Imidazolin binding sites
Many publications have shown that imidazoline derivatives such as clonidine, moxonidine or rilmenidine reduce sympathetic tone via a central mechanism and that as a result they reduce plasma catecholamines and blood pressure (Reid et al., 1995). This reduction in blood pressure appears not to be regulated via peripheral, presynaptically localized receptors, since neither catecholamine depletion by reserpine, or destruction of the nerve endings with 6-hydroxydopamine produces a notable weakening of the clonidine-induced blood pressure reduction (Haeusler, 1974a,1974b; Kobinger & Pichler, 1976; Finch et al., 1975). In contrast, selective α2-adrenoceptor antagonists such as rauwolscine dose-dependently block hypotension induced by intravertebral application of clonidine. While some classical α2-adrenoceptor antagonists such as SKF86466 (in contrast to the imidazoline derivatives efaroxan and idazoxan) did not inhibit the clonidine-induced hypotension in the CNS (Ernsberger et al., 1988b; 1994; Haxhiu et al., 1994), this was discussed to due to underdosage of the antagonist (Bock et al., 1999). Hence the effects of clonidine are due to a central α2-adrenoceptor-mediated mechanism. The first signs that imidazoline derivatives might also work via non-adrenergic binding sites stem from Ruffolo (1977); the imadazoline derivative tetrahydrozoline was able to antagonize oxymetazoline-induced contraction, but not the contractile response induced by phenylethylamine derivatives such as noradrenaline, methoxamine or phenylephrine. Clear indications for a novel receptor type came from Bousquet et al. (1984), who reported hypotension after microinjection of clonidine into the rostroventrolateral medulla (RVLM). α-methylnoradrenaline showed no blood pressure reducing effect in the same model. The authors therefore assumed that binding sites must be present in the RVLM which preferentially bind imidazolines. Radioligand binding studies on RVLM membranes showed selective binding sites for imidazolines (Ernsberger et al., 1987). These data confirmed the assumptions of Ernsberger et al. (1988a; 1992); Buccafusco et al. (1995) and Bousquet et al. (1984) that the C1 region of the RVLM appeared to be the decisive area involved (Reis et al., 1989).
Since then, two different imidazoline binding site subtypes have been identified (Michel & Insel, 1989; Michel & Ernsberger, 1992; Ernsberger et al., 1992). The I1-binding site, which shows a high affinity binding to [3H]-clonidine, is localized in the frontal cortex and the ventrolateral medulla (Bricca et al., 1989; Ernsberger et al., 1990a; 1992; Gomez et al., 1991), an area associated with central blood pressure regulation. Functionally, the I1-binding site seems to be involved in central blood pressure regulation (Ernsberger et al., 1988b; 1994; Haxhiu et al., 1994; Hamilton, 1992a,1992b; Hamilton et al., 1992; Hieble & Ruffolo, 1992), even though its importance remains largely unclarified, since in functional α2A-adrenoceptor 'knock out‘ mice (D79N; Macmillan et al., 1996) no evidence of I1-imidazoline binding site-mediated effects was revealed (Zhu et al., 1999). Its amino acid sequence and the DNA coding for it also remain to be determined, although an imidazoline binding site antisera cDNA has been isolated and characterized as encoding a 1504 amino acid protein (IRAS-1) showing properties of an I1-binding site (Piletz et al., 2000). I1-binding sites have also been demonstrated in the spinal cord, kidney and pancreas (Regunathan et al., 1993; Ernsberger et al., 1995; Schulz & Hasselblatt, 1989a), but not in the left ventricle (Raasch et al., 2000).
Unlike the I1-binding site, the role of the I2-binding sites has been better characterized, whereby I2-binding sites can be further differentiated amongst I2A- and I2B-binding sites depending on their amiloride sensitivity. I2 binding sites have been demonstrated in various tissues such as brain (Brown et al., 1990), liver (Tesson & Parini, 1991) and kidney (Michel et al., 1989), whereby studies by Tesson et al. (1991) concluded that they are associated with mitochondria. Further studies focused their localization to the outer mitochondrial membrane. Functionally, the I2-binding site has been characterized as a regulatory subunit of monoamine oxidase (MAO, Figure 1), and later on it became clear that both MAO A and B share the same I2-binding site as a novel domain on the protein (Limon et al., 1992; Olmos et al., 1993). Moreover, studies on MAO A- and MAO B-deficient mice indicate that (1) the I2 binding sites identified by [3H]-idazoxan reside solely on MAO B, and (2) the binding sites on MAO A and a 28-kDa protein identified in livers of MAO A- and MAO B-deficient mice by photolabelling with 2-[3-azido-4-[(125)l]iodophenoxyl]methylimidazoline ([125I]-AZIPI) may represent additional subtypes of the imidazoline-binding site family (Remaury et al., 2000). In vitro studies have shown that selective ligands of the I2-binding site reduce MAO activity (Carpene et al., 1995; Tesson et al., 1995; Raasch et al., 1996; 1999). Further on, no correlation with a reduced oxygen utilization could be shown in vitro (Raasch et al., 1999). After protein molecular studies showed that I2-binding sites could be found on various MAO isoenzymes with varying molecular weights (Escriba et al., 1996), and that a binding domain (Escriba et al., 1999) for imadazoline derivatives could be identified in the MAO-B (Raddatz & Lanier, 1997; Raddatz et al., 1997; 1999; 2000), the functional role of the I2-binding site, unlike the I1-binding site, could be considered as established. Chronic treatment of rats with specific I2-ligands reduces MAO activity in various organs and catecholamines increase as a consequence (Raasch et al., 1999). In pathophysiological states such as heroin dependency or neurodegenerative disorders such as Alzheimer's disease or Huntington's chorea, the I2-binding site density is reduced (Garcia-Sevilla et al., 1999), which indicates that I2-binding sites might also be of clinical significance. In addition to this correlation to MAO, the I2-binding site has also been suggested to be involved in cell growth (Regunathan et al., 1996a) or analgesic effects (Olmos et al., 1994; Sastre et al., 1996a). However, both are isolated findings and require confirmation with more detailed investigations. In this view, the 28-kDa protein identified in livers of MAO A- and MAO B-deficient mice (Olmos et al., 1994; Remaury et al., 2000) may be of some importance (see Figure 1; for detailed information see corresponding section of this review).
Figure 1.
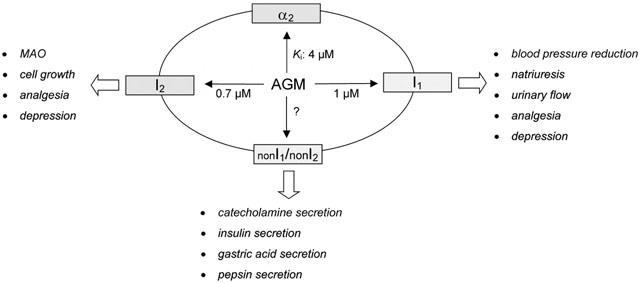
Imidazoline binding sites and their suggested functions. Agmatine binds with a moderate affinity (Ki values are taken from Li et al.) to α2-adrenoceptors as well as to I1 and I2 binding sites. Some authors (Chan, 1998; Molderings et al., 1998a; 1999a) attributed functions to binding sites (nonl1/non I2-binding sites) the affinity profile of which is consistent with neither the I1- nor the I2-binding sites.
Furthermore, some authors have attributed specific functions such as noradrenaline release (Fuder & Schwarz, 1993; Molderings & Göthert, 1995; Likungu et al., 1996; Molderings et al., 1999b), secretion of gastric acid and pepsin (Molderings et al., 1998a; 1999a) and insulin from β-cells (Chan, 1998) to binding sites for which the affinity profile is not consistent with those of I1- or I2-binding sites (nonI1/non I2-binding sites see Figure 1).
Clonidine displacing substance
Apart from a specific, saturable, high affinity and reversible binding, the corresponding anatomical, histological and subcellular distribution of the putative binding sites as well as the identification of a physiological function, the establishment of the protein sequence, DNA structure, signal transduction and the identification of endogenous ligands are decisive criteria for the establishment of a new receptor system (Ernsberger, 1999).
Isolation, chemical characterization and receptor specificity of CDS
On the basis of the fact that the phenylethyl derivative noradrenaline and the imadazoline derivative clonidine influence blood pressure via central α2-adrenoceptors and/or imidazoline binding site-mediated mechanisms, it had to be asked whether other non-catecholaminergic and until now unidentified substances participate in the regulation of blood pressure and heart rate. Atlas (Atlas & Burstein, 1984a,1984b; Atlas et al., 1987) isolated a substance from rat and calf brain by ion exchange chromatography, electrophoresis and HPLC. This isolate bound specifically to α2-adrenoceptors and displaced clonidine, but not the α1-ligand prazosin or the β-ligand cyanopindolol. Because of this property, the substance was named ‘Clonidine Displacing Substance' (CDS). Occasionally, this CDS is referred to as 'classical CDS‘ (cCDS) to emphasize its detection by radioligand binding studies. Even though Atlas (Atlas & Burstein, 1984a,1984b; Atlas et al., 1987) did not clarify its structure, CDS was characterized as a hydrophobic substance with a molecular weight of 587 Da1 stable to heat, acid hydrolysis and proteolytic enzymes such as trypsin, chymotrypsin, pronase, papain and pyroglutamase. Moreover it was postulated that CDS was not an amino acid and that it possessed no amino groups as shown by a negative ninhydrin and fluorecamine reaction. Due to its electrophoretic properties it was claimed that CDS was positively charged. Wavelengths of 224 and 276 nm represented its absorption maxima, which suggested the presence of aromatic residues in the molecule (see also Table 1; Atlas & Burstein, 1984a,1984b; Meeley et al., 1988a,1988b). Meeley et al. (1988a,1988b) developed a specific antibody directed against the clonidine analogue p-aminoclonidine. Since this antibody revealed a cross-reactivity with CDS, the authors concluded that there were structural similarities between clonidine and CDS and claimed that a phenyl and imidazole ring were mandatory structural characteristics for CDS, which confirmed the findings of Atlas & Burstein (1984a,1984b) regarding the relative hydrophobic character and the positive charge at neutral pH. CDS determined by radioimmunoassays is referred to in some studies as 'immunoreactive CDS‘ (irCDS) in order to emphasize its mode of determination. Since the distribution of irCDS in various organs of rats is directly correlated with biological activity attributed to cCDS, both cCDS and irCDS have been suggested to be similar (Meeley et al., 1988a). For this reason we have not distinguished between cCDS and irCDS in later sections of this review; only the term CDS is used irrespective of the way in which it was determined.
Table 1.
Comparison of physicochemical and physiological properties of CDS and agmatine (according to Atlas (1994; 1995); Meeley et al. (1992); Raasch et al. (1995a)). The adrenal gland was identified as the organ with the highest CDS, but low agmatine tissue levels, whereas the small intestine contains high agmatine but very low CDS levels

Later on, CDS could be characterized by radioligand binding studies as being 30 fold selective for imidazoline binding sites compared to α2-adrenoceptors (Table 1), which strengthened the hypothesis that CDS might be an endogenous ligand for the imidazoline binding site (Ernsberger et al., 1988a; 1990b). Studies showing that CDS has only a weak affinity towards the inhibitory G-protein also fit in with this idea (Atlas, 1991). Unlike clonidine, CDS was incapable of influencing basal adenylate cyclase activity in human platelets or the noradrenaline-induced inhibition of adenylate cyclase at a concentration able to displace clonidine binding, findings which certainly do not support an α2-adrenoceptor-mediated mechanism of action for CDS.
Distribution of CDS in central and peripheral tissues
After CDS had initially been isolated from the brains of several species (Atlas & Burstein, 1984a,1984b; Meeley et al., 1986; Ernsberger et al., 1988a; Regunathan et al., 1991a), CDS was also shown in various peripheral tissues by a specific antiserum directed against CDS (see Table 1; Meeley et al., 1988b; 1992; Dontenwill et al., 1988; Hensley et al., 1989). The existence of peripheral CDS was confirmed by a specific bioassay, which is based on the ability of CDS to induce contraction of the vas deferens or gastric fundus (Diamant & Atlas, 1986; Felsen et al., 1987). CDS, which has been isolated from brain, gastric fundus, heart, small intestine, kidney, liver, skeletal muscle and serum, contracted the gastric funds in a manner completely or at least partially antagonizable by verapamil. CDS isolated from the adrenal glands, however, relaxed the gastric fundus. The different extent of antagonism as well as the relaxation by adrenal CDS was explained by the possible co-extraction of other, effect-masking substances. Finally, Synetos et al. (1991) isolated CDS from human plasma and showed its biological effectiveness through the contraction of rat aortal vascular rings. Because of the significantly reduced plasma concentrations of CDS in adrenalectomized rats compared to sham-operated animals, Meeley et al. (1992) suggested the adrenals as the possible source of CDS circulating in the blood.
Biological function of CDS
Cardiovascular effects of CDS
Since CDS and clonidine compete for a common binding site, it was questioned whether CDS would have agonistic or antagonistic activity in a functional test. After central application of CDS, arterial blood pressure increases significantly without any change in heart rate both in the cat and the rat (Bousquet et al., 1986; 1987), which in this way is opposite to the effects seen after central dosing of clonidine (see Table 2; Bousquet et al., 1984). Intracisternal application of CDS produces no change in blood pressure in anaesthetized rabbits (Bousquet et al., 1987). Furthermore, CDS can antagonize clonidine-stimulated hypotension directly, i.e. the blood pressure reduction is reduced (Bousquet et al., 1986) and the dose-response curve for clonidine is clearly shifted to the right by CDS. This finding completely contradicts the results of Meeley et al. (1986), who observed a clear drop in blood pressure and heart rate after injection of CDS into the C1-region of the rat RVLM. Combination experiments with clonidine were not performed in this study. The reasons underlying these discrepant results may lie in the various solvents used for isolating and purifying the CDS (Bousquet et al., 1987). Alternatively, effects from CDS-extract impurities such as aminoacids, CDS-fragments, catecholamines, histamine or potassium might also have lead to these inconsistencies (Reis et al., 1992; Szabo et al., 1995; Singh et al., 1995).
Table 2.
Biological functions of CDS, agmatine and imidazoline derivatives on various effector systems
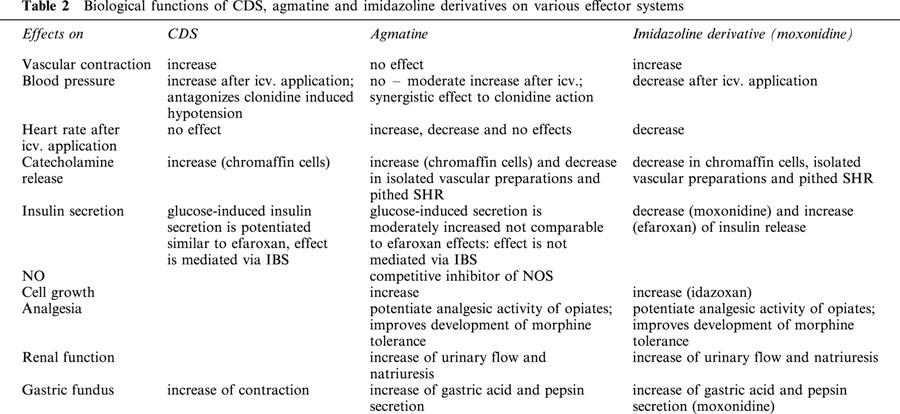
CDS stimulates catecholamine release
Apart from the above described property of CDS to contract various organ preparations, CDS has also been identified as a catecholamine releasing substance (Table 2). CDS binds with a high affinity to membranes of bovine chromaffin cells, whereby the displacement of [3H]-idazoxan by CDS was not impeded by guanosine 5′-(β,γ-imido)triphosphate, indicating that the corresponding imidazoline binding site was not coupled to a GTP binding protein (Regunathan et al., 1991a). It should be noted that this was an I2 like site labelled by [3H]-idazoxan and that I2-binding sites are consistently unaffected by guanine nucleotides. The concentration-dependent adrenaline release from chromaffin cells in response to CDS was comparable to that in response to nicotine, while the CDS-stimulated noradrenaline release was only about a quarter of the noradrenaline release induced by nicotine. Unlike the nicotine response, the release of either catecholamine following CDS can not be blocked by hexamethonium, which suggests a nicotine receptor-independent mechanism (Regunathan et al., 1991a). Since the catecholamine release is also not inhibited by the specific α2-antagonist SKF-86466, which shows no affinity towards imidazoline binding sites (Ernsberger et al., 1990b), but is influenced by cobalt (Regunathan et al., 1991a), a specific imidazoline binding site-dependent and calcium-dependent release mechanism induced by CDS is suggested.
Insulinotropic effects of CDS
For the further functional characterization of CDS, its influence on glucose stimulated insulin release was investigated in isolated Langerhans cells (Table 2 and Figure 2). The existence of imidazoline binding sites was shown in the pancreas (Schulz & Hasselblatt, 1989a,1989b), but the imidazoline binding sites of the β-cells of the pancreas appear to differ from the known I1- and I2-binding sites (Figures 1 and 2; Brown et al., 1993a; Chan et al., 1994; 1995; Morgan et al., 1995), and are also not identical with the binding site for sulphonylurea derivatives (Brown et al., 1993b; Rustenbeck et al., 1997). For this reason Morgan et al. (1999) have speculated about the existence of a pancreas specific I3-binding site. Moreover, it could be shown that imadazoline derivatives such as efaroxan or phentolamine increase the release of insulin by influencing the K-ATP channel (Figure 2; Chan & Morgan, 1990; Dunne et al., 1995; Plant & Henquin, 1990). CDS isolated from rat brain potentiated the glucose (6 mM) induced secretory insulin response concentration-dependently to a similar extent as efaroxan, and reversed the inhibitory effects of diazoxide on glucose-stimulated insulin release just as other similar imadazoline derivatives do. That this CDS effect is possibly mediated via imidazoline binding sites can be concluded from the observation that imadazoline derivatives such as RX801080 and KU14R antagonize the insulin-releasing effect of CDS. In addition, the effects of CDS on insulin secretion were not altered by pretreatment of the CDS extract (protease incubation as well 3000 Da molecular filtration centrifugation; Chan et al., 1997), which confirms the structural properties of CDS postulated by Atlas & Burstein (1984a,1984b), i.e. that CDS is not a peptide, but rather a low molecular weight substance. In closing, efaroxan-pretreated islet cells appear to be desensitized to CDS concerning insulin release, which correlates with observations obtained for efaroxan itself (Chan, 1998).
Figure 2.
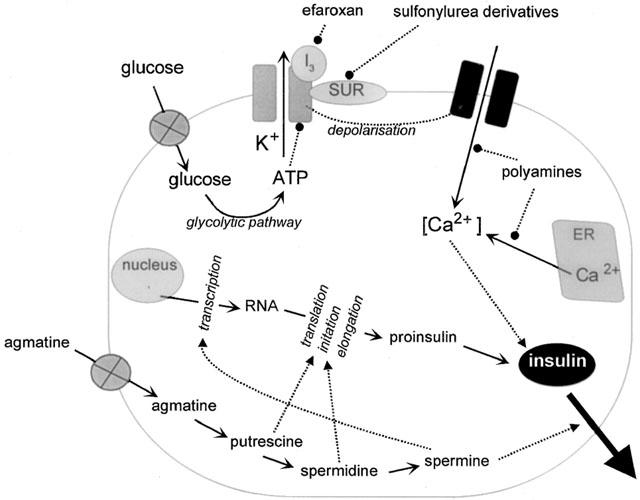
Current working hypothesis depicting mechanisms regulating insulin secretion from pancreatic β-cells and proposed sites for interference by agmatine. ATP generated by glucose metabolism shuts down K+-channels, resulting in depolarization and subsequent influx of Ca2+ through voltage-activated Ca2+-channels. This influx of Ca2+ increases cytosolic Ca2+ concentration, which is accompanied by mobilization of Ca2+ from the endoplasmic reticulum (ER), an event triggering secretory granule translocation and exocytotic release of insulin. The respective binding of imidazolines (such as efaroxan) and sulphonylurea derivatives (such as glibenclamide) to I3-binding sites and the sulphonylurea receptor, also closes the K+-channels. Agmatine does not bind to I3-binding sites. However, agmatine may enhance insulin secretion via its metabolites, after it is taken up by specific transporters. Putrescine, spermidine are necessary for proinsulin biosynthesis, whereas spermine may exert a stimulatory or permissive role in RNA transcription and long-term insulin release. Polyamines are also probably involved in regulation of cytosolic Ca2+-concentration by blocking Ca2+-influx and its release from intracellular stores. Abbreviations: …▪quot; : stimulation; …•: inhibition.
Agmatine
Synthesis and metabolism of agmatine
Biosynthesis of agmatine
Li et al. (1994) succeeded in identifying and characterizing mammalian agmatine by ion and molecular weight exclusion chromatography, high pressure liquid chromatography and mass spectroscopy, as a candidate for CDS. Agmatine, the decarboxylation product of the amino acid arginine, was first identified in 1910 by Kossel in herring sperm and is known as an intermediate in the polyamine metabolism of various bacteria, fungi, parasites and marine fauna (Tabor & Tabor, 1984; Yamamoto et al., 1988; Ramakrishna & Adiga, 1975), where polyamines have been attributed an important function in cellular growth. Agmatine is chemically characterized as follows (Table 1): its molecular mass is 130 Da, the UV absorption maximum of 200 nm suggests an aliphatic structure and a ninhydrin positive reaction confirms the existence of an amino group. Radioligand binding studies on membranes of bovine cerebral cortex, the ventrolateral medulla and on chromaffin cells have revealed Kds towards α2-adrenoceptors, I1- and I2-binding sites of 4, 0.7 and 1 μM, respectively (Figures 1 and 3; Li et al., 1994a). It had low affinity for the α1- and β-adrenoceptors, 5−HT3 serotonin and D2 dopamine binding sites (Li et al., 1994a), or the κ opioid and adenosine A1-receptors (Szabo et al., 1995). An interaction with the sigma3 binding site has been shown on murine neuroblastoma cells (Molderings et al., 1996). As a functional correlate to CDS, agmatine concentration-dependently releases adrenaline and noradrenaline from chromaffin cells (Table 2). Since chromaffin cells express imidazoline binding sites, but not α2-adrenoceptors (Regunathan et al., 1993), this can be considered as an indication for an agonistic function of agmatine at these binding sites. However, since there is a lack of proof that agmatine-induced catecholamine release can be blocked by antagonists of the I1-binding site, it is not certain whether these binding sites mediate this effect. Moreover, data showing an inhibitory potency or no effect of agmatine on noradrenaline release (Häuser & Dominiak, 1995; Häuser et al., 1995; Molderings & Göthert, 1995; Molderings et al., 1997; 2000; Schäfer et al., 1999b) fuels doubts as to whether this catecholamine releasing effect is really mediated via a direct mechanism whereby imidazoline binding sites are involved. On the other hand, it was suggested that agmatine influences noradrenaline release via a dual interaction, namely a competitive antagonism and an allosteric activation of the rat α2D-adrenoceptor, since (1); noradrenaline, moxonidine- or clonidine-induced noradrenaline release in segments of rat vena cava was dose-dependently enhanced or inhibited by agmatine, and (2); binding of clonidine and rauwolscine was inhibited, the rate of association and dissociation of clonidine was altered, and [14C]-agmatine was inhibited from binding to its specific recognition site by agmatine (Molderings et al., 2000).
