Abstract
The hypotensive and vasorelaxant effect of dioclein in resistance mesenteric arteries was studied in intact animals and isolated vessels, respectively.
In intact animals, initial bolus administration of dioclein (2.5 mg kg−1) produced transient hypotension accompanied by an increase in heart rate. Subsequent doses of dioclein (5 and 10 mg kg−1) produced hypotensive responses with no significant change in heart rate. NG-nitro-L-arginine methyl ester (L-NAME) did not affect the hypotensive response.
In endothelium-containing or -denuded vessels pre-contracted with phenylephrine, dioclein (5 and 10 mg kg−1 produced a concentration-dependent vasorelaxation (IC50=0.3±0.06 and 1.6±0.6 μM, respectively) which was not changed by 10 μM indomethacin. L-NAME (300 μM) produced a shift to the right.
Dioclein was without effect on contraction of vessels induced by physiological salt solution (PSS) containing 50 mM KCl and the concentration dependence of dioclein's effect on phenylephrine induced contraction was shifted to the right in vessels bathed in PSS containing 25 mM KCl.
Tetraethylammonium (10 mM) and BaCl2 (1 mM) increased the IC50 for dioclein-induced vasorelaxation without affecting the maximal response (Emax). Charybdotoxin (100 nM), 4-aminopyridine (1 mM) and iberiotoxin (100 nM) increased the IC50 and reduced the Emax. Apamin (1 μM) reduced the Emax without affecting the IC50.
Dioclein produced a hyperpolarization in smooth muscle of mesenteric arteries with or without endothelium (7.7±1.4 mV and 12.3±3.6 mV, respectively).
In conclusion dioclein lowered arterial pressure probably through a decrease in peripheral vascular resistance. The underling mechanism implicated in the vasorelaxant effect of dioclein appears to be the opening of KCa and Kv channels and subsequent membrane hyperpolarization.
Keywords: Dioclein, hypotension, resistance arteries, vasorelaxation, K+ channels, hyperpolarization
Introduction
Vascular tone of small arteries and arterioles underlies the maintenance of peripheral resistance in the circulation and is a major contributor to the control of blood pressure (White et al., 1996). Potassium channels (K+ channels) appear to play a crucial role in controlling the cellular membrane potential and have an important role in the control of vascular tone (Jackson, 2000). They comprise the most diverse group of ion channels so far investigated and a large number of agents have been shown to modulate their activities. Potassium channel openers exert their biological effects by increasing the K+ channel open probability and a number of substances that act on K+ channels have been shown to dilate arteries by causing hyperpolarization in vascular smooth muscle cells (Nelson et al., 1990; Quast, 1993). These effects of K+ channel openers could be useful in the treatment of hypertension (Quast, 1992; Kuriyama et al., 1995; Lawson, 2000).
Flavonoids constitute a large group of low molecular weight polyphenolic compounds derived from plants, and currently consumed in large amounts in the daily diet. Interestingly, consumption of flavonoids in the diet has been shown to be inversely associated with morbidity and mortality from coronary heart disease (Hertog et al., 1993, 1995; Knekt et al., 1996; Muldoon & Kritchevsky, 1996). In addition, flavonoids inhibit platelet aggregation (Seigneur et al., 1990; Fitzpatrick et al., 1993) and their antioxidant properties may delay the onset of atherogenesis by reducing peroxidative reactions and decreasing thrombotic tendency (Rice-Evans et al., 1997; Aviram & Fuhrman, 1998; Stanley & Mazier, 1999). Furthermore, flavonoids have been widely described in the literature as vasodilator compounds (Duarte et al., 1993; Fitzpatrick et al., 1993; Herrera et al., 1996). Inhibition of protein kinase C, voltage-dependent calcium channels, phosphodiesterases as well as increase in nitric oxide content in endothelial vascular cells (Andriambeloson et al., 1997; 1998) have been reported as the main mechanisms implicated in the vasorelaxant effect of flavonoids.
Dioclein (Figure 1) is a new flavonoid isolated from the roots of Dioclea grandiflora Mart. ex Benth. (Bhattacharyya et al., 1995), a vine that grows in the coastal plain of northeastern Brazil. Very recently we have reported a nitric oxide- and endothelium-dependent vasorelaxant effect of dioclein in the rat aorta (Lemos et al., 1999). We now present evidence for a new mechanism of flavonoid-induced vasodilatation. On rat small mesenteric artery, dioclein produces a vasodilator effect by opening K+ channels and inducing membrane hyperpolarization. This effect of dioclein on K+ channels may underlie its hypotensive actions when injected in conscious freely moving rats.
Figure 1.
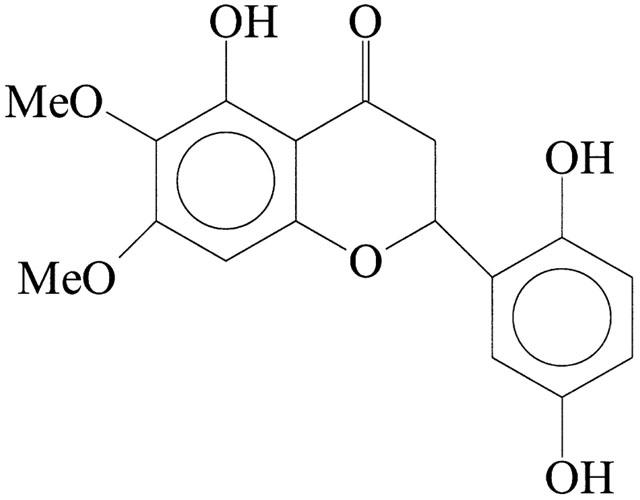
Structure of dioclein (5, 2′, 5′-trihydroxy-6-7-dimethoxyflavanone).
