Abstract
The effects of intravenous sufentanil and pre-administration of N-methyl-D-aspartate (NMDA) receptor antagonists were tested on a reflex triggered by C-fibre activation. The reflex was elicited by electrical stimulation of the sural nerve and recorded from the ipsilateral biceps femoris muscle in halothane anaesthetized rats either (1) with an intact neuraxis or (2) in which the brain had previously been transected at the level of the obex.
All four doses of sufentanil (0.33, 0.6, 1 and 2 μg kg−1) elicited a depression of the reflex in a dose-dependent manner. However, following the expected depression, all doses of sufentanil elicited both facilitation of the reflex and tonic inter-stimulus discharges.
The C-fibre reflex was not modified following intravenous ketamine (1 mg kg−1) or (+)-HA966 (5 or 10 mg kg−1) but, when administered 5 min before sufentanil, these drugs enhanced both the extent and the duration of the depression and strongly reduced the facilitations.
In the obex-transected rats, the depressive effect of 1 μg kg−1 sufentanil increased, while the facilitation of the C-fibre reflex and the tonic inter-stimulus discharges disappeared. Pre-administration of 10 mg kg−1 (+)-HA966 reinforced and prolonged the depressive effect of sufentanil.
These results extend previous studies suggesting the involvement of NMDA receptors in the spinal transmission of nociceptive signals. They illustrate the potential of spinal NMDA receptor blockade to both enhance the analgesic, and prevent the pro-nociceptive, effects of sufentanil.
Keywords: Nociception, C-fibre reflex, sufentanil, NMDA receptor antagonists, obex-transection
Introduction
Pharmacological activities of opioids can be modulated by drugs affecting N-methyl-D-aspartate (NMDA) receptors (Celerier et al., 2000). High frequency stimulation of C-fibres induces the phenomenon of wind-up either on dorsal horn neurones (Mendell & Wall, 1965) or on flexion reflexes (Schouenborg & Sjölund, 1983). Morphine reduces the initial response but not wind-up, whereas an antagonist of the NMDA receptor reduces wind-up but not the initial response; combination of the two drugs abolishes both phenomena and prolongs analgesia (Chapman & Dickenson, 1992). In behavioural models of pain involving prolonged noxious stimulation, NMDA receptor antagonists potentiated the effects of morphine (Yamamoto et al., 1993; Hunter et al., 1994). However conflicting results have been reported in models of acute pain (see Mao, 1999). In other words, the ability of NMDA receptor antagonists to modify the acute analgesic effects of opioids remains open to question.
Clinically, high- and low-doses of ketamine exhibit anaesthetic and analgesic properties respectively. In human volunteers, ketamine (0.1–0.3 mg kg−1) reduced both hyperalgesia and a nociceptive reflex elicited by temporal summation of inputs from Aδ-fibres (Guirimand et al., 2000). In surgical patients, the adjunction of ketamine to morphine treatment, decreased postoperative pain and wound-induced hyperalgesia (Javery et al., 1996; Stubhaug et al., 1997; Adriaenssens et al., 1999). The analgesic effects of low-doses of ketamine probably resulted from a non-competitive NMDA antagonist effect. However, the molecule interacts with multiple binding sites, including NMDA, muscarinic, monoaminergic and opioid receptors (Hirota & Lambert, 1996).
The partial agonist at the modulatory strychnine-insensitive glycine site of the NMDA receptor complex, (+)-HA966, has been shown to cross the blood-brain barrier and to be associated with fewer motor side-effects than other NMDA receptor antagonists (Kemp & Leeson, 1993). (+)-HA966 potentiated morphine antinociception in a behavioural model of peripheral neuropathy (Christensen et al., 1998) and the reduction of carragenin-induced c-Fos expression in the spinal cord (Honore et al., 1996).
Sufentanil is a potent opioid agonist widely used in anaesthesiology. It is characterized by a high affinity for μ-receptors and a high lipid solubility (Leysen et al., 1983). Following intravenous administration, it produces immediate, but short-lived, analgesic effects because it is rapidly distributed throughout the brain and other tissues.
The aim of the present study was to evaluate on the C-fibre reflex, the ability of NMDA antagonists, namely ketamine a clinically available drug, and (+)-HA966, to modulate the antinociceptive action of sufentanil. The relative contributions of spinal and supraspinal mechanisms to any effects were also investigated.
Methods
The Ethical Guidelines (1983) of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (1983) were adhered to in these studies.
Animals
Experiments were performed on 133 male albino Sprague–Dawley rats (Charles River, France), weighing 250–350 g, housed in groups of 3–4 per cage, allowed free access to food and water with a 12 h alternating light-dark cycle, and acclimatized to the laboratory for at least 1 week before the experiment.
Animal preparation
During the surgical procedures, the animals were deeply anaesthetized with 2.5% halothane in 100% oxygen. The animals were artificially ventilated through a tracheal cannula after tracheotomy. Intra-arterial and intravenous catheters were inserted into the common carotid artery and jugular vein.
The procedure for transection at the level of the obex consisted of fixing the rat in a stereotaxic frame with the head ventro-flexed using a metal bar and exposing the brainstem by a slit into the dura mater overlying the cisterna magna. The transection at the level of the obex was then made by electrocoagulation. After this preparation, animals were removed from the head-holder and the experimental protocol was followed.
On completion of the surgery, the concentration of halothane was lowered to 1.2% in 100% oxygen. The arterial blood pressure was monitored continuously via the intra-arterial catheter, which was connected via a transducer to a computer. Throughout the experiments, the animals were artificially ventilated and the heart rate was monitored. Respiratory rate (50 counts min−1), O2, end-tidal CO2 (36–40 mmHg) and halothane level (1.2%) were monitored continuously using a capnometer (Capnomac II, Datex Instruments, Helsinki, Finland). Body temperature was maintained at 37±0.5°C by means of a homeothermic blanket system.
