Abstract
The mechanism of α2 adrenoceptor-mediated vasoconstriction is unknown, but may involve activation of voltage-sensitive calcium channels, and/or a protein tyrosine kinase. Recently the extracellular signal-regulated kinase (Erk) cascade, often an event downstream of tyrosine kinase activation, has been shown to mediate vasoconstriction to a variety of agents. The aim of this present study was to determine the involvement of the Erk signal transduction cascade in α2 adrenoceptor-mediated vasoconstriction, and to confirm the involvement of activation of voltage-sensitive calcium channels, and protein tyrosine kinase.
Contractions to the α2 adrenoceptor agonist UK14304 in the porcine palmar lateral vein in vitro were reduced 70 – 80% by the MEK inhibitors PD98059 (10 – 50 μM) and U0126 (10 – 50 μM), indicating the involvement of the Erk signal transduction cascade. Immunoblots also demonstrated an increase in the phosphorylated (activated) form of Erk in palmar lateral vein segments after contraction with UK14304, which was inhibited by PD98059 and U0126.
The calcium channel blockers nifedipine and verapamil, or removal of extracellular calcium inhibited UK14304-induced contractions and phosphorylation of Erk, demonstrating the importance of an influx of extracellular calcium. UK14304-induced contractions were inhibited by PP2 (1 – 10 μM), a selective inhibitor of Src tyrosine kinases, but not by PP3, an inactive analogue. PP2 also prevented the phosphorylation of Erk by UK14304.
These data demonstrate that α2 adrenoceptor-mediated vasoconstriction in the porcine palmar lateral vein is dependent upon activation of the Erk signal transduction cascade, which is downstream of an influx of extracellular calcium, and activation of Src tyrosine kinases.
Keywords: Erk, α2 adrenoceptors, vasoconstriction, calcium influx, tyrosine kinase
Introduction
Noradrenaline and adrenaline produce vasoconstriction through both α1 and α2 adrenoceptors (see Ruffolo et al., 1993). Although the signal transduction mechanism behind α1 adrenoceptor-mediated vasoconstriction is well established, little is known about the mechanisms of α2 adrenoceptor-mediated vasoconstriction. α2 Adrenoceptors are Gi-protein coupled receptors, and, in general, activation of these receptors results in a decrease in intracellular adenosine 3′:5′-cyclic monophosphate (cyclic AMP) levels through inhibition of adenylyl cyclase (Bylund et al., 1994). An increase in intracellular cyclic AMP causes relaxation of vascular smooth muscle (Abe & Karaki, 1989; McDaniel et al., 1991; Yamagishi et al., 1994; Rembold & Chen, 1998). Therefore a reduction of cyclic AMP levels by activation of vascular α2 adrenoceptors would be expected to lead to an increase in blood vessel tone. This certainly appears to be the case when intracellular cyclic AMP levels are raised, for example, in the presence of forskolin (Roberts et al., 1998). However, direct α2 adrenoceptor-mediated vasoconstriction does not seem to be due to inhibition of cyclic AMP production. For example, in the porcine palmar lateral vein, activation of α2 adrenoceptors alone results in a large contraction, but does not decrease intracellular cyclic AMP levels (i.e. contraction occurs through an adenylyl cyclase-independent pathway; Wright et al., 1995). Although the identity of this adenylyl cyclase-independent pathway is unknown, α2-adrenoceptor-mediated vasoconstriction appears to be dependent upon an influx of extracellular calcium through voltage-sensitive calcium channels (McGrath et al., 1989; Dunn et al., 1991; Lepretre et al., 1994). Other possible signalling events include activation of protein tyrosine kinases (Jinsi & Deth, 1995). However, the relationship of these signal transduction events to each other is unclear.
α2 Adrenoceptors can stimulate the extracellular signal-regulated kinase (Erk) mitogen-activated protein (MAP) kinase signal transduction cascade in cells grown in culture (Della Rocca et al., 1997; Kribben et al., 1997). In the latter study, activation of Erk by endogenous α2 adrenoceptors was shown to be independent of adenylyl cyclase (Kribben et al., 1997). Although traditionally associated with gene transcription (Lopez-Ilasaca, 1998), recent studies in isolated blood vessels have demonstrated that the Erk signal transduction cascade can mediate vasoconstriction in response to a number of different agents (Katoch & Moreland, 1995; Dessy et al., 1998; Banes et al., 1999). Erk activation is often an event downstream of protein tyrosine kinases (Della Rocca et al., 1997), and in certain cell types, including vascular smooth muscle cells, activation of Erk is dependent upon an increase in intracellular calcium (Della Rocca et al., 1997; Seewald et al., 1998; Muthalif et al., 1998). Taking into account the previous observations that α2 adrenoceptor-mediated vasoconstriction is dependent upon an influx of extracellular calcium and activation of protein tyrosine kinases (McGrath et al., 1989; Dunn et al., 1991; Jinsi & Deth, 1995), the Erk signal transduction cascade is a possible pathway behind the α2 adrenoceptor-mediated, adenylyl cyclase-independent vasoconstriction. The aim of this present study was to explore this possibility, and to determine whether opening of calcium channels and activation of protein tyrosine kinases, as indicated in other studies, are part of the same pathway.
Methods
Isometric tension recordings
Porcine trotters were obtained from a local abattoir and transported to the laboratory on ice. Palmar lateral veins were dissected out and placed in Krebs – Henseleit buffer containing 2% Ficoll, which had been pre-gassed with 95% O2/5% CO2, and stored overnight at 4°C (see Wright et al., 1995). The following day veins were carefully cleaned of fat and connective tissue, dissected into 5 mm ring segments, and suspended in a 5 ml isolated tissue bath containing Krebs – Henseleit buffer maintained at 37°C and constantly gassed with 95% O2/5% CO2. The lower support was fixed and the upper support was connected to a force transducer (World Precision Instruments, Sarasota, FL, U.S.A.) linked to a MacLab data acquisition system (AD Instruments Ltd., Hastings, U.K.) via an amplifier. After a 20 min equilibration period, tension was applied to the tissue which was allowed to relax to a final resting tension of between 0.5 – 1.0 g wt. Before each experiment the tissues were contracted with 60 mM KCl, until the final two responses differed by less than 10%.