Methods
Measurement of arterial blood pressure in conscious unrestrained rats
For the measurement of arterial blood pressure, rats were anaesthetized with halothane (1.5 – 2% in oxygen), and a polyethylene (PE) catheter (heat-stretched PE-10 fused to a PE-50 extension) was inserted into the lower abdominal aorta via the left femoral artery. Stretching of the PE-10 intravascular part minimized the risk of ischaemia in the catheterized leg, without altering the arterial pressure signal (Oliveira et al., 1996). Another catheter (PE-10 fused to PE-50) was inserted into the inferior vena cava via the left femoral vein for the administration of drugs. Both catheters were filled with heparinized saline and led under the skin to exit between the scapulae. After catheterization, rats were placed in large individual recording cages. Two days later, experiments were performed in conscious freely moving rats accustomed to their environment, and provided with food and water ad libitum. The arterial catheter was connected to a precalibrated pressure transducer (BLPR transducer, World Precision Instruments, Inc., Sarasota, FL, U.S.A.). Pressure outputs were fed to an amplifier-recorder (Model TMB-4; World Precision Instruments, Inc., Sarasota, FL, U.S.A.) and to a personal computer (486 DX 2/66) equipped with an analogue-to-digital converter board (AD16JR; World Precision Instruments, Inc., Sarasota, FL, U.S.A.). Using CVMS data acquisition/recording software (World Precision Instruments, Inc., Sarasota, FL, U.S.A.), data were sampled every 2 ms and stored on CD-ROM. Beat-to-beat time series were generated and processed off-line. For each cardiac cycle, the computer calculated mean arterial pressure (MAP) and pulse interval (referred to as heart rate (HR) in beats min−1).
Systemic haemodynamic effects of dioclein in conscious rats
After haemodynamic parameters had stabilized, sodium nitroprusside (10 μg kg−1 i.v.) was injected to check the patency of the venous catheter. Ten to 15 min later, different doses of dioclein (2.5, 5 and 10 mg kg−1 i.v.) were randomly administered. Successive injections were separated by a time interval sufficient to allow full recovery of arterial pressure, usually 35 – 45 min.
Effect of NO-synthase inhibition on the hypotensive response to dioclein in conscious rats
Following a 60-min stabilization period, a bolus dose (20 mg kg−1) of NG-Nitro-L-arginine Methyl Ester (L-NAME) sufficient to inhibit acetylcholine (Ach)-induced hypotensive effects (Boussairi & Sassard, 1994) was injected intravenously. Dioclein (2.5, 5 and 10 mg kg−1) administration was then repeated after waiting 30 min. Changes in MAP and HR induced by dioclein were compared before and after L-NAME administration.
Small mesenteric artery preparation and mounting
Male Wistar albino rats were killed by cervical dislocation and exsanguinated. The viscera were exposed, and a proximal segment of the small bowel was removed and pinned in a dissecting dish containing physiological salt solution (PSS) of the following composition (in mmol/l): NaCl, 119; KCl, 4.7; KH2PO4, 0.4; NaHCO3, 14.9; MgSO4, 1.17; CaCl2, 2.5; glucose, 5.5. Branch II or III resistance arteries were cleaned of fat and connective tissue, and a segment 1.6 to 2.0 mm in length was removed. In some experiments, the endothelial layer was removed immediately after dissection by intraluminal perfusion with 0.5 % CHAPS (in PSS) for 25 s followed by repeated washings with PSS. The segment was then mounted on a previously described myograph (Mulvany & Halpern, 1977) using two tungsten wires (20 μm in diameter) inserted through the lumen of the vessel. Mechanical activity was recorded isometrically by a force transducer (Kistler-Morse, DSG BE4). After mounting, the vessel was placed in PSS, kept at 37°C and gassed continuously with 95% O2 and 5% CO2 (pH 7.4). After an equilibration period of 60 min, the vessel was stretched to a length that yields a circumference equivalent to 90% of that given by an internal pressure of 100 mmHg; this required a load of about 200 mg. After setting the vessel to its working length, it was challenged twice with 3 μM phenylephrine to elicit reproducible contractile responses. The presence of functional endothelium was assessed by the ability of ACh (1 μM) to induce more than 50% relaxation of vessels pre-contracted with phenylephrine (3 μM). The absence of a relaxation response to ACh was taken as evidence that the vessel segments were functionally denuded of endothelium.
Vasorelaxant activity of dioclein in pre-contracted vessels
The vasorelaxant activity of dioclein was measured in vessels with or without functional endothelium pre-contracted with phenylephrine (3 μM). The removal of endothelium did not change the maximal contraction induced by phenylephrine (0.88±0.10 and 0.81±0.11 g, in the presence and in the absence of functional endothelium, respectively; n=6). Dioclein was added in increasing cumulative concentrations once the response to phenylephrine had stabilized. In order to verify the participation of endothelium-derived products in the relaxant effect of dioclein, experiments were performed in the presence of L-NAME (300 μM), indomethacin (10 μM) or PSS containing 25 or 50 mM KCl (equimolar replacement of NaCl with KCl). To achieve blockade of specific K+ channels, endothelium-denuded vessels were exposed to: 10 mM tetraethylammonium (TEA), 100 μM or 1 mM BaCl2, 1 mM 4-aminopyridine, 10 μM glybenclamide, 0.1 μM charybdotoxin, 0.1 μM iberiotoxin and 1 μM apamin. Maximal tension developed by 3 μM phenylephrine was not affected by pre-treatment of vessels with 25 mM KCl or the K+ channel blockers used (data not shown), which is in accordance with previous reports (Côrtes et al., 1996; Taylor & Benoit, 1999). The maximal tension developed in response to 50 mM KCl was not different from the maximal tension developed in response to phenylephrine (1.11±0.23 g and 1.12±0.19 g, respectively). L-NAME, indomethacin and all the K+ channel blockers were added to the bath 20 min prior to the addition of phenylephrine. As a control for all the above-mentioned protocols, another vessel fragment from each rat was simultaneously monitored for dioclein alone.