Figure 3.
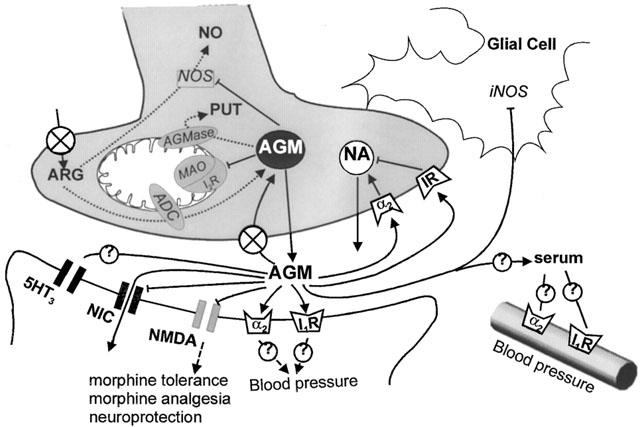
Schematic representation of an agmatinergic synapse: L-arginine enters the nerve ending via a transporter and is decarboxylated by the mitochondrial arginine decarboxylase (ADC) to agmatine (AGM), which is stored in vesicles and metabolized to putrescine (PUT) by agmatinase (AGMase). Agmatine inhibits NO synthase (NOS) as well as monoamine oxidase (MAO) since it was demonstrated that I2-binding site (I2-BS) is a regulative binding site of MAO. After agmatine is released from the neuron it is subject for a specific uptake or it interacts with various pre- and postsynaptic receptors including the I1-binding site (I1-BS), α2 adrenoceptor (α2-R), NMDA, nicotinic cholineric (NIC), 5−HT3 (via the sigma-2 binding site) receptor. Furthermore, agmatine enters postsynaptic neurons via nicotinic and possibly NMDA ion channels. Whether such released agmatine represents a source for serum agmatine has not yet been determined. Peripheral effects of agmatine on blood pressure and cell growth are also a matter of debate. Released agmatine binds to presynaptic imidazoline binding sites and α2 adrenoceptors and in this way is involved in the regulation of catecholamines. Agmatine penetrates glial cells where it also modulates the expression and activity of iNOS.
Agmatine arises enzymatically from the activity of arginine decarboxylase (ADC) on arginine (Figures 3 and 4) and is not supplied from nutritional components or bacterial colonization. ADC isolated from rat brain differs from plant or bacteria-derived ADC concerning localization, since ADC is associated with the mitochondria rather than the cytoplasm (as is typical for bacteria). The second difference concerns its substrate specificity. In contrast to bacterial ADC, mammalian ADC uses ornithine in addition to arginine, whereby it is not a typical ornithine decarboxylase, since it is neither cytosolic nor inhibited by diflouromethylornithine, a universal and irreversible inhibitor of all isoforms of ornithine decarboxylase (ODC) (Hunter et al., 1991). Finally, the optimum temperature of mammalian ADC is 30°C. At the bacterial temperature optimum of 37°C the enzyme activity of the mammalian ADC is only one third as active as it is at 30°C. Only the pH optimum (8.25) is similar between mammalian and bacterial ADC (Li et al., 1994a; 1995; Regunathan & Reis, 2000). Inhibition experiments in macrophages with lipopolysaccharides (LPS), transforming growth factor-β (TGF-β) and Interleukin-10 (IL-10) showed that ADC activity is subject to physiological control (Sastre et al., 1998). The co-localization of I2-binding sites and ADC on mitochondria has been discussed as a potential intracellular receptor-controlled regulatory loop for endogenous biosynthesis (Figure 3; Li et al., 1995). However, the organ specific distribution of ADC in rats (Regunathan & Reis, 2000) differs from that of agmatine (Raasch et al., 1995a), revealing some doubt that there is a close correlation between agmatine and its biosynthetic enzyme.
Figure 4.
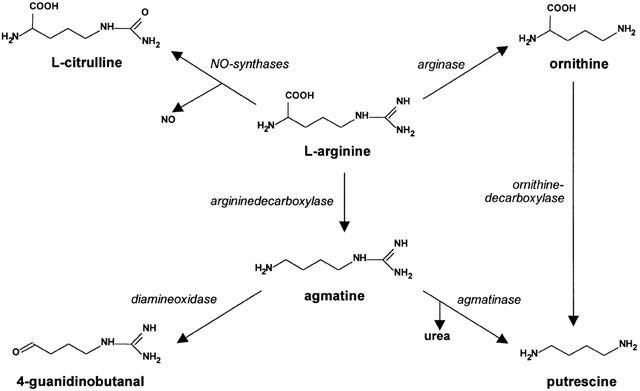
Metabolism of L-arginine in the mammalian organism.
Organ-specific, cellular and subcellular distribution of agmatine
Using high pressure liquid chromatography, agmatine has been demonstrated in nearly all organs of the rat (Table 1), whereby the highest concentrations are found in the stomach (71 ng g−1 wet weight), followed by the aorta, small and large intestine, and spleen; it is found in lower concentrations (<10 ng g−1 wet weight) in the lungs, vas deferens, adrenals, kidneys, heart, liver, skeletal muscle, brain and testes (Raasch et al., 1995a,1995b). Gas chromatography studies by Stickle et al. (1996) confirmed an organ specific distribution of agmatine. This distribution pattern of agmatine in various organs (Raasch et al., 1995a) differs widely from that of CDS (Table 1; Meeley et al., 1992). As an example, high concentrations of CDS but only low concentrations of agmatine are found in the adrenal gland. Moreover, the low correlation (r=0.2193) between agmatine and CDS tissue levels in both studies indicates clearly that agmatine can not exclusively represent CDS, but that, if at all, it only represents a member of a whole CDS family.
The concentration of agmatine in rat plasma is only 0.45 ng ml−1 (Raasch et al., 1995a,1995b), which renders it doubtful that agmatine acts as a circulating hormone since the Kd values for the I1- (0.7 μM) and I2-binding sites (1 μM; (Li et al., 1994a) are approximately 200 – 300 fold higher compared to rat plasma concentrations. In addition, the source for circulating agmatine remains unidentified, since (1) ADC has not been detected in plasma until now, and (2) the adrenals, which were identified as sources for CDS (Meeley et al., 1992), contain only minimal amounts of agmatine (see Table 1; Raasch et al., 1995a). Stimulation experiments on animals designed to investigate this question have not been performed until now. In humans, substantially higher plasma concentrations (47 ng ml−1) were determined when compared to rats (Feng et al., 1997). The reasons underlying this large difference remain to be clarified. An age-dependency for agmatine tissue concentrations could not be established, with the exception of the cerebral cortex, where concentrations declined by nearly 50% with age (Raasch et al., 1995a).
Using immunohistochemistry with specific antibodies against agmatine (Wang et al., 1995), agmatine was found to be regionally distributed in the cerebral cortex, the lower brain stem, the midbrain, frontal brain, thalamus and the hypothalamus in rat brain (Otake et al., 1998). In this way the distribution of agmatine-containing neurones correlates with the distribution pattern of α2-adrenoceptors and imidazoline binding sites to the extent that (1) in most agmatine containing regions, α2-adrenoceptors and imidazoline binding sites are also expressed (Kamisaki et al., 1990; de vos et al., 1991; 1994; Bricca et al., 1993; Nicholas et al., 1993; 1996; King et al., 1995; Ruggiero et al., 1995) and (2) agmatinergic neurones are concentrated in brain regions (e.g. cerebral cortex), which project to areas (e.g. striatum and midline thalamus), which contain α2-adrenoceptors and imidazoline binding sites (Berendse & Groenewegen, 1991; Jones & Yang, 1985; Saper et al., 1986). There also appear to be a multitude of interactions between agmatinergic cells and receptor areas in the corticothalamostriatal regulatory loops.
At the cellular level, agmatine could be shown in smooth muscle and endothelial cells. Since ADC is expressed only in endothelial cells and not in smooth vascular muscle cells, it was concluded that agmatine either originates in the serum or is taken up from the endothelial cells and stored in the smooth vascular muscle cells, although a corresponding transporter for agmatine has not yet been identified in the vasculature (Regunathan et al., 1996a). There are, however, reports on an agmatine-transport system of bacterial (Kashiwagi et al., 1986; Driessen et al., 1988) and neuronal origin (Sastre et al., 1997). Glial cells not only express imidazoline binding sites, they also synthesize agmatine (Regunathan et al., 1995a), whereby the agmatine concentration and ADC activity in the cultivated cells are substantially higher than the concentrations in brain, which might indicate that glial cells represent the main site for synthesis and storage. It is well known that cells under culture conditions can undergo alterations in distinct features such as receptor population, enzyme activity and this might also alter agmatine content. This could be why there are differences in agmatine content between neuronal and glial cells. In this respect, interferon-gamma (IFN-γ) was able to increase ADC activity without inducing nitric oxide synthase (iNOS) activity significantly in astrocytes, whereas LPS stimulated iNOS but not ADC activity. These data suggest that the ADC activity in neuronal tissue is subject to regulation, and that two different stimuli influence two pathways of arginine metabolism in entirely different ways.
At the subcellular level, agmatine was shown by immuncytochemistry to be localized mainly in large dense-core vesicles in the cytoplasm and in the immediate vicinity of the endoplasmic reticulum and the mitochondria (Figure 3; Otake et al., 1998; Reis et al., 1998), which correlates well with the demonstration of a mitochondria-associated ADC (Li et al., 1995). Moreover, an association of agmatine with small synaptic vesicles was shown in the rat hippocampus within the nerve endings, whereby the function of these endings has not yet been clarified. The co-transmitter function to L-glutamate has been speculated, since inhibitory effects at the N-methyl-D-aspartate (NMDA) receptor have been attributed to agmatine (Yang & Reis, 1999), which would suggest a regulation of the excitatory effect of L-glutamate via co-transmission.
Release of agmatine
In rat brain slices and synaptosomes, a release of agmatine, but not of putrescine could be shown in response to depolarization with 55 mM KCl (Figure 3; Regunathan et al., 1996b; Reis & Regunathan, 1998). In the absence of Ca2+, the release of agmatine was significantly reduced, suggesting a calcium dependent mechanism. Significant quantities of radiolabelled agmatine could be released from bovine chromaffin cells by 55 mM KCl and 10 pM nicotine induced depolarization (Tabor & Tabor, 1984). Immunocytochemical studies on the storage of agmatine support the findings of a stimulation-receptive agmatine release from neuronal tissue, since agmatine-like immuno-reactivity is primarily associated with small synaptic vesicles (20 – 30 nm diameter) in nerve endings that form asymmetric contacts to the spines of pyramidal cell dendrites. While the adrenals were identified as a source of circulating CDS (Meeley et al., 1992), no data exists about the release of endogenous agmatine into the circulation.
Inactivation of agmatine
The existence of a specific transporter system working against a concentration gradient (Kashiwagi et al., 1986) as well as an agmatine-putrescine antiporter (Driessen et al., 1988) have been shown in prokaryotes. In synaptosomes from rat brain, a selective ATP- and temperature dependent as well as Na-independent agmatine transporter could be shown (Figure 3; Sastre et al., 1997). The affinity of agmatine to this transporter is extremely low with a Km of 18.8 mM. Other polyamine transporters, however, have also shown Km values in the millimolar range (Seiler & Dezeure, 1990). The fact that agmatine uptake can not be inhibited by amino acids, catecholamines or polyamines at concentrations (1 mM) which can be judged as high (with the exception of amino acids) underscores the specificity of the transporter. Combination experiments with carbachol and nicotine have excluded the possibility that the agmatine-transporter is a nicotine-associated ion channel; earlier studies showed that [3H]-agmatine can act as a marker for ion flux through nicotinergic ion channels (Quik, 1985) and that agmatine acts as an antagonist of the retinal nicotine receptor (Loring, 1990). The transporter is also no K+ATP- channel. However, agmatine-uptake can be suppressed by CoCl2, CdCl2 or verapamil and doubled by application of a calcium-free medium, an indication that agmatine is transported through a calcium channel, although it could be excluded that the calcium channels were of an L- or T-type (Sastre et al., 1997). Finally, agmatine-uptake into synaptosomes is blocked by the imidazoline derivatives idazoxan and phentolamine, but not by clonidine, moxonidine, rilmenidine or p-aminoclonidine, so that apart from a guanidine or imidazoline partial structure, other structural properties also contribute to the inhibitory effect. Recently another agmatine uptake system was identified and pharmacologically characterized in human glioma cells; it was energy dependent, and saturable with a Km of 8.6 μM and a Vmax of 64.3 nmol min−1 mg protein−1 but distinct from the putrescine transporter and known amino acid or monoamine carriers and not associated with a calcium- or 5-HT3 receptor channel or an organic cation transporter (Molderings et al., 2001). Those features, especially the much higher affinity (∼2200 fold), clearly indicate its difference from the agmatine transporter identified by Sastre et al. (1997). From all these findings it seems reasonable to suspect that this transporter might be involved in the regulation of extracellular agmatine concentrations.
From bacterial polyamine metabolism it is well known that agmatine can be degraded enzymatically by agmatinase (Satishchandran & Boyle, 1986; Panagiotidis et al., 1987) or agmatine deaminase (Mercenier et al., 1980). The question is now whether the same metabolization pattern can be demonstrated in mammals, and whether an alternative means for putrescine biosynthesis apart from decarboxylation of ornithine (Tabor & Tabor, 1984) exists. The first evidence for a breakdown of agmatine to putrescine with cleavage of urea (Figures 3 and 4) in rat brain was obtained by Gilad et al. (1996a). Later, Sastre et al. (1996b) identified a mitochondrial agmatinase in rat brain with a low affinity for agmatine (Km=5.3 mM), and a Vmax of 530 nmol h−1 mg−1 protein. These enzyme kinetic parameters are comparable with those of the bacterial agmatinase (Satishchandran & Boyle, 1986). When one considers the relatively low concentrations of agmatine in rat brain (Raasch et al., 1995a) and the very low affinity of agmatine to the enzyme, one can reasonably question the extent to which agmatinase is of any physiological relevance. Since agmatinase is co-localized with ADC (Li et al., 1995; Regunathan & Reis, 2000), however, one can not rule out the presence of mM agmatine concentrations in intracellular compartments, which would then represent an adequate substrate concentration for the agmatinase. The high Vmax might also compensate for the lower affinity. A regional heterogeneous distribution of agmatinase has indeed been shown in rat brain (Sastre et al., 1996b), with the highest activity in the hypothalamus, followed by the medulla oblongata and hippocampus, and the lowest activity in the striatum and cerebral cortex. This distribution pattern is largely consistent with the imidazoline binding sites (Mallard et al., 1992), but less so with the α2-adrenoceptors (Ruggiero et al., 1995). Moreover, the distribution of agmatinase seems not to be consistent with the regional pattern of agmatinergic neurones in the CNS as determined by immunocytochemistry (Wang et al., 1995).
Outside of the CNS, agmatinase could also be demonstrated in macrophages (Sastre et al., 1998), where its activity can be stimulated by LPS, but inhibited by TGF-β and IL-10. Since ADC in macrophages is inhibited by LPS, TGF-β, and IL-10, and the activity of iNOS is also regulated by these substances (Wang et al., 1995), it seems reasonable to suggest that arginine might be metabolized by these three enzymes, and possibly other enzymes, to agmatine, nitric oxide (NO) and polyamines. Aside from agmatinase-induced degradation, agmatine can also be metabolized in the kidney by diamine oxidase (DAO; Figure 4; Holt & Baker, 1995; Lortie et al., 1996), where the affinity of agmatine to this enzyme is in the micromolar range and therefore substantially higher than that of agmatinase. Studies with specific DAO inhibitors underscore the importance of DAO in agmatine metabolism. Indeed, it has been speculated that specific inhibition of DAO by the antidepressant drug phenelzine might lead to an increase in serum and tissue concentrations of agmatine and in this way contribute to its antidepressive efficacy (Holt & Baker, 1995). However, this hypothesis is weakened by the fact that (1) no changes in plasma or tissue levels of agmatine were found during chronic DAO blockade; and (2) no DAO activity could be demonstrated in the brain (Lortie et al., 1996), suggesting an organ specific metabolism of agmatine, i.e. degradation by DAO in the kidney and agmatinase in central tissues. Moreover, it seems feasible that an increase in histamine due to DAO blockade might participate in antidepressive effects, since an antidepressant-like effect, via activation of H1-receptors, has been suggested elsewhere (Lamberti et al., 1998).
In studies by Holt & Baker (1995), agmatine (50 μM) had no influence on either the semicarbazide-sensitive aminoxidase (SSAO) or MAO. By using specific substrates it could be shown that agmatine does not modulate either MAO-A or MAO-B; hardly surprising to many, given that other polyamines such as putrescine are not substrates for MAO (Blaschko, 1974). These results are somewhat inconsistent with the fact that the I2-binding site has been characterized as a regulatory subunit of MAO (Tesson & Parini, 1991; Tesson et al., 1991; 1995; Carpene et al., 1995; Raasch et al., 1996; Raddatz et al., 1995; 1997; Escriba et al., 1996; Raddatz & Lanier, 1997) and that agmatine has been identified as a ligand at imidazoline binding sites (Li et al., 1994a; Piletz et al., 1995; 1996a). In this respect, the fact that agmatine is able, just as other I2-ligands, to inhibit MAO isolated from rat liver, does not seem completely surprising (Figure 3; Raasch et al., 1996; 1999). The IC50 value of 167 μM reported by Raasch et al. (1999) is about 3 fold higher than the maximal concentration used by Holt & Baker (1995) in their study. At 50 μM only a minor inhibition of MAO could be seen, even in Raasch et al.'s (1999) study. However, it seems more than doubtful that the agmatine-induced inhibition of MAO in vitro should have some relevance in vivo, since plasma and tissue concentrations of agmatine are approximately 500 – 40,000 fold lower than the EC50 values in the in vitro assays. Furthermore, an interaction between endogenous agmatine and MAO inhibitors is unlikely because of their different sites of action.
Biological functions of agmatine
Effects of agmatine on the central nervous system (Antinociceptive effects of agmatine)
Since the mid 80's it has been known (Gossop, 1988) that clonidine potentiates the analgesic activity of opiates, an effect which is purportedly mediated via α2-adrenoceptors (Maze et al., 1988; Quan et al., 1993). Studies on α2A-knock-out mice have clearly revealed the participation of α2A-adrenoceptors in analgesia (Hein et al., 1999; Hein, 2001). In this context, Fairbanks & Wilcox (1999) demonstrated that the centrally acting antihypertensive drug moxonidine produces antinociception in mice with dysfunctional α2A-adrenoceptors (α2A-D79N not a null mutation; Macmillan et al., 1996), whereby the selective α2A-adrenoceptor antagonist SK&F86466 (Hieble et al., 1986) and the I1-binding site/α2A-adrenoceptor mixed antagonist efaroxan (Haxhiu et al., 1994) antagonizes the moxonidine effects. The following mechanisms may underlie these results: (1) other substypes of α2A-adrenoceptor – either α2B or α2C – may participate in analgesia (Fairbanks & Wilcox, 1999); (2) since gene expression of the α2A-adrenoceptor is reduced by 80% via targeted mutation in the α2A-D79N mice (Macmillan et al., 1996), there is still the possibility of a residual activity of the α2A-adrenoceptor. This conclusion is authoritatively confirmed by the observation of different physiological effects between mice with fully disrupted α2A-adrenoceptors (α2A-adrenoceptor knock out mice) and those with gene-targeted mutation receptors (α2A-D79N, Altman et al., 1999); (3) imidazoline binding sites may induce antinociceptive effects (Figure 1), which raises the question of the biological relevance of agmatine mediating such effects, since agmatine was shown to be synthesized, stored, released and metabolized either by uptake or by enzymatic breakdown in tissue of neuronal origin (for detailed information see sections above).
Agmatine alone (0.1 – 10 mg kg−1) was ineffective in the mouse tailflick assay, but after intravenous or intrathecal application it potentiated the analgesic efficacy of morphine dose-dependently by factors of five or nine, respectively, without affecting morphine-induced gastrointestinal transit (Kolesnikov et al., 1996). Such results were confirmed by using slightly modified study protocols of Bradley & Headley (1997) and Horváth et al. (1999). The potentiation of morphine activity by agmatine seems to be mediated via a δ- rather than a κ1- or κ3 opiate receptor mechanism. Moreover, chronic studies also showed that agmatine, at a dose (0.1 mg kg−1) which did not potentiate morphine activity, reduced the development of tolerance during a 10-day morphine regime. It is not unlikely that this effect is mediated via I2-binding sites, since selective I2-ligands such as idazoxan or 2-benzofuranylimidazoline also suppressed the morphine-induced development of tolerance, while selective I1-ligands or α2-adrenoceptor antagonists did not (Boronat et al., 1998). How this binding site mediates suppression of the development of tolerance is still not completely understood, even considering the fact that I2-binding sites have been implicated as a regulatory binding site on MAO. Moreover, results using idazoxan, showing increases in cerebral levels of 3-methoxy-4-hydroxyphenylethyleneglycol (MOPEG) and 3,4,dihydroxy-phenylacetic acid (DOPAC) in morphine-withdrawn mice and an enhancement of morphine's elevating effects on MAO (Airio & Ahtee, 1999) suggests rather an interaction with MAO than an increase in cerebral noradrenaline turnover and release as a mechanism underlying idazoxan's overcoming of morphine tolerance. However, such a noradrenaline release can not be mediated classically via presynaptic α2-adrenoceptor, since it has repeatedly been shown that idazoxan inhibits noradrenaline release by this mechanism (Molderings et al., 1997). Another I2-mediated mechanism, possibly attributed to the 28-kDa protein which is present in MAO deficient mice (Remaury et al., 2000), was speculated to be involved in the reduction of morphine tolerance, since an astrocyte hyperplasia following chronic dosing of I2-selective imadazoline derivatives (Olmos et al., 1994; Alemany et al., 1995) can antagonize the morphine-induced suppression of astrocyte growth (Stiene-Martin et al., 1991; Stiene-Martin & Hauser, 1993). Since astrocytes play an important role in the regulation of synaptic density (Meshul et al., 1987), the astrocyte growth can modulate synaptic plasticity and appears to be associated with chronic morphine dosing (Nestler et al., 1996). Recently, a functional interaction between opioid- receptors and I2-binding sites could be shown. The first results on a so-called Gi-Go transducer protein have also been obtained, a protein which appears to play some role in this interaction (Sanchez-Blazquez et al., 2000). However, the low affinity of agmatine to the I2-binding sites compared to the selective I2 ligands provides reason to doubt this concept. Moreover, the concept of an I2-mediated mechanism for agmatine's antinociceptive effects contradicts the conclusion derived from the findings using α2A-adrenoceptor knock out mice, in which if anything a participation of I1-binding sites was suggested. Overall, it must be determined whether endogenous agmatine would participate in such an antinociceptive effect. To answer this, physiological or pathophysiological conditions have to be identified whereby agmatine levels (e.g. depression; Halaris et al., 1999) as well as morphine's effects are altered.