Effect of inhibitors on UK14304 responses
Tissues were incubated for 1 h with one of the following inhibitors: the MEK inhibitors PD98059 (1 – 50 μM), or U0126 (10 or 50 μM); the L-type calcium channel blockers nifedipine (0.1 – 50 μM), or verapamil (1 – 50 μM); the general protein tyrosine kinase inhibitor genistein (5 or 10 μM), or its inactive analogue daidzein (5 or 10 μM); the selective Src tyrosine kinase inhibitor PP2 (1 or 10 μM), or its inactive analogue PP3 (10 μM). Control tissues received just vehicle (0.1% DMSO for PD98059, U0126, genistein, daidzein, PP2, and PP3; 0.1% ethanol for nifedipine and verapamil). Cumulative concentration response curves to UK14304 (1 nM to 10 μM) were then performed. In experiments in which UK14304 response curves were performed in the absence of extracellular calcium, the Krebs – Henseleit buffer was replaced with calcium-free Krebs – Henseleit in which the calcium was replaced with 2 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 min before the UK14304 concentration-response curves were performed.
Effect of enzyme inhibitors on 60 mM KCl responses
Porcine palmar lateral vein segments were set up as above. Tissues were incubated with 50 μM PD98059, 50 μM U0126, 10 μM PP2, or 10 μM genistein, or vehicle control, for 1 h. KCl (60 mM) was then added to the tissues. Responses were expressed as a percentage of the response to 60 mM KCl obtained prior to incubation with the inhibitors.
Immunoblotting
Segments of porcine palmar lateral vein were set up in tissue baths as above, in the absence or presence of inhibitors. Tissues were then exposed to a maximal concentration of UK14304 (10 μM). Control tissues were not exposed to any compound (basal conditions). When the contractions to UK14304 reached a plateau (3 – 4 min after addition of the agonist), the segments were quickly removed from the tissue baths, and immediately frozen on dry ice. Frozen segments were then homogenized in ice-cold buffer (80 mM sodium β-glycerophosphate, 20 mM imidazole [pH 7.0], 1 mM dithiothreitol, 1 mM sodium fluoride, 500 μM 4-(2-aminoethyl)benzenesulphonyl fluoride (AEBSF), 1 μM trans-epoxysuccinyl-L-leucylamide-(4-guanidino) butane (E-64), 10 μg ml−1 aprotonin, 1 μM leupeptin, 500 μM EDTA). After removal of a sample for a protein assay, the homogenate was diluted 1 : 1 in 2×Laemmli sample suffer, and heated at 95°C for 5 min. Equal amounts of protein from each sample were separated on 10% SDS – PAGE gels, and then transferred onto nitrocellulose membranes by Western blotting. After incubating in blocking solution (5% powdered milk in tris-buffered saline containing 0.1% Tween-20 (TBS-T)), nitrocellulose blots were incubated overnight at 4°C with an antibody that recognises the double phosphorylated (activated) forms of both isoforms of Erk (Erk1 and 2) (New England Biolabs). After washing in TBS-T, the blots were incubated with the appropriate, hydrogen peroxidase-conjugated secondary antibody. Proteins were visualized using the ECl system (Amersham Life Sciences). Blots were then stripped of antibodies by immersing the blot in a solution containing 100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl (pH 6.7), and incubating at 50°C with gentle shaking for 30 min. After washing in TBS-T, and blocking as above, blots were re-probed with an antibody against total Erk (New England Biolabs). Bands were visualized as before. Both phosphorylated and total Erk bands were analysed by densitometry.
Drugs
5-bromo-6-[2-imidazolin-2-ylamine]-quinoxaline bitartrate (UK14304), (Pfizer); 2-amino-3-methoxyflavone (PD98059), (Calbiochem); genistein (Alexis Chemicals); daidzein (Alexis Chemicals); nifedipine (Alexis Chemicals); 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) (Calbiochem); 4-amino-7-phenylpyrazolol[3,4]pyrimidine (PP3) (Calbiochem); 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126) (Tocris). All other compounds were obtained from Sigma, Poole, U.K.
Data analysis
Contractile responses were expressed as a percentage of the response to 60 mM KCl, and results expressed as mean±s.e.mean. Bands obtained by immunoblotting were analysed by densitometry. Multiple comparisons between treatment groups were performed using analysis of the variance (ANOVA) followed by a Bonferroni test.
Results
Role of the Erk MAP kinase cascade in α2 adrenoceptor-mediated contractions
The MEK inhibitor PD98059 inhibited the responses to UK14304 in a concentration-dependent manner (Figure 1a). Although 1 μM PD98059 had no significant effect on UK14304 responses, both 10 and 50 μM PD98059 significantly inhibited the vasoconstriction (response to 3 μM UK14304 22.2±8.9% of 60 mM KCl response in the presence of 50 μM PD98059 compared to 97.4±13.1% in the presence of 0.1% DMSO only; P<0.005, ANOVA followed by a Bonferroni test, n=8. The response to 60 mM KCl in porcine palmar lateral vein segments was 3.9±0.1 g wt, n=73). The effect of U0126 (another, structurally dissimilar inhibitor of MEK) on UK14304 responses was also studied. Like PD98059, U0126 (10 or 50 μM) caused significant inhibition of UK14304-induced contractions in segments of porcine palmar lateral vein (Figure 1b).
Figure 1.