Measurement of membrane potential
Segments of the small bowel mesenteric artery (1 cm in length) removed as described above, was pinned down to the bottom of an organ chamber (2 ml) continuously superfused with PSS solution (37°C, aerated with a 95% O2, 5% CO2 gas mixture; pH 7.4). The experiments were performed in endothelium containing or denuded vessels. When necessary the endothelium was removed, as described above. Endothelium cell removal was considered to be successful when the hyperpolarization induced by ACh did not exceed 5 mV. Transmembrane potentials were recorded with a glass microelectrodes filled with KCl 3 M (tip resistance 20 – 40 MΩ). The microelectrode was mounted on a sliding micromanipulator (Leitz (St Gallen, Switzerland)). The membrane potential was amplified by means of an Axopatch 200B and digitalized with a Digidata 1200 (Axon Instruments, Foster City, U.S.A.). The signal was monitored and acquired on the scope mode of pClamp7 software. Impalements were accepted when a sudden negative change in voltage were kept constant for at least 3 min at the point the membrane potential had stabilized, and returned instantaneously to the previous voltage level after withdrawal. The impalements were performed from the adventitial side for experiments in the presence of functional endothelium and from the intimal side for experiments in endothelium-denuded vessels.
Drugs
Acetylcholine chloride, indomethacin, L-NAME, L-phenylephrine chloride, sodium nitroprusside, glybenclamide, tetraethylammonium, 4-aminopyridine and BaCl2, were purchased from Sigma. Charybdotoxin, iberiotoxin and apamin from Calbiochem. The isolation and identification of dioclein is described in detail elsewhere (Bhattacharyya et al., 1995). Dioclein was solubilized in a mixture of distilled water/chremophor at a concentration of 10 mM and diluted to the desired concentration with distilled water just before use. Charybdotoxin and apamin were dissolved in dimethyl sulphoxide (DMSO). The final concentration of chremophor and DMSO in the bath never exceeded 0.01% and was without effect when tested in control preparations (data not shown). Indomethacin was dissolved in 0.5% w v−1 sodium bicarbonate. The other compounds were freely dissolved in distilled water.
Data analysis
Results are expressed as means±s.e.mean. Results from contractile experiments are expressed as percentage decreases in the maximal contraction induced by phenylephrine, and the point when the basal line was reached was considered 100% relaxation. The concentration of dioclein that produced 50% inhibition of agonist-induced contraction (IC50) was defined by non-linear curve fitting of data from concentration response experiments. Student's t-test and two-way analysis of variance (ANOVA) were used to analyse the data, and results were considered significant when P<0.05.
Results
Systemic haemodynamic effects of dioclein in conscious rats
In 10 conscious rats, baseline values of MAP and heart rate were 110±3 mmHg and 390±5 beats min−1, respectively. Dioclein (2.5, 5 and 10 mg kg−1 i.v.) induced hypotensive responses (Figure 2) that were rapid in onset and short-lasting. Peak changes in MAP were observed at 3±1 min and MAP usually returned to baseline within 15 – 20 min. The hypotensive response to dioclein was accompanied by an increase in HR for the first dose (2.5 mg kg−1 i.v.) and no significant changes induced by the second and third doses, respectively (Figure 2).
Figure 2.
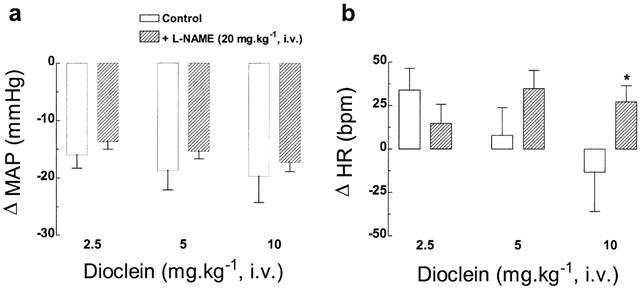
Bar graphs show (a) peak changes in mean arterial pressure (MAP), and (b) heart rate (HR) induced by the acute administration of increasing doses of dioclein in conscious unrestrained normotensive rats, before (control) and after NO-synthase inhibition (after L-NAME). Results are means±s.e.mean. (n=10). *P<0.05 vs control values.
Effect of NO-synthase inhibition on the hypotensive response to dioclein in conscious rats
In nine conscious rats, L-NAME (20 mg kg−1 i.v.) induced a sustained increase in MAP (138±4 from 110±3 mmHg, P<0.01) accompanied by bradycardia (363±18 from 390±5 beats min−1, P<0.05). After NO-synthase inhibition, the hypotensive response to dioclein (2.5, 5 and 10 mg kg−1 i.v.) remained unchanged compared to the responses before L-NAME administration (Figure 2). As illustrated in Figure 2, dioclein also induced changes in HR. We observed that under NO-synthase blockade, dioclein produced tachycardia with all doses tested.
Effect of dioclein on isolated resistance mesenteric arteries with and without functional endothelium
In endothelium containing mesenteric arteries pre-contracted with 3 μM phenylephrine, dioclein produced a concentration-dependent vasorelaxation, with an IC50 value of 0.3±0.06 μM (Figure 3). In endothelium-denuded vessels, the concentration-response curve for dioclein was shifted to the right (IC50=1.6±0.6 μM; P<0.05). However, maximal values for the relaxant effect (Emax) did not change (Figure 3; Table 1).