Aside from improvements in development of tolerance and morphine's analgesic action, agmatine also dose-dependently improved (20 – 40 mg kg−1) acute morphine withdrawal symptoms under naloxone in rats, such as jumping, wet dog shakes, writhing, defecation, ptosis, teeth chattering and diarrhoea (Aricioglu-Kartal & Uzbay, 1997). However, this behavioural pattern could not be induced by giving agmatine alone. A lacking impediment of locomotor activity under agmatine alone and in combination with naloxone suggests that the observed agmatine effects were not due to sedation or muscle relaxation. The authors of this study also associated the effects observed less not so much with an interaction at α2-adrenoceptors, but much more with an interaction at imidazoline binding sites or NOS.
(Effects of agmatine on the NMDA receptor) After it was recognized that NMDA receptors participate in the development of opiate dependency and development of tolerance (Elliott et al., 1995; Trujillo, 1995), it had to be asked whether ligands of imidazoline binding sites modulate the above discussed morphine tolerance via an NMDA receptor-mediated mechanism. The binding of the NMDA ligand [H]-(+)-MK-801 to cerebral cortex membranes can be reduced by various imidazolines with weak potencies (Ki) of 37 – 190 μM, whereby the potencies of the I1-ligand (moxonidine), the I2-ligand (idazoxan) and the α2 agonist (RX821002) were not different (Boronat et al., 1998). This suggests a lack of a correlation between the potency at NMDA receptors and the ability to prevent opiate tolerance. Compared to the other substances, agmatine had the lowest affinity for the I2-binding site, but the highest affinity for the NMDA receptor (Boronat et al., 1998). Moreover, agmatine differed from the other substances in this study since it had a significantly shallower Hill-Slope and the curve-fitting characteristics were different. All these signs could mean that agmatine differs from other imadazoline derivatives in its binding behaviour to NMDA receptors, and that even if this hypothesis were to be rejected for the imadazoline derivatives, the agmatine effects on morphine tolerance and potentiation could be mediated via NMDA receptors (Figure 3).
Against this background, whole cell patch clamp studies on cultivated hippocampal neurones showed that agmatine specifically induces a voltage and concentration-dependent block of the NMDA current, but not the AMPA- (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) or kainate flux (Yang & Reis, 1999). This inhibition was reversible and most potent at hyperpolarizing membrane potentials, and less effective at positive potentials. Even in the presence of 10 μM glycine, agmatine (100 μM) showed no measurable whole-cell flux, which does not support an NMDA-agonistic effect of agmatine. As a NMDA antagonist, (±)-2-amino-5-phosphonopentanoic acid (AP5) inhibits both, the inward and outward directed NMDA current. In the presence of agmatine there was an additional inhibition of the AP5-inhibited NMDA current, comparable to the inhibition under agmatine alone, making it reasonable to presume that agmatine is not a competitive NMDA antagonist. It could be shown, however, that agmatine interacts with the NMDA-pore directly at a site part-way across the membrane electric field (Yang & Reis, 1999). By investigating various agmatine structural analogues (arcaine, putrescine, spermine, arginine) regarding their ability to alter NMDA current, it could be shown that the guanidine structure of agmatine appears to be essential for suppressing NMDA current. A similar structure-activity relationship was also reported concerning the MAO inhibitory activity of agmatine (Raasch et al., 1999). Since the agmatine concentration (100 μM) required for an effective NMDA receptor blockade is relatively high, one has to question the physiological significance of this effect, especially against the background of published agmatine tissue concentrations (Raasch et al., 1995a; Feng et al., 1997; Stickle et al., 1996). However, since agmatine is not uniformly distributed in the CNS, but preferentially distributed in certain areas and subcellular structures (Otake et al., 1998; Reis et al., 1998), a concentration adequate to block NMDA might be reached, providing that the agmatine becomes released.
Glutamate, the most important excitatory neurotransmitter in the brain, also is potentially neurotoxic (Choi et al., 1988). Glutamate exposure to primary cultures of cerebellar granule cells has been characterized as a model for neurotoxic effects, which correlates with effects at NMDA receptors (Lysko et al., 1989). NMDA antagonists are consequently able to suppress such neurotoxic glutamate effects. In this context, imadazoline derivatives revealed some neuroprotective efficacy (Gustafson et al., 1990; Maiese et al., 1992; Olmos et al., 1996; 1999; Degregorio-Rocasolano et al., 1999). It therefore seemed natural to study whether agmatine, as an endogenous ligand of imidazoline binding sites with NMDA antagonistic activity (see above), possesses any neuroprotective activity. In cultivated cerebellar neurones, agmatine suppresses the NMDA-induced neurotoxic effects at concentrations of between 10 – 100 μM, but at higher concentrations it becomes toxic itself (LD50: 700 μM; Gilad et al., 1996b), perhaps by inhibiting growth effects as discussed later. Similar neuroprotective effects in cell cultures were shown by Olmos et al. (1999), who associated this effect of agmatine directly with an antagonistic activity at the NMDA receptor. In vivo studies on gerbils revealed neuroprotection after a global frontal brain ischaemia following an intraperitoneal application of agmatine (10 – 100 mg kg−1). These results occurred in line with a complete (after agmatine treatment) or partial (controls) recovery of the neurological deficit within 72 h, as revealed by the motor performance of the animals (Gilad et al., 1996b). The importance of endogenous agmatine during cerebral ischaemia is also stressed by the fact that the activity of ADC is increased transiently and in parallel to ODC by about 7 fold (Gilad et al., 1996c). This study seems to be a very important study, since it was shown that due to pathophysiological events the rate limiting enzyme of agmatine's biosynthesis is enhanced, suggesting a biological function for agmatine. As in many other functional studies, however, the applied dose of agmatine was indeed very high. Also, the extent of the agmatine transport is unknown in the brain and might indeed be limited under normal conditions. However, it is known that the blood-brain barrier after an ischaemic insult is damaged very early on and in a long-lasting manner (Dietrich et al., 1991), which could allow better access to the brain for exogenous substances. Nevertheless, circulating agmatine concentrations are very low (Raasch et al., 1995a), so that it is not very likely that endogenous agmatine from the periphery will contribute to neuroprotective effects. Apart from this it appears questionable, especially considering the relatively low affinity of agmatine to the NMDA receptor (Ki=219 μM, Olmos et al., 1999) and the endogenous tissue concentrations, whether endogenous agmatine could be held accountable for the survival-promoting effects observed in vivo (Gilad et al., 1996b). But at this point we must refer once again to the non-uniform distribution of agmatine in the CNS (Otake et al., 1998) which could theoretically approach efficacious concentrations at the NMDA receptor, provided of course that it is released.
(Agmatine's significance in depression) Various findings indicate a potential pathophysiological role of imidazoline binding sites in the development of depression (Figure 1): the density of I1-binding sites in human platelet plasma membranes is upregulated in depressive patients and normalized by antidepressive therapy (Piletz & Halaris, 1995; Piletz et al., 1996b,1996c). A similar increase in I1-binding sites was observed in untreated women with dysphoric premenstrual syndrome (Halbreich et al., 1993). Alterations in various imidazoline binding site proteins (45-kDa, 29/30-kDa) could be shown by Western blotting in membranes from platelets and brains of unipolar depressive patients, and from cortical autopsy samples from suicidal individuals (Sastre et al., 1995; Garcia-Sevilla et al., 1996; 1998a). The concentration of agmatine was raised in the plasma of depressed patients compared to a control group (70.6±6.5 vs 38.5±5.4 ng ml−1, P<0.05). After treatment with the antidepressant bupropion, agmatine plasma concentrations normalized to 57.0±6.7 ng ml−1, but no correlation between the bupropion and agmatine plasma concentrations was found (Halaris et al., 1999). At the same time the density of I1-binding sites and the immunodensity of a 33-kDa band in platelet membranes from depressive patients increased compared to controls. Both parameters fell to control levels with bupropion treatment, whereby the number (Bmax) of I1-binding sites correlated well with the agmatine plasma concentration (r=0.800, P=0.005). The finding regarding elevated levels of agmatine and I1-binding sites is surprising, since upregulation of an endogenous ligand normally occurs in line with a downregulation of its receptor, and vice versa. Whatever causes this uniform elevation of those parameters remains unclear, but the course of disease might play a significant role. Hence longer-lasting, disease-following studies on plasma agmatine concentrations and receptor densities in platelets are necessary to provide a satisfactory and plausible explanation. For other neurodegenerative disorders (e.g. Parkinson's disease, Alzheimer's disease, glial tumours), alterations in the expression of imidazoline binding sites in the brain or platelets could also be shown (Garcia-Sevilla et al., 1998b; 1999; Ulibarri et al., 1999). However, until today no studies have been performed, analogous to the depression studies, which have pursued the question of changes in agmatine plasma concentrations and their potential role in those diseases.
Cardiovascular properties of agmatine
(Effects of agmatine on isolated vessels and atria (for overview see Table 3)) Agmatine exerted no effect of its own and failed to alter the concentration-dependent contractile effects of the α2-adrenoceptor agonist UK14304 on the KCI-precontracted porcine palmar lateral vein, and unlike clonidine, agmatine did not increase contractility in the endothelium-denuded thoracic artery of the rat (Pinthong et al., 1995a). These results were confirmed both in intact and endothelium-denuded thoracic aortal segments (Gonzalez et al., 1996; Schäfer et al., 1999a). Agmatine also did not influence the contractile responses either to clonidine or phenylephrine (Schäfer et al., 1999a). Similarly, agmatine failed to impart any direct inotropic activity in the isolated, electrically stimulated atrium. In contrast, the imidazoline derivatives cirazoline and moxonidine produced significant increases in contractility, which were found to be due to an α1-adrenoceptor-mediated mechanism (Raasch et al., 2000). Such negative results on contraction were confirmed in studies where agmatine (0.1 – 100 μM) was ineffective at inhibiting electrically stimulated contraction of the rat isolated vas deferens and isolated guinea-pig ileum (Pinthong et al., 1995a). This is inconsistent with the effects of CDS on these preparations (Diamant & Atlas, 1986; Felsen et al., 1987; Meeley et al., 1992
Table 3.
Effects of agmatine on cardiovascular functions
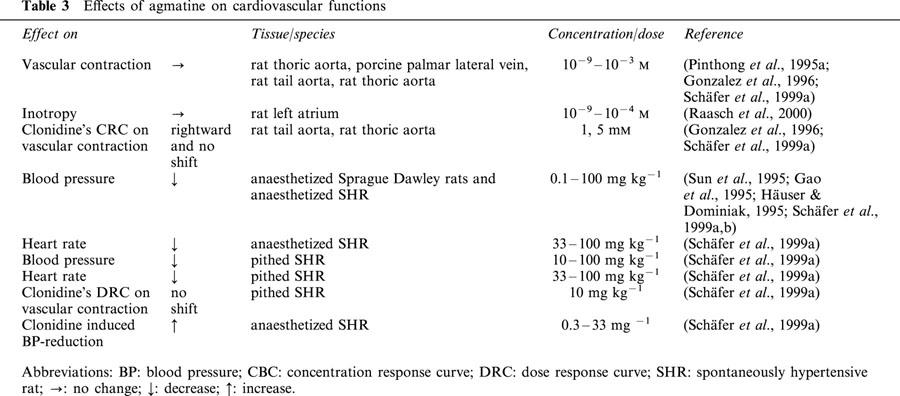
It is also plausible, but rather unlikely, that a null effect of agmatine on isolated vp>essels may represent the sum of a contracting and a dilating effect. Regarding potential interactions with relaxing neurotransmitters, an interaction with NO (Auguet et al., 1995; Galea et al., 1996; Schwartz et al., 1997) could be shown, but not with acetylcholine (Colucci et al., 1998) or bradykinin (Gao et al., 1995). Concerning interactions with vasoconstrictive neurotransmitters, some evidence exists for angiotensin II (Regunathan & Reis, 1997) and catecholamines (Gonzalez et al., 1996; Schäfer et al., 1999b; Molderings et al., 2000).
At first it was speculated whether agmatine might function as an alternative substrate for endothelial NOS (Ishikawa et al., 1995), and in this way contribute towards vasodilatation. This hypothesis was confirmed by the fact that agmatine provokes no relaxation of isolated vessel rings denuded of endothelium or pretreated with L-NAME. Agmatine itself is not a precurser for NO-synthase, but is rather a weak competitive inhibitor of various NOS isoenzymes (Figure 3; Auguet et al., 1995; Galea et al., 1996). The clearest effect was an inhibition of iNOS, whereby eNOS was maximally inhibited by 60% with 10 mM agmatine (Galea et al., 1996). However, even though agmatine has been detected in endothelium (Regunathan et al., 1996a), such high concentrations in the millimolar range are rather unlikely in vivo (Raasch et al., 1995a). Therefore, the relevance of this in vitro finding for the in vivo situation remains dubious.
The stimulation of presynaptic α2-adrenoceptors leads to vasodilatation, mediated via inhibition of noradrenaline release from sympathetic varicosities (Langer & Hicks, 1984). Agmatine suppresses noradrenergic neurotransmission in rat tail arteries, since it inhibits contraction after transmural nerve stimulation for about 10 min. However, contraction by exogenous noradrenaline was not inhibited, so that the authors concluded a presynaptic effect of agmatine (Gonzalez et al., 1996). This was confirmed by dosing with idazoxan or rauwolscine which antagonized the agmatine-mediated effect and resulted even in a delayed potentiation of the contraction response. The unchanged [3H]-noradrenaline uptake into chromaffin cells showed that this hypothetical presynaptic effect was not mediated by an inhibition of noradrenaline reuptake into the sympathetic nerve endings (Gonzalez et al., 1996). An inhibition of noradrenaline release by presynaptic imidazoline binding sites with a subsequent vasodilatation could be demonstrated in various vascular preparations and in the pithed SHR (Figure 3; Göthert & Molderings, 1991; Molderings et al., 1991; Göthert et al., 1995; Molderings & Göthert, 1995; Häuser et al., 1995; Raasch et al., 1999; Schäfer et al., 1999b). Nevertheless, the mechanism by which agmatine modulates noradrenaline release remains unclear: Firstly, it was demonstrated (Schäfer et al., 1999b) that agmatine failed to reduce noradrenaline release when α2-adrenoceptors were blocked reversibly and irreversibly by rauwolscine and phenoxybenzamine, respectively, but not under selective blockade of I1-binding sites with AGN192403 (Munk et al., 1996). These data contradict at least in part (Schäfer et al., 1998) the only sparse findings published until now regarding AGN192403 (Munk et al., 1996), which have characterized this agent as a high affinity I1-ligand with no affinity for α2-adrenoceptors, showing neither agonistic nor antagonistic effects on blood pressure and the sympathetic nervous system. Similar effects of agmatine on noradrenaline release modulated by presynaptic imidazoline binding sites were also demonstrated in various vascular preparations (Molderings & Göthert, 1995). This study suggested that the presynaptic activity of agmatine is probably related to a regulation of noradrenaline release by presynaptic imidazoline binding sites. However, a ganglionic mechanism can not be excluded since (1) I1-binding sites are present at the cell bodies of sympathetic ganglia and adrenal medulla (Molderings et al., 1993); and (2) agmatine blocks nicotinic-cholinergic transmission in sympathetic ganglia (Loring, 1990). Furthermore, since activation of I1-binding sites releases prostaglandins (Ernsberger et al., 1995) and histamine (Molderings et al., 1999a), which were both shown to diminish noradrenaline release (Wennmalm & Junstad, 1976; Göthert et al., 1999), it also seems likely that agmatine reduces noradrenaline overflow via such an indirect mechanism. Secondly, agmatine was suggested to act as an antagonist at the ligand recognition site of the α2D-adrenoceptor and enhances the effects of the α2-adrenoceptor agonist moxonidine probably by binding to an allosteric binding site on the α2D-adrenoceptor (Molderings et al., 2000). However, all those effects (Schäfer et al., 1999b; Molderings et al., 2000) were obtained with agmatine concentrations in the millimolar range, which generates serious doubts of any in vivo relevance. This dual interaction of agmatine at α2D-adrenoceptors probably explains the inconsistent effects of agmatine on catecholamine release, since data also exists concerning a prosecretory effect on adrenaline and noradrenaline release from chromaffin cells (Li et al., 1994a), adrenal medulla (Häuser et al., 1995) and vas deferens (Jurkiewicz et al., 1996). It is conspicuous, however, that divergent results have also been published for other imidazoline derivatives concerning a catecholamine releasing effect (Regunathan et al., 1991a,1991b; Reis et al., 1992; Ohara-Imaizumi & Kumakura, 1992; Steffen & Dominiak, 1996; Jurkiewicz et al., 1996). Overall, the inconsistent literature emphasizes that the effects of imidazoline derivatives and especially agmatine on the sympathetic nervous system are complex.
(Effects of agmatine on blood pressure and heart rate in vivo (for overview see Tables 3 and 4 and Figure 3)) After intravenous administration, agmatine reduces the blood pressure of anaesthetized SHR or Sprague Dawley rats at a dose of 100 μg kg−1 (Sun et al., 1995; Gao et al., 1995; Häuser & Dominiak, 1995; Schäfer et al., 1999a); although doses of 10 mg kg−1 and more showed long-lasting blood pressure effects (until 25 min), no reflex tachycardia but rather a significantly reduced heart rate were observed. Compared to pithed SHR, agmatine clearly exerts a higher blood pressure reducing potency in anaesthetized animals (Schäfer et al., 1999a). After intravenous application, agmatine exerted no influence on the pressor activity of clonidine. Even though clonidine has an approximately 700 fold higher affinity for I1-binding sites and a 115 – 246 fold higher affinity for all three α2-adrenoceptor subtypes, agmatine even at a 10,000 fold higher dose compared to clonidine had no effect on clonidine-induced blood pressure increases in pithed rats. Hence, antagonistic or CDS-like effects at the α2-adrenoceptors and I1-binding sites in the vessel musculature can be excluded. In complete contrast, an additive blood pressure and heart rate reduction could be seen in anaesthetized SHR during continuous clonidine infusion with agmatine doses as low as 100 μg kg−1, which suggests in fact a synergistic effect to clonidine (Schäfer et al., 1999a,1999b).
Table 4.
Blood pressure and heart rate changes after central agmatine-dosing
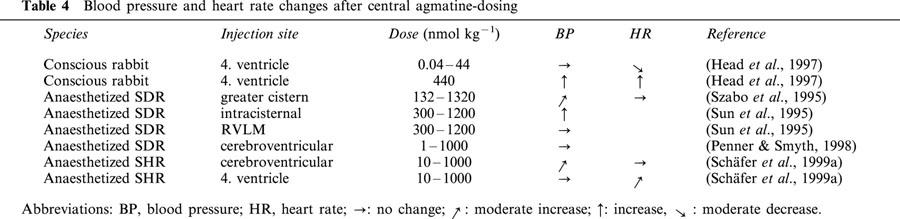
The lack of agmatine effect on isolated vessels (Gonzalez et al., 1996; Pinthong et al., 1995a; Schäfer et al., 1999a), as well as the markedly lower dose required to produce a comparable reduction of blood pressure in anaesthetized compared to pithed SHR (Schäfer et al., 1999a), suggests a central regulation of blood pressure (see Table 4 for an overview). However, no effects of an intracerebroventricular application of agmatine (1 – 1000 nmol 5 μl−1) on blood pressure and heart rate were observed (Penner & Smyth, 1996). Also, Head et al. (1997) saw no increase in heart rate in conscious rabbits, except at high doses (100 μg kg−1) that caused agitation, tachypnoea, increase in blood pressure and a reversal of the dose-dependent bradycardia effect (0.01 – 10 μg kg−1). These negative findings on blood pressure and heart rate were also confirmed by Sun et al. (1995) after an injection of agmatine into the RVLM of anaesthetized rats. In contrast, when agmatine was injected into the greater cisterna of anaesthetized rats (Sun et al., 1995) and conscious rabbits (Szabo et al., 1995), blood pressure and sympathetic nerve activity increased, indicating a site dependency of central application which might be due to differences in the pattern of imidazoline binding sites and α2-adrenoceptors with different species or different modes of application. This hypothesis is confirmed by findings of Schäfer et al. (1999a), whereby agmatine caused a dose-dependent, significant increase in blood pressure without changing the heart rate after its intracerebroventricular injection to SHR, whereas no change in blood pressure but an increase in heart rate was observed after its injection into the IV ventricle. A comparison between moxonidine and agmatine after injection into the IV-ventricle of conscious rabbits revealed that both substances caused a similar bradycardial effect (Head et al., 1997). These effects were attenuated by efaroxan (I1- and α2-adrenoceptor antagonist) and 2-methoxy-idazoxan (α2-adrenoceptor antagonist). From these results Head et al. (1997) concluded that agmatine might be an α2-adrenoceptor agonist. The fact that agmatine exerts no hypotensive effect as other agonists for central α2-adrenoceptors contradicts this. The authors saw an indication that agmatine possessed a simultaneous hypertensive activity which might mask an α2-adrenoceptor-induced hypotension. The dual interaction of agmatine on noradrenaline release via an allosteric activation or a competitive antagonism at α2D-adrenoceptors (Molderings et al., 2000) could probably contribute to this effect and strengthen the hypothesis of Head et al. (1997). Both in conscious rabbits and anaesthetized SHR, ventricularly applied agmatine shows no effects on blood pressure after induction of hypotension by clonidine or moxonidine (Head et al., 1997; Schäfer et al., 1999a), while bradycardia is potentiated by agmatine (Schäfer et al., 1999a). If the functional data for CDS and agmatine after central administration are compared, there appears to be absolutely no correlation between the two substances.