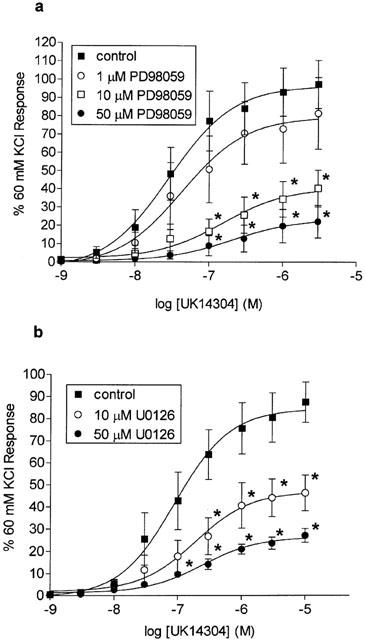
Log concentration-response curves to UK14304 in segments of porcine palmar lateral vein expressed as per cent 60 mM KCl response, and shown as means±s.e.mean. (a) Responses in the absence (control), or presence of 1, 10 or 50 μM PD98059. *Indicates significant difference from control responses, P<0.01, ANOVA followed by Bonferroni test, n=8. (b) Responses in the absence (control) or presence of 10 or 50 μM U0126. *Indicates significant difference from control responses, P<0.01, ANOVA followed by Bonferroni test, n=8.
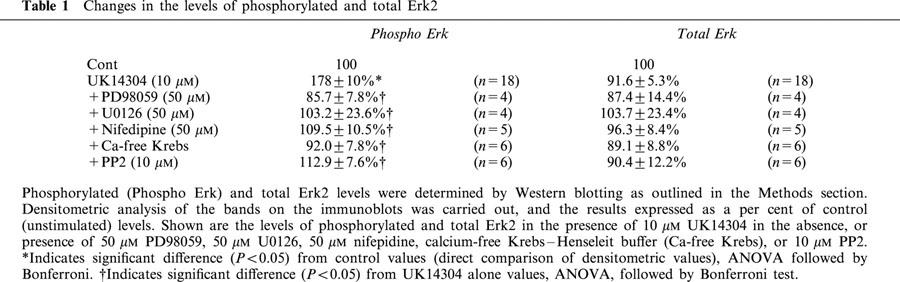
Segments of porcine palmar lateral vein were contracted with a single concentration of UK14304 (10 μM), and then rapidly frozen, homogenized, and the proteins separated by SDS – PAGE. The proteins were transferred on to nitrocellulose membranes, and the nitrocellulose blots probed with an antibody against the double phosphorylated (activated) form of Erk. Development of the immunoblots revealed just two bands of around 40 – 45 kDa in size (see Figure 2). The lower band, equivalent to the p42 isoform of Erk (Erk2), was a lot stronger than the top band, equivalent to the p44 isoform of Erk (Erk1). Densitometric analysis of the bands revealed that there was a significant increase in the amount of phosphorylated Erk1 and Erk2 in UK14304-contracted palmar lateral vein segments compared to control tissues (tissue segments not stimulated with UK14304; see Figure 2). Table 1 shows the densities of the Erk2 bands expressed as a per cent of control values. The densities of the Erk1 bands in the presence of UK14304 were 141.7±10.4% of control levels (n=16). After the antibodies had been stripped off the nitrocellulose, the blots were re-probed with an antibody against total Erk. There was no apparent difference in the levels of total Erk between control and UK14304-stimulated palmar lateral vein segments. The densities of total Erk2 bands expressed as a per cent of control values are shown in Table 1. The density of total Erk1 bands in the presence of UK14304 was 102.7±2.2% of control levels (n=14).
Figure 2.
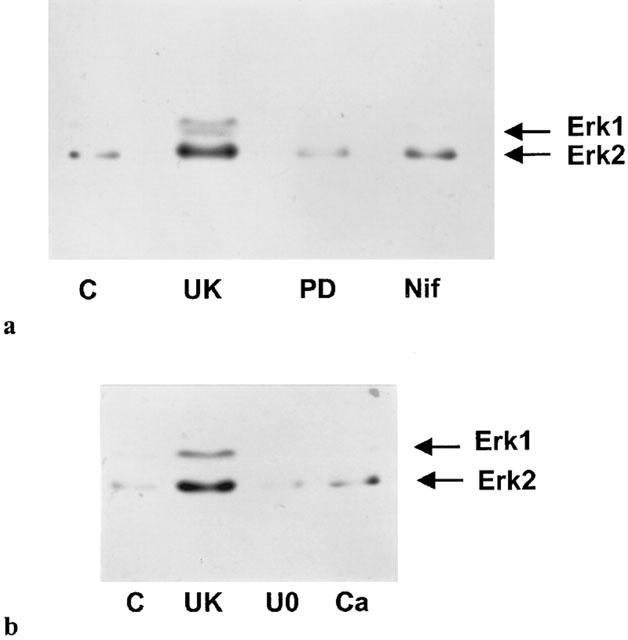
(a and b) Representative immunoblots of porcine palmar lateral vein proteins separated by SDS – PAGE, transferred onto nitrocellulose membranes, and incubated with a primary antibody against phosphorylated Erk1/2. Segments of porcine palmar lateral vein were set up in a tissue bath and contracted with 10 μM UK14304 in the absence (UK) or presence of 50 μM PD98059 (PD), 50 μM nifedipine (Nif), 50 μM U0126 (U0), or calcium-free Krebs – Henseleit buffer (Ca). Non-stimulated segments kept under basal conditions were also obtained (C). Tissues were rapidly frozen, homogenized, and then subjected to SDS – PAGE.
Table 1.
Changes in the levels of phosphorylated and total Erk2
Role of extracellular calcium on UK14304 responses
The L-type calcium channel blocker verapamil (1 – 50 μM) inhibited UK14304 responses in the porcine palmar lateral vein in a concentration dependent manner (Figure 3a). Nifedipine also inhibited UK14304 responses, with 0.1 and 1 μM nifedipine causing significant inhibition. However, there was no difference in the degree of inhibition obtained with these two concentrations (Figure 3b). Higher concentrations of nifedipine (10 – 50 μM) produced further inhibition of the UK14304 responses, with 50 μM nifedipine causing around 70% inhibition (Figure 3b). Replacement of normal Krebs – Henseleit buffer with Ca2+-free Krebs – Henseleit buffer 5 min before UK14304 response curves were performed almost completely inhibited the responses to UK14304 (Figure 3b).
Figure 3.