Electrophysiological recordings
The electrophysiological methods for recording have been described previously (Falinower et al., 1994). Recordings of the reflex activities evoked by electrical stimulation of C-fibres within the receptive field of the sural nerve were made from the ipsilateral biceps femoris muscle. A pair of non-insulated, platinum-iridium needle electrodes was inserted subcutaneously into the fourth and the fifth toe. Reflex responses were recorded electromyographically by another pair of non-insulated platinum-iridium needles, inserted through the skin into the biceps femoris muscle.
The electrical stimuli were 2 ms duration single square-wave shocks delivered once every 6 s (0.17 Hz) from a constant current stimulator. The stimulus intensities and the electromyographic (EMG) responses were fed to a computerized system (PLS, Notocord) for on-line digitization and continuous monitoring. The digitized EMG responses were full-wave rectified and the C-fibre evoked responses were integrated within a 150–600 ms post-stimulus time window. In order to quantify the continuous inter-stimulus discharges (which sometimes followed the C-fibre reflex), the EMG responses were also integrated within a 2–6 s post-stimulus time window. The individual reflex responses were plotted against time to allow the study of their temporal evolution. The integrals were expressed in millivolts×milliseconds and the current intensities in milliamperes.
Characteristics of the reflex and general procedures
All the individual experiments started with a control period during which the characteristics of the reflex were determined. This took place 20–30 min after the end of surgery and decrease in the level of anaesthesia (or after 1 h in the rats with transections at the level of the obex). At this stage, the application of 15 mA stimuli to the sural nerve territory resulted in stable reflex responses, which showed minimal spontaneous fluctuations. This preliminary finding was regarded as a prerequisite before starting the pharmacological procedures.
After the stabilization period, a control recruitment curve was built by increasing the stimulus intensity. The threshold of the C-fibre reflex was determined as being the intersection of the polynomial regression curve and the abscissa. Thereafter, constant-current stimuli (three times threshold) were applied. During the first 10 min, the stability of EMG responses was checked. The mean of the 20 successive reflex responses in the 2 min period immediately preceding the first injection was taken as the mean control value. The constant current stimuli were applied over a 95 min period following the first intravenous injection. The mean arterial pressure was calculated during the same period.
Drugs
The following drugs were used: sufentanil citrate (Janssen, Boulogne-Billancourt, France), ketamine chlorhydrate (Parke-Davis, Courbevoie, France) and (+)-HA966 (Tocris Cookson, Bristol, U.K.). Saline was used as a control. Doses are expressed in mg and μg. All drugs were diluted in saline and administered intravenously in a constant volume of 1 ml kg−1. Each animal (5–7 animals group−1) received just a single dose of sufentanil and of an NMDA antagonist. The rats were sacrificed with an overdose of pentobarbitone at the end of the experiments.
Pharmacological procedures
In a pilot series of experiments, the effects of single intravenous injections of ketamine (1, 5 and 10 mg kg−1) or (+)-HA966 (5 or 10 mg kg−1) were studied on the C-fibre reflex.
In the first series of experiments, the effects of intravenous saline, ketamine (1 mg kg−1) and (+)-HA966 (5 and 10 mg kg−1) injected 5 min before intravenous sufentanil (0.33, 0.6, 1 and 2 μg kg−1) were studied on the C-fibre reflex. The 1 mg kg−1 dose of ketamine was chosen because it was found to only elicit moderate and transitory changes in the C-fibre reflex. Higher doses depressed both the reflex and the mean arterial blood pressure.
In the second series of experiments, the effects of 10 mg kg−1 (+)-HA966 or saline, injected 5 min before 1 μg kg−1 intravenous sufentanil or saline, were compared between sham-operated rats and rats with transections of the brain at the level of the obex.
Processing of data and statistical analysis
Each individual EMG response was expressed as a percentage of the mean control value. The extent of the effects of sufentanil was quantified by the areas under the curves (AUC) of the time course of the reflex calculated during the 10 min following its injection. AUCs for each group were compared by a 2-way analysis of variance. Post-hoc comparisons were made using Bonferroni tests. These AUCs were also used to investigate the dose-effect relationship according to a least squares linear regression. Fieller's theorem was used to determine the 95% confidence interval for the ED50.
To quantify the duration of any effect, each individual EMG response was expressed as a percentage of the mean control value calculated during the 2 min period immediately preceding the first injection. The final individual results were expressed as means of 10 successive responses, each corresponding to 1 min of the procedure. A significant variation (depressive or facilitatory) was defined as any change of more than two standard deviations of the control value. The durations of such variations were calculated with respect to this limit of two standard deviations and were compared by analysis of variance.
The post-discharges were expressed in terms of the percentage of the mean control value of the C-fibre reflex and were assessed in a temporal window from 2–6 s after stimulation. Their occurrences were compared with a χ2 test.
The arterial blood pressure was also expressed as percentage of the mean value calculated during the same control period. Hyper- or hypotension was defined as a variation in the mean arterial blood pressure of more than twice the standard deviation during the control period.
Statistics were performed with the statistical software Sigmastat® 2.0 SPSS®. Results were considered significant at P<0.05. Data are expressed as means±s.e.mean.
Results
Characteristics of the reflex in the control period
Electrical stimulation within the receptive field of the sural nerve elicited a two-component flexion reflex in the ipsilateral biceps femoris muscle. The first component had a short latency (10–20 ms) and low threshold. The second component had a longer latency (160–180 ms) and higher threshold (7.3±1.6 mA) elicited by activation of unmyelinated afferent fibres; accordingly it has been termed the C-fibre reflex (Falinower et al., 1994).
Effects of intravenous sufentanil
Figure 1A shows an individual example of a reflex response elicited by a stimulus intensity of three times threshold (trace 1), which was depressed immediately after 1 μg kg−1 sufentanil (trace 2). Following the depressive effect, tonic inter-stimulus discharges appeared after the C-fibre reflex and they continued throughout the 6 s interval between successive stimuli (trace 3).