Figure 3.
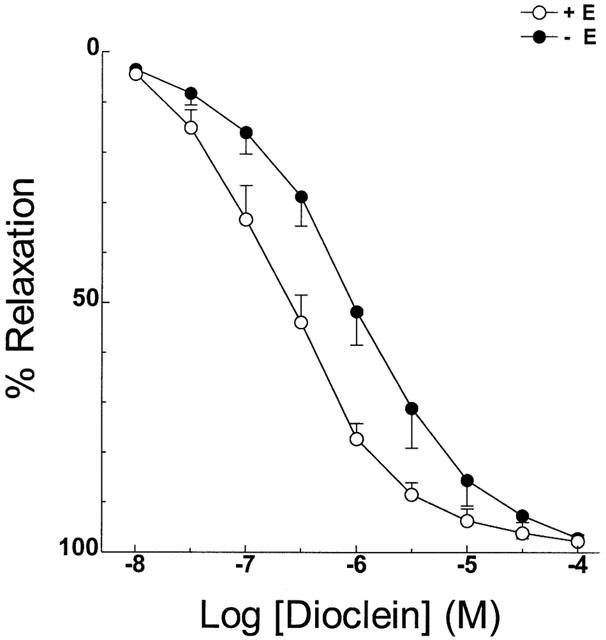
Vasodilator effect of dioclein in resistance mesenteric arteries with or without functional endothelium. The values are means±s.e.mean of at least nine experiments.
Table 1.
IC50 and Emax values for dioclein in the different experimental conditions
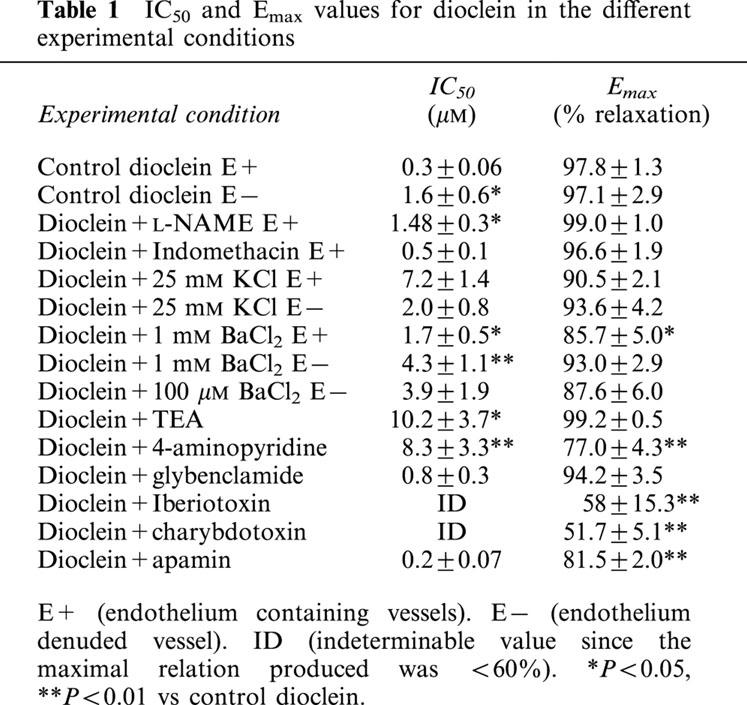
Pre-treatment of endothelium-intact vessels with indomethacin, an inhibitor of cyclo-oxygenase did not change the vasorelaxant effect of dioclein (Figure 4a). In addition, L-NAME, an inhibitor of nitric oxide synthase, produced only a minor shift to the right in the concentration-response curve for dioclein (Figure 4b, Table 1). Similarly to endothelium-denuded vessels, the Emax value for dioclein was not changed (Table 1).
Figure 4.
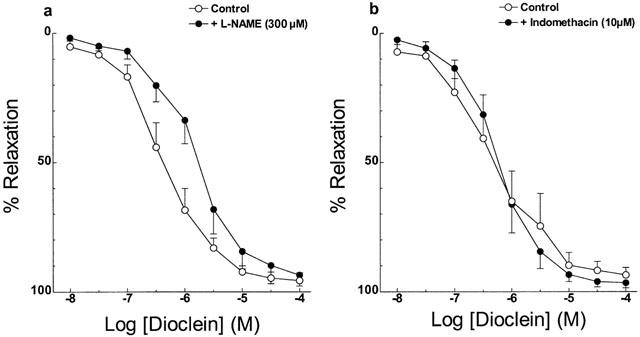
Vasodilator effect of dioclein in rat aortic rings (a) in the absence or in the presence of L-NAME; (b) in the absence or in the presence of indomethacin. The values are means±s.e.mean of at least five experiments.
When the vessels were stimulated with phenylephrine in the presence of a PSS solution containing 25 mM KCl, the concentration-response curve for dioclein was shifted to the right (Figure 5, Table 1). The IC50 for dioclein in this condition was unchanged whether endothelium intact or endothelium-denuded vessels were used (Figure 5, Table 1). Dioclein had no vasorelaxant effect when small mesenteric arteries were stimulated with 50 mM KCl (Figure 5). Table 1 summarizes the IC50 and Emax values for dioclein in control vessels and under different experimental conditions.
Figure 5.