Agmatine and its potency on cell growth
Since polyamines participate in DNA replication and cellular proliferation (Pegg & Mccann, 1982), it was a plausible hypothesis that agmatine might also be involved in cellular growth processes. Regunathan et al. (1996; 1997; 1999) demonstrated a partial inhibition of agmatine (100 – 1000 μM) on foetal calf serum-stimulated thymidine incorporation in endothelial cells, vascular smooth muscle cells and asterocytes, whereby measurements of lactate dehydrogenase release and morphological examination ruled out a cytotoxic effect being responsible for the inhibition of growth (Regunathan et al., 1999). The low potency of agmatine compared to idazoxan regarding proliferation inhibition might be due to its lower affinity towards I2-binding sites compared to other imadazoline derivatives. However, the manner by which I2-binding sites (which are associated with MAO and located in mitochondria) should mediate growth effects has not yet been clarified. Due to its rapid metabolism to putrescine (Gilad et al., 1996a), it might be speculated whether the antiproliferative effects of agmatine can be attributed to other polyamines. However, putrescine acts as a proliferation promoter, since it doubled thymidine incorporation (Regunathan et al., 1996a; Regunathan & Reis, 1997), and would therefore act antagonistically to agmatine. The antiproliferative effects of agmatine and idazoxan in vascular smooth muscle cells could also be observed following other stimulatory conditions such as noradrenaline, angiotensin II or platelet derived growth factor (PDGF; Regunathan & Reis, 1997). Growth factors, especially PDGF, activate primarily membrane receptor-associated tyrosine kinases, which trigger intracellular processes such as activation of protein kinase C (PKC) and various mitogen-activated protein kinases (MAP-kinases), which ultimately result in expression of the immediate-early genes c-fos and c-myc are involved in DNA-synthesis and cell division (Somlyo & Somlyo, 1994; van biesen et al., 1995). Since both angiotensin II and noradrenaline activate PKC and MAP kinases via a G-protein coupled mechanism, it has to be assumed that the antiproliferative effect of agmatine is a mechanism occurring further downstream (Figure 5) since the effects of both G-protein-coupled agents and PDGF (tyrosine kinase mediated) could not be inhibited. It is not known whether this interaction occurs within the cytosolic signal transduction cascade (e.g. Ca2+, PKC, MAP kinase) or at a transcriptional level. Satriano et al. (1998) also showed that agmatine suppresses growth of MTC-cells (mouse kidney proximal tubule cells) by thymidine incorporation experiments, an effect that could be antagonized by putrescine. The authors, however, pursued a completely different hypothesis to explain the agmatine-mediated cell growth, which was based on the following findings: (1) Agmatine time-dependently reduced the intracellular content of putrescine and spermidine. (2) Agmatine was not converted intracellularly to polyamines (in MTC cells). (3) Exogenous agmatine reduced ODC activity dose- and time-dependently in MTC cells, where even high agmatine concentrations (1 mM) exert no cytotoxic effects; the specificity of this effect could also be seen with other cell lines, and such findings were also confirmed by Vargiu et al. (1999). (4) Agmatine (1 mM) time-dependently suppressed the polyamine transporter in MTC cells, and this results in a reduction of the intracellular content (c. 15% of control values) of exogenously applied [3H]-putrescine. This result could also be confirmed by others, where it was shown that intracellular spermidine content in rat hepatocytes can be reduced by an agmatine-induced inhibition of uptake (Vargiu et al., 1999). (5) Agmatine induced antizyme which regulates the synthesis and transport of polyamines. This was established by (a) an agmatine-dependent translational frame-shift of antizyme mRNA to produce a full-length protein and (b) a suppression of agmatine-dependent inhibitory activity by either anti-antizyme IgG or antizyme inhibitor. Satriano et al. (1998) therefore deduced the following hypothesis (Figure 4): the intracellular polyamine content, which plays an important role in DNA-replication and cell division, is regulated either by endogenous cellular biosynthesis from arginine to ornithine and further on under ODC catalysis to putrescine, or by the uptake of exogenous polyamines. Both the membrane-located polyamine transporter as well as ODC are subject to regulation by antizyme, whereby this protein exerts an inhibitory effect on both proteins. Agmatine acts as a regulator of antizyme, which means that agmatine is able to control synthesis.
Figure 5.
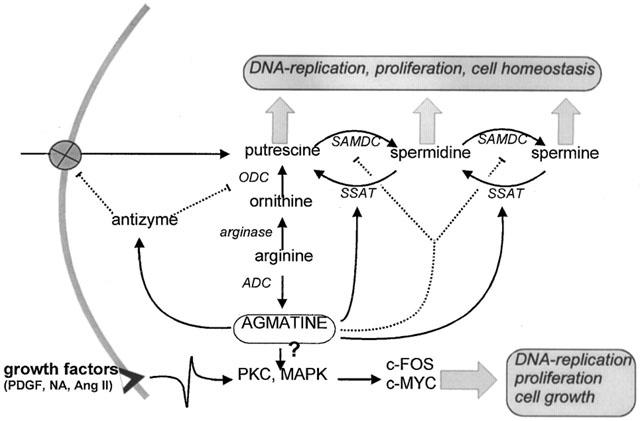
Influence of agmatine on cell growth: Control of cell growth can be attributed to two different pathways, namely a membrane receptor controlled pathway, and a pathway dependent on cellular polyamine content. Putrescine content is regulated by an active tranport mechanism as well as by arginine metabolism. Agmatine acts by stimulating an antizyme that inhibits both processes, so that a reduction in putrescine content occurs which contributes to an antiproliferative activity for agmatine. Agmatine also stimulates the spermidine/spermine acetyltransferase (SSAT), the key rate-limiting enzyme for polyamine intraconversion, and simultaneously inhibits S-adenosylmethionine decarboxylase (SAMDC), an enzyme which also has a modulating effect on intracellular polyamine content. Growth factors also stimulate membrane-located receptors. Following stimulation of protein kinase C (PKC) and MAP kinase (MAPK), expression of c-FOS and c-MYC occurs which eventually leads to DNA replication, cell proliferation and cell growth. Whether agmatine exerts an influence on this signal cascade has not yet been established.
A third hypothesis for agmatine's antiproliferative effects is that agmatine regulates the interconversion pathway of polyamine metabolism at the level of the rate-limiting enzyme (Figure 5). In hepatocyte cultures agmatine (0.5 mM) inhibits cell growth and increases the activity of the spermidine/spermine acetyltransferase (SSAT) about 10 – 25 fold in a manner dependent on oxygen saturation (5 vs 21%). This result was confirmed at the protein level, i.e. the increase in activity was due to an increased expression of SSAT (Vargiu et al., 1999). The content of putrescine simultaneously increases in line with reductions in spermine and spermidine, whereby putrescine-accumulation was considered to be responsible for the reduced ODC activity, since it is also known that the biosynthesis of putrescine is regulated via a negative feedback mechanism (Persson et al., 1986). The observed increase in activity of S-adenosylmethionine decarboxylase (SAMDC), which catalyses the breakdown of putrescine to spermidine and spermine, appears to be a consequence of the increased intracellular putrescine content. The authors attributed the observed inhibition of cell growth mainly to the depletion of spermidine and spermine. However, since cellular putrescine levels increase concomitantly, it seems somehow unclear whether cell growth should be inhibited, since putrescine causes the opposite effects (Regunathan et al., 1996a; Regunathan & Reis, 1997). Moreover, Vargiu et al. (1999) attributed the inhibition of cell growth solely to an agmatine-induced modification of putrescine metabolism. However, they ignored the observation that total polyamine content in cells increased by 40% depending on the agmatine concentration, which would indicate an alteration in uptake or biosynthesis, although both were in fact shown to be reduced (Satriano et al., 1998; Vargiu et al., 1999). Some questions, therefore, remain to be answered.
Taken together, all three hypotheses failed more or less to explain whether agmatine's mediated growth effects would be mediated via imidazoline binding sites, a fact which would strengthen the hypothesis of an endogenous ligand. In case of a growth control of agmatine via a feedback regulation of enzyme activity, as shown for ODC (Vargiu et al., 1999), agmatine would indeed be biologically active, possibly as an intermediate in the metabolism of arginine and ornithine to the polyamines, but it would not be a compulsory endogenous ligand for imidazoline binding sites. Another limitation of all three hypotheses is the fact that agmatine inhibits growth at concentrations (0.1 – 1 mM) which although non-cytotoxic (Vargiu et al., 1999), are much higher than circulating agmatine and even tissue concentrations. Therefore, the biological significance of this agmatine effect remains uncertain.
Effects of agmatine on renal function
Renal sodium regulation is important both under normal and pathological conditions for circulatory regulation. A range of different central and peripheral parameters influence sodium excretion and reabsorption. The role of α-adrenoceptors in this context has been demonstrated (Michel & Rump, 1996). Radioligand binding studies succeeded in demonstrating both α2-adrenoceptors as well as imidazoline binding sites, where different distributions could be shown within the kidney (Coupry et al., 1990; Evans & Haynes, 1995; Ernsberger et al., 1995). Moxonidine studies suggested a functional participation of imidazoline binding sites in natriuresis (Allan et al., 1993; 1996), but it is not yet clear whether moxonidine influences sodium excretion and urinary flow via a central and/or peripheral mechanism (reviewed by Smyth & Penner, 1999). In this context the question arises as to the renal function of agmatine, especially considering its demonstration in both brain and kidney (Raasch et al., 1995a; Lortie et al., 1996).
At concentrations that induce no changes in haemo-dynamics or creatinine clearance, agmatine as well as the I1-ligands moxonidine, clonidine and 2,6-dimethyl-clonidine increase urinary flow-rate, whereby agmatine first shows significant changes at ∼10 fold higher doses (Allan et al., 1993; Li et al., 1994b; Ernsberger et al., 1995; Penner & Smyth, 1996). The increase in urinary flow under moxonidine, 2,6-dimethylclonidine and agmatine results from an increased osmotic clearance, while under clonidine it is due to an increased clearance of free water, consistent with the imidazoline-mediated inhibition of the Na+/H+-exchange in isolated renal tubular cells (Bidet et al., 1990). This suggests that the osmotic clearance is possibly an imidazoline binding site-mediated effect, but that free water clearance might be due to an α2-receptor-related mechanism (Smyth & Penner, 1995), since compared to clonidine the relative affinities of agmatine, moxonidine and dimethyl clonidine are higher for imidazoline binding sites than they are for α2-adrenoceptors (Ernsberger et al., 1995).
In the perfused isolated kidneys of Wistar-Frömter rats, agmatine dose-dependently and significantly increases the single nephron glomerular filtration rate (SNGFR) and the absolute proximal reabsorption (APR), after application into the renal interstitium and the urinary space of surface glomeruli. In denervated kidneys microperfused in exactly the same way, the SNGFR, but not the APR, was increased after agmatine application. The agmatine effects were transient, possibly due to metabolization by DAO (Lortie et al., 1996). While the I2-ligand BU-224 exerts a similar effect to agmatine, moxonidine was ineffective (Lortie et al., 1996). The effects of the synthetic and DAO-stable I2-ligand BU-224 were, however, longer lasting, which corresponds well with the reversible activity of agmatine and its degradation in the kidney. Because of the application of agmatine into the urinary space, one can assume that agmatine increases the SNGFR due to an effect on the glomerulus as well as the associated vessels. The negative results of agmatine on isolated vessels (Pinthong et al., 1995a; Schäfer et al., 1999a), however, contradict such a participation. The simultaneous increase in APR could be directly mediated, a supposition supported by the finding that agmatine increases the Na+/K+ ATPase in renal membranes (Bidet et al., 1990). The fact that agmatine increases SNGFR after denervation to the same extent as that seen in innervated kidneys, excludes the possibility of a presynaptic mechanism. In contrast to innervated kidneys, the APR in denervated kidneys was not raised after agmatine, which allows us to conclude either (1) that the glomerular and tubular effects of agmatine are mediated by different mechanisms; or (2) that from denervation and the resulting removal of presynaptic α2-adrenoceptors, the glomerular balance as well as the APR response to agmatine are destroyed. It has also been shown that the agmatine-induced increase in glomerular filtration might be mediated via a NO-synthase-dependent mechanism (Schwartz et al., 1997).
Metabolic effects of agmatine
It has long been known that agmatine increases insulin release from rat pancreatic islets of Langerhans cells (Alberti et al., 1973; Sener et al., 1989) glucose uptake into the isolated rat respiratory diaphragm (Frank, 1927; Weitzel et al., 1971; 1972a,1972b), glucose oxidation in isolated fat cells, and the glycogen content in the respiratory diaphragm (Weitzel et al., 1971; 1972a,1972b). In all these cases the agmatine effect appeared to be receptor-independent. Since those studies, however, the existence of a specific imidazoline binding site has been demonstrated in the pancreas (Schulz & Hasselblatt, 1989a,1989b), where the imidazoline binding sites located on the pancreatic β-cells is not identical to either of the known I1- and I2 binding sites (Figures 1 and 2; Chan et al., 1994; 1995; Morgan et al., 1995; Tsoli et al., 1995) or the binding site for sulphonylurea derivatives (Brown et al., 1993b; Ishida-Takahashi et al., 1996; Rustenbeck et al., 1997), even though I2-binding sites are expressed abundantly (Brown et al., 1993a). However, the archetypal I2-ligand idazoxan is not an insulin secretagogue and displays negligible antagonist activity towards other imidazoline secretagogues. Equally, there is no convincing evidence that I1-binding sites are involved in insulin secretion, especially since few if any I1-binding sites were detected on pancreatic β-cells (Brown et al., 1993a; Rustenbeck et al., 1997). For this reason, the existence of a pancreas specific I3-binding site was postulated (Morgan et al., 1999). It could also be shown that imadazoline derivatives such as clonidine or moxonidine inhibit insulin release from isolated pancreatic cells (Langer et al., 1983; Hillaire-Buys et al., 1985; Skoglund et al., 1988; Tsoli et al., 1995), although efaroxan or phentolamine increase insulin release by influencing the K-ATP channels (Figure 2; Chan & Morgan, 1990; Chan et al., 1991; Plant & Henquin, 1990; Jonas et al., 1992; Berdeu et al., 1994) and exert vasoconstrictive effects on isolated perfused rat pancreas (Berdeu et al., 1994). Considering that CDS and even imadazoline derivatives seem to possess insulin pro-secretory activity, one might ask whether agmatine possesses insulin regulatory activity.
Under a slightly stimulating glucose concentration (8.3 mM) and unchanged pancreatic flow rate, agmatine (0.1 – 3 mM) induced a moderate increase in insulin secretion. In comparable studies, efaroxan was substantially more effective, also with combined stimulation by arginine (10 mM) and glucose (5 mM; Berdeu et al., 1996). Unlike efaroxan (0.1 mM), even the highest concentration of agmatine (3 mM) was without effect on the vascular flow rate in isolated pancreas. The lacking insulin secretion under glucose/arginine stimulation and the moderate release of insulin under higher glucose concentrations allow us to presume different mechanisms of release governed by agmatine and efaroxan. Hence, the hypothesis was forwarded that the secretolytic effect of agmatine (unlike efaroxan) is not mediated via imidazoline binding sites, as previously suggested by Weitzel et al. (1971; 1972), but rather by metabolic products. Polyamines in particular are suggested to mediate agmatine-mediated insulin secretion, assuming that agmatine is subject to uptake into β-cells as insinuated by Sastre et al. (1997) and Molderings et al. (2001) for neuronal tissue. The polyamines themselves possess no affinity towards imidazoline binding sites but were shown to be involved in regulation and proliferation of pancreatic β-cells and hormone production by insulin-secreting cells (Figure 2; Sjoholm, 1993). From the lack of secretolytic and vasopressive activities of agmatine, consistent with the findings of other authors (Piletz et al., 1995), Berdeu et al. (1996) concluded that agmatine cannot function as the endogenous ligand at the imidazoline binding sites of the pancreas or blood vessels.
Agmatine and its function in the gastrointestinal tract
The following facts suggest a potential role of agmatine in the gastrointestinal tract: (1) Imidazoline binding sites have been demonstrated in the stomachs of various animal species, identified as I2- or non I1-/non I2 binding sites (Houi et al., 1987; Tesson et al., 1992; Molderings et al., 1995; 1998b; 1999c); (2) Clonidine and clonidine-like substances at high concentrations stimulate gastric acid secretion (Houi et al., 1987; Medgett & Mcculloch, 1979; del Tacca et al., 1982a,1982b; Bhandare et al., 1991). However, clonidine may exert this effect by modulating vagal cholinergic stimulation via α2-adrenoceptors (Blandizzi et al., 1990a,1990b; 1995); (3) In isolated organ preparations of the gastric fundus, CDS leads to a concentration-dependent contraction, due to an interaction with imidazoline binding sites (Felsen et al., 1987); (4) Stomach, small and large intestine have been characterized as the organs with the highest agmatine tissue concentrations (Raasch et al., 1995a,1995b). However, since ADC has not been yet been demonstrated in gastric fundus or the intestine, there remain some doubts as to whether agmatine in fact originates from arginine metabolism. Indeed, it may originate from bacterial colonization or food intake after adsorption by a transporter (which also remains to be identified in gastric tissue).
Glavin et al. (1995) were the first to show that agmatine possesses pro-secretory and ulcerogenic activities, since it increases the secretion of gastric acid and pepsin, reduces mucus thickness, and exacerbates stress-induced mucosal lesions (Table 5). Hence, it appears that agmatine-mediated effects are contrary to those mediated by moxonidine (Glavin & Smyth, 1995a,1995b), consistent with the differing activities of moxonidine and agmatine on peristalsis (Table 5; Liu & Coupar, 1997). Since agmatine has been characterized as an endogenous ligand for imidazoline binding sites, one should actually expect agonistic effects such as those seen for moxonidine. Since this is not the case, different mechanisms are probably inferred. For this reason, an inverse agonism for agmatine at the imidazoline binding sites has been suggested (Glavin et al., 1995). Secondly, it seems feasible that moxonidine-like substances stimulate gastric acid secretion via α2-adrenoceptors (Daly, 1984), whereas agmatine acts at imidazoline binding sites of the non I1-/non I2 subtype (Molderings et al., 1998b; 1999c). Against this background it has been purported that imadazoline derivatives do not stimulate acid secretion directly, but rather indirectly, i.e. by altering endogenous histamine release from enterochromaffin-like cells (Table 5; Molderings et al., 1999a). This is further supported by the facts that imidazoline-induced acid secretion (Molderings et al., 1999c) can be blocked by an H2-receptor antagonist (Houi et al., 1987), and that polyamines can regulate histamine release (Purcell et al., 1994). That prostaglandins contribute to this indirect agmatine effect on gastric function seems rather unlikely, since (1) an activation of I1-binding sites releases prostaglandins (Ernsberger et al., 1995); (2) I1-binding sites were not determined in stomach (Molderings et al., 1999c); and (3) prostaglandins decrease gastric acid release and enhance mucus thickness, which are opposite to agmatine's effects (Glavin et al., 1995).
Table 5.
Effects of agmatine on gastric function
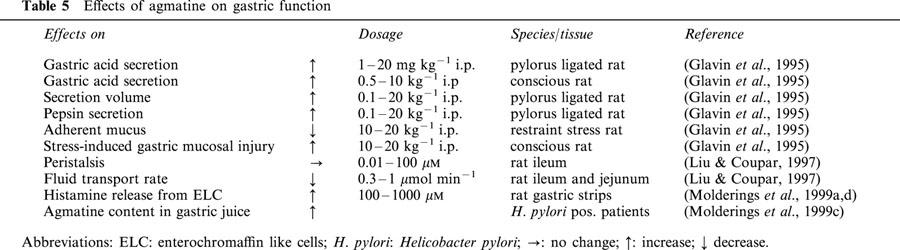
Whether agmatine reveals gastric functions as an endogenous neurotransmitter is uncertain, especially since Helicobacter pylori has been shown to generate and release agmatine (Molderings et al., 1999d). Moreover, H. pylori expresses ADC and contains adequate arginine (Tomb et al., 1997). Therefore, it seems reasonable to hypothesize that agmatine may be responsible for stimulating stomach acid release during gastrointestinal H. pylori colonization, especially since H. pylori-induced acid release is not yet completely understood (el Omar et al., 1995; Courillon-Mallet et al., 1995; Sobhani et al., 1996). More recently it was shown that the growth of H. pylori is dependent on agmatine concentrations in the culture medium and that cell lines from different stomach biopsies synthesize different quantities of agmatine (Molderings et al., 1999d). Furthermore, the agmatine content in the gastric juice of H. pylori positive patients (83.5±18.7 ng ml−1) is double that of gastric juice isolated from H. pylori negative patients (46.8±18.7 ng ml−1; Molderings et al., 1999d, Table 5). Considering the mechanism of an agmatine-induced secretion of gastric acid, a route via the stimulated release of histamine from enterochromaffin cells seems the most probable. It can not, however, be ruled out that an increased gastrin secretion leads to the increased quantity of acid, particularly since H. pylori infection is associated with a raised plasma concentration of gastrin (Peterson, 1996; Molderings et al., 1999a). However, since there is no correlation between plasma gastrin concentration and agmatine concentration in gastric juice, this hypothesis appears doubtful according to our current state of knowledge. The significance of agmatine in the gastric juice of H. pylori negative patients remains unclear. Whether agmatine is subject to degradation to putrescine in both the gastric juice and fundus itself has not yet been demonstrated. In H. pylori positive patients, however, an increased putrescine and spermidine content could be shown in biopsies, supporting the hypothesis that agmatine can contribute to the carcinogenic actions of H. pylori by this route, since polyamines have been attributed an important role in cell proliferation and carcinogenesis (Berdinskikh et al., 1991; Leveque et al., 1998; Blachier et al., 1995).
Is agmatine a CDS?
As the preceding paragraphs have described, agmatine can be characterized as a substance synthesized by an ADC in mammals (Li et al., 1994a), which is heterogeneously distributed in various organs (Raasch et al., 1995a) and subcellular compartments (Otake et al., 1998), released (Reis & Regunathan, 1998) and inactivated by reuptake (Sastre et al., 1997; Molderings et al., 2001) or metabolism via agmatinase (Sastre et al., 1996b) or DAO (Holt & Baker, 1995). Moreover, effects on various organ systems have been shown, even though very high agmatine concentrations were needed to provoke various biological effects. Now we are faced with the question as to whether agmatine represents the active principle in the CDS isolated by Atlas (Atlas & Burstein, 1984a,1984b; Atlas et al., 1987).
Comparison between CDS and agmatine regarding their molecular and physiological properties (overview in Tables 1 and 2)
A molecular mass of 587 daltons has been determined for CDS (Atlas & Burstein, 1984a,1984b; Atlas et al., 1987). Its UV spectrum shows two absorption maxima at 224 and 276 nm, which suggests an aromatic structure. A ninhydrin-negative reaction excludes the possibility that it contains an amino group. The physicochemical properties of agmatine on the other hand seem to contradict those of CDS completely, i.e. a molecular weight of 130 daltons, an absorption maximum of 200 nm (suggesting an aliphatic structure), as well as a ninhydrin-positive reaction confirming an amine structure. These chemical differences alone, combined with the different abundance in the brain and the widely differing affinities for imidazoline binding sites and α2-adrenoceptors, have already lead Atlas (1994; 1995) to declare that agmatine and CDS are not identical. Piletz et al. (1995) and Pinthong et al. (1995b) made similar arguments, when they demonstrated differing affinity profiles for agmatine and CDS to imidazoline binding sites and α2-adrenoceptors by radioligand binding studies and contradicted the findings of Li et al. (1994a). In the response from Reis et al. (1994) published in Science to Atlas's publication (Atlas, 1994), the authors quoted various, primarily methodological and preparative reasons to explain the physicochemical inconsistencies. In addition, the authors emphasized once again that agmatine does not represent CDS exclusively, but that CDS might represent a family of substances, of which agmatine is only one member. This was quite deliberately expressed in the title of their publication, in which the authors talked of agmatine as ‘one‘ and not 'the‘ CDS.