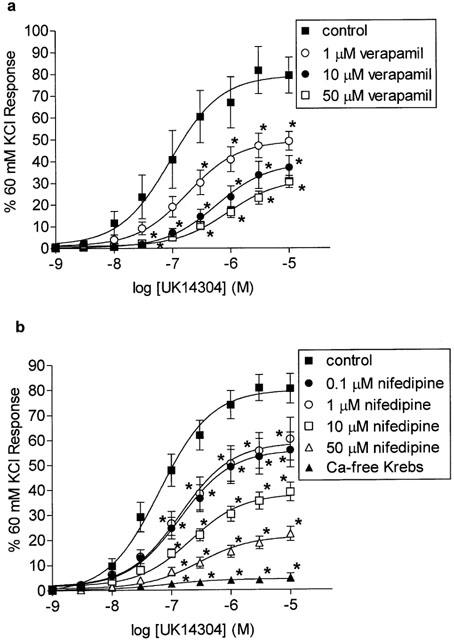
(a) Log concentration-response curves to UK14304 in segments of porcine palmar lateral vein in the absence (control), or presence of 1, 10 or 50 μM verapamil. Responses are expressed as per cent 60 mM KCl response, and are means±s.e.mean of 10 experiments. *Indicates significant difference from control responses (P<0.01, ANOVA followed by Bonferroni test). (b) Log concentration-response curves to UK14304 in segments of porcine palmar lateral vein in the absence (control), or presence of 0.1, 1, 10 or 50 μM nifedipine, or in the presence of calcium-fee Krebs – Henseleit buffer (Ca-free Krebs). Responses are expressed as per cent 60 mM KCl response, and are means±s.e.mean of 6 – 18 experiments. *Indicates significant difference from control responses (P<0.005, ANOVA followed by Bonferroni test).
Effect of protein tyrosine kinase inhibitors on UK14304 responses
Neither the non-specific protein tyrosine kinase inhibitor genistein nor its inactive analogue daidzein inhibited UK14304 responses when used at a concentration of 5 μM (Figure 4a). However, when the concentrations were increased to 10 μM, both genistein and daidzein inhibited the UK14304 responses in the palmar lateral vein (Figure 4b), with genistein causing a slightly greater inhibition.
Figure 4.
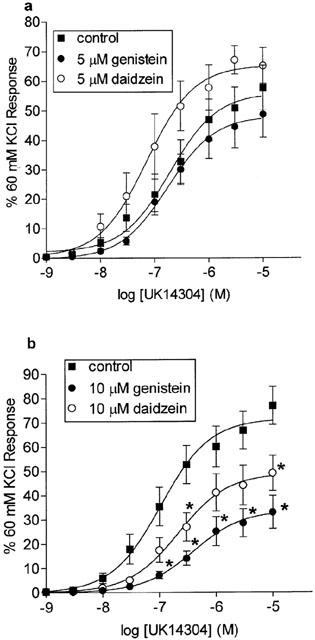
Log concentration-response curves to UK14304 in segments of porcine palmar lateral vein expressed as per cent 60 mM KCl response, and shown as means±s.e.mean. (a) Responses in the absence (control), or presence of 5 μM genistein, or 5 μM daidzein (n=8). (b) Responses in the absence (control) or presence of 10 μM genistein or 10 μM daidzein. *Indicates significant difference from control responses, P<0.01, ANOVA followed by Bonferroni test, n=9.
PP2 (1 and 10 μM), a selective inhibitor of the Src family of tyrosine kinases (Hanke et al., 1996), produced a significant inhibition of UK14304 responses in a concentration-dependent manner (Figure 5). On the other hand, PP3 (10 μM), which is a compound with a similar structure to PP2, but does not inhibit Src kinases, had no effect on UK14304 responses (Figure 5).
Figure 5.
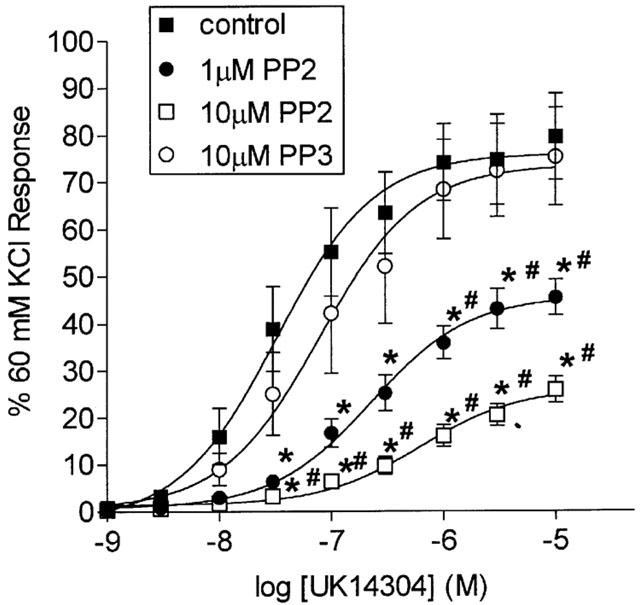
Log concentration-response curves to UK14304 in segments of porcine palmar lateral vein in the absence (control), or presence of 1 or 10 μM PP2, or 10 μM PP3. Responses are expressed as per cent 60 mM KCl response, and are means±s.e.mean of eight experiments. *Indicates significant difference from control responses (P<0.01, ANOVA followed by Bonferroni test). #Indicates significant difference from responses in the presence of PP3 (P<0.01 ANOVA followed by Bonferroni test).
Effect of inhibitors on phosphorylation of Erk
The increase in phosphorylation of Erk1 and Erk2 observed in the presence of 10 μM UK14304 was inhibited by pre-incubation of the tissues with 50 μM PD98059, 50 μM U0126, 50 μM nifedipine, calcium-free Krebs – Henseleit buffer, or 10 μM PP2 (see Figure 2). The levels of phosphoryation of Erk2 under these conditions are shown in Table 1. Total Erk levels were unchanged in the presence of the inhibitors (Table 1).