Figure 1.
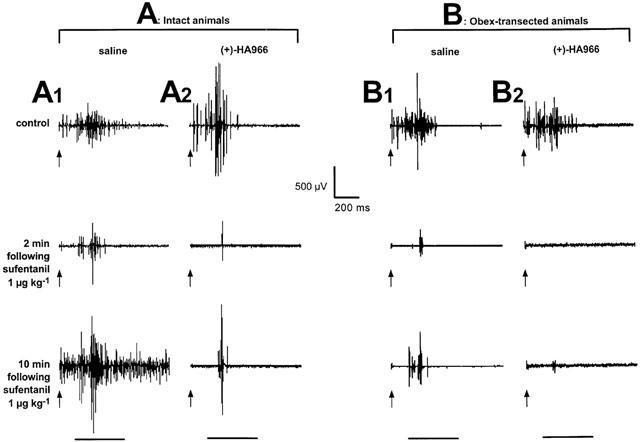
Individual examples of reflex responses to C-fibre activation. Individual EMG recordings from the biceps femoris. The responses were elicited by electrical stimulation (2 ms pulses, 3×threshold) within the sural nerve territory at the time indicated by the arrows. In the following figures, the responses were analysed within a time-window from 150–600 ms following the stimulus (horizontal bar). The upper traces were recorded during the control period, the middle and lower traces were recorded 2 and 10 min following sufentanil 1 μg kg−1. A1, intact animal pre-treated with saline. Following the expected depression, sufentanil elicited facilitations of the reflex. A2, intact animal pre-treated with (+)-HA966 10 mg kg−1. (+)-HA966 enhanced the depressive effects of sufentanil and inhibited the facilitations. B1, obex-transected animal pre-treated with saline. Compared to intact animal, the sufentanil-induced depression increased. B2, obex-transected animal pre-treated with (+)-HA966 10 mg kg−1. (+)-HA966 enhanced the depressive effects of sufentanil.
No changes in the reflex occurred following injections of saline. Both duration and extent of the depressive effects on the C-fibre reflex increased with the dose of sufentanil. However, following the expected depression, sufentanil elicited a facilitation of the C-fibre reflex (Figure 2Aa, shaded area), which was particularly pronounced at the lower doses (0.33, 0.6 and 1 μg kg−1) but almost absent at the highest dose (2 μg kg−1). In addition, following any dose of sufentanil, a continuous EMG discharge was observed during the 6 s interval between successive stimuli (Figure 2Ba). Only the 2 μg kg−1 dose elicited a statistically significant hypotension lasting over 20 min (not shown).
Figure 2.
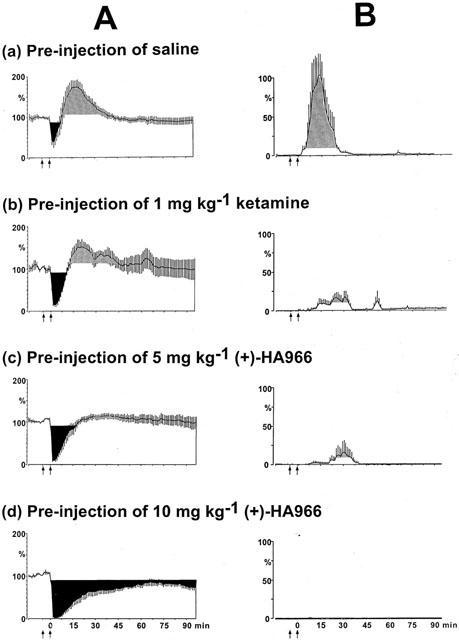
Time course of the effects of 1 μg kg−1 sufentanil following saline (a), ketamine 1 mg kg−1 (b), (+)-HA966 5 (c) and 10 mg kg−1 (d) pre-treatment on the C-fibre responses (A), and on the tonic inter-stimulus discharges (B). Ordinate: (A) C-fibre evoked EMG responses integrated in a 150–600 ms time window after the stimulus (see horizontal bar in Figure 1), and (B) inter-stimulus EMG responses recorded within a 2–6 s time window, elicited by a constant stimulus intensity (3×threshold). The responses are expressed as percentages of the mean control values calculated during the 2-min period immediately preceding the first injection. Saline was administered after a 10-min control period (first arrow), and 5 min before saline or sufentanil injection (second arrow). The shaded areas represent the variations on the reflex of more than two standard deviations of the control value. (A) Following the expected depression, sufentanil elicited a facilitation of the reflex. The prior administration of NMDA antagonists enhanced both the extent and the duration of the depressive effect of sufentanil. (B) Tonic inter-stimulus discharges were observed during the 6-s interval between two stimuli, NMDA antagonists reduced or blocked the tonic inter-stimulus discharges.
Effects of intravenous NMDA receptor antagonists
In the 1–10 mg kg−1 range, ketamine depressed the C-fibre reflex in a dose-dependent manner (not shown). The highest doses (5 and 10 mg kg−1) also depressed the mean arterial blood pressure. Facilitations or inter-stimulus discharges were never observed. The 1 mg kg−1 dose of ketamine was chosen to study the interaction with sufentanil because it was the highest dose that had only minor depressive effects on the reflex and did not alter the mean arterial blood pressure.
Following 5 or 10 mg kg−1 (+)-HA966, neither the C-fibre reflex nor the mean arterial blood pressure were affected (not shown).