Since 1994, numerous papers have been published which have concerned themselves with the physiological and/or pathophysiological functions of agmatine and discussed the hypothesis that agmatine might be a neurotransmitter or neuromodulator (see Table 2, Figure 3). However, the following findings and considerations contradict this hypothesis: (1) Findings with agmatine against CDS are in some cases completely contradictory to those published for CDS. This applies particularly to the cardiovascular system with respect to agmatine's effects on isolated vessels (Table 4) and after its i.c.v. application (Table 5). After central application, CDS shows clear, blood pressure-increasing effects and acts antagonistically to clonidine (Bousquet et al., 1986; 1987; Pinthong et al., 1995b). Central agmatine dosing leads on the other hand to hypertensive effects, whereby individual findings from different publications appear to be inconsistent, and as mentioned above, such discrepancies are usually attributed to the use of different species and/or sites of application (Sun et al., 1995; Szabo et al., 1995; Penner & Smyth, 1996; Head et al., 1997; Schäfer et al., 1999a). Unlike clonidine and CDS (Synetos et al., 1991), at most weak and usually no effects could be shown in isolated organs (Pinthong et al., 1995a; Gonzalez et al., 1996; Schäfer et al., 1999a). A clonidine-antagonistic activity could not be demonstrated. After peripheral agmatine-application, a hypotensive effect was even observed, albeit at extremely high and non-physiological concentrations (Gao et al., 1995; Schäfer et al., 1999a). In addition to the cardiovascular actions, contrary effects between CDS and agmatine have been determined for catecholamine and insulin secretion which predispose a negation of a CDS-like activity for agmatine. Clearer effects of agmatine could be shown for gastrointestinal (Table 5) and renal functions, analgesia, as well as cell growth (Figure 5), whereby CDS was not investigated for its ability to modulate all these functions. (2) From a mechanistic viewpoint there is inconsistency regarding the correlation between agmatine's function on various biological systems (e.g. growth effects, insulin release; see Figures 2 and 5) and its mediation via imidazoline binding sites. Although biological effects were indeed observed, they were rather attributed to polyamines derived from the metabolic breakdown of agmatine. This contradicts a function as an endogenous ligand for imidazoline binding sites. (3) Another important objection is the distribution pattern of agmatine in various organs (Raasch et al., 1995a) which differs widely from that of CDS (Meeley et al., 1992, see Table 1). As an example, the highest concentrations of CDS are found in the adrenal gland where only low concentrations of agmatine exist. Moreover, the low correlation (r=0.2193) between agmatine and CDS tissue levels in both studies clearly indicates that agmatine can not exclusively represent CDS, but that it represents rather a member of a whole CDS family. In addition, since agmatine is not recognized by the CDS-antibody (Wang et al., 1997), irCDS can not be agmatine. If one considers that cCDS is similar to irCDS (Meeley et al., 1992), one therefore has to conclude that CDS is not agmatine. Whether antibodies against agmatine (Wang et al., 1995) recognize CDS has not been investigated until now. (4) The most serious doubt as to whether agmatine is a CDS is that all in vitro and in vivo reactions of agmatine, as described in preceding sections, only occurred at heroic concentrations, usually much higher than those endogenously present in the corresponding tissues. For example, the agmatine doses (i.v.) required to cause a significant blood pressure reduction were approximately 10% of the lethal dose in the experimental model. Furthermore, when one compares agmatine with other vasoactive substances such as bradykinin, noradrenaline or angiotensin II, only a 1000 – 10,000 fold higher dosage brings about the same blood pressure effect (Gao et al., 1995), which excludes agmatine as being an endogenous neuromodulator or neurotransmitter. The extremely high concentrations of agmatine are probably necessary because of its moderate affinity in the micromolar range towards I1- and I2-binding sites as well as towards α2-adrenoceptors. This differs grossly (25 – 330 fold) from the affinities of CDS observed at the same binding sites and receptors.
Identification of other CDS's from the CDS family
Because of the fact that agmatine appears in the majority of publications, and that it is seen as a substance within a whole CDS family, we should now ask whether other substances with CDS similar properties have been identified.
Arginine is known to be metabolized by NOS to NO and citrulline, whereby NO acts as a potent vasodilator. As an alternative to this route it could be shown that arginine can be degraded to hydroxyarginine, and that this can also be degraded to NO in a second step, whereby hydroxyarginine itself has relaxant properties (Zembowicz et al., 1991; 1992a,1992b,1992c). As an analogue to this metabolic path, it has also been shown that the hydroxylation product of agmatine, just like agmatine itself, is able to dilate aortal rings, whereby no relaxing effect could be seen following preincubation with a NOS inhibitor (L-NAME) or denudation of the epithelium (Ishikawa et al., 1995). Such vasoactive properties of agmatine, however, contradict studies where no relaxing effects for agmatine have been shown. From these observations the authors concluded that agmatine and hydroxyagmatine represent substrates for NOS and in this way possess endothelium-dependent relaxing activity. However, until now neither the existence of this substance nor the metabolic path itself have been confirmed in the tissue. These findings also contradict those of others (Galea et al., 1996) who characterized agmatine as an inhibitor rather than a substrate for NOS.
Modified protocols for CDS preparations have resulted in the isolation of another 'clonidine-displacing substance‘, for which it has not yet been definitely clarified whether it is different or identical to the substance described by Atlas (Atlas & Burstein, 1984a,1984b; Atlas et al., 1987). Spectroscopic studies and HPLC have shown that this isolated CDS is not noradrenaline, adrenaline, histamine, agmatine, guanosine, GMP, GDP or GDP, but that it represents a guanosine derivative (Grigg et al., 1998; Parker et al., 1999a,1999b). Functional studies and binding data, however, remain to be performed.
To this extent there is some evidence that CDS might comprise a family of structurally and functionally similar substances, of which agmatine is one, but specific evidence, especially at a functional level, is outstanding for other CDSs.
Acknowledgments
The authors would like to thank Dr J.P. Keogh for his linguistic assistance in preparing the manuscript.
Abbreviations
- ADC
arginine decarboxylase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP5
(±-2-amino-5-phosphonopentanoic acid
- APR
absolute proximal reabsorption
- cCDS
classical CDS
- CDS
Clonidine Displacing Substance
- DAO
diamine oxidase
- IFN-γ
interferon-gamma
- IL-10
Interleukin-10
- irCDS
immunoreactive CDS
- LPS
lipopolysaccharide
- MAO
monoamine oxidase
- MAP-kinases
mitogen-activated protein kinases
- MTC cells
mouse kidney proximal tubule cells
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NOS
NO synthase
- ODC
ornithine decarboxylase
- PDGF
platelet derived growth factor
- PKC
protein kinase C
- RVLM
rostroventrolateral medulla
- SAMDC
S-adenosylmethionine decarboxylase
- SHR
spontaneously hypertensive rats
- SNGFR
single nephron glomerular filtration rate
- SSAO
semicarbazide-sensitive aminoxidase
- SSAT
spermidine/spermine acetyltransferase
- TGF-β
transforming growth factor-β
References
- AIRIO J., AHTEE L. The involvement of noradrenergic transmission in the morphine-induced locomotor hyperactivity in mice withdrawn from repeated morphine treatment. Br. J. Pharmacol. 1999;126:1609–1619. doi: 10.1038/sj.bjp.0702485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERTI K., WOODS H., WHALLEY M. Mechanism of action of the monoguanidine hypoglaemic agents, galegine and agmatine. Eur. J. Clin. Invest. 1973;3:208. [Google Scholar]
- ALEMANY R., OLMOS G., ESCRIBA P.V., MENARGUES A., OBACH R., GARCIA-SEVILLA J.A. LSL 60101, a selective ligand for imidazoline I2 receptors, on glial fibrillary acidic protein concentration. Eur. J. Pharmacol. 1995;280:205–210. doi: 10.1016/0014-2999(95)00214-6. [DOI] [PubMed] [Google Scholar]
- ALLAN D.R., PENNER S.B., SMYTH D.D. Renal imidazoline preferring sites and solute excretion in the rat. Br. J. Pharmacol. 1993;108:870–875. doi: 10.1111/j.1476-5381.1993.tb13480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLAN D.R., PENNER S.B., SMYTH D.D. Antagonism by idazoxan at low dose but not high dose, of the natriuretic action of moxonidine. Br. J. Pharmacol. 1996;117:29–34. doi: 10.1111/j.1476-5381.1996.tb15150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTMAN J.D., TRENDELENBURG A.U., MACMILLAN L., BERNSTEIN D., LIMBIRD L., STARKE K., KOBILKA B.K., HEIN L. Abnormal regulation of the sympathetic nervous system in alpha2A- adrenergic receptor knockout mice. Mol. Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- ARICIOGLU-KARTAL F., UZBAY I.T. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci. 1997;61:1775–1781. doi: 10.1016/s0024-3205(97)00801-1. [DOI] [PubMed] [Google Scholar]
- ATLAS D. Clonidine-displacing substance (CDS) and its putative imidazoline receptor. New leads for further divergence of alpha 2-adrenergic receptor activity. Biochem. Pharmacol. 1991;41:1541–1549. doi: 10.1016/0006-2952(91)90152-u. [DOI] [PubMed] [Google Scholar]
- ATLAS D. Identifying clonidine-displacing substance. Science. 1994;266:462–464. doi: 10.1126/science.7939689. [DOI] [PubMed] [Google Scholar]
- ATLAS D. Molecular and physiological properties of clonidine-displacing substance. Ann. N.Y. Acad. Sci. 1995;763:314–324. doi: 10.1111/j.1749-6632.1995.tb32417.x. [DOI] [PubMed] [Google Scholar]
- ATLAS D., BURSTEIN Y. Isolation and partial purification of a clonidine-displacing endogenous brain substance. Eur. J. Biochem. 1984a;144:287–293. doi: 10.1111/j.1432-1033.1984.tb08462.x. [DOI] [PubMed] [Google Scholar]
- ATLAS D., BURSTEIN Y. Isolation of an endogenous clonidine-displacing substance from rat brain. FEBS Lett. 1984b;170:387–390. doi: 10.1016/0014-5793(84)81350-2. [DOI] [PubMed] [Google Scholar]
- ATLAS D., DIAMANT S., FALES H.M., PANNELL L. The brain's own clonidine: purification and characterization of endogenous clonidine displacing substance from brain. J. Cardiovasc. Pharmacol. 1987;10 Suppl 12:S122–S127. [PubMed] [Google Scholar]
- AUGUET M., VIOSSAT I., MARIN J.G., CHABRIER P.E. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn. J. Pharmacol. 1995;69:285–287. doi: 10.1254/jjp.69.285. [DOI] [PubMed] [Google Scholar]
- BERDEU D., GROSS R., RIBES G., LOUBATIERES-MARIANI M.M., BERTRAND G. Effects of imidazolines and derivatives on insulin secretion and vascular resistance in perfused rat pancreas. Eur. J. Pharmacol. 1994;254:119–125. doi: 10.1016/0014-2999(94)90378-6. [DOI] [PubMed] [Google Scholar]
- BERDEU D., PUECH R., LOUBATIERES-MARIANI M.M., BERTRAND G. Agmatine is not a good candidate as endogenous ligand for imidazoline sites of pancreatic B cells and vascular bed. Eur. J. Pharmacol. 1996;308:301–304. doi: 10.1016/0014-2999(96)00329-9. [DOI] [PubMed] [Google Scholar]
- BERDINSKIKH N.K., IGNATENKO N.A., ZALETOK S.P., GANINA K.P., CHORNIY V.A. Ornithine decarboxylase activity and polyamine content in adenocarcinomas of human stomach and large intestine. Int. J. Cancer. 1991;47:496–498. doi: 10.1002/ijc.2910470404. [DOI] [PubMed] [Google Scholar]
- BERENDSE H.W., GROENEWEGEN H.J. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- BHANDARE P.N., RATABOLI P.V., D'SOUZA R.S. Dual action of clonidine on ethanol-induced gastric lesions: is the imidazoline-preferring receptor involved. Eur. J. Pharmacol. 1991;199:243–245. doi: 10.1016/0014-2999(91)90464-2. [DOI] [PubMed] [Google Scholar]
- BIDET M., TAUC M., KOECHLIN N., POUJEOL P. Video microscopy of intracellular pH in primary cultures of rabbit proximal and early distal tubules. Pflugers Arch. 1990;416:270–280. doi: 10.1007/BF00392063. [DOI] [PubMed] [Google Scholar]
- BLACHIER F., SELAMNIA M., ROBERT V., M'RABET-TOUIL H., DUEE P.H. Metabolism of L-arginine through polyamine and nitric oxide synthase pathways in proliferative or differentiated human colon carcinoma cells. Biochim. Biophys. Acta. 1995;1268:255–262. doi: 10.1016/0167-4889(95)00083-5. [DOI] [PubMed] [Google Scholar]
- BLANDIZZI C., BERNARDINI M.C., NATALE G., DEL TACCA M. Alpha 2-adrenoceptor-mediated inhibitory and excitatory effects of detomidine on rat gastric acid secretion. J. Pharm. Pharmacol. 1990a;42:685–688. doi: 10.1111/j.2042-7158.1990.tb06559.x. [DOI] [PubMed] [Google Scholar]
- BLANDIZZI C., BERNARDINI M.C., VIZI E.S., DEL TACCA M. Modulation of gastric acid secretion by peripheral presynaptic alpha 2- adrenoceptors at both sympathetic and parasympathetic pathways. J. Auton. Pharmacol. 1990b;10:305–312. doi: 10.1111/j.1474-8673.1990.tb00030.x. [DOI] [PubMed] [Google Scholar]
- BLANDIZZI C., NATALE G., COLUCCI R., CARIGNANI D., LAZZERI G., DEL TACCA M. Characterization of alpha 2-adrenoceptor subtypes involved in the modulation of gastric acid secretion. Eur. J. Pharmacol. 1995;278:179–182. doi: 10.1016/0014-2999(95)00170-p. [DOI] [PubMed] [Google Scholar]
- BLASCHKO H. The natural history of amine oxidases. Rev. Physiol. Biochem. Pharmacol. 1974;70:83–148. doi: 10.1007/BFb0034294. [DOI] [PubMed] [Google Scholar]
- BOCK C., NIEDERHOFFER N., SZABO B. Analysis of the receptor involved in the central hypotensive effect of rilmenidine and moxonidine. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:262–271. doi: 10.1007/pl00005351. [DOI] [PubMed] [Google Scholar]
- BORONAT M.A., OLMOS G., GARCIA-SEVILLA J.A. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other 12-imidazoline ligands. Br. J. Pharmacol. 1998;125:175–185. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSQUET P., FELDMAN J., ATLAS D. An endogenous, non-catecholamine clonidine antagonist increases mean arterial blood pressure. Eur. J. Pharmacol. 1986;124:167–170. doi: 10.1016/0014-2999(86)90138-x. [DOI] [PubMed] [Google Scholar]
- BOUSQUET P., FELDMAN J., ATLAS D. Central cardiovascular effects of a noncatecholamine endogenous ligand for clonidine receptors. J. Cardiovasc. Pharmacol. 1987;10 Suppl 12:S167–S171. [PubMed] [Google Scholar]
- BOUSQUET P., FELDMAN J., SCHWARTZ J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J. Pharmacol. Exp. Ther. 1984;230:232–236. [PubMed] [Google Scholar]
- BRADLEY K.J., HEADLEY P.M. Effect of agmatine on spinal nociceptive reflexes: lack of interaction with alpha2-adrenoceptor or mu-opioid receptor mechanisms. Eur. J. Pharmacol. 1997;331:133–138. doi: 10.1016/s0014-2999(97)01043-1. [DOI] [PubMed] [Google Scholar]
- BRICCA G., DONTENWILL M., MOLINES A., FELDMAN J., BELCOURT A., BOUSQUET P. The imidazoline preferring receptor: binding studies in bovine, rat and human brainstem. Eur. J. Pharmacol. 1989;162:1–9. doi: 10.1016/0014-2999(89)90597-9. [DOI] [PubMed] [Google Scholar]
- BRICCA G., ZHANG J., GRENEY H., DONTENWILL M., STUTZMANN J., BELCOURT A., BOUSQUET P. Relevance of the use of [3H]-clonidine to identify imidazoline receptors in the rabbit brainstem. Br. J. Pharmacol. 1993;110:1537–1543. doi: 10.1111/j.1476-5381.1993.tb13998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.A., CHAN S.L., STILLINGS M.R., SMITH S.A., MORGAN N.G. Antagonism of the stimulatory effects of efaroxan and glibenclamide in rat pancreatic islets by the imidazoline, RX801080. Br. J. Pharmacol. 1993b;110:1017–1022. doi: 10.1111/j.1476-5381.1993.tb13915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.A., LOWETH A.C., SMITH S.A., MORGAN N.G. Stimulation of insulin secretion by imidazoline compounds is not due to interaction with non-adrenoceptor idazoxan binding sites. Br. J. Pharmacol. 1993a;108:312–317. doi: 10.1111/j.1476-5381.1993.tb12801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.M., MACKINNON A.C., MCGRATH J.C., SPEDDING M., KILPATRICK A.T. Alpha 2-adrenoceptor subtypes and imidazoline-like binding sites in the rat brain. Br. J. Pharmacol. 1990;99:803–809. doi: 10.1111/j.1476-5381.1990.tb13010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCCAFUSCO J.J., LAPP C.A., WESTBROOKS K.L., ERNSBERGER P. Role of medullary I1-imidazoline and alpha 2-adrenergic receptors in the antihypertensive responses evoked by central administration of clonidine analogs in conscious spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1995;273:1162–1171. [PubMed] [Google Scholar]
- CARPENE C., COLLON P., REMAURY A., CORDI A., HUDSON A., NUTT D., LAFONTAN M. Inhibition of amine oxidase activity by derivatives that recognize imidazoline 12 sites. J. Pharmacol. Exp. Ther. 1995;272:681–688. [PubMed] [Google Scholar]
- CHAN S.L. Clonidine-displacing substance and its putative role in control of insulin secretion: a minireview. Gen. Pharmacol. 1998;31:525–529. doi: 10.1016/s0306-3623(98)00052-4. [DOI] [PubMed] [Google Scholar]
- CHAN S.L., ATLAS D., JAMES R.F., MORGAN N.G. The effect of the putative endogenous imidazoline receptor ligand, clonidine-displacing substance, on insulin secretion from rat and human islets of Langerhans. Br. J. Pharmacol. 1997;120:926–932. doi: 10.1038/sj.bjp.0700964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN S.L., BROWN C.A., SCARPELLO K.E., MORGAN N.G. The imidazoline site involved in control of insulin secretion: characteristics that distinguish it from I1- and I2-sites. Br. J. Pharmacol. 1994;112:1065–1070. doi: 10.1111/j.1476-5381.1994.tb13191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN S.L., BROWN C.A., SCARPELLO K.E., MORGAN N.G. Pancreatic beta-cells express an imidazoline binding site that is distinct from I1 and I2 sites. Ann. N.Y. Acad. Sci. 1995;763:153–156. doi: 10.1111/j.1749-6632.1995.tb32400.x. [DOI] [PubMed] [Google Scholar]
- CHAN S.L., DUNNE M.J., STILLINGS M.R., MORGAN N.G. The alpha 2-adrenoceptor antagonist efaroxan modulates K+ATP channels in insulin-secreting cells. Eur. J. Pharmacol. 1991;204:41–48. doi: 10.1016/0014-2999(91)90833-c. [DOI] [PubMed] [Google Scholar]
- CHAN S.L., MORGAN N.G. Stimulation of insulin secretion by efaroxan may involve interaction with potassium channels. Eur. J. Pharmacol. 1990;176:97–101. doi: 10.1016/0014-2999(90)90137-u. [DOI] [PubMed] [Google Scholar]
- CHOI D.W., KOH J.Y., PETERS S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J. Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLUCCI R., BLANDIZZI C., CARIGNANI D., PLACANICA G., LAZZERI G., DEL TACCA M. Effects of imidazoline derivatives on cholinergic motility in guinea-pig ileum: involvement of presynaptic alpha2-adrenoceptors or imidazoline receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357:682–691. doi: 10.1007/pl00005225. [DOI] [PubMed] [Google Scholar]
- COUPRY I., ATLAS D., PODEVIN R.A., UZIELLI I., PARINI A. Imidazoline-guanidinium receptive site in renal proximal tubule: asymmetric distribution, regulation by cations and interaction with an endogenous clonidine displacing substance. J. Pharmacol.. Exp. Ther. 1990;252:293–299. [PubMed] [Google Scholar]
- COURILLON-MALLET A., LAUNAY J.M., ROUCAYROL A.M., CALLEBERT J., EMOND J.P., TABUTEAU F., CATTAN D. Helicobacter pylori infection: physiopathologic implication of N alpha-methyl histamine. Gastroenterology. 1995;108:959–966. doi: 10.1016/0016-5085(95)90190-6. [DOI] [PubMed] [Google Scholar]
- DALY M.J. The classification of adrenoceptors and their effects on gastric acid secretion. Scand. J. Gastroenterol. 1984;89 Suppl.:3–9. [PubMed] [Google Scholar]