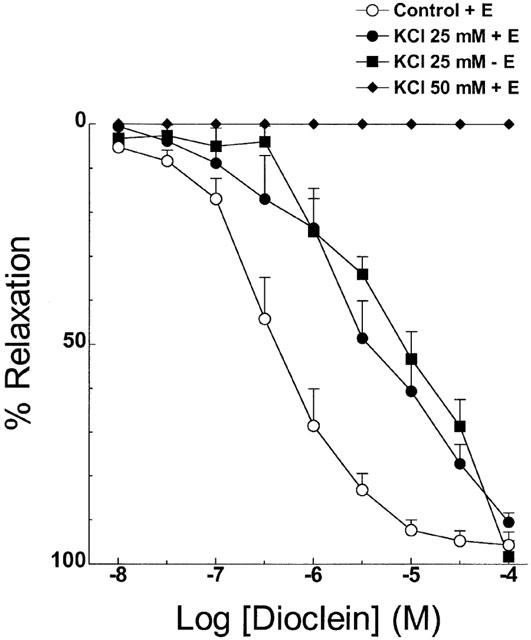
Vasodilator effect of dioclein in resistance mesenteric arteries pre-contracted with phenylephrine in normal PSS solution, after isotonic replacement by 25 mM KCl in endothelium-containing or -denuded vessels and vasorelaxant effect of dioclein in vessels pre-contracted with 50 mM KCl. The results are means±s.e.mean of at least five experiments.
Effect of K+ channel blockers in the vasorelaxant effect of dioclein
Pre-treatment of endothelium containing small mesenteric arteries with 10 mM TEA (Figure 6a) and 1 mM BaCl2 (Figure 6b), two non-selective K+ channel blockers, produced a rightward shift in the concentration-response curve to dioclein with no change in the Emax values (Table 1). Removal of endothelium did not change the effect of 1 mM BaCl2 (Figure 6b). Glybenclamide, a selective ATP-sensitive K+ (KATP) channel blocker, and 100 μM BaCl2 which blocks selectively the inward rectifier K+ (KIR) channel in rat mesenteric artery had no effect on the vasorelaxant effect of dioclein (Figure 6c, Table 1). However, charybdotoxin and iberiotoxin, which selectively blocks large-conductance Ca2+-activated K+ (BKCa) channels and 4-aminopyridine, which inhibits voltage-activated K+ (Kv) channel, effectively inhibited the vasorelaxant effect of dioclein with a strong reduction on Emax values (Figure 6d, Table 1). The effect of co-administration of iberiotoxin and 4-Ap was not additive (not shown). Finally, apamin a selective blocker of small-conductance Ca2+-activated K+ (SKCa) channels did not change IC50 value for dioclein but produced a significant reduction of Emax value (Figure 6d, Table 1). Table 1 shows the IC50 and Emax values for dioclein in control condition and after K+ channel blockade. None of the K+ channel blockers used had any significant vasoconstrictor effects on small mesenteric artery by themselves (not shown).
Figure 6.
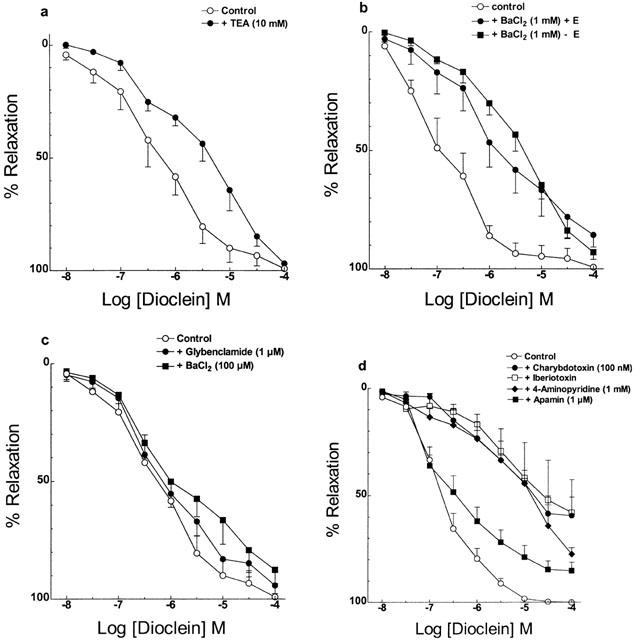
Effect of potassium channel blockers on the vasorelaxant effect of dioclein in resistance mesenteric arteries. The experiments were performed in endothelium containing vessels, except where indicated. Vasorelaxant effect of dioclein, (a) in the absence or in the presence of 10 mM TEA; (b) in the absence or in the presence of 1 mM BaCl2, in endothelium containing or denuded vessels; (c) in the absence or in the presence of 1 μM glybenclamide, or 100 μM BaCl2 (d) in the absence or in the presence of 100 nM charybdotoxin, 100 nM iberiotoxin, 1 mM 4-aminopyridine or 1 μM apamin. The results are means±s.e.mean of at least five experiments.
Effect of dioclein in the membrane potential
Stimulation of endothelium containing mesenteric arteries with 1 μM ACh produced a transient hyperpolarization of the membrane of vascular smooth muscle cells (Figure 7a). This hyperpolarization was not seen in endothelium-denuded vessel (Figure 7d). On the other hand, dioclein (10 μM) induced a slower onset hyperpolarization, which was comparable in both endothelium containing and denuded vessels (Figure 7b,d). Dioclein also hyperpolarized vessels pre-contracted with phenylephrine, and iberiotoxin reversed dioclein-induced hyperpolarization (Figure 7e,f). The hyperpolarizing effect of dioclein was of long duration and was not recovered even after 5 – 15 min washing.
Figure 7.