Effects of association of sufentanil with ketamine
Figure 2Ab illustrates the evolution of the C-fibre reflex when 1 mg kg−1 ketamine was administrated 5 min before 1 μg kg−1 sufentanil. The prior administration of ketamine enhanced both the extent and the duration of the depressive effect of sufentanil (black areas). Figure 2Bb shows that ketamine also reduced the inter-stimulus discharges observed during the 2–6 s post-stimulus time window. The mean arterial blood pressure was not affected by this dose of ketamine (not shown). It is also worth pointing out that ketamine did not enhance the hypotension produced by 2 μg kg−1 sufentanil. Thus, the reinforcement of the depression of the reflex was not related to variations in mean arterial blood pressure. On a semi-logarithmic plot, the relationship was linear in the 0.6–2 μg kg−1 range (Figure 3), with an ED50 (95% CL) of 1.4 (1.1–2.0) μg kg−1. This relationship was shifted to the left by ketamine (ED50=0.8 (0.6–1.0) μg kg−1) without any change in the slope of the curve. The potency ratio was 1.5.
Figure 3.
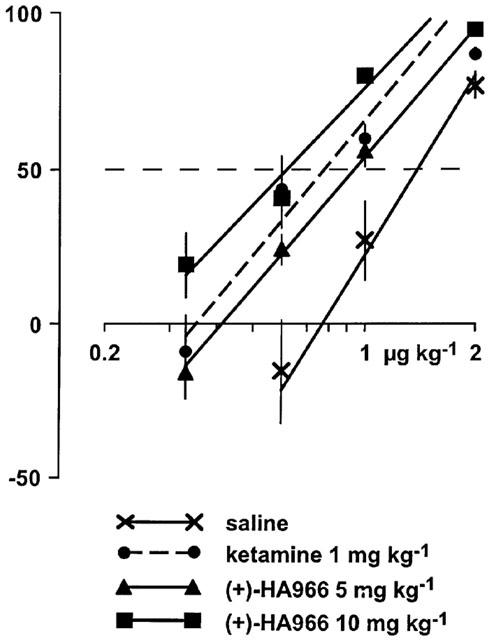
Dose-response curves for sufentanil following pre-treatment by saline or NMDA receptor antagonists. The dose-response curves were built by plotting on a semilogarithmic scale, the percentage of depressive (positive) or facilitatory (negative) effects of sufentanil on the C-fibre responses (ordinate) elicited 10 min following different doses of the drug (abscissa). By comparison with saline, the dose-response curves for NMDA receptor antagonists are shifted to the left. The ED50 (95% CL) of the effects of sufentanil for these curves are: 1.4 (1.1–2.0) μg kg−1 for saline, 0.8 (0.6–1.0) μg kg−1 for 1 mg kg−1 of ketamine, 0.9 (0.9–1.0) μg kg−1 for 5 mgkg−1 of (+)-HA966, and 0.6 (0.5–0.8) μg kg−1 for 10 mg kg−1 of (+)-HA966.
Effects of association of sufentanil with (+)-HA966
Figure 2Ac and Ad show the time-courses of the effects of 1 μg kg−1 sufentanil following the administration of 5 and 10 mg kg−1 (+)-HA966, respectively. The prior administration of 5 mg kg−1 (+)-HA966: (1) significantly enhanced both the extent and duration of the depressive effects; and (2) reduced the facilitatory effects of sufentanil. Following 10 mg kg−1 (+)-HA966, the depressions elicited by sufentanil were further enhanced while facilitations were blocked completely (Figure 2Ad).
As shown in Figure 2Bc and Bd, (+)-HA966 reduced (5 mg kg−1) or blocked (10 mg kg−1) the discharges observed during the inter-stimulus period. The prior administration of (+)-HA966 did not modify the variations in the mean arterial blood pressure induced by sufentanil. Thus, changes in the C-fibre reflex were not related to any variations in mean arterial blood pressure.
Both 5 and 10 mg kg−1 (+)-HA966 shifted the dose-response curve for sufentanil to the left, without any change in the slope of the curve (Figure 3). There was no statistically significant differences between the left shift induced by ketamine and 5 mg kg−1 (+)-HA966 [ED50=0.9 (0.9–1.0) μg kg−1]. There was a statistically significant difference between both ketamine and 5 mg kg−1 (+)-HA966, and 10 mg kg−1 (+)-HA966 (ED50=0.6 (0.5–0.8) μg kg−1). Five and 10 mg kg−1 (+)-HA966 enhanced the potency of sufentanil by 1.5 and 2.3 times, respectively.
Effects of sufentanil in obex-transected animals
A section at this level avoids both spinal shock and the rigidity of decerebration because the obex is caudal to the vestibular nuclei (Lundberg, 1982). Indeed, there was no obvious differences in our experimental conditions, between the C-fibre reflexes of obex-transected animals and those seen in sham operated animals: thresholds (4.8±0.6 and 5.2±0.7 mA, respectively), latencies and durations were similar.
No changes in the reflex occurred when saline was injected in sham-operated or obex-transected rats (not shown). Results between intact and sham-operated animals were roughly similar. The dose of 1 μg kg−1 sufentanil clearly depressed the reflex for a longer period of time in obex-transected animals (Figure 4Ab) than in sham-operated rats (Figure 4Aa). Interestingly, in obex-transected rats, only depressive effects were observed: significant facilitations (Figure 4Ab) or inter-stimulus discharges (Figure 4Bb) were never recorded at any time. Although in these animals, the blood pressure was lower than in sham-operated controls (91.7±5.1 and 104.5±10.9 mmHg, respectively), no change in blood pressure occurred following 1 μg kg−1 sufentanil (not shown).
Figure 4.