- DE VOS H., BRICCA G., DE KEYSER J., DE BACKER J.P., BOUSQUET P., VAUQUELIN G. Imidazoline receptors, non-adrenergic idazoxan binding sites and alpha 2-adrenoceptors in the human central nervous system. Neuroscience. 1994;59:589–598. doi: 10.1016/0306-4522(94)90179-1. [DOI] [PubMed] [Google Scholar]
- DE VOS H., CONVENTS A., DE KEYSER J., DE BACKER J.P., VAN MEGEN I.J., EBINGER G., VAUQUELIN G. Autoradiographic distribution of alpha 2 adrenoceptors, NAIBS, and 5- HT1A receptors in human brain using [3H]idazoxan and [3H]rauwolscine. Brain Res. 1991;566:13–20. doi: 10.1016/0006-8993(91)91675-q. [DOI] [PubMed] [Google Scholar]
- DEGREGORIO-ROCASOLANO N., OLMOS G., GASULL T., BORONAT M.A., TRULLAS R., GARCIA-SEVILLA J.A. Protection by imidazol(ine) compounds of L-glutamate neurotoxicity through NMDA receptor blockade. Ann. N.Y. Acad. Sci. 1999;881:452. doi: 10.1111/j.1749-6632.1999.tb09393.x. [DOI] [PubMed] [Google Scholar]
- DEL TACCA M., SOLDANI G., BERNARDINI C., MARTINOTTI E., IMPICCIATORE M. Pharmacological studies on the mechanisms underlying the inhibitory and excitatory effects of clonidine on gastric acid secretion. Eur. J. Pharmacol. 1982a;81:255–261. doi: 10.1016/0014-2999(82)90443-5. [DOI] [PubMed] [Google Scholar]
- DEL TACCA M., SOLDANI G., POLLONI A., BERNARDINI C., MARTINOTTI E. The effect of pirenzepine on gastric acid secretion and gastrin release induced by bombesin and meal in the dog. Scand. J. Gastroenterol. 1982b;72 Suppl.:105–110. [PubMed] [Google Scholar]
- DIAMANT S., ATLAS D. An endogenous brain substance, CDS (clonidine-displacing-substance), inhibits the twitch response of rat vas deferens. Biochem. Biophys. Res. Commun. 1986;134:184–190. doi: 10.1016/0006-291x(86)90545-0. [DOI] [PubMed] [Google Scholar]
- DIETRICH W.D., HALLEY M., VALDES I., BUSTO R. Interrelationships between increased vascular permeability and acute neuronal damage following temperature-controlled brain ischemia in rats. Acta Neuropathol. 1991;81:615–625. doi: 10.1007/BF00296371. [DOI] [PubMed] [Google Scholar]
- DONTENWILL M., BRICCA G., MOLINES A., BOUSQUET P., BELCOURT A. Production and characterization of anti-clonidine antibodies not cross-reacting with catecholamines. Eur. J. Pharmacol. 1988;149:249–255. doi: 10.1016/0014-2999(88)90655-3. [DOI] [PubMed] [Google Scholar]
- DRIESSEN A.J., SMID E.J., KONINGS W.N. Transport of diamines by Enterococcus faecalis is mediated by an agmatine-putrescine antiporter. J. Bacteriol. 1988;170:4522–4527. doi: 10.1128/jb.170.10.4522-4527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNNE M.J., HARDING E.A., JAGGAR J.H., SQUIRES P.E., LIANG R., KANE C., JAMES R.F., LONDON N.J. Potassium channels, imidazolines, and insulin-secreting cells. Ann. N.Y. Acad. Sci. 1995;763:243–261. doi: 10.1111/j.1749-6632.1995.tb32410.x. [DOI] [PubMed] [Google Scholar]
- EL OMAR E.M., PENMAN I.D., ARDILL J.E., CHITTAJALLU R.S., HOWIE C., MCCOLL K.E. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- ELLIOTT K., KEST B., MAN A., KAO B., INTURRISI C.E. N-methyl-D-aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology. 1995;13:347–356. doi: 10.1016/0893-133X(95)00083-P. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P. The I1-imidazoline receptor and its cellular signaling pathways. Ann. N.Y. Acad. Sci. 1999;881:35–53. doi: 10.1111/j.1749-6632.1999.tb09339.x. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., FEINLAND G., MEELEY M.P., REIS D.J. Characterization and visualization of clonidine-sensitive imidazole sites in rat kidney which recognize clonidine-displacing substance. Am. J. Hypertens. 1990b;3:90–97. doi: 10.1093/ajh/3.2.90. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., GIULIANO R., WILLETTE R.N., GRANATA A.R., REIS D.J. Hypotensive action of clonidine analogues correlates with binding affinity at imidazole and not alpha-2-adrenergic receptors in the rostral ventrolateral medulla. J. Hypertens. 1988b;6 Suppl.:S554–S557. doi: 10.1097/00004872-198812040-00174. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., GIULIANO R., WILLETTE R.N., REIS D.J. Role of imidazole receptors in the vasodepressor response to clonidine analogs in the rostral ventrolateral medulla. J. Pharmacol. Exp. Ther. 1990a;253:408–418. [PubMed] [Google Scholar]
- ERNSBERGER P., GRAVES M.E., GRAFF L.M., ZAKIEH N., NGUYEN P., COLLINS L.A., WESTBROOKS K.L., JOHNSON G.G. I1-imidazoline receptors. Definition, characterization, distribution, and transmembrane signaling. Ann. N.Y. Acad. Sci. 1995;763:22–42. doi: 10.1111/j.1749-6632.1995.tb32388.x. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., HAXHIU M.A., GRAFF L.M., COLLINS L.A., DRESHAJ I., GROVE D.L., GRAVES M.E., SCHÄFER S.G., CHRISTEN M.O. A novel mechanism of action for hypertension control: moxonidine as a selective I1-imidazoline agonist. Cardiovasc. Drugs Ther. 1994;8 Suppl.1:27–41. doi: 10.1007/BF00877082. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., MEELEY M.P., MANN J.J., REIS D.J. Clonidine binds to imidazole binding sites as well as alpha 2- adrenoceptors in the ventrolateral medulla. Eur. J. Pharmacol. 1987;134:1–13. doi: 10.1016/0014-2999(87)90125-7. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., MEELEY M.P., REIS D.J. An endogenous substance with clonidine-like properties: selective binding to imidazole sites in the ventrolateral medulla. Brain Res. 1988a;441:309–318. doi: 10.1016/0006-8993(88)91409-6. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., WESTBROOKS K.L., CHRISTEN M.O., SCHÄFER S.G. A second generation of centrally acting antihypertensive agents on putative I1-imidazoline receptors. J. Cardiovasc. Pharmacol. 1992;20 Suppl.4:S1–S10. [Google Scholar]
- ESCRIBA P.V., ALEMANY R., SASTRE M., OLMOS G., OZAITA A., GARCIA-SEVILLA J.A. Pharmacological modulation of immunoreactive imidazoline receptor proteins in rat brain: relationship with non-adrenoceptor [3H]-idazoxan binding sites. Br. J. Pharmacol. 1996;118:2029–2036. doi: 10.1111/j.1476-5381.1996.tb15640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCRIBA P.V., OZAITA A., GARCIA-SEVILLA J.A. Pharmacologic characterization of imidazoline receptor proteins identified by immunologic techniques and other methods. Ann. N.Y. Acad. Sci. 1999;881:8–25. doi: 10.1111/j.1749-6632.1999.tb09336.x. [DOI] [PubMed] [Google Scholar]
- EVANS R.G., HAYNES J.M. Alpha 2 adrenoceptor- and Imidazoline-prefering binding sites in the dog kidney. Ann. N.Y. Acad. Sci. 1995;763:357–360. doi: 10.1111/j.1749-6632.1995.tb32423.x. [DOI] [PubMed] [Google Scholar]
- FAIRBANKS C.A., WILCOX G.L. Moxonidine, a selective alpha2-adrenergic and imidazoline receptor agonist, produces spinal antinociception in mice. J. Pharmacol. Exp. Ther. 1999;290:403–412. [PubMed] [Google Scholar]
- FELSEN D., ERNSBERGER P., MEELEY M.P., REIS D.J. Clonidine displacing substance is biologically active on smooth muscle. Eur. J. Pharmacol. 1987;142:453–455. doi: 10.1016/0014-2999(87)90087-2. [DOI] [PubMed] [Google Scholar]
- FENG Y., HALARIS A.E., PILETZ J.E. Determination of agmatine in brain and plasma using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1997;691:277–286. doi: 10.1016/s0378-4347(96)00458-6. [DOI] [PubMed] [Google Scholar]
- FINCH L., BUCKINGHAM R.E., MOORE R.A., BUCHER T.J. Evidence for a central alpha-sympathomimetic action of clonidine in the rat. J. Pharm. Pharmacol. 1975;27:181–186. doi: 10.1111/j.2042-7158.1975.tb09434.x. [DOI] [PubMed] [Google Scholar]
- FRANK E. Über eine synthetische Substanz (Synthalin) mit insulinartiger Wirkung. Die Naturwissenschaften. 1927;9:213–215. [Google Scholar]
- FUDER H., SCHWARZ P. Desensitization of inhibitory prejunctional alpha 2-adrenoceptors and putative imidazoline receptors on rabbit heart sympathetic nerves. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:127–133. doi: 10.1007/BF00164788. [DOI] [PubMed] [Google Scholar]
- GALEA E., REGUNATHAN S., ELIOPOULOS V., FEINSTEIN D.L., REIS D.J. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem. J. 1996;316:247–249. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO Y., GUMUSEL B., KOVES G., PRASAD A., HAO Q., HYMAN A., LIPPTON H. Agmatine: a novel endogenous vasodilator substance. Life Sci. 1995;57:L83–L86. doi: 10.1016/0024-3205(95)02011-7. [DOI] [PubMed] [Google Scholar]
- GARCIA-SEVILLA J.A., ESCRIBA P.V., BUSQUETS X., WALZER C., GUIMON J. Platelet imidazoline receptors and regulatory G proteins in patients with major depression. Neuroreport. 1996;8:169–172. doi: 10.1097/00001756-199612200-00034. [DOI] [PubMed] [Google Scholar]
- GARCIA-SEVILLA J.A., ESCRIBA P.V., GUIMON J. Imidazoline receptors and human brain disorders. Ann. N.Y. Acad. Sci. 1999;881:392–409. doi: 10.1111/j.1749-6632.1999.tb09388.x. [DOI] [PubMed] [Google Scholar]
- GARCIA-SEVILLA J.A., ESCRIBA P.V., OZAITA A., WALZER C., GUIMON J. Density of imidazoline receptors in platelets of euthymic patients with bipolar affective disorder and in brains of lithium-treated rats. Biol. Psychiatry. 1998a;43:616–618. doi: 10.1016/s0006-3223(97)00398-3. [DOI] [PubMed] [Google Scholar]
- GARCIA-SEVILLA J.A., ESCRIBA P.V., WALZER C., BOURAS C., GUIMON J. Imidazoline receptor proteins in brains of patients with Alzheimer's disease. Neurosci. Lett. 1998b;247:95–98. doi: 10.1016/s0304-3940(98)00265-1. [DOI] [PubMed] [Google Scholar]
- GILAD G.M., GILAD V.H., RABEY J.M. Arginine and ornithine decarboxylation in rodent brain: coincidental changes during development and after ischemia. Neurosci. Lett. 1996c;216:33–36. doi: 10.1016/0304-3940(96)12996-7. [DOI] [PubMed] [Google Scholar]
- GILAD G.M., SALAME K., RABEY J.M., GILAD V.H. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996b;58:L-6. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- GILAD G.M., WOLLAM Y., IAINA A., RABEY J.M., CHERNIHOVSKY T., GILAD V.H. Metabolism of agmatine into urea but not into nitric oxide in rat brain. Neuroreport. 1996a;7:1730–1732. doi: 10.1097/00001756-199607290-00007. [DOI] [PubMed] [Google Scholar]
- GLAVIN G.B., CARLISLE M.A., SMYTH D.D. Agmatine, an endogenous imidazoline receptor agonist, increases gastric secretion and worsens experimental gastric mucosal injury in rats. J. Pharmacol. Exp. Ther. 1995;274:741–744. [PubMed] [Google Scholar]
- GLAVIN G.B., SMYTH D.D. Moxonidine decreases gastric secretion and gastric mucosal injury in rats. Ann. N.Y. Acad. Sci. 1995a;763:433–435. doi: 10.1111/j.1749-6632.1995.tb32432.x. [DOI] [PubMed] [Google Scholar]
- GLAVIN G.B., SMYTH D.D. Effects of the selective I1 imidazoline receptor agonist, moxonidine, on gastric secretion and gastric mucosal injury in rats. Br. J. Pharmacol. 1995b;114:751–754. doi: 10.1111/j.1476-5381.1995.tb13268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMEZ R.E., ERNSBERGER P., FEINLAND G., REIS D.J. Rilmenidine lowers arterial pressure via imidazole receptors in brainstem C1 area. Eur. J. Pharmacol. 1991;195:181–191. doi: 10.1016/0014-2999(91)90534-w. [DOI] [PubMed] [Google Scholar]
- GONZALEZ C., REGUNATHAN S., REIS D.J., ESTRADA C. Agmatine, an endogenous modulator of noradrenergic neurotransmission in the rat tail artery. Br. J. Pharmacol. 1996;119:677–684. doi: 10.1111/j.1476-5381.1996.tb15726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSSOP M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21:253–259. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M., BRÜSS M., BÖNISCH H., MOLDERINGS G.J. Presynaptic imidazoline receptors. New developments in characterization and classification. Ann. N.Y. Acad. Sci. 1999;881:171–184. doi: 10.1111/j.1749-6632.1999.tb09356.x. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M., MOLDERINGS G.J. Involvement of presynaptic imidazoline receptors in the alpha 2- adrenoceptor-independent inhibition of noradrenaline release by imidazoline derivatives. Naunyn Schmiedebergs Arch. Pharmacol. 1991;343:271–282. doi: 10.1007/BF00251126. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M., MOLDERINGS G.J., FINK K., SCHLICKER E. Alpha 2-adrenoceptor-independent inhibition by imidazolines and guanidines of noradrenaline release from peripheral, but not central noradrenergic neurons. Ann. N.Y. Acad. Sci. 1995;763:405–419. doi: 10.1111/j.1749-6632.1995.tb32430.x. [DOI] [PubMed] [Google Scholar]
- GRIGG M., MUSGRAVE I.F., BARROW C.J. Isolation and partial structure determination of a clonidine-displacing substance from bovine lung and brain. J. Auton. Nerv. Syst. 1998;72:86–93. doi: 10.1016/s0165-1838(98)00092-7. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON I., WESTERBERG E., WIELOCH T. Protection against ischemia-induced neuronal damage by the alpha 2- adrenoceptor antagonist idazoxan: influence of time of administration and possible mechanisms of action. J. Cereb. Blood Flow Metab. 1990;10:885–894. doi: 10.1038/jcbfm.1990.145. [DOI] [PubMed] [Google Scholar]
- HÄUSER W., DOMINIAK P. Effects of agmatine on catecholamine release and blood pressure of spontaneously hypertensive rats. A possible role for imidazoline receptors. Pharm. Pharmacol. Lett. 1995;5:87–89. [Google Scholar]
- HÄUSER W., GÜTTING J., NGUYEN T., DOMINIAK P. Influence of imidazolines on catecholamine release in pithed spontaneously hypertensive rats. Ann. N.Y. Acad. Sci. 1995;763:573–579. doi: 10.1111/j.1749-6632.1995.tb32452.x. [DOI] [PubMed] [Google Scholar]
- HAEUSLER G. Proceedings: Sympathetic nerve activity after noradrenaline depletion and its alteration by clonidine. Naunyn Schmiedebergs Arch. Pharmacol. 1974a;282 Suppl.:R29. [PubMed] [Google Scholar]
- HAEUSLER G. Clonidine-induced inhibition of sympathetic nerve activity: no indication for a central presynaptic or an indirect sympathomimetic mode of action. Naunyn Schmiedebergs Arch. Pharmacol. 1974b;286:107–111. doi: 10.1007/BF00499107. [DOI] [PubMed] [Google Scholar]
- HALARIS A., ZHU H., FENG Y., PILETZ J.E. Plasma agmatine and platelet imidazoline receptors in depression. Ann. N.Y. Acad. Sci. 1999;881:445–451. doi: 10.1111/j.1749-6632.1999.tb09392.x. [DOI] [PubMed] [Google Scholar]
- HALBREICH U., PILETZ J.E., CARSON S., HALARIS A., ROJANSKY N. Increased imidazoline and alpha 2 adrenergic binding in platelets of women with dysphoric premenstrual syndromes. Biol. Psychiatry. 1993;34:676–686. doi: 10.1016/0006-3223(93)90040-k. [DOI] [PubMed] [Google Scholar]
- HAMILTON C.A. Adrenergic and nonadrenergic effects of imidazoline and related antihypertensive drugs in the brain and periphery. Am. J. Hypertens. 1992a;5:58S–63S. doi: 10.1093/ajh/5.4.58s. [DOI] [PubMed] [Google Scholar]
- HAMILTON C.A. The role of imidazoline receptors in blood pressure regulation. Pharmacol. Ther. 1992b;54:231–248. doi: 10.1016/0163-7258(92)90001-g. [DOI] [PubMed] [Google Scholar]
- HAMILTON C.A., YAKUBU M.A., HOWIE C.A., REID J.L. Do centrally-acting antihypertensive drugs act at non-adrenergic as well as alpha-2 adrenoceptor sites. Clin. Exp. Hypertens. [A] 1992;14:815–835. doi: 10.3109/10641969209036221. [DOI] [PubMed] [Google Scholar]
- HAXHIU M.A., DRESHAJ I., SCHÄFER S.G., ERNSBERGER P. Selective antihypertensive action of moxonidine is mediated mainly by I1-imidazoline receptors in the rostral ventrolateral medulla. J. Cardiovasc. Pharmacol. 1994;24 Suppl.1:S1–S8. doi: 10.1097/00005344-199424001-00002. [DOI] [PubMed] [Google Scholar]
- HEAD G.A., CHAN C.K., GODWIN S.J. Central cardiovascular actions of agmatine, a putative clonidine- displacing substance, in conscious rabbits. Neurochem. Int. 1997;30:37–45. doi: 10.1016/s0197-0186(96)00044-7. [DOI] [PubMed] [Google Scholar]
- HEIN L. Transgenic models of alpha 2-adrenergic receptor subtype function. Rev. Physiol. Biochem. Pharmacol. 2001;142:161–185. doi: 10.1007/BFb0117493. [DOI] [PubMed] [Google Scholar]
- HEIN L., LIMBIRD L.E., EGLEN R.M., KOBILKA B.K. Gene substitution/knockout to delineate the role of alpha 2- adrenoceptor subtypes in mediating central effects of catecholamines and imidazolines. Ann. N.Y. Acad. Sci. 1999;881:265–271. doi: 10.1111/j.1749-6632.1999.tb09368.x. [DOI] [PubMed] [Google Scholar]
- HENSLEY M.L., MEELEY M.P., MCCAULEY P.M., ERNSBERGER P., REIS D.J. Clonidine-displacing substance is present in peripheral tissues of the rat. Am. J. Hypertens. 1989;2:917–919. doi: 10.1093/ajh/2.12.917. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., DEMARINIS R.M., FOWLER P.J., MATTHEWS W.D. Selective alpha-2 adrenoceptor blockade by SK&F 86466: in vitro characterization of receptor selectivity. J. Pharmacol. Exp. Ther. 1986;236:90–96. [PubMed] [Google Scholar]
- HIEBLE J.P., RUFFOLO R.R., , JR Imidazoline receptors: historical perspective. Fundam. Clin. Pharmacol. 1992;6 Suppl.1:7S–13S. doi: 10.1111/j.1472-8206.1992.tb00136.x. [DOI] [PubMed] [Google Scholar]
- HILLAIRE-BUYS D., GROSS R., BLAYAC J.P., RIBES G., LOUBATIERES-MARIANI M.M. Effects of alpha-adrenoceptor agonists and antagonists on insulin secreting cells and pancreatic blood vessels: comparative study. Eur. J. Pharmacol. 1985;117:253–257. doi: 10.1016/0014-2999(85)90610-7. [DOI] [PubMed] [Google Scholar]
- HOLT A., BAKER G.B. Metabolism of agmatine (clonidine-displacing substance) by diamine oxidase and the possible implications for studies of imidazoline receptors. Prog. Brain Res. 1995;106:187–197. doi: 10.1016/s0079-6123(08)61215-7. [DOI] [PubMed] [Google Scholar]
- HORVATH G., KEKESI G., DOBOS I., SZIKSZAY M., KLIMSCHA W., BENEDEK G. Effect of intrathecal agmatine on inflammation-induced thermal hyperalgesia in rats. Eur. J. Pharmacol. 1999;368:197–204. doi: 10.1016/s0014-2999(99)00060-6. [DOI] [PubMed] [Google Scholar]
- HOUI N., KAMISAKI Y., ITOH T. Effects of histamine H2 receptor antagonists on acid secretion stimulated by imidazoline derivatives in isolated parietal cells. Eur. J. Pharmacol. 1987;144:67–76. doi: 10.1016/0014-2999(87)90010-0. [DOI] [PubMed] [Google Scholar]
- HUNTER K.J., STROBOS C.A., FAIRLAMB A.H. Inhibition of polyamine biosynthesis in Crithidia fasciculata by D,L-alpha-difluoromethylornithine and D,L-alpha-difluoromethylarginine. Mol. Biochem. Parasitol. 1991;46:35–43. doi: 10.1016/0166-6851(91)90196-d. [DOI] [PubMed] [Google Scholar]
- ISHIDA-TAKAHASHI A., HORIE M., TSUURA Y., ISHIDA H., AI T., SASAYAMA S. Block of pancreatic ATP-sensitive K+ channels and insulinotrophic action by the antiarrhythmic agent, cibenzoline. Br. J. Pharmacol. 1996;117:1749–1755. doi: 10.1111/j.1476-5381.1996.tb15349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA T., MISONOU T., IKENO M., SATO K., SAKAMAKI T. N omega-hydroxyagmatine: a novel substance causing endothelium- dependent vasorelaxation. Biochem. Biophys. Res. Commun. 1995;214:145–151. doi: 10.1006/bbrc.1995.2268. [DOI] [PubMed] [Google Scholar]
- JONAS J.C., PLANT T.D., HENQUIN J.C. Imidazoline antagonists of alpha 2-adrenoceptors increase insulin release in vitro by inhibiting ATP-sensitive K+ channels in pancreatic beta-cells. Br. J. Pharmacol. 1992;107:8–14. doi: 10.1111/j.1476-5381.1992.tb14456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES B.E., YANG T.Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- JURKIEWICZ N.H., GARCEZ DO C.L., HIRATA H., DA COSTA S.W., JURKIEWICZ A. Functional properties of agmatine in rat vas deferens. Eur. J. Pharmacol. 1996;307:299–304. doi: 10.1016/0014-2999(96)00274-9. [DOI] [PubMed] [Google Scholar]
- KAMISAKI Y., ISHIKAWA T., TAKAO Y., OMODANI H., KUNO N., ITOH T. Binding of [3H]p-aminoclonidine to two sites, alpha 2-adrenoceptors and imidazoline binding sites: distribution of imidazoline binding sites in rat brain. Brain Res. 1990;514:15–21. doi: 10.1016/0006-8993(90)90430-j. [DOI] [PubMed] [Google Scholar]
- KASHIWAGI K., KOBAYASHI H., IGARASHI K. Apparently unidirectional polyamine transport by proton motive force in polyamine-deficient Escherichia coli. J. Bacteriol. 1986;165:972–977. doi: 10.1128/jb.165.3.972-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING P.R., GUNDLACH A.L., LOUIS W.J. Quantitative autoradiographic localization in rat brain of alpha 2-adrenergic and non-adrenergic I-receptor binding sites labelled by [3H]rilmenidine. Brain Res. 1995;675:264–278. doi: 10.1016/0006-8993(95)00083-3. [DOI] [PubMed] [Google Scholar]