Effect of enzyme inhibitors on 60 mM KCl responses
PD98059 (50 μM), PP2 (10 μM) and genistein (10 μM) had no effect on the contractile responses to 60 mM KCl in the porcine palmar lateral vein (102.6±7.0% (n=4); 98.2±5.8% (n=4); and 92.9±4.1% (n=6) of control responses to 60 mM KCl, respectively). On the other hand, the response to KCl in the presence of 50 μM U0126 was slightly reduced (76.5±6.5% (n=6) of control response to 60 mM KCl; P<0.005, ANOVA followed by Bonferroni).
Discussion
The aim of this present study was to determine whether the Erk MAP kinase signal transduction cascade is involved in the α2 adrenoceptor-mediated, adenylyl cyclase-independent mechanism of vasoconstriction. The Erk signal transduction cascade has traditionally only been associated with growth related processes such as activation of gene transcription (Lopez-Ilasaca, 1998). However, recent studies have shown that this pathway can be involved in other, seemingly unrelated processes, such as vasoconstriction (Katoch & Moreland, 1995; Dessy et al., 1998; Banes et al., 1999). As α2 adrenoceptors have been shown to stimulate the Erk signal transduction cascade in cells grown in culture (Della Rocca et al., 1997; Kribben et al., 1997), it is possible that a similar activation in blood vessels mediates vasoconstriction.
The selective α2 adrenoceptor agonist UK14304 (Cambridge, 1981) produced a large, concentration-dependent contraction of the porcine palmar lateral vein. These contractions were inhibited by both PD98059, and U0126. These compounds are structurally dissimilar, but are both selective inhibitors of MEK (Alessi et al., 1995; Favata et al., 1998). MEK phosphorylates Erk MAP kinases on both a threonine and a tyrosine residue. This dual phosphorylation by MEK is the only way in which Erk is activated. MEK appears to be a dedicated kinase in that no other substrate has yet been identified. Therefore, inhibiting the Erk signal transduction cascade at MEK prevents activation of Erk itself (Cobb & Goldsmith, 1995). The selectivity of PD98059, in particular, for MEK is indicated by the fact that 50 μM of the compound has little effect on the activities of a wide range of other purified protein kinases in vitro (Alessi et al., 1995). U0126 also shows a great deal of selectivity for MEK over other kinases (Favata et al., 1998). These compounds are therefore useful tools for investigating the Erk signal transduction cascade. The degree of inhibition of the UK14304 response in the porcine palmar lateral vein by these agents (approximately 70 – 80% inhibition of the response to 3 μM UK14304 by 50 μM PD98059 or 50 μM U0126) suggests that the Erk signal transduction cascade is a major pathway involved in α2 adrenoceptor-mediated vasoconstriction. This contrasts with other receptor-induced vasoconstriction such as 5-HT2A and α1-adrenoceptor-mediated vasoconstriction in which the Erk signal transduction cascade appears to be a relatively minor mechanism (Dessy et al., 1998; Florian & Watts, 1998).
Further evidence for the involvement of Erk in the α2 adrenoceptor-mediated vasoconstriction comes from immunoblotting experiments. As mentioned above, Erk is activated after it has been phosphorylated on both a threonine and a tyrosine residue. Using an antibody against these dual phosphorylation sites it was shown that the degree of phosphorylation of both isoforms of Erk (Erk1 and Erk2) in porcine palmar lateral vein segments is increased after contraction with UK14304. Total Erk levels appear to be unaffected, indicating that α2 adrenoceptor-mediated vasoconstriction is associated with an increase in Erk activation. Furthermore, phosphorylation of Erk1 and Erk2 was inhibited in tissues pre-incubated with either PD98059 or U0126 demonstrating that these compounds do prevent activation of Erk.
A recent study suggested that PD98059 might have non-specific effects on voltage sensitive calcium channels as it inhibited KCl-induced vasoconstriction in rat middle cerebral arteries (Lagaud et al., 1999). As the α2 adrenoceptor-mediated response is sensitive to inhibition by voltage-sensitive calcium channel blockers, the inhibition of the vasoconstriction seen in the presence of PD98059 could be due to inhibition of calcium influx, rather than inhibition of MEK. However, 50 μM PD98059, which caused a large inhibition of the UK14304 response, had no effect on the 60 mM KCl-induced contraction in the porcine palmar lateral vein suggesting that the effects of PD98059 in this tissue are not due to non-specific inhibition of calcium channels. Further evidence in support of this comes from a study of calcium currents stimulated by platelet-derived growth factor in rabbit colonic smooth muscle cells. PD98059 (30 μM) had no effect on these calcium currents indicating that it does not block calcium channels (Hu et al., 1998). The fact that PD98059 also prevented the phosphorylation of Erk (which is mediated through activation of MEK) is further evidence that this compound is inhibiting the Erk MAP kinase signal transduction cascade.
Although PD98059 had no effect on KCl-induced contractions, 50 μM U0126 did cause a slight inhibition of the 60 mM KCl response, although this was only minor compared to the inhibition of the UK14304 response. U0126 has also been shown to inhibit KCl-induced vasoconstriction in rat thoracic aorta, although this was not thought to be due to calcium channel blockade (Banes et al., 1999). In swine carotid artery, KCl can lead to phosphorylation of Erk, with a time course similar to that for KCl-induced contractions (Katoch & Moreland, 1995). This could explain the effects of U0126 on KCl-induced contractions of the porcine palmar lateral vein, although this does not tie in with a lack of effect with PD98059. However, U0126 is able to inhibit both isoforms of MEK (MEK1 and MEK2) (Favata et al., 1998), whereas PD98059 shows selectivity for MEK1 over MEK2 (Alessi et al., 1995). If KCl induces activation of Erk through activation of MEK2 in the porcine palmar lateral vein, the difference in the selectivity of the compounds for MEK 1 and 2 could explain the difference in the effects against the KCl responses. Again, the fact that U0126 prevented the phosphorylation of Erk by UK14304 indicates that this compound is inhibiting the Erk MAP kinase signal transduction cascade.