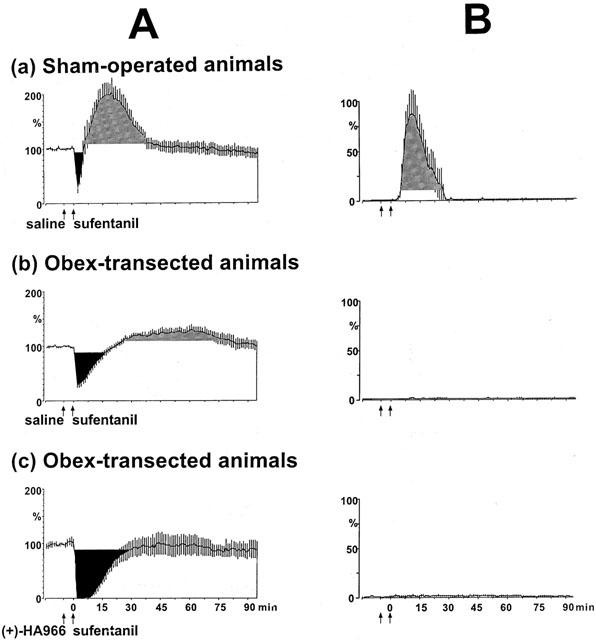
Time course of the effects of 1 μg kg−1 sufentanil on the C-fibre responses (A) and on the tonic inter-stimulus discharges (B) in sham-operated and obex-transected animals. Presentation as in Figure 2. In sham-operated animals the depressive (Aa) and facilitatory effects (Ba) of 1 μg kg−1 sufentanil were similar to that seen in intact rats (Figure 2). In obex-transected animals the sufentanil-induced depression increased (Ab) and the tonic inter-stimulus discharges (Bb) seen in sham-operated rats were never observed. In obex-transected animals the prior administration of 10 mg kg−1 (+)-HA966 (Ac) potentiated and prolonged the depressive effect of 1 μg kg−1 sufentanil.
Effects of association of sufentanil and (+)-HA966 in obex-transected animals
In both sham-operated and obex-transected rats, the reflex did not change following 10 mg kg−1 (+)-HA966 (not shown). In obex-transected animals, 10 mg kg−1 (+)-HA966 reinforced and prolonged the depressive effect of 1 μg kg−1 sufentanil (Figure 4Ac), without changing the blood pressure (not shown). Both in terms of the extent and the duration of the effects, the interaction between (+)-HA966 and sufentanil were essentially similar in intact animals and obex-transected animals.
Discussion
Effects of sufentanil on the C-fibre reflex in intact rats
This matter has been discussed in a previous paper (Adam et al., 2001) and only a brief outline will be given here. The intravenous administration of sufentanil resulted in a dose-dependent depression of a nociceptive flexion reflex elicited by activation of C-fibre afferents. Following the expected depressive effect, sufentanil elicited a facilitation of the reflex. This facilitation occurred together with tonic inter-stimulus discharges and was reversed by naloxone. In obex-transected rats, the tonic inter-stimulus discharges disappeared, suggesting that they resulted from a supraspinal effect of the drug. Such effects could be due to a modulation of the descending pathways that control the motor and/or the sensory facets of the reflex arc (see below).
Interactions between sufentanil and NMDA receptor antagonists in intact rats
In a pilot study, we confirmed that systemic ketamine dose-dependently depressed the nociceptive flexor reflex (Hao et al., 1998). NMDA receptor antagonists were reported to exhibit minimal effects on acute nociception but to impair sensitized states of nociceptive processing (Coderre & Van Empel, 1994).
Conflicting results have been reported regarding the involvement of NMDA receptors in opioid-mediated antinociception, depending on both animal species and experimental procedures (see Mao, 1999). We chose the 1 mg kg−1 dose of ketamine because it was the highest dose that induced only minor depressive effects on the reflex and did not alter the mean arterial blood pressure. (+)-HA966 did not alter these parameters. The administration of either ketamine or (+)-HA966 potentiated and prolonged the antinociceptive action of sufentanil, and reduced or blocked the sustained inter-stimulus discharges. The potentiation of the early depressive effects of sufentanil by ketamine or (+)-HA966 reflects a synergy between sufentanil and NMDA receptor antagonists. The prolongation of the duration of the sufentanil-induced depression, notably with (+)-HA966, can be explained either by: (1) an increase in the antinociceptive effect; (2) a prevention of acute tolerance to this effect; and/or (3) the blockade of the excitatory actions on the motor system.
Regarding this last hypothesis, we have previously discussed one possible explanation for the motor effect of sufentanil (Adam et al., 2001). Indeed high doses of opioids elicit muscular rigidity. As with morphine-induced muscular rigidity (Kuschinsky et al., 1977), the sufentanil-induced facilitations of the reflex and inter-stimulus discharges are eliminated either by naloxone or by spinal cord transection. The doses of sufentanil used in our study were clearly too low to produce any spontaneous rigidity, but were sufficient to slightly modify the muscular tone elicited by iterative peripheral stimulation. In this respect it is important to note that spontaneous discharges were never observed in the absence of stimuli. We clearly showed that NMDA receptor antagonists reduced or blocked the tonic inter-stimulus discharges. However, using behavioural tests, it has been found that various NMDA receptor antagonists potentiate rather than attenuate morphine-induced catalepsy (Trujillo & Akil, 1991b; Ben-Eliyahu et al., 1992). By contrast, intrathecal administration of NMDA receptor antagonists reduced fentanyl-induced muscular rigidity, as quantified by electromyography (Fu et al., 1997).
The prolongation of the duration of the depressive effect of sufentanil by NMDA receptor antagonists might be due to prevention of acute tolerance (Kissin et al., 1991). NMDA receptor antagonists have been reported to inhibit both acute and long-lasting opiate tolerance (Trujillo & Akil, 1991a; Ben-Eliyahu et al., 1992; Larcher et al., 1998). Several mechanisms could be involved in the development of opioid tolerance, from uncoupling of the opioid receptors from their second messengers to adaptative changes in parallel facilitatory pain transmission systems (Mao et al., 1995). However these are beyond the topic of the present paper. Various drugs prevent the development of opiate tolerance (Collin & Cesselin, 1991) but NMDA receptor antagonists appear to be the most effective (Mao, 1999). Thus, the mechanism by which NMDA receptor antagonists inhibit opioid tolerance might be similar to that underlying their ability to attenuate the development of hyperalgesia following tissue injury (Mao et al., 1995). It has been shown in animal models of pain that fentanyl, a potent μ-opioid receptor agonist used in human surgery, induces in a dose-dependent fashion, a delayed long-lasting hyperalgesia which is prevented by ketamine (Celerier et al., 2000). Such behavioural observations are in keeping with the present electrophysiological data.