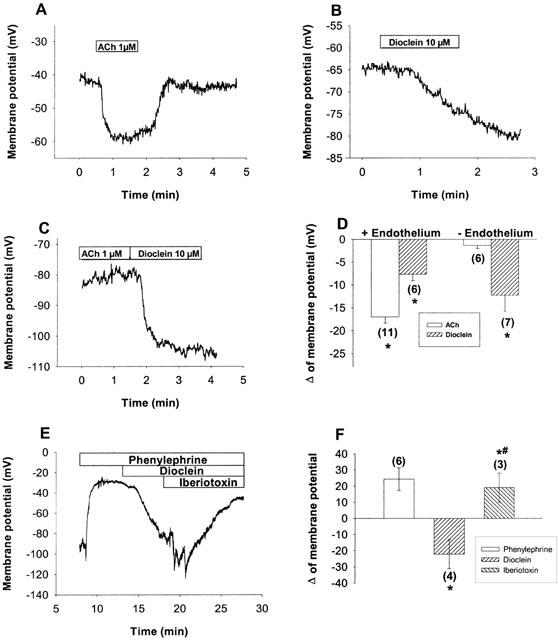
Changes in membrane potential of smooth muscle cells of resistance mesenteric arteries induced by acetylcholine and dioclein. The resting membrane potential of endothelium-containing and denuded vessels were −46.7±5 and −47±3.9 mV, respectively. Representative examples of the hyperpolarization induced by 1 μM ACh (a) or dioclein (b) in endothelium-containing vessels. While in endothelium-denuded vessels ACh no more induces hyperpolarizations, the hyperpolarizing effect of dioclein is not changed (c). Average values±s.e.mean of the hyperpolarizing effect of dioclein and ACh in endothelium-containing and denuded vessels (d). A representative example of hyperpolarization induced by dioclein in a cell pre-stimulated with phenylephrine and its reversion by iberiotoxin (e) and their respective average values±s.e.mean (f). The average values *P<0.05. # Statistically significant in relation to dioclein. (n) Number of experiments.
Discussion
In the present study we found that the new flavonoid dioclein had a marked hypotensive effect when injected into freely moving rats. As dioclein had little effect on HR and appears to have no negative inotropic effect (data not shown), dioclein-induced hypotension is most likely associated with diminution of peripheral resistance. In vitro, dioclein caused marked vasodilatation. These effects of dioclein were largely endothelium-independent and were inhibited by K+ channels blockers. In addition, dioclein induced significant membrane hyperpolarization even in the absence of a functional endothelium, which was reversed by iberiotoxin. These effects point to an important role of K+ channels activation of smooth muscle cells as the underlying mechanism implicated in the vasorelaxant effects of dioclein.
We chose to measure haemodynamic parameters in conscious freely moving rats so as to minimize the influence of anaesthesia and surgical stress (Smith & Hutchins, 1980; Fluckiger et al., 1985). On the other hand, we also used isolated preparations, which are more suitable to study specific effects, in the absence of neurohumoral influences. Administration of dioclein in conscious unrestrained normotensive rats lead to a marked hypotension, accompanied by a slight tachycardia (with the dose of 2.5 mg kg−1, i.v.) or no significant changes in heart rate (with 5 and 10 mg kg−1, i.v., respectively). In a previous work, we demonstrated that dioclein produced an endothelium/NO-dependent vasorelaxant effect in the rat aorta (Lemos et al., 1999). As endothelium-derived NO plays a major role in the regulation of blood pressure and vascular tone (Rees et al., 1989; Moncada et al., 1991) it was possible that the decrease in blood pressure induced by dioclein was due to NO release from the vascular endothelium. Nevertheless, in the presence of NO-synthase inhibition (L-NAME 20 mg kg−1, i.v.), hypotension induced by dioclein (2.5, 5 and 10 mg kg−1, i.v.) was similar to that induced by the flavonoid given alone. These results suggest that the hypotensive action of dioclein in conscious normotensive rats is independent of NO release from the vascular endothelium. In contrast to the effects on mean arterial pressure, in presence of NO-synthase inhibition, dioclein induced tachycardia for all tested doses (2.5, 5 and 10 mg kg−1, i.v.), indicating that changes in heart rate do not play any role in the hypotensive effect of the flavonoid.
To test the hypothesis that the flavonoid acts directly on resistance vessels, we used the model of small mesenteric arteries. We found that in resistance small mesenteric arteries dioclein also induced relaxation. Removal of endothelium or pre-treatment of vessels with the NO-synthase inhibitor L-NAME only produced a slight change in IC50 value for dioclein with no modification in Emax values. In addition, blockade of cyclo-oxygenase by indomethacin did not affect dioclein-induced relaxation. Therefore, it is clear that endothelium-derived vasorelaxant factors as NO or prostacyclins do not account for the vasorelaxant effect of dioclein in small mesenteric arteries.
It is well known that in this type of vessel there is an additional component to endothelium-dependent relaxation that has been attributed to the endothelium-derived hyperpolarizing factor (EDHF; Mombouli & Vanhoutte, 1997). Changing the bath from normal PSS containing 4.7 to 25 mM KCl is known to blunt the vasorelaxant effect of EDHF (Andriantsitohaina et al., 1996). When the vasorelaxant effect of dioclein was accessed in PSS solution containing 25 mM KCl vasorelaxation was not impaired although a shift to the right of the concentration-response curve to dioclein was seen. However, the IC50 value for dioclein in endothelium-denuded vessels in the presence of a PSS solution containing 25 mM KCl did not change, ruling out the participation of EDHF in the vasorelaxant effect of dioclein. Altogether, our results suggest that the response to dioclein is primarily due to a direct effect on vascular smooth muscle cells. Endothelium-dependent NO-induced vasorelaxation appears to play only a minor role.
White et al. (1996) suggested that the resistance vasculature, which is critical to the control of peripheral resistance, might not show the same functional alterations as those expressed in large conductance arteries. Thus, considering that in our previous study (Lemos et al., 1999) we employed aortic preparations which are conductance arteries (Magliola & Jones, 1999) and likely would contribute little to the decrease in peripheral resistance seen after dioclein, it is plausible to propose that the hypotensive effect produced by the flavonoid in conscious normotensive rats is, at least, secondary to a vasorelaxant effect on resistance vessels.