- KOBINGER W., PICHLER L. Centrally induced reduction in sympathetic tone-a postsynaptic alpha- adrenoceptor-stimulating action of imidazolines. Eur. J. Pharmacol. 1976;40:311–320. doi: 10.1016/0014-2999(76)90068-6. [DOI] [PubMed] [Google Scholar]
- KOLESNIKOV Y., JAIN S., PASTERNAK G.W. Modulation of opioid analgesia by agmatine. Eur. J. Pharmacol. 1996;296:17–22. doi: 10.1016/0014-2999(95)00669-9. [DOI] [PubMed] [Google Scholar]
- KOSSEL A. Über das Agmatin. Hoppe Seylers. Z. Physiol. Chem. 1910;66:257–261. [Google Scholar]
- LAMBERTI C., IPPONI A., BARTOLINI A., SCHUNACK W., MALMBERG-AIELLO P. Antidepressant-like effects of endogenous histamine and of two histamine H1 receptor agonists in the mouse forced swim test. Br. J. Pharmacol. 1998;123:1331–1336. doi: 10.1038/sj.bjp.0701740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER J., PANTEN U., ZIELMANN S. Effects of alpha-adrenoceptor antagonists on clonidine-induced inhibition of insulin secretion by isolated pancreatic islets. Br. J. Pharmacol. 1983;79:415–420. doi: 10.1111/j.1476-5381.1983.tb11014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER S.Z., HICKS P.E. Alpha-adrenoreceptor subtypes in blood vessels: physiology and pharmacology. J. Cardiovasc. Pharmacol. 1984;6 Suppl 4:S547–S558. doi: 10.1097/00005344-198406004-00001. [DOI] [PubMed] [Google Scholar]
- LEVEQUE J., BURTIN F., CATROS-QUEMENER V., HAVOUIS R., MOULINOUX J.P. The gastrointestinal polyamine source depletion enhances DFMO induced polyamine depletion in MCF-7 human breast cancer cells in vivo. Anticancer Res. 1998;18:2663–2668. [PubMed] [Google Scholar]
- LI G., REGUNATHAN S., BARROW C.J., ESHRAGHI J., COOPER R., REIS D.J. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994a;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- LI G., REGUNATHAN S., REIS D.J. Agmatine is synthesized by a mitochondrial arginine decarboxylase in rat brain. Ann. N.Y. Acad. Sci. 1995;763:325–329. doi: 10.1111/j.1749-6632.1995.tb32418.x. [DOI] [PubMed] [Google Scholar]
- LI P., PENNER S.B., SMYTH D.D. Attenuated renal response to moxonidine and rilmenidine in one kidney- one clip hypertensive rats. Br. J. Pharmacol. 1994b;112:200–206. doi: 10.1111/j.1476-5381.1994.tb13052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIKUNGU J., MOLDERINGS G.J., GÖTHERT M. Presynaptic imidazoline receptors and alpha 2-adrenoceptors in the human heart: discrimination by clonidine and moxonidine. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:689–692. doi: 10.1007/BF00170847. [DOI] [PubMed] [Google Scholar]
- LIMON I., COUPRY I., LANIER S.M., PARINI A. Purification and characterization of mitochondrial imidazoline- guanidinium receptive site from rabbit kidney. J. Biol. Chem. 1992;267:21645–21649. [PubMed] [Google Scholar]
- LIU L., COUPAR I.M. Involvement of alpha-2 adrenoceptors in the effects of moxonidine on intestinal motility and fluid transport. J. Pharmacol. Exp. Ther. 1997;283:1367–1374. [PubMed] [Google Scholar]
- LORING R.H. Agmatine acts as an antagonist of neuronal nicotinic receptors. Br. J. Pharmacol. 1990;99:207–211. doi: 10.1111/j.1476-5381.1990.tb14680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORTIE M.J., NOVOTNY W.F., PETERSON O.W., VALLON V., MALVEY K., MENDONCA M., SATRIANO J., INSEL P., THOMSON S.C., BLANTZ R.C. Agmatine, a bioactive metabolite of arginine. Production, degradation, and functional effects in the kidney of the rat. J. Clin. Invest. 1996;97:413–420. doi: 10.1172/JCI118430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYSKO P.G., COX J.A., VIGANO M.A., HENNEBERRY R.C. Excitatory amino acid neurotoxicity at the N-methyl-D-aspartate receptor in cultured neurons: pharmacological characterization. Brain Res. 1989;499:258–266. doi: 10.1016/0006-8993(89)90773-7. [DOI] [PubMed] [Google Scholar]
- MACMILLAN L.B., HEIN L., SMITH M.S., PIASCIK M.T., LIMBIRD L.E. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- MAIESE K., PEK L., BERGER S.B., REIS D.J. Reduction in focal cerebral ischemia by agents acting at imidazole receptors. J. Cereb. Blood Flow Metab. 1992;12:53–63. doi: 10.1038/jcbfm.1992.7. [DOI] [PubMed] [Google Scholar]
- MALLARD N.J., HUDSON A.L., NUTT D.J. Characterization and autoradiographical localization of non- adrenoceptor idazoxan binding sites in the rat brain. Br. J. Pharmacol. 1992;106:1019–1027. doi: 10.1111/j.1476-5381.1992.tb14450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZE M., SEGAL I.S., BLOOR B.C. Clonidine and other alpha2 adrenergic agonists: strategies for the rational use of these novel anesthetic agents. J. Clin. Anesth. 1988;1:146–157. doi: 10.1016/0952-8180(88)90034-7. [DOI] [PubMed] [Google Scholar]
- MEDGETT I.C., MCCULLOCH M.W. Effects of clonidine, guanfacine and three imidazolidine derivatives related to clonidine on blood pressure, heart rate and gastric acid secretion in the anaesthetized rat. Arch. Int. Pharmacodyn. Ther. 1979;240:158–168. [PubMed] [Google Scholar]
- MEELEY M.P., ERNSBERGER P.R., GRANATA A.R., REIS D.J. An endogenous clonidine-displacing substance from bovine brain: receptor binding and hypotensive actions in the ventrolateral medulla. Life Sci. 1986;38:1119–1126. doi: 10.1016/0024-3205(86)90248-1. [DOI] [PubMed] [Google Scholar]
- MEELEY M.P., ERNSBERGER P., MCCAULEY P.M., REIS D.J. Clonidine-specific antibodies as models for imidazole and alpha 2-adrenergic receptor binding sites: implications for the structure of clonidine-displacing substance. J. Hypertens. 1988a;6 Suppl:S490–S493. [PubMed] [Google Scholar]
- MEELEY M.P., HENSLEY M.L., ERNSBERGER P., FELSEN D., REIS D.J. Evidence for a bioactive clonidine-displacing substance in peripheral tissues and serum. Biochem. Pharmacol. 1992;44:733–740. doi: 10.1016/0006-2952(92)90410-k. [DOI] [PubMed] [Google Scholar]
- MEELEY M.P., TOWLE A.C., ERNSBERGER P., REIS D.J. A specific antiserum recognizes clonidine-displacing substance: implications for the structure of the brain's own clonidine. Neurosci. Lett. 1988b;84:84–90. doi: 10.1016/0304-3940(88)90342-4. [DOI] [PubMed] [Google Scholar]
- MERCENIER A., SIMON J.P., HAAS D., STALON V. Catabolism of L-arginine by Pseudomonas aeruginosa. J. Gen. Microbiol. 1980;116:381–389. doi: 10.1099/00221287-116-2-381. [DOI] [PubMed] [Google Scholar]
- MESHUL C.K., SEIL F.J., HERNDON R.M. Astrocytes play a role in regulation of synaptic density. Brain Res. 1987;402:139–145. doi: 10.1016/0006-8993(87)91056-0. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BRODDE O.E., SCHNEPEL B., BEHRENDT J., TSCHADA R., MOTULSKY H.J., INSEL P.A. [3H]idazoxan and some other alpha 2-adrenergic drugs also bind with high affinity to a nonadrenergic site. Mol. Pharmacol. 1989;35:324–330. [PubMed] [Google Scholar]
- MICHEL M.C., ERNSBERGER P. Keeping an eye on the I site: imidazoline-preferring receptors. Trends Pharmacol. Sci. 1992;13:369–370. [PubMed] [Google Scholar]
- MICHEL M.C., INSEL P.A. Are there multiple imidazoline binding sites. Trends Pharmacol. Sci. 1989;10:342–344. doi: 10.1016/0165-6147(89)90002-3. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., RUMP L.C. alpha-Adrenergic regulation of human renal function. Fundam. Clin. Pharmacol. 1996;10:493–503. doi: 10.1111/j.1472-8206.1996.tb00606.x. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., BÖNISCH M., GÖTHERT, BRÜSS H. Agmatine and putrescine uptake in the human glioma cell line SK-MG-1. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:671–679. doi: 10.1007/s002100100418. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., BURIAN M., HOMANN J., NILIUS M., GÖTHERT M. Potential relevance of agmatine as a virulence factor of Helicobacter pylori. Dig. Dis. Sci. 1999d;44:2397–2404. doi: 10.1023/a:1026662316750. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., BURIAN M., MENZEL S., DONECKER K., HOMANN J., NILIUS M., GÖTHERT M. Imidazoline recognition sites and stomach function. Ann. N.Y. Acad. Sci. 1999c;881:332–343. doi: 10.1111/j.1749-6632.1999.tb09377.x. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., DONECKER K., BURIAN M., SIMON W.A., SCHRODER D.W., GÖTHERT M. Characterization of I2 imidazoline and sigma binding sites in the rat and human stomach. J. Pharmacol. Exp. Ther. 1998b;285:170–177. [PubMed] [Google Scholar]
- MOLDERINGS G.J., DONECKER K., GÖTHERT M. Characterization of non-adrenergic [3H]clonidine binding sites in rat stomach: high affinity of imidazolines, guanidines and sigma ligands. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:561–564. doi: 10.1007/BF00171049. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., GÖTHERT M. Inhibitory presynaptic imidazoline receptors on sympathetic nerves in the rabbit aorta differ from I1- and I2-imidazoline binding sites. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:507–516. doi: 10.1007/BF00171042. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., GÖTHERT M., HOMANN J., NILIUS M. Potential relevance of agmatine for the pathogenesis of gastric mucosal lesions. Naunyn Schmiedebergs Arch. Pharmacol. 1998a;357 Suppl:R166. [Google Scholar]
- MOLDERINGS G.J., HENTRICH F., GÖTHERT M. Pharmacological characterization of the imidazoline receptor which mediates inhibition of noradrenaline release in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch. Pharmacol. 1991;344:630–638. doi: 10.1007/BF00174746. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., LIKUNGU J., GÖTHERT M. Presynaptic cannabinoid and imidazoline receptors in the human heart and their potential relationship. Naunyn Schmiedebergs Arch. Pharmacol. 1999b;360:157–164. doi: 10.1007/s002109900043. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., LIKUNGU J., JAKSCHIK J., GÖTHERT M. Presynaptic imidazoline receptors and non-adrenoceptor [3H]-idazoxan binding sites in human cardiovascular tissues. Br. J. Pharmacol. 1997;122:43–50. doi: 10.1038/sj.bjp.0701343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G.J., MENZEL S., GÖTHERT M. Imidazoline derivatives and agmatine induce histamine release from the rat stomach. Naunyn Schmiedebergs Arch. Pharmacol. 1999a;360:711–714. doi: 10.1007/s002109900129. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., MENZEL S., KATHMANN M., SCHLICKER E., GÖTHERT M. Dual interaction of agmatine with the rat alpha(2D)-adrenoceptor: competitive antagonism and allosteric activation. Br. J. Pharmacol. 2000;130:1706–1712. doi: 10.1038/sj.bjp.0703495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G.J., MOURA D., FINK K., BÖNISCH H., GÖTHERT M. Binding of [3H]clonidine to I1-imidazoline sites in bovine adrenal medullary membranes. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:70–76. doi: 10.1007/BF00168539. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., SCHMIDT K., BÖNISCH H., GÖTHERT M. Inhibition of 5-HT3 receptor function by imidazolines in mouse neuroblastoma cells: potential involvement of sigma 2 binding sites. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:245–252. doi: 10.1007/BF00171054. [DOI] [PubMed] [Google Scholar]
- MORGAN N.G., CHAN S.L., BROWN C.A., TSOLI E. Characterization of the imidazoline binding site involved in regulation of insulin secretion. Ann. N.Y. Acad. Sci. 1995;763:361–373. doi: 10.1111/j.1749-6632.1995.tb32424.x. [DOI] [PubMed] [Google Scholar]
- MORGAN N.G., CHAN S.L., MOURTADA M., MONKS L.K., RAMSDEN C.A. Imidazolines and pancreatic hormone secretion. Ann. N.Y. Acad. Sci. 1999;881:217–228. doi: 10.1111/j.1749-6632.1999.tb09364.x. [DOI] [PubMed] [Google Scholar]
- MUNK S.A., LAI R.K., BURKE J.E., ARASASINGHAM P.N., KHARLAMB A.B., MANLAPAZ C.A., PADILLO E.U., WIJONO M.K., HASSON D.W., WHEELER L.A., GARST M.E. Synthesis and pharmacologic evaluation of 2-endo-amino-3-exo-isopropylbicyclo[2.2.1]heptane: a potent imidazoline 1 receptor specific agent. J. Med. Chem. 1996;39:1193–1195. doi: 10.1021/jm960012o. [DOI] [PubMed] [Google Scholar]
- NESTLER E.J., BERHOW M.T., BRODKIN E.S. Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol. Psychiatry. 1996;1:190–199. [PubMed] [Google Scholar]
- NICHOLAS A.P., HOKFELT T., PIERIBONE V.A. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol. Sci. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- NICHOLAS A.P., PIERIBONE V., HOKFELT T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J. Comp. Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- OHARA-IMAIZUMI M., KUMAKURA K. Effects of imidazole compounds on catecholamine release in adrenal chromaffin cells. Cell. Mol. Neurobiol. 1992;12:273–283. doi: 10.1007/BF00712931. [DOI] [PubMed] [Google Scholar]
- OLMOS G., ALEMANY R., ESCRIBA P.V., GARCIA-SEVILLA J.A. The effects of chronic imidazoline drug treatment on glial fibrillary acidic protein concentrations in rat brain. Br. J. Pharmacol. 1994;111:997–1002. doi: 10.1111/j.1476-5381.1994.tb14842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMOS G., DEGREGORIO-ROCASOLANO N., PAZ R.M., GASULL T., ASSUMPCIO B.M., TRULLAS R., VILLARROEL A., LERMA J., GARCIA-SEVILLA J.A. Protection by imidazol(ine) drugs and agmatine of glutamate-induced neurotoxicity in cultured cerebellar granule cells through blockade of NMDA receptor. Br. J. Pharmacol. 1999;127:1317–1326. doi: 10.1038/sj.bjp.0702679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMOS G., GABILONDO A.M., MIRALLES A., ESCRIBA P.V., GARCIA-SEVILLA J.A. Chronic treatment with the monoamine oxidase inhibitors clorgyline and pargyline down-regulates non-adrenoceptor [3H]-idazoxan binding sites in the rat brain. Br. J. Pharmacol. 1993;108:597–603. doi: 10.1111/j.1476-5381.1993.tb12848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMOS G., RIBERA J., GARCIA-SEVILLA J.A. Imidazoli(di)ne compounds interact with the phencyclidine site of NMDA receptors in the rat brain. Eur. J. Pharmacol. 1996;310:273–276. doi: 10.1016/0014-2999(96)00519-5. [DOI] [PubMed] [Google Scholar]
- OTAKE K., RUGGIERO D.A., REGUNATHAN S., WANG H., MILNER T.A., REIS D.J. Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain Res. 1998;787:1–14. doi: 10.1016/s0006-8993(97)01200-6. [DOI] [PubMed] [Google Scholar]
- PANAGIOTIDIS C.A., BLACKBURN S., LOW K.B., CANELLAKIS E.S. Biosynthesis of polyamines in ornithine decarboxylase, arginine decarboxylase, and agmatine ureohydrolase deletion mutants of Escherichia coli strain K-12. Proc. Natl. Acad. Sci. U.S.A. 1987;84:4423–4427. doi: 10.1073/pnas.84.13.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER C.A., HUDSON A.L., NUTT D.J., DILLON M.P., EGLEN R.M., CHAN S.L., MORGAN N.G., CROSBY J. Extraction of active clonidine-displacing substance from bovine lung and comparison with clonidine-displacing substance extracted from other tissues. Eur. J. Pharmacol. 1999a;378:213–221. doi: 10.1016/s0014-2999(99)00449-5. [DOI] [PubMed] [Google Scholar]
- PARKER C.A., HUDSON A.L., NUTT D.J., DILLON M.P., EGLEN R.M., CROSBY J. Comparison of crude methanolic CDS extracts from various tissues. Ann. N.Y. Acad. Sci. 1999b;881:92–96. doi: 10.1111/j.1749-6632.1999.tb09345.x. [DOI] [PubMed] [Google Scholar]
- PEGG A.E., MCCANN P.P. Polyamine metabolism and function. Am. J. Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- PENNER S.B., SMYTH D.D. Natriuresis following central and peripheral administration of agmatine in the rat. Pharmacology. 1996;53:160–169. doi: 10.1159/000139427. [DOI] [PubMed] [Google Scholar]
- PERSSON L., HOLM I., HEBY O. Translational regulation of ornithine decarboxylase by polyamines. FEBS Lett. 1986;205:175–178. doi: 10.1016/0014-5793(86)80892-4. [DOI] [PubMed] [Google Scholar]
- PETERSON W.L. Gastrin and acid in relation to Helicobacter pylori. Aliment. Pharmacol. Ther. 1996;10 Suppl 1:97–102. doi: 10.1046/j.1365-2036.1996.22164010.x. [DOI] [PubMed] [Google Scholar]
- PILETZ J.E., CHIKKALA D.N., ERNSBERGER P. Comparison of the properties of agmatine and endogenous clonidine- displacing substance at imidazoline and alpha-2 adrenergic receptors. J. Pharmacol. Exp. Ther. 1995;272:581–587. [PubMed] [Google Scholar]
- PILETZ J.E., HALARIS A. Involvement of I1-imidazoline receptors in mood disorders. Ann. N.Y. Acad. Sci. 1995;763:510–519. doi: 10.1111/j.1749-6632.1995.tb32443.x. [DOI] [PubMed] [Google Scholar]
- PILETZ J.E., HALARIS A., NELSON J., QU Y., BARI M. Platelet I1-imidazoline binding sites are elevated in depression but not generalized anxiety disorder. J. Psychiatr. Res. 1996c;30:147–168. doi: 10.1016/0022-3956(96)00005-2. [DOI] [PubMed] [Google Scholar]
- PILETZ J.E., HALARIS A.E., CHIKKALA D., QU Y. Platelet I1-imidazoline binding sites are decreased by two dissimilar antidepressant agents in depressed patients. J. Psychiatr. Res. 1996b;30:169–184. doi: 10.1016/0022-3956(96)00019-2. [DOI] [PubMed] [Google Scholar]
- PILETZ J.E., IVANOV T.R., SHARP J.D., ERNSBERGER P., CHANG C.H., PICKARD R.T., GOLD G., ROTH B., ZHU H., JONES J.C., BALDWIN J., REIS D.J. Imidazoline receptor antisera-selected (IRAS) cDNA: cloning and characterization. DNA Cell. Biol. 2000;19:319–329. doi: 10.1089/10445490050043290. [DOI] [PubMed] [Google Scholar]
- PILETZ J.E., ZHU H., CHIKKALA D.N. Comparison of ligand binding affinities at human I1-imidazoline binding sites and the high affinity state of alpha-2 adrenoceptor subtypes. J. Pharmacol. Exp. Ther. 1996a;279:694–702. [PubMed] [Google Scholar]
- PINTHONG D., HUSSAIN J.F., KENDALL D.A., WILSON V.G. Comparison of the interaction of agmatine and crude methanolic extracts of bovine lung and brain with alpha 2-adrenoceptor binding sites. Br. J. Pharmacol. 1995b;115:689–695. doi: 10.1111/j.1476-5381.1995.tb14988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTHONG D., WRIGHT I.K., HANMER C., MILLNS P., MASON R., KENDALL D.A., WILSON V.G. Agmatine recognizes alpha 2-adrenoceptor binding sites but neither activates nor inhibits alpha 2-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1995a;351:10–16. doi: 10.1007/BF00169058. [DOI] [PubMed] [Google Scholar]
- PLANT T.D., HENQUIN J.C. Phentolamine and yohimbine inhibit ATP-sensitive K+ channels in mouse pancreatic beta-cells. Br. J. Pharmacol. 1990;101:115–120. doi: 10.1111/j.1476-5381.1990.tb12099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURCELL W.M., DOYLE K.M., BAGGA L., DERKS M. Histamine release from mast cells by polyamines: an NMDA receptor- mediated event. Biochem. Soc. Trans. 1994;22:398S. doi: 10.1042/bst022398s. [DOI] [PubMed] [Google Scholar]
- QUAN D.B., WANDRES D.L., SCHROEDER D.J. Clonidine in pain management. Ann. Pharmacother. 1993;27:313–315. doi: 10.1177/106002809302700313. [DOI] [PubMed] [Google Scholar]
- QUIK M. Inhibition of nicotinic receptor mediated ion fluxes in rat sympathetic ganglia by BGT II-S1 a potent phospholipase. Brain Res. 1985;325:79–88. doi: 10.1016/0006-8993(85)90304-x. [DOI] [PubMed] [Google Scholar]
- RAASCH W., CHUN K.R.J., DENDORFER A., DOMINIAK P. Positive inotropic effects of imidazoline derivatives are not mediated via imidazoline binding sites but alpha1-adrenergic receptors. Jpn. J. Pharmacol. 2000;84:1–6. doi: 10.1254/jjp.84.1. [DOI] [PubMed] [Google Scholar]
- RAASCH W., LIST B., HÄUSER W., SCHÄFER U., DOMINIAK P. Presynaptic release of noradrenaline is mediated not only through α2-adrenoceptors but also through imidazoline binding sites. Dtsch. Med. Wschr. 1999;124:S114. [Google Scholar]
- RAASCH W., MUHLE H., DOMINIAK P. Monoamine oxidase (MAO) is inhibited by imidazoline binding site I2 specific ligands in vitro. Pharm. Pharmacol. Lett. 1996;6:42–45. [Google Scholar]
- RAASCH W., MUHLE H., DOMINIAK P. Modulation of MAO activity by imidazoline and guanidine derivatives. Ann. N.Y. Acad. Sci. 1999;881:313–331. doi: 10.1111/j.1749-6632.1999.tb09376.x. [DOI] [PubMed] [Google Scholar]
- RAASCH W., REGUNATHAN S., LI G., REIS D.J. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 1995a;56:2319–2330. doi: 10.1016/0024-3205(95)00226-v. [DOI] [PubMed] [Google Scholar]
- RAASCH W., REGUNATHAN S., LI G., REIS D.J. Agmatine is widely and unequally distributed in rat organs. Ann. N.Y. Acad. Sci. 1995b;763:330–334. doi: 10.1111/j.1749-6632.1995.tb32419.x. [DOI] [PubMed] [Google Scholar]
- RADDATZ R., LANIER S.M. Relationship between imidazoline/guanidinium receptive sites and monoamine oxidase A and B. Neurochem. Int. 1997;30:109–117. doi: 10.1016/s0197-0186(96)00036-8. [DOI] [PubMed] [Google Scholar]