Both the potentiation of antinociceptive effects and the blockade of tolerance to opioids, are of potential clinical importance. It was reported recently that remifentanil, a short acting opioid, elicits acute tolerance both in volunteers (Vinick & Kissin, 1998) and in patients with postoperative pain after major abdominal surgery (Guignard et al., 2000). In the latter case, the patients who received the larger intra-operative dose of remifentanil, suffered from more pain in the post-operative period, which required a higher consummation of morphine. As discussed recently, one of the most pertinent objectives for anaesthetists is probably the pre-emption of opioid-induced hyperalgesia (Eisenach, 2000). The blockade of anti-opioid systems might explain both the prevention of sufentanil-induced facilitation by NMDA antagonists in our study and the reduction of postoperative need for opiates by co-administration of ketamine and sufentanil during surgery.
Effects of sufentanil on the C-fibre reflex in obex-transected rats
The obex is caudal to the vestibular nuclei and a section at this level avoids both spinal shock and the rigidity of decerebration (Lundberg, 1982). One hour after transection, a C-fibre reflex could be elicited easily and electromyographic responses were similar to sham-operated animals. By comparison with intact and sham-operated animals, the sufentanil-induced depression increased in obex-transected rats. This unexpected increase suggests that the effect of sufentanil was influenced by the transection. It is conceivable that the direct spinal depressive effect of sufentanil was partially inhibited in the intact rats by descending controls with supraspinal origins; thus with such inhibitory processes removed by the obex transection, there was a potentiation of the antinociceptive effect.
The effects of opioids on spinal cord nociceptive transmission in the presence and absence of spinal cord transections has resulted in conflicting results (Fields & Basbaum, 1994; Le Bars et al., 1980). Interestingly, intrathecal morphine has been reported to be more effective in spinal than in intact rats (Siuciak & Advokat, 1989). In addition, following systemic administration, the morphine concentration in the central nervous system was found to be significantly altered by spinal transection (Advokat & Gulati, 1991). Taken together, these data make one cautious about drawing conclusions as to the effects of morphine on descending modulation.
The sufentanil-induced tonic inter-stimulus discharges seen in intact and sham-operated animals disappeared in obex-transected rats. This result suggests the involvement of supraspinal opioid receptors in these effects. Thus, the facilitation of the C-fibre reflex elicited by sufentanil was probably related to an action on the bulbo-spinal influences that control the motor limb of the reflex arc. Interestingly, morphine facilitates the C-fibre reflex elicited by high intensity electrical stimulation in intact rats but not in animals whose rostral ventromedial medulla has previously been lesioned (Gozariu et al., 2000). Thus several pieces of evidence are consistent with the notion that opioids facilitate spinal reflexes through bulbo-spinal mechanisms. It is possible that such activity partially masks their direct spinal depressive effect in non-transected animals.
Interactions between (+)-HA966 and sufentanil in obex-transected rats
When studying the interaction between sufentanil and (+)-HA966 in obex-transected rats, we chose the dose of 10 mg kg−1 which completely blocked the tonic inter-stimulus discharges in the ‘intact' preparation. (+)-HA966 did not affect the C-fibre reflex but increased and prolonged the antinociceptive action of sufentanil. This result suggested that (+)-HA966 potentiated the sufentanil-induced antinociception by a spinal action. Such an effect could not result from dysfacilitation since sufentanil did not produce any facilitation in the obex-transected preparation. Interestingly, dextrorphan was reported to increase the antinociceptive effect of morphine assessed by the tail-flick test in spinal rats (Advokat & Rhein, 1995).
The spinal cord is probably a major site for the action of NMDA receptor antagonists in attenuating morphine tolerance. In chronic spinal rats, infusion of MK-801 inhibited the development of morphine tolerance and increased the antinociceptive effect of morphine pellets (Gutstein & Trujillo, 1993). Intrathecal administration of MK-801 attenuated morphine tolerance produced by intrathecal administration of morphine with no apparent direct effect on morphine-induced antinociception (Mao et al., 1994). It is commonly recognized that stimulation of μ opioid receptors, triggers the activation of NMDA receptors by reducing Mg2+ blocking via intracellular protein kinase C activation (Chen & Huang, 1992). This might be one of the mechanisms by which opioids interact with the NMDA receptor during the development of tolerance (Mao et al., 1995). GM1 ganglioside, a substance that inhibits the translocation of protein kinase C, prevented morphine tolerance and the presumably related increase in membrane-bound protein kinase C in the superficial layers of the spinal cord (Mayer et al., 1995).
Although the results of these studies do not eliminate a potential role for supraspinal NMDA receptors in the development of opioid tolerance, they suggest that NMDA receptor antagonists act predominantly at a spinal level to block tolerance. Thus, the enhancement of sufentanil antinociception by (+)-HA966 in obex-transected animals may have resulted from the prevention of acute tolerance via a spinal mechanism. It is therefore possible that acute tolerance is an additional factor that partially masks the depressive effect of sufentanil in intact animals.
In conclusion, the present study showed that sufentanil depresses the C-fibre reflex in a dose-dependent manner and that NMDA receptor antagonists can increase these effects. These increases persisted in a preparation devoid of supraspinal controls, suggesting a direct spinal mechanism of action. Sufentanil also elicited facilitations of the reflex by a supraspinal action and these were reduced by NMDA receptor antagonists. These findings offer a promising therapeutic alternative in clinical pain management, not only for patients requiring long-term opioid therapy but also, perhaps more commonly, to provide high quality analgesia both during and after surgery.