Vascular smooth muscle K+ channels are known to play an important role in the regulation of vascular tone (Nelson & Quayle, 1995; Jackson, 2000) and under the influence of vasoconstrictor stimuli, the regulation of K+ current through these channels may be important in determining the magnitude and duration of contraction. The evidence to suggest an effect of dioclein on K+ channels is presented below. The vasorelaxant effect of K+ channel openers is known to be extremely sensitive to the degree of cell membrane depolarization (Magnon et al., 1998). A reduction in potency of dioclein occurred when the vessels were exposed to a PSS solution containing 25 mM KCl and potency was virtually zero when the vessels were pre-contracted with 50 mM KCl. Also, the concentration-response curve to dioclein was shifted to the right by 10 mM TEA and 1 mM BaCl2, two unspecific K+ channel blockers. IC50 values for dioclein in the presence of BaCl2 were not different in vessels with or without endothelium. Finally, dioclein produced a hyperpolarization of resistance mesenteric arteries, which was independent on the presence of functional endothelium and was reversed by treatment with a K+ channel blocker. Together, these results strongly suggest the participation of smooth muscle K+ channels in the vasorelaxant effect of dioclein.
At least four different K+ channels have been identified in vascular smooth muscle of the microcirculation (Jackson, 1998; 2000): KATP channel, Kv channel, KCa channels and KIR channel. The effect of dioclein on phenylephrine responses was then evaluated in the presence of various K+ channel selective blockers to determine the role of each one in the dioclein-induced inhibition of vasoconstriction. Blockade of KATP or KIR with glybenclamide or 100 μM Ba2+, respectively, did not change vasorelaxant effect of dioclein. On the other hand, blockade of large-conductance KCa by charybdotoxin and iberiotoxin and Kv channels by 4-aminopyridine, respectively, strongly inhibited dioclein- induced vasorelaxation. Blockade of small-conductance KCa channels by apamin diminished Emax values for dioclein. Together, these findings suggest that dioclein vasorelaxant effect may be mediated via opening of smooth muscle KCa and Kv. However, iberiotoxin virtually abrogated the hyperpolarizations induced by dioclein, suggesting that activation of KCa channels appears to play a greater role in the vasorelaxant effect of dioclein. In addition, direct measurements of single channel activity using patch clamp techniques (inside-out configuration) in GH-3 cells showed a direct effect of dioclein on KCa but not on Kv channels (not shown). However, we cannot exclude the possibility of an indirect effect of dioclein on Kv or even on KCa channels through a signalling cascade.
As iberiotoxin did not completely inhibit vasorelaxation by dioclein and the effect of iberiotoxin plus 4-AP was not additive, we cannot exclude the possibility that mechanisms other than activation of K+ channels are contributing to the vasorelaxant effect of dioclein. However, our results clearly demonstrate a major role for BKca in the vasodilatory effects of dioclein.
In conclusion, the present study, using a combined in vivo and in vitro approach, demonstrates that dioclein lowers arterial pressure in normotensive rats through a decrease in peripheral vascular resistance. The vasorelaxant action of dioclein is mediated mainly by potassium channel opening and membrane hyperpolarization.
Acknowledgments
V.S. Lemos, S.F. Côrtes and M.M. Teixeira were financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and B.A. Rezende was supported by FAPEMIG (Fundação de Apoio a Pesquisa do Estado de Minas Gerais). This work was partially supported by grants from Pró-Reitoria de Pesquisa/UFMG.
Abbreviations
- ACh
acetylcholine
- ANOVA
two-way analysis of variance
- BKca
large conductance Ca2+-activated potassium channel
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulphonate
- DMSO
dimethyl sulphoxide
- Emax
maximal effect
- HR
heart rate
- IC50
inhibitory concentration 50%
- K+ channels
potassium channels
- KATP
ATP-sensitive potassium channel
- Kir
inward rectifier potassium channel
- Kv
voltage activated potassium channel
- L-NAME
NG-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
- PE
polyethylene
- PSS
physiological salt solution
- s.e.mean
standard error of the mean
- SKca
small conductance Ca2+-activated potassium channel
- TEA
tetraethylammonium
References
- ANDRIAMBELOSON E., MAGNIER C., HAAN-ARCHIPOFF G., LOBSTEIN A., ANTON R., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998;128:2324–2333. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRIANTSITOHAINA R., OKRUHLICOVA L., CÔRTES S.F., LAGAUD G.J.L., RANDRIAMBOAVONJY V., MÜLLER B., STOCLET J.C. Role of endothelial-nitric oxide in the response to angiotensin II of rat small mesenteric arteries. J. Vas. Res. 1996;33:386–394. doi: 10.1159/000159167. [DOI] [PubMed] [Google Scholar]
- AVIRAM M., FUHRMAN B. Polyphenolic flavonoids inhibit macrophage-mediated oxidation of LDL and attenuate atherogenesis. Atherosclerosis. 1998;137:S45–S50. doi: 10.1016/s0021-9150(97)00306-7. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYYA J., BATISTA J.S., ALMEIDA R.N. Dioclein, a flavanone from the roots of Dioclea grandiflora. Phytochemistry. 1995;38:277–278. [Google Scholar]
- BOUSSAIRI H., SASSARD J. Cardiovascular effects of basic fibroblast growth factor in rats. J. Cardiovasc. Pharmacol. 1994;23:99–102. doi: 10.1097/00005344-199401000-00013. [DOI] [PubMed] [Google Scholar]
