALAM J., SOARES M., TAO L.H., WYSK M., DAVIS R.J., FLAVELL R.A., CHOI A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., KOLLS J.K., MANTELL L.L., COOK J.L., ALAM J., CHOI A.M. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRACHE I., OTTERBEIN L.E., ALAM J., WIEGAND G.W., CHOI A.M. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- POSS K.D., TONEGAWA S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U.S.A. 1997a;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSS K.D., TONEGAWA S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997b;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBAHARA S., MULLER R.M., TAGUCHI H. Transcriptional control of rat heme oxygenase by heat shock. J. Biol. Chem. 1987;262:12889–12892. [PubMed] [Google Scholar]
- SHIMIZU H., TAKAHASHI T., SUZUKI T., YAMASAKI A., FUJIWARA T., ODAKA Y., HIRAKAWA M., FUJITA H., AKAGI R. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit. Care Med. 2000;28:809–817. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- SUTTNER D.M., DENNERY P.A. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- TERRY C.M., CLIKEMAN J.A., HOIDAL J.R., CALLAHAN K.S. Effect of tumor necrosis factor-α and interleukin-1 α on heme oxygenase-1 expression in human endothelial cells. Am. J. Physiol. 1998;274:H883–H891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- WILLIS D., MOORE A.R., FREDERICK R., WILLOUGHBY D.A. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nature Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- YACHIE A., NIIDA Y., WADA T., IGARASHI N., KANEDA H., TOMA T., OHTA K., KASAHARA Y., KOIZUMI S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]