Acknowledgments
The authors thank Dr S.W. Cadden for advice in the preparation of the manuscript. This work was supported by l'Institut National de la Santé et de la Recherche Médicale (INSERM), by l'Institut UPSA de la Douleur and by la Direction Régionale de la Recherche Clinique de l'Assistance-Publique Hôpitaux de Paris (CRC 96028). Anne Gairard was supported by a grant from the Fondation pour la Recherche Médicale (FRM). Presented at the 9th World Congress on Pain, August 22–27, 1999, Vienna, Austria.
Abbreviations
- EMG
electromyographic
- NMDA
N-methyl-D-aspartate
References
- ADAM F., LE BARS D., CHAUVIN M., GUIRIMAND F. Effects of intravenous and intrathecal sufentanil on a C-fibre reflex elicited by a wide range of stimulus intensities in the rat. Eur. J. Pharmacol. 2001;411:93–106. doi: 10.1016/s0014-2999(00)00881-5. [DOI] [PubMed] [Google Scholar]
- ADRIAENSSENS G., VERMEYEN K.M., HOFFMANN V.L.H., MERTENS E., ADRIAENSSENS H.F. Postoperative analgesia with i.v. patient-controlled morphine: effect of adding ketamine. Br. J. Anaesth. 1999;83:393–396. doi: 10.1093/bja/83.3.393. [DOI] [PubMed] [Google Scholar]
- ADVOKAT C., GULATI A. Spinal transection reduces both spinal antinociception and CNS concentration of systemically administered morphine in rats. Brain Res. 1991;555:251–258. doi: 10.1016/0006-8993(91)90349-z. [DOI] [PubMed] [Google Scholar]
- ADVOKAT C., RHEIN F.Q. Potentiation of morphine-induced antinociception in acute spinal rats by the NMDA antagonist dextrorphan. Brain Res. 1995;699:157–160. doi: 10.1016/0006-8993(95)01023-o. [DOI] [PubMed] [Google Scholar]
- BEN-ELIYAHU S., MAREK P., VACCARINO A.L., MOGIL J.S., STERNBERG W.F., LIEBESKIND J.C. The NMDA receptor antagonist MK-801 prevents long-lasting non-associative morphine tolerance in the rat. Brain Res. 1992;575:304–308. doi: 10.1016/0006-8993(92)90094-p. [DOI] [PubMed] [Google Scholar]
- CELERIER E., RIVAT C., JUN Y., LAULIN J.P., LARCHER A., REYNIER P., SIMMONET G. Long-lasting hyperalgesia induced by fentanyl in rats. Preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- CHAPMAN V., DICKENSON A.H. The combination of NMDA antagonism and morphine produces profound antinociception in the rat dorsal horn. Brain Res. 1992;573:321–323. doi: 10.1016/0006-8993(92)90780-d. [DOI] [PubMed] [Google Scholar]
- CHEN L., HUANG L.Y.M. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN D., IDANPAAN-HEIKKILA J.J., GUILBAUD G., KAYSER V. The antinociceptive effect of combined systemic administration of morphine and the glycine/NMDA receptor antagonist, (+)-HA966 in a rat model of peripheral neuropathy. Br. J. Pharmacol. 1998;126:1641–1650. doi: 10.1038/sj.bjp.0702240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CODERRE T.J., VAN EMPEL I. The utility of excitatory amino acid (EAA) antagonists as analgesic agents. I. Comparison of the antinociceptive activity of various classes of EAA antagonists in mechanical, thermal and chemical nociceptive tests. Pain. 1994;59:345–352. doi: 10.1016/0304-3959(94)90020-5. [DOI] [PubMed] [Google Scholar]
- COLLIN E., CESSELIN F. Neurobiological mechanisms of opioid tolerance and dependence. Clin. Neuropharmacol. 1991;14:465–488. doi: 10.1097/00002826-199112000-00001. [DOI] [PubMed] [Google Scholar]
- COMMITTEE FOR RESEARCH AND ETHICAL ISSUES OF THE IASP Ethical standards for investigations of experimental pain in animals. Pain. 1983;16:109–110. [Google Scholar]
- EISENACH J.C. Preemptive hyperalgesia, not analgesia. Anesthesiology. 2000;92:308–309. doi: 10.1097/00000542-200002000-00009. [DOI] [PubMed] [Google Scholar]
- FALINOWER S., WILLER J.C., JUNIEN J.L., LE BARS D. A C-fiber reflex modulated by heterotopic noxious somatic stimuli in the rat. J. Neurosci. 1994;72:194–213. doi: 10.1152/jn.1994.72.1.194. [DOI] [PubMed] [Google Scholar]
- FIELDS H.L., BASBAUM A.L.Central nervous system mechanisms of pain modulation Textbook of Pain 1994Edinburgh: Churchill Livingstone; 243–257.Ed. Wall, P.D., Melzack, R. pp [Google Scholar]
- FU M.J., TSEN L.Y., LEE T.Y., LUI P.W., CHAN S.H.H. Involvement of cerulospinal glutamatergic neurotransmission in fentanyl-induced muscular rigidity in the rat. Anesthesiology. 1997;87:1450–1459. doi: 10.1097/00000542-199712000-00024. [DOI] [PubMed] [Google Scholar]
- GOZARIU M., BOUHASSIRA D., WILLER J.C., LE BARS D. Temporal summation and a C-fibre reflex in the rat: effects of morphine on facilitatory and inhibitory mechanisms. Eur. J. Pharmacol. 2000;394:75–84. doi: 10.1016/s0014-2999(00)00114-x. [DOI] [PubMed] [Google Scholar]
- GUIGNARD B., BOSSARD A.E., COSTE C., SESSLER D.I., LEBRAULT C., ALFONSI P., FLETCHER D., CHAUVIN M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- GUIRIMAND F., DUPONT X., BRASSEUR L., CHAUVIN M., BOUHASSIRA D. The effects of ketamine on the temporal summation (wind-up) of the RIII nociceptive flexion reflex and pain in humans. Anesth. Analg. 2000;90:408–414. doi: 10.1097/00000539-200002000-00031. [DOI] [PubMed] [Google Scholar]