The contractions to UK14304 in the porcine palmar lateral vein were also inhibited by nifedipine and verapamil, structurally dissimilar L-type calcium channel blockers (Fleckenstein, 1977), in keeping with previous studies using other blood vessels (McGrath et al., 1989; Dunn et al., 1991). However, a degree of caution must be used with verapamil as this compound has been has been shown to bind to α2 adrenoceptors (Motulsky et al., 1983) and could, therefore, be acting as an antagonist at these receptors. On the other hand, nifedipine does not bind to α2 adrenoceptors (Motulsky et al., 1983), and so the inhibition of the responses observed in the presence of this compound is unlikely to be due to antagonism at α2 adrenoceptors. Interestingly, although nifedipine at 0.1 μM caused significant inhibition of the UK14304 response, 1 μM nifedipine had no further effect. However, increasing the concentration of nifedipine to 10 and 50 μM did reduce the response further. This suggests that nifedipine may be acting at another set of channels at the higher concentrations. In rat mesenteric arteries pre-contracted with the α1 adrenoceptor agonist methoxamine, nifedipine concentration-relaxation curves showed a similar effect, suggesting two components (Sarsero et al., 1998). One possible explanation for this is that nifedipine at the higher concentrations inhibits T-type, voltage-sensitive calcium channels. Nifedipine has been shown to inhibit T-type calcium channels in neuronal cells (Akaike et al., 1989; Wu et al., 1992), although this effect seems to be dependent upon nifedipine being dissolved in DMSO, not ethanol (Wu et al., 1992). Although in the rat mesenteric artery study nifedipine was dissolved in DMSO (Sarsero et al., 1998), in this present study nifedipine was dissolved in ethanol. Whichever voltage-sensitive calcium channel is involved, what is clear is that the α2 adrenoceptor responses are extremely dependent upon an influx of extracellular calcium as the responses to UK14304 were almost completely inhibited by removal of extracellular calcium.
Previously it has been shown that the general protein tyrosine kinase inhibitor genistein can inhibit α2 adrenoceptor-mediated vasoconstriction in rabbit saphenous vein (Jinsi & Deth, 1995) and rat aorta (Jinsi et al., 1996), with near complete inhibition with 100 μM genistein. In this present study, genistein at 10 μM, but not at 5 μM, caused significant inhibition of UK14304-induced contractions in the porcine palmar lateral vein. However, daidzein, which is an inactive or weak analogue of genistein, also caused a significant inhibition of UK14304-induced contractions at the same concentration (10 μM). Daidzein is thought to be unable to inhibit protein tyrosine kinases, but shares other, non-tyrosine kinase mediated effects with genistein (Higashi & Ogawara, 1992; Paillart et al., 1997). One non-tyrosine kinase effect of genistein and daidzein is the inhibition of the L-type calcium channel current in rat ventricular cells (Yokoshiki et al., 1996), which could explain the inhibition of the UK14304-response in the porcine palmar lateral vein. On the other hand, 10 μM genistein had no effect on KCl-induced contractions in the porcine palmar lateral vein, which does not indicate an effect on voltage-sensitive calcium channels. In the studies carried out by Jinsi and colleagues, daidzein was not used as a non-selective control (Jinsi & Deth, 1995; Jinsi et al., 1996). Furthermore, 100 μM genistein inhibited the response to both 60 mM KCl, and the L-type calcium channel opener BAYK8644 in the rat aorta (Jinsi et al., 1996) which could be explained by the ability of genistein to inhibit the L-type calcium channel current (Yokoshiki et al., 1996). Therefore, at least part of the inhibition seen with genistein in the studies by Jinsi and colleagues and this present study could be due to non-tyrosine kinase effects.
An involvement of protein tyrosine kinases in the α2 adrenoceptor-mediated vasoconstriction in the porcine palmar lateral vein cannot be deduced from the experiments using genistein. However, evidence for the involvement of the Src family of tyrosine kinases comes from experiments using PP2, a specific inhibitor of this family of tyrosine kinases (Hanke et al., 1996). PP2 (1 or 10 μM) caused a concentration-dependent inhibition of UK14304-induced contractions in the porcine palmar lateral vein. Furthermore, an inactive structural analogue of PP2, PP3 (10 μM), had no effect on UK14304 responses. Src tyrosine kinases have been shown to mediate rabbit smooth muscle cell contraction (Ibitayo et al., 1998), and have recently been implicated in 5-HT2A-mediated vasoconstriction (Banes et al., 1999). Also, Src kinases have been shown to be involved in Gi protein-mediated Erk activation (Igishi & Gutkind, 1998) suggesting that a Src kinase could be an upstream component of the Erk cascade involved in α2 adrenoceptor-mediated vasoconstriction. In fact, 10 μM PP2 prevented the UK14304-induced increase in Erk phosphorylation indicating that this is the case. The relationship of Src kinase to calcium influx, however, is not clear. In rabbit colonic smooth muscle cells, Src kinase activity appears to be calcium dependent (Ibitayo et al., 1998). On the other hand, Src kinase has been shown to increase voltage-sensitive calcium channel currents in vascular smooth muscle cells and so could be upstream of calcium influx instead (Wijetunge & Hughes, 1995). The α2 adrenoceptor-mediated activation of Erk in the porcine palmar lateral vein appears to be dependent upon influx of extracellular calcium as phosphorylation of Erk was prevented in the presence of nifedipine, or in the absence of extracellular calcium. Therefore, both the influx of calcium, and activation of Src tyrosine kinase seem to be events upstream of the activation of Erk, although further work is required to determine their relationship to one another.