- CÔRTES S.F., ANDRIANTSITOHAINA R., STOCLET J.C. Alterations of cyclo-oxygenase products and NO in responses to angiotensin II of resistance arteries from the spontaneously hypertensive rat. Br. J. Pharmacol. 1996;119:1635–1641. doi: 10.1111/j.1476-5381.1996.tb16083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUARTE J., VIZCAÍNO F.P., UTRILLA P., JIMÉNEZ J., TAMARGO J., ZARZUELO A. Vasodilatatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen. Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK D.F., HIRSCHFIELD S.L., COFFEY R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993;65:774–778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- FLUCKIGER J.P., SONNAY M., BOILLAT N., ATKINSON J. Attenuation of the barorreceptor reflex by general anaesthetic agent in the normotensive rat. Eur. J. Pharmacol. 1985;109:105–109. doi: 10.1016/0014-2999(85)90545-x. [DOI] [PubMed] [Google Scholar]
- HERRERA M.D., ZARZUELO A., JIMÉNEZ J., MARHUENDA E., DUARTE J. Effects of flavonoids on rat aortic smooth muscle contractility: structure-activity relationships. Gen. Pharmacol. 1996;27:273–277. doi: 10.1016/0306-3623(95)02010-1. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G.L., KROMHOUT D., ARAVANIS C., BLACKBURN H., BUZINA R., FIDANZA F., GIAMPAOLI S., JANSEN A., MENOTTI A., NEDELJKOVIC S., PEKKARINEN M., SIMIC B.S., TOSHIMA H., FESKENS E.J.M., HOLLMAN P.C.H., KATAN M.B. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. [PubMed] [Google Scholar]
- HERTOG M.G.L., FESKENS E.J.M., HOLLMAN P.C.H., KATAN M.B., KROMHOUT D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- JACKSON W.F. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON W.F. Potassium channels and regulation of microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- KNEKT P., JARVINEN R., REUNANEN A., MAATELA J. Flavonoid intake and coronary mortality in Finland: a cohort study. Br. Med. J. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., NABATA H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol. Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- LAWSON K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000;57:838–845. doi: 10.1046/j.1523-1755.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- LEMOS V.S., FREITAS M.R., MULLER B., LINO Y.D., QUEIROGA C.E.G., CÔRTES S.F. Dioclein, a new nitric oxide- and endothelium-dependent vasodilator flavonoid. Eur. J. Pharmacol. 1999;386:41–46. doi: 10.1016/s0014-2999(99)00747-5. [DOI] [PubMed] [Google Scholar]
- MAGLIOLA L., JONES A.W. Size dependence of functional alterations in mesenteric arteries from the aldosterone-salt hypertensive rat. J. Vasc. Res. 1999;36:404–414. doi: 10.1159/000025680. [DOI] [PubMed] [Google Scholar]
- MAGNON M., CALDERONE V., FLOCH A., CAVERO I. Influence of depolarization on vasorelaxant potency and efficacy of Ca2+ entry blockers, K+ channel openers, nitrate derivatives, salbutamol and papaverine in the aortic rings. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:452–463. doi: 10.1007/pl00005278. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor(s): updating the unknown. Trends Pharmacol. Sci. 1997;18:252–256. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MULDOON F.M., KRITCHEVSKY S.B. Flavonoids and heart disease: evidence of benefit still fragmentary. Br. Med. J. 1996;312:458–459. doi: 10.1136/bmj.312.7029.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W.S. Contractile properties of small arterial resistance vessels in spontaneously hypertensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., PATLAK J.B., WORLEY J.F., STANDEN N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA E.J., MEDEIROS I.A., MUKEERJEE R. Hypotensive and spasmolytic effects of normacusine B from Strychnos atlantica root. Phytomedicine. 1996;3:45–49. doi: 10.1016/S0944-7113(96)80009-2. [DOI] [PubMed] [Google Scholar]
- QUAST U. Do the K+ channels openers relax smooth muscle by opening K+ channels. Trends. Pharmacol. Sci. 1993;14:332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- QUAST U. Potassium channel openers: pharmacological and clinical aspects. Fundam. Clin. Pharmacol. 1992;6:279–293. doi: 10.1111/j.1472-8206.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., MONCADA S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE-EVANS C., MILLER N.J., PAGANGA G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- SEIGNEUR M., BONNET J., DORIAN B., BENCHIMOL D., DROUILLET F., GOUVERNEUR G., LARRUE J., CROCKETT R., BOISSEAU M., GAYON R., BRICAUS H. Effect of the consumption of alcohol, white wine, and red wine on platelets function and serum lipids. J. Appl. Cardiol. 1990;5:215–222. [Google Scholar]
- SMITH T.L., HUTCHINS P.M. Anaesthetic effects on hemodynamics of spontaneously hypertensive and Wistar-Kyoto rats. Am. J. Physiol. 1980;238:H539–H544. doi: 10.1152/ajpheart.1980.238.4.H539. [DOI] [PubMed] [Google Scholar]
- STANLEY L.L., MAZIER M.J.P. Potential explanations for the French paradox. Nutrition Res. 1999;19:3–15. [Google Scholar]
- TAYLOR M.S., BENOIT J.N. Effect of milrinone on small mesenteric artery vasoconstriction: role of K+ channels. Am. J. Physiol. 1999;277:G69–G78. doi: 10.1152/ajpgi.1999.277.1.G69. [DOI] [PubMed] [Google Scholar]
- WHITE R.M., RIVERA C.O., DAVISON C.B. Differential contribution of endothelial function to vascular reactivity in conduit and resistance arteries from deoxycorticosterone-salt hypertensive rats. Hypertension. 1996;27:1245–1253. doi: 10.1161/01.hyp.27.6.1245. [DOI] [PubMed] [Google Scholar]