- GUTSTEIN H.B., TRUJILLO K.A. MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res. 1993;626:332–334. doi: 10.1016/0006-8993(93)90597-g. [DOI] [PubMed] [Google Scholar]
- HAO J.X., SJOLUND B.H., WIESENFELD-HALLIN Z. Electrophysiological evidence for an antinociceptive effect of ketamine in the rat spinal cord. Acta Anesthesiol. Scand. 1998;42:435–441. doi: 10.1111/j.1399-6576.1998.tb05138.x. [DOI] [PubMed] [Google Scholar]
- HIROTA K., LAMBERT D.G. Ketamine: its mechanism(s) of action and unusual clinical uses. Br. J. Anaesth. 1996;77:441–444. doi: 10.1093/bja/77.4.441. [DOI] [PubMed] [Google Scholar]
- HONORE P., CHAPMAN V., BURITOVA J., BESSON J.M. Concomitant administration of morphine and an N-methyl-D-aspartate receptor antagonist profoundly reduces inflammatory evoked spinal c-Fos expression. Anesthesiology. 1996;85:150–160. doi: 10.1097/00000542-199607000-00021. [DOI] [PubMed] [Google Scholar]
- HUNTER J.C., ATWAL P., WOODRUFF G.N., SINGH L. Differential modulation of κ and μ opioid antinociception by the glycine/NMDA receptor agonist D-serine. Br. J. Pharmacol. 1994;112:1002–1003. doi: 10.1111/j.1476-5381.1994.tb13181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAVERY K.B., USSERY T.W., STEGER H.G., COLCLOUGH G.W. Comparison of morphine and morphine with ketamine for postoperative analgesia. Can. J. Anaesth. 1996;43:212–215. doi: 10.1007/BF03011736. [DOI] [PubMed] [Google Scholar]
- KEMP J.A., LEESON P.D. The glycine site of the NMDA receptor five years on. Trends Pharmacol. Sci. 1993;14:20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- KISSIN I., BROWN P.T., BRADLEY E.L., Jr Magnitude of acute tolerance to opioids is not related to their potency. Anesthesiology. 1991;75:813–816. doi: 10.1097/00000542-199111000-00013. [DOI] [PubMed] [Google Scholar]
- KUSCHINSKY K., ROPTE H., MESEKE R., CREMER H., SONTAG K.H. Specific action of narcotics on reflex activation of rat alpha-motoneurones. Naunyn-Schmiedeberg's Arch. Pharmacol. 1977;296:249–254. doi: 10.1007/BF00498690. [DOI] [PubMed] [Google Scholar]
- LARCHER A., LAULIN J.P., CELERIER E., LE MOAL M., SIMMONET G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- LE BARS D., GUILBAUD G., CHITOUR D., BESSON J.M. Does systemic morphine increase descending inhibitor controls of dorsal horn neurones involved in nociception. Brain Res. 1980;202:223–228. doi: 10.1016/0006-8993(80)90659-9. [DOI] [PubMed] [Google Scholar]
- LEYSEN J.E., GOMMEREN W., NIEMEGEERS C.J.E. [3H] sufentanil, a superior ligand for μ-opiate receptors: binding properties and regional distribution in the rat brain and spinal cord. Eur. J. Pharmacol. 1983;87:209–225. doi: 10.1016/0014-2999(83)90331-x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A.Inhibitory control from the brain stem of transmission from primary afferents to motoneurons, primary afferent terminals and ascending pathways Brain Stem Control of Spinal Mechanisms 1982Amsterdam: Elsevier; 179–224.Ed. Sjölund, B., Björklund, A. pp [Google Scholar]
- MAO J., PRICE D.D., MAYER D.J. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J. Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAO J., PRICE D.D., MAYER D.J. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- MAO J. NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res. Reviews. 1999;30:289–304. doi: 10.1016/s0165-0173(99)00020-x. [DOI] [PubMed] [Google Scholar]
- MAYER D.J., MAO J., PRICE D.D. The development of morphine tolerance and dependance is associated with translocation of protein kinase C. Pain. 1995;61:365–374. doi: 10.1016/0304-3959(95)00023-L. [DOI] [PubMed] [Google Scholar]
- MENDELL L.M., WALL P.D. Response of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- SCHOUENBORG J., SJÖLUND B.H. Activity evoked by A- and C-afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J. Neurophysiol. 1983;50:1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- SIUCIAK J.A., ADVOKAT C. Antinociceptive effect of intrathecal morphine in tolerant and non-tolerant spinal rats. Pharmacol. Biochem. Behav. 1989;34:445–452. doi: 10.1016/0091-3057(89)90539-x. [DOI] [PubMed] [Google Scholar]
- STUBHAUG A., BREIVIK H., EIDE P.K., KREUNEN M., FOSS A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anesthesiol. Scand. 1997;41:1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- TRUJILLO K.A., AKIL H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991a;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- TRUJILLO K.A., AKIL H. The NMDA antagonist MK-801 increases morphine catalepsy and lethality. Pharmacol. Biochem. Behav. 1991b;38:673–675. doi: 10.1016/0091-3057(91)90032-w. [DOI] [PubMed] [Google Scholar]
- VINICK H.R., KISSIN I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth. Analg. 1998;86:1307–1311. doi: 10.1097/00000539-199806000-00033. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., SHIMOYAMA N., MIZUGUCHI T. The effects of morphine, MK-801, an NMDA antagonist, and CP-96,345, an NK1 antagonist, on the hyperesthesia evoked by carageenan injection in the rat paw. Anesthesiology. 1993;78:124–133. doi: 10.1097/00000542-199301000-00018. [DOI] [PubMed] [Google Scholar]