This study has demonstrated an involvement of the Erk signal transduction cascade in the α2 adrenoceptor-mediated, adenylyl cyclase-independent vasoconstriction of the porcine palmar lateral vein. The Erk signal transduction cascade has also been shown to be involved in vasoconstriction mediated by histamine, α1 adrenoceptors, and 5-HT2A (Katoch & Moreland, 1995; Dessy et al., 1998; Banes et al., 1999). However, there are major differences between the receptor systems. The Erk signal transduction cascade is a relatively minor mechanism of α1 adrenoceptor- and 5-HT2A receptor-mediated vasoconstriction. It only mediates the minor, calcium-independent phase of α1 adrenoceptor-mediated vasoconstriction (Dessy et al., 1998), and only seems to account for around 20% of the 5-HT2A receptor-mediated vasoconstriction (Florian & Watts, 1998). On the other hand, as shown in this present study, inhibition of the Erk signal transduction cascade reduced α2 adrenoceptor-mediated vasoconstriction by 70 – 80% suggesting it is a major mediator of this response. Further differences include the role of calcium influx. Influx of calcium appears to be independent of the Erk cascade in α1 adrenoceptor- and 5-HT2A receptor-mediated vasoconstriction (Dessy et al., 1998; Florian & Watts, 1998), whereas influx of extracellular calcium appears to be necessary for α2 adrenoceptor activation of the Erk cascade.
In summary, α2 adrenoceptor-mediated vasoconstriction in the porcine palmar lateral vein is dependent upon activation of the Erk signal transduction cascade. Activation of Erk is dependent upon an influx of extracellular calcium, and activation of Src tyrosine kinase. However, further work is required to understand the exact mechanism of Erk activation, and how Erk activation results in contraction of the blood vessel.
Acknowledgments
This study was supported by The Wellcome Trust. Porcine tissues were obtained from G. Woods & Sons Ltd. abattoir (Clipstone, Nottinghamshire).
Abbreviations
- AEBSF
4-(2-aminoethyl)benzenesulphonyl fluoride
- ANOVA
analysis of the variance
- cyclic AMP
adenosine 3′:5′-cyclic monophosphate
- E-64
trans-epoxysuccinyl-L-leucylamide-(4-guanidino) butane
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- Erk
extracellular signal-regulated kinase
- MAP kinase
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- TBS-T
tris-buffered saline containing 0.1% Tween-20
References
- ABE A., KARAKI H. Effect of forskolin on cytosolic Ca++ level and contraction in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1989;249:895–900. [PubMed] [Google Scholar]
- AKAIKE N., KOSTYUK P.G., OSIPCHUK Y.V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J. Physiol. 1989;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD 098059 is a specific inhibitor of the acitvation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- BANES A., FLORIAN J.A., WATTS S.W. Mechanisms of 5-hydroxytryptamine2A receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1999;291:1179–1187. [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBURG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R., TRENDELENBURG U. International Union of Pharmacology nomenclature on adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- CAMBRIDGE D. UK-14304, a potent and selective α2-agonist for the characterisation of α-adrenoceptor subtypes. Eur. J. Pharmacol. 1981;72:413–415. doi: 10.1016/0014-2999(81)90588-4. [DOI] [PubMed] [Google Scholar]
- COBB M.H., GOLDSMITH E.J. How MAP kinases are regulated. J. Biol. Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- DELLA ROCCA G.J., VAN BIESEN T., DAAKA Y., LUTTRELL D.K., LUTTRELL L.M., LEFKOVITZ R.J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. J. Biol. Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- DESSY C., KIM I., SOUGNEZ C.L., LAPORTE R., MORGAN K.G. A role for MAP kinase in differentiated smooth muscle contraction evoked by α-adrenoceptor stimulation. Am. J. Physiol. 1998;275:C1081–C1086. doi: 10.1152/ajpcell.1998.275.4.C1081. [DOI] [PubMed] [Google Scholar]
- DUNN W.R., DALY C.J., MCGRATH J.C., WILSON V.G. Effects of nifedipine on α2-adrenoceptor-mediated contractions in several isolated blood vessels from the rabbit. Br. J. Pharmacol. 1991;103:1493–1499. doi: 10.1111/j.1476-5381.1991.tb09816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVATA M.F., HORIUCHI K.Y., MANOS E.J., DAULERIO A.J., STRADLEY D.A., FEESER W.S., VAN DYK D.E., PITTS W.J., EARL R.A., HOBBS F., COPELAND R.A., MAGOLDA R.L., SCHERLE P.A., TRZASKOS J.M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- FLECKENSTEIN A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Ann. Rev. Pharmacol. Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- FLORIAN J.A., WATTS S.W. Integration of mitogen-activated protein kinase kinase activation in vascular 5-hydroxytryptamine2A receptor signal transduction. J. Pharmacol. Exp. Ther. 1998;284:346–355. [PubMed] [Google Scholar]
- HANKE J.H., GARDNER J.P., DOW R.L., CHANGELIAN P.S., BRISSETTE W.H., WERINGER E.J., POLLOK B.A., CONNELLY P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- HIGASHI K., OGAWARA H. Effects of isoflavone compounds on the activation of phospholipase C. Chem. Pharm. Bull. 1992;40:157–160. doi: 10.1248/cpb.40.157. [DOI] [PubMed] [Google Scholar]
- HU X-Q, , SINGH N., MUKHOPADHYAY D., AKBARALI H.I. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J. Biol. Chem. 1998;273:5337–5342. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- IBITAYO A.I., TSUNODA Y., NOZU F., OWYANG C., BITAR K.N. Src kinase and PI 3-kinase as a transduction pathway in ceramide-induced contraction of colonic smooth muscle. Am. J. Physiol. 1998;275:G705–G711. doi: 10.1152/ajpgi.1998.275.4.G705. [DOI] [PubMed] [Google Scholar]