- RADDATZ R., PARINI A., LANIER S.M. Imidazoline/guanidinium binding domains on monoamine oxidases. Relationship to subtypes of imidazoline-binding proteins and tissue-specific interaction of imidazoline ligands with monoamine oxidase B. J. Biol. Chem. 1995;270:27961–27968. doi: 10.1074/jbc.270.46.27961. [DOI] [PubMed] [Google Scholar]
- RADDATZ R., PARINI A., LANIER S.M. Localization of the imidazoline binding domain on monoamine oxidase B. Mol. Pharmacol. 1997;52:549–553. doi: 10.1124/mol.52.4.549. [DOI] [PubMed] [Google Scholar]
- RADDATZ R., SAVIC S.L., BAKTHAVACHALAM V., LESNICK J., JASPER J.R., MCGRATH C.R., PARINI A., LANIER S.M. Imidazoline-binding domains on monoamine oxidase B and subpopulations of enzyme. J. Pharmacol. Exp. Ther. 2000;292:1135–1145. [PubMed] [Google Scholar]
- RADDATZ R., SAVIC S.L., LANIER S.M. Imidazoline binding domains on MAO-B. Localization and accessibility. Ann. N.Y. Acad. Sci. 1999;881:26–31. doi: 10.1111/j.1749-6632.1999.tb09337.x. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNA S., ADIGA P.R. Arginine decarboxylase from Lathyrus sativus seedlings. Purification and properites. Eur. J. Biochem. 1975;59:377–386. doi: 10.1111/j.1432-1033.1975.tb02465.x. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., EVINGER M.J., MEELEY M.P., REIS D.J. Effects of clonidine and other imidazole-receptor binding agents on second messenger systems and calcium influx in bovine adrenal chromaffin cells. Biochem. Pharmacol. 1991b;42:2011–2018. doi: 10.1016/0006-2952(91)90602-2. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., FEINSTEIN D.L., RAASCH W., REIS D.J. Agmatine (decarboxylated arginine) is synthesized and stored in astrocytes. Neuroreport. 1995a;6:1897–1900. doi: 10.1097/00001756-199510020-00018. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., FEINSTEIN D.L., REIS D.J. Anti-proliferative and anti-inflammatory actions of imidazoline agents. Are imidazoline receptors involved. Ann. N.Y. Acad. Sci. 1999;881:410–419. doi: 10.1111/j.1749-6632.1999.tb09389.x. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., MEELEY M.P., REIS D.J. Clonidine-displacing substance from bovine brain binds to imidazoline receptors and releases catecholamines in adrenal chromaffin cells. Mol. Pharmacol. 1991a;40:884–888. [PubMed] [Google Scholar]
- REGUNATHAN S., MEELEY M.P., REIS D.J. Expression of non-adrenergic imidazoline sites in chromaffin cells and mitochondrial membranes of bovine adrenal medulla. Biochem. Pharmacol. 1993;45:1667–1675. doi: 10.1016/0006-2952(93)90308-j. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., REIS D.J. Stimulation of imidazoline receptors inhibits proliferation of human coronary artery vascular smooth muscle cells. Hypertension. 1997;30:295–300. doi: 10.1161/01.hyp.30.2.295. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., REIS D.J. Characterization of arginine decarboxylase in rat brain and liver: distinction from ornithine decarboxylase. J. Neurochem. 2000;74:2201–2208. doi: 10.1046/j.1471-4159.2000.0742201.x. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., SASTRE M., REIS D.J.Agmatine is released from synaptosomes and adrenal chromaffin cells by depolarization Soc. Neurosci. 1996b1564(Abstr. 22)
- REGUNATHAN S., YOUNGSON C., RAASCH W., WANG H., REIS D.J. Imidazoline receptors and agmatine in blood vessels: a novel system inhibiting vascular smooth muscle proliferation. J. Pharmacol. Exp. Ther. 1996a;276:1272–1282. [PubMed] [Google Scholar]
- REGUNATHAN S., YOUNGSON C., WANG H., REIS D.J. Imidazoline receptors in vascular smooth muscle and endothelial cells. Ann. N.Y. Acad. Sci. 1995b;763:580–590. doi: 10.1111/j.1749-6632.1995.tb32453.x. [DOI] [PubMed] [Google Scholar]
- REID J.L., PANFILOV V., MACPHEE G., ELLIOTT H.L. Clinical pharmacology of drugs acting on imidazoline and adrenergic receptors. Studies with clonidine, moxonidine, rilmenidine, and atenolol. Ann. N.Y. Acad. Sci. 1995;763:673–678. doi: 10.1111/j.1749-6632.1995.tb32461.x. [DOI] [PubMed] [Google Scholar]
- REIS D.J., REGUNATHAN S. Agmatine: a novel neurotransmitter. Adv. Pharmacol. 1998;42:645–649. doi: 10.1016/s1054-3589(08)60834-0. [DOI] [PubMed] [Google Scholar]
- REIS D.J., LI G., REGUNATHAN S., BARROW C.J., COOPER R. Identifying clonidine-displacing substance. Science. 1994;266:462–464. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- REIS D.J., REGUNATHAN S., MEELEY M.P. Imidazole receptors and clonidine-displacing substance in relationship to control of blood pressure, neuroprotection, and adrenomedullary secretion. Am. J. Hypertens. 1992;5:51S–57S. doi: 10.1093/ajh/5.4.51s. [DOI] [PubMed] [Google Scholar]
- REIS D.J., RUGGIERO D.A., MORRISON S.F. The C1 area of the rostral ventrolateral medulla oblongata. A critical brainstem region for control of resting and reflex integration of arterial pressure. Am. J. Hypertens. 1989;2:363S–374S. [PubMed] [Google Scholar]
- REIS D.J., YANG X.C., MILNER T.A. Agmatine containing axon terminals in rat hippocampus form synapses on pyramidal cells. Neurosci. Lett. 1998;250:185–188. doi: 10.1016/s0304-3940(98)00466-2. [DOI] [PubMed] [Google Scholar]
- REMAURY A., RADDATZ R., ORDENER C., SAVIC S., SHIH J.C., CHEN K., SEIF I., DE MAEYER E., LANIER S.M., PARINI A. Analysis of the pharmacological and molecular heterogeneity of I(2)- imidazoline-binding proteins using monoamine oxidase-deficient mouse models. Mol. Pharmacol. 2000;58:1085–1090. doi: 10.1124/mol.58.5.1085. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R., TUROWSKI B.S., PATIL P.N. Lack of cross-desensitization between structurally dissimilar alpha- adrenoceptor agonists. J. Pharm. Pharmacol. 1977;29:378–380. doi: 10.1111/j.2042-7158.1977.tb11344.x. [DOI] [PubMed] [Google Scholar]
- RUGGIERO D.A., REGUNATHAN S., WANG H., MILNER T.A., REIS D.J. Distribution of imidazoline receptor binding protein in the central nervous system. Ann. N.Y. Acad. Sci. 1995;763:208–221. doi: 10.1111/j.1749-6632.1995.tb32408.x. [DOI] [PubMed] [Google Scholar]
- RUSTENBECK I., HERRMANN C., RATZKA P., HASSELBLATT A. Imidazoline/guanidinium binding sites and their relation to inhibition of K(ATP) channels in pancreatic B-cells. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:410–417. doi: 10.1007/pl00005070. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-BLAZQUEZ P., BORONAT M.A., OLMOS G., GARCIA-SEVILLA J.A., GARZON J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br. J. Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAPER C.B., AKIL H., WATSON S.J. Lateral hypothalamic innervation of the cerebral cortex: immunoreactive staining for a peptide resembling but immunochemically distinct from pituitary/arcuate alpha-melanocyte stimulating hormone. Brain Res. Bull. 1986;16:107–120. doi: 10.1016/0361-9230(86)90018-3. [DOI] [PubMed] [Google Scholar]
- SASTRE M., ESCRIBA P.V., REIS D.J., GARCIA-SEVILLA J.A. Decreased number and immunoreactivity of I2-imidazoline receptors in the frontal cortex of suicide victims. Ann. N.Y. Acad. Sci. 1995;763:520–522. doi: 10.1111/j.1749-6632.1995.tb32444.x. [DOI] [PubMed] [Google Scholar]
- SASTRE M., GALEA E., FEINSTEIN D., REIS D.J., REGUNATHAN S. Metabolism of agmatine in macrophages: modulation by lipopolysaccharide and inhibitory cytokines. Biochem. J. 1998;330:1405–1409. doi: 10.1042/bj3301405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASTRE M., REGUNATHAN S., GALEA E., REIS D.J. Agmatinase activity in rat brain: a metabolic pathway for the degradation of agmatine. J. Neurochem. 1996b;67:1761–1765. doi: 10.1046/j.1471-4159.1996.67041761.x. [DOI] [PubMed] [Google Scholar]
- SASTRE M., REGUNATHAN S., REIS D.J. Uptake of agmatine into rat brain synaptosomes: possible role of cation channels. J. Neurochem. 1997;69:2421–2426. doi: 10.1046/j.1471-4159.1997.69062421.x. [DOI] [PubMed] [Google Scholar]
- SASTRE M., VENTAYOL P., GARCIA-SEVILLA J.A. Decreased density of I2-imidazoline receptors in the postmortem brain of heroin addicts. Neuroreport. 1996a;7:509–512. doi: 10.1097/00001756-199601310-00032. [DOI] [PubMed] [Google Scholar]
- SATISHCHANDRAN C., BOYLE S.M. Purification and properties of agmatine ureohydrolyase, a putrescine biosynthetic enzyme in Escherichia coli. J. Bacteriol. 1986;165:843–848. doi: 10.1128/jb.165.3.843-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATRIANO J., MATSUFUJI S., MURAKAMI Y., LORTIE M.J., SCHWARTZ D., KELLY C.J., HAYASHI S., BLANTZ R.C. Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J. Biol. Chem. 1998;273:15313–15316. doi: 10.1074/jbc.273.25.15313. [DOI] [PubMed] [Google Scholar]
- SCHÄFER U., RAASCH W., CHUN J.K.R., DENDORFER A., QADRI F., DOMINIAK P. The cardiovascular effects of agmatine are not mediated via peripheral binding sites but via blockade of the ganglionic transmission. Naunyn Schmiedebergs Arch. Pharmacol. 1999b;359 Suppl:R25. [Google Scholar]
- SCHÄFER, RAASCH W., DENDORFER A., QADRI F., DOMINIAK P. Effects of agmatine on the cardiovascular system. Kidney Blood Press Res. 1998;21:A131. [Google Scholar]
- SCHÄFER U., RAASCH W., QADRI F., CHUN J., DOMINIAK P. Effects of agmatine on the cardiovascular system of spontaneously hypertensive rats. Ann. N.Y. Acad. Sci. 1999a;881:97–101. doi: 10.1111/j.1749-6632.1999.tb09346.x. [DOI] [PubMed] [Google Scholar]
- SCHULZ A., HASSELBLATT A. Dual action of clonidine on insulin release: suppression, but stimulation when alpha 2-adrenoceptors are blocked. Naunyn Schmiedebergs Arch. Pharmacol. 1989a;340:712–714. doi: 10.1007/BF00717749. [DOI] [PubMed] [Google Scholar]
- SCHULZ A., HASSELBLATT A. An insulin-releasing property of imidazoline derivatives is not limited to compounds that block alpha-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1989b;340:321–327. doi: 10.1007/BF00168517. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ D., PETERSON O.W., MENDONCA M., SATRIANO J., LORTIE M., BLANTZ R.C. Agmatine affects glomerular filtration via a nitric oxide synthase- dependent mechanism. Am. J. Physiol. 1997;272:F597–F601. doi: 10.1152/ajprenal.1997.272.5.F597. [DOI] [PubMed] [Google Scholar]
- SEILER N., DEZEURE F. Polyamine transport in mammalian cells. Int. J. Biochem. 1990;22:211–218. doi: 10.1016/0020-711x(90)90332-w. [DOI] [PubMed] [Google Scholar]
- SENER A., LEBRUN P., BLACHIER F., MALAISSE W.J. Stimulus-secretion coupling of arginine-induced insulin release. Insulinotropic action of agmatine. Biochem. Pharmacol. 1989;38:327–330. doi: 10.1016/0006-2952(89)90044-0. [DOI] [PubMed] [Google Scholar]
- SINGH G., HUSSAIN J.F., MACKINNON A., BROWN C.M., KENDALL D.A., WILSON V.G. Evidence for the presence of a non-catecholamine, clonidine-displacing substance in crude, methanolic extracts of bovine brain and lung. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:17–26. doi: 10.1007/BF00169059. [DOI] [PubMed] [Google Scholar]
- SJOHOLM A. Role of polyamines in the regulation of proliferation and hormone production by insulin-secreting cells. Am. J. Physiol. 1993;264:C501–C518. doi: 10.1152/ajpcell.1993.264.3.C501. [DOI] [PubMed] [Google Scholar]
- SKOGLUND G., LUNDQUIST I., AHREN B. Selective alpha 2-adrenoceptor activation by clonidine: effects on 45Ca2+ efflux and insulin secretion from isolated rat islets. Acta Physiol Scand. 1988;132:289–296. doi: 10.1111/j.1748-1716.1988.tb08332.x. [DOI] [PubMed] [Google Scholar]
- SMYTH D.D., PENNER S.B. Renal I1-imidazoline receptor-selective compounds mediate natriuresis in the rat. J. Cardiovasc. Pharmacol. 1995;26 Suppl 2:S63–S67. [PubMed] [Google Scholar]
- SMYTH D.D., PENNER S.B. Peripheral and central imidazoline receptor-mediated natriuresis in the rat. Ann. N.Y. Acad. Sci. 1999;881:344–357. doi: 10.1111/j.1749-6632.1999.tb09378.x. [DOI] [PubMed] [Google Scholar]
- SOBHANI I., BADO A., CHERIFI Y., MOIZO L., LAIGNEAU J.P., POSPAI D., MIGNON M., LEWIN Helicobacter pylori stimulates gastric acid secretion via platelet activating factor. J. Physiol Pharmacol. 1996;47:177–185. [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- STEFFEN G., DOMINIAK P. Significance of imidazolines for catecholamine release and for voltage-gated calcium channels in the bovine chromaffin cell. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353 Suppl:R4. [Google Scholar]
- STICKLE D., BOHRER A., BERGER R., MORRISSEY J., KLAHR S., TURK J. Quantitation of the putative neurotransmitter agmatine as the hexafluoroacetylacetonate derivative by stable isotope dilution gas chromatography and negative-ion chemical ionization mass spectrometry. Anal. Biochem. 1996;238:129–136. doi: 10.1006/abio.1996.0265. [DOI] [PubMed] [Google Scholar]
- STIENE-MARTIN A., GURWELL J.A., HAUSER K.F. Morphine alters astrocyte growth in primary cultures of mouse glial cells: evidence for a direct effect of opiates on neural maturation. Brain Res. Dev. Brain Res. 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STIENE-MARTIN A., HAUSER K.F. Morphine suppresses DNA synthesis in cultured murine astrocytes from cortex, hippocampus and striatum. Neurosci. Lett. 1993;157:1–3. doi: 10.1016/0304-3940(93)90628-x. [DOI] [PubMed] [Google Scholar]
- SUN M.K., REGUNATHAN S., REIS D.J. Cardiovascular responses to agmatine, a clonidine-displacing substance, in anesthetized rat. Clin. Exp. Hypertens. 1995;17:115–128. doi: 10.3109/10641969509087059. [DOI] [PubMed] [Google Scholar]
- SYNETOS D., MANOLOPOULOS V.G., ATLAS D., PIPILI-SYNETOS E. Human plasma-derived material with clonidine displacing substance (CDS)- like properties contracts the isolated rat aorta. J. Auton. Pharmacol. 1991;11:343–351. doi: 10.1111/j.1474-8673.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- SZABO B., URBAN R., LIMBERGER N., STARKE K. Cardiovascular effects of agmatine, a ”clonidine-displacing substance“, in conscious rabbits. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:268–273. doi: 10.1007/BF00233246. [DOI] [PubMed] [Google Scholar]
- TABOR C.W., TABOR H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- TESSON F., LIMON-BOULEZ I., URBAN P., PUYPE M., VANDEKERCKHOVE J., COUPRY I., POMPON D., PARINI A. Localization of I2-imidazoline binding sites on monoamine oxidases. J. Biol. Chem. 1995;270:9856–9861. doi: 10.1074/jbc.270.17.9856. [DOI] [PubMed] [Google Scholar]
- TESSON F., LIMON I., PARINI A. Tissue-specific localization of mitochondrial imidazoline-guanidinium receptive sites. Eur. J. Pharmacol. 1992;219:335–338. doi: 10.1016/0014-2999(92)90316-v. [DOI] [PubMed] [Google Scholar]
- TESSON F., PARINI A. Identification of an imidazoline-guanidinium receptive site in mitochondria from rabbit cerebral cortex. Eur. J. Pharmacol. 1991;208:81–83. doi: 10.1016/0922-4106(91)90055-m. [DOI] [PubMed] [Google Scholar]
- TESSON F., PRIP-BUUS C., LEMOINE A., PEGORIER J.P., PARINI A. Subcellular distribution of imidazoline-guanidinium-receptive sites in human and rabbit liver. Major localization to the mitochondrial outer membrane. J. Biol. Chem. 1991;266:155–160. [PubMed] [Google Scholar]
- TOMB J.F., WHITE O., KERLAVAGE A.R., CLAYTON R.A., SUTTON G.G., FLEISCHMANN R.D., KETCHUM K.A., KLENK H.P., GILL S., DOUGHERTY B.A., NELSON K., QUACKENBUSH J., ZHOU L., KIRKNESS E.F., PETERSON S., LOFTUS B., RICHARDSON D., DODSON R., KHALAK H.G., GLODEK A., MCKENNEY K., FITZEGERALD L.M., LEE N., ADAMS M.D., VENTER J.C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- TRUJILLO K.A. Effects of noncompetitive N-methyl-D-aspartate receptor antagonists on opiate tolerance and physical dependence. Neuropsychopharmacology. 1995;13:301–307. doi: 10.1016/0893-133X(95)00088-U. [DOI] [PubMed] [Google Scholar]
- TSOLI E., CHAN S.L., MORGAN N.G. The imidazoline I1 receptor agonist, moxonidine, inhibits insulin secretion from isolated rat islets of Langerhans. Eur. J. Pharmacol. 1995;284:199–203. doi: 10.1016/0014-2999(95)00455-t. [DOI] [PubMed] [Google Scholar]
- ULIBARRI I., SOTO J., RUIZ J., BALLESTEROS J., JAUREGUI J.V., MEANA J.J. I2-imidazoline receptors in platelets of patients with Parkinson's disease and Alzheimer's type dementia. Ann. N.Y. Acad. Sci. 1999;881:199–202. doi: 10.1111/j.1749-6632.1999.tb09360.x. [DOI] [PubMed] [Google Scholar]
- VAN BIESEN T., HAWES B.E., LUTTRELL D.K., KRUEGER K.M., TOUHARA K., PORFIRI E., SAKAUE M., LUTTRELL L.M., LEFKOWITZ R.J. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- VARGIU C., CABELLA C., BELLIARDO S., CRAVANZOLA C., GRILLO M.A., COLOMBATTO S. Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur. J. Biochem. 1999;259:933–938. doi: 10.1046/j.1432-1327.1999.00126.x. [DOI] [PubMed] [Google Scholar]
- WANG H., REGUNATHAN S., MCGOWAN D., BRAMWELL S., REIS D.J. An antiserum to idazoxan recognizes an immunoreactive substance in human serum and cerebral spinal fluid which is not agmatine. Neurochem. Int. 1997;30:85–94. doi: 10.1016/s0197-0186(96)00041-1. [DOI] [PubMed] [Google Scholar]
- WANG H., REGUNATHAN S., YOUNGSON C., BRAMWELL S., REIS D.J. An antibody to agmatine localizes the amine in bovine adrenal chromaffin cells. Neurosci. Lett. 1995;183:17–21. doi: 10.1016/0304-3940(94)11104-q. [DOI] [PubMed] [Google Scholar]
- WEITZEL G., RENNER R., GUGLIELMI H. Metabolic effects of arginine derivatives. I. Insulin like activity of arginyl compounds in vitro. Hoppe Seylers. Z. Physiol Chem. 1971;352:1617–1630. [PubMed] [Google Scholar]
- WEITZEL G., RENNER R., GUGLIELMI H. Metabolic effects of arginine derivatives. 2. Antilipolytic action of arginyl compounds. Hoppe Seylers. Z. Physiol Chem. 1972b;353:535–539. [PubMed] [Google Scholar]
- WEITZEL G., STOCK W., GUGLIELMI H. Metabolic effects of arginine derivatives. 3. Insulin-like activity of L-arginine-N,N-dialkylamides in vitro. Hoppe Seylers. Z. Physiol Chem. 1972a;353:1661–1670. [PubMed] [Google Scholar]
- WENNMALM A., JUNSTAD M. Prostaglandin-mediated inhibition of noradrenaline release: its resistance to variations in rate of prostaglandine synthesis. Acta Physiol Scand. 1976;97:60–65. doi: 10.1111/j.1748-1716.1976.tb10235.x. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO S., NAKAO H., YAMASAKI K., TAKASHINA K., SUEMOTO Y., SHINODA S. Activities and properties of putrescine-biosynthetic enzymes in Vibrio parahaemolyticus. Microbiol. Immunol. 1988;32:675–687. doi: 10.1111/j.1348-0421.1988.tb01429.x. [DOI] [PubMed] [Google Scholar]
- YANG X.C., REIS D.J. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J. Pharmacol Exp. Ther. 1999;288:544–549. [PubMed] [Google Scholar]
- ZEMBOWICZ A., CHLOPICKI S., RADZISZEWSKI W., VANE J.R., GRYGLEWSKI R.J. NG-hydroxy-L-arginine and hydroxyguanidine potentiate the biological activity of endothelium-derived relaxing factor released from the rabbit aorta. Biochem. Biophys. Res. Commun. 1992a;189:711–716. doi: 10.1016/0006-291x(92)92259-z. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., HECKER M., MACARTHUR H., SESSA W.C., VANE J.R. Nitric oxide and another potent vasodilator are formed from NG-hydroxy- L-arginine by cultured endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:11172–11176. doi: 10.1073/pnas.88.24.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEMBOWICZ A., SWIERKOSZ T.A., SOUTHAN G.J., HECKER M., GRYGLEWSKI R.J., VANE J.R. Mechanisms of the endothelium-dependent relaxation induced by NG-hydroxy-L-arginine. J. Cardiovasc. Pharmacol. 1992c;20 Suppl 12:S57–S59. doi: 10.1097/00005344-199204002-00017. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., SWIERKOSZ T.A., SOUTHAN G.J., HECKER M., VANE J.R. Potentiation of the vasorelaxant activity of nitric oxide by hydroxyguanidine: implications for the nature of endothelium-derived relaxing factor. Br. J. Pharmacol. 1992b;107:1001–1007. doi: 10.1111/j.1476-5381.1992.tb13398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU Q.M., LESNICK J.D., JASPER J.R., MACLENNAN S.J., DILLON M.P., EGLEN R.M., BLUE D.R. Cardiovascular effects of rilmenidine, moxonidine and clonidine in conscious wild-type and D79N alpha2A-adrenoceptor transgenic mice. Br. J. Pharmacol. 1999;126:1522–1530. doi: 10.1038/sj.bjp.0702429. [DOI] [PMC free article] [PubMed] [Google Scholar]