- IGISHI T., GUTKIND J.S. Tyrosine kinases of the Src family participate in signaling to MAP kinase from both G(q) and G(i)-coupled receptors. Biochem. Biophys. Res. Commun. 1998;244:5–10. doi: 10.1006/bbrc.1998.8208. [DOI] [PubMed] [Google Scholar]
- JINSI A., DETH R.C. α2-Adrenoceptor-mediated vasoconstriction requires a tyrosine kinase. Eur. J. Pharmacol. 1995;277:29–34. doi: 10.1016/0014-2999(95)00053-n. [DOI] [PubMed] [Google Scholar]
- JINSI A., PARADISE J., DETH R.C. A Tyrosine kinase regulates α-adrenoceptor-stimulated contraction and phospholipase D activation in the rat aorta. Eur. J. Pharmacol. 1996;302:183–190. doi: 10.1016/0014-2999(96)00049-0. [DOI] [PubMed] [Google Scholar]
- KATOCH S., MORELAND R.S. Agonist and membrane depolarization induced activation of MAP kinase in the swine carotid artery. Am. J. Physiol. 1995;269:H222–H229. doi: 10.1152/ajpheart.1995.269.1.H222. [DOI] [PubMed] [Google Scholar]
- KRIBBEN A., HERGET-ROSENTHAL S., LANGE B., ERDBRUGGER W., PHILIPP T., MICHEL M.C. α2-Adrenoceptors in opossum kidney cells couple to stimulation of mitogen-activated protein kinase independently of adenylyl cyclase. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:225–232. doi: 10.1007/pl00005045. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J.L., LAM E., LUI A., VAN BREEMEN C., LAHER I. Nonspecific inhibition of myogenic tone by PD98059, a MEK1 inhibitor, in rat middle cerebral arteries. Biochem. Biophys. Res. Commun. 1999;257:523–527. doi: 10.1006/bbrc.1999.0350. [DOI] [PubMed] [Google Scholar]
- LEPRETRE N., MIRONNEAU J., MOREL J.-L. Both α1A- and α2A-adrenoceptor subtypes stimulate voltage-operated L-type calcium channels in rat portal vein myocytes. J. Biol. Chem. 1994;269:29546–29552. [PubMed] [Google Scholar]
- LOPEZ-ILASACA M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem. Pharmacol. 1998;56:269–277. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- MCDANIEL N.L., REMBOLD C.M., RICHARD H.M., MURPHY R.A. Cyclic AMP relaxes swine arterial smooth muscle predominantly by decreasing cell Ca2+ concentration. J. Physiol. 1991;439:147–160. doi: 10.1113/jphysiol.1991.sp018661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGRATH J.C., BROWN C.M., WILSON V.G. α-Adrenoceptors: a critical review. Med. Res. Rev. 1989;9:407–535. doi: 10.1002/med.2610090403. [DOI] [PubMed] [Google Scholar]
- MOTULSKY H.J., SNAVELY M.D., HUGHES R.J., INSEL P.A. Interaction of verapamil and other calcium channel blockers with α1- and α2-adrenergic receptors. Circ. Res. 1983;52:226–231. doi: 10.1161/01.res.52.2.226. [DOI] [PubMed] [Google Scholar]
- MUTHALIF M.M., BENTER I.F., KARZOUN N., FATIMA S., HARPER J., UDDIN M.R., MALIK K.U. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAILLART C., CARLIER E., GUEDIN D., DARGENT B., COURAUD F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J. Pharmacol. Exp. Therap. 1997;280:521–526. [PubMed] [Google Scholar]
- REMBOLD C.M., CHEN X.-L. Mechanisms responsible for forskolin-induced relaxation of rat tail artery. Hypertension. 1998;31:872–877. doi: 10.1161/01.hyp.31.3.872. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R., NICHOLS A.J., STADEL J.M., HIEBLE J.P. Pharmacologic and therapeutic applications of α2-adrenoceptor subtypes. Ann. Rev. Pharmacol. Toxicol. 1993;32:243–279. doi: 10.1146/annurev.pa.33.040193.001331. [DOI] [PubMed] [Google Scholar]
- ROBERTS R.E., TOMLINSON A.E., KENDALL D.A., WILSON V.G. α2-Adrenoceptor-mediated contractions of the porcine isolated ear artery: evidence for a cyclic AMP-dependent and a cyclic AMP-independent mechanism. Br. J. Pharmacol. 1998;124:1107–1114. doi: 10.1038/sj.bjp.0701935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARSERO D., FUJIWARA T., MOLENAAR P., ANGUS J.A. Human vascular to cardiac tissue selectivity of L- and T-type calcium channel antagonists. Br. J. Pharmacol. 1998;125:109–119. doi: 10.1038/sj.bjp.0702045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEEWALD S., SEUL C., KETTENHOFEN R., BOKEMEYER D., KO Y., VETTER H., SACHINIDIS A. Role of mitogen-activated protein kinase in the angiotensin II-induced DNA synthesis in vascular smooth muscle cells. Hypertension. 1998;31:1151–1156. doi: 10.1161/01.hyp.31.5.1151. [DOI] [PubMed] [Google Scholar]
- WIJETUNGE S., HUGHES A.D. PP60 (c-src) increases voltage-operated calcium channel currents in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1995;217:1039–1044. doi: 10.1006/bbrc.1995.2874. [DOI] [PubMed] [Google Scholar]
- WRIGHT I.K., HARLING R., KENDALL D.A., WILSON V.G. Examination of the role of inhibition of cyclic AMP in α2-adrenoceptor mediated contractions of the porcine isolated palmar lateral vein. Br. J. Pharmacol. 1995;114:157–165. doi: 10.1111/j.1476-5381.1995.tb14920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU L., KARPINSKI E., WANG R., PANG P.K.T. Modification by solvents of the action of nifedipine on calcium channel currents in neuroblastoma cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;345:478–484. doi: 10.1007/BF00176628. [DOI] [PubMed] [Google Scholar]
- YAMAGISHI T., YANAGISAWA T., SATOH K., TAIRA N. Relaxant mechanisms of cyclic AMP-increasing agents in porcine coronary artery. Eur. J. Pharmacol. 1994;251:253–262. doi: 10.1016/0014-2999(94)90407-3. [DOI] [PubMed] [Google Scholar]
- YOKOSHIKI H., SUMII K., SPERELAKIS N. Inhibition of L-type calcium current in rat ventricular cells by the tyrosine kinase inhibitor, genistein and its inactive analogue, daidzein. J. Mol. Cell. Cardiol. 1996;28:807–814. doi: 10.1006/jmcc.1996.0075. [DOI] [PubMed] [Google Scholar]


