Abstract
Activated hepatic stellate cells play a major role in the pathophysiology of chronic liver disease. They can influence the metabolism of hepatocytes by producing a variety of cytokines and growth factors. Upon stimulation with endotoxin, stellate cells also synthesize nitric oxide (NO), a potent mediator of growth of several cell types including hepatocytes.
We investigated the effect of serum-free medium conditioned by activated stellate cells in the absence and presence of endotoxin on NO and DNA synthesis in hepatocytes. Stellate cells and hepatocytes were isolated by enzymatic digestion of the liver. Stellate cells were cultured for 10 days after which the majority exhibited α-smooth muscle actin (a marker for activated cells); hepatocytes were used after overnight culture.
While the medium conditioned by stellate cells in the absence of endotoxin stimulated DNA synthesis in hepatocytes, medium conditioned in its presence inhibited this process in an endotoxin concentration-dependent manner (10–1000 ng ml−1). Endotoxin-conditioned stellate cell medium also stimulated NO synthesis in hepatocytes; the effect was consistent with increased protein and mRNA expression of inducible NO synthase (iNOS). However, inhibition of DNA synthesis in hepatocytes caused by endotoxin-conditioned stellate cell medium was unaffected by the NOS inhibitor, L-NG-monomethylarginine (L-NMMA), guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), and neutralizing antibodies for TGF-β, IL-1β, IL-6 and TNF-α.
These results indicate that factors other than these cytokines produced by activated stellate cells upon stimulation with endotoxin or by hepatocytes challenged with endotoxin-conditioned stellate cell medium inhibit DNA synthesis in hepatocytes.
Keywords: Cytokines, DNA synthesis, endotoxin, growth, hepatic stellate cells, hepatocytes, liver, nitric oxide
Introduction
Hepatic stellate cells (fat-storing cells, perisinusoidal cells, Ito cells) are located in the space of Disse. The contractile stellate cells produce components of extracellular matrix and play a central role in the maintenance of sinusoidal blood flow and hepatic architecture (Blomhoff & Wake, 1991; Geerts et al., 1994; Ramadori, 1994). During chronic liver injury, the quiescent stellate cells transform into proliferating α-smooth muscle actin expressing cells (activated stellate cells) (Blomhoff & Wake, 1991; Geerts et al., 1994; Ramadori, 1994). Apart from their strong fibrogenic activity and contractility, activated stellate cells also produce a variety of biologically active mediators such as transforming growth factor (TGF)-α and -β, interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α, but they lose the ability to express hepatocyte growth factor (HGF) found in the quiescent stellate cells (Ramadori et al., 1992; Schirmacher et al., 1992). Endotoxin was recently reported to stimulate the expression of inducible nitric oxide synthase (iNOS) and the synthesis of nitric oxide (NO) in the stellate cells (Helyar et al., 1994; Kawada et al., 1998). The concentration of endotoxin is increased during acute and chronic liver injury (Triger et al., 1978; Nolan, 1981; Lumsden et al., 1988; Bomzon & Blendis, 1994). In Kupffer cells, endotoxin stimulated production of cytokines such as IL-1β, TNF-α and interferon (IFN)-γ (Nathan, 1968; Adams & Hamilton, 1984; Decker, 1990; Klocker et al., 2000) that cause induction of NO synthesis in hepatocytes (Curran et al., 1990; Nussler et al., 1992). It is conceivable that endotoxin exerts similar effect on hepatocytes via activated stellate cells. Thus the mediators produced by activated stellate cells with or without stimulation with endotoxin can be of critical significance in the regulation of the metabolism of hepatocytes during pathological developments. Therefore, the aim of this study was to investigate the effects of the medium conditioned by activated stellate cells in the absence and presence of endotoxin on hepatocytes. The results demonstrate that medium conditioned by activated stellate cells stimulates DNA but not NO synthesis in hepatocytes. Conversely, although the medium conditioned by stellate cells in the presence of endotoxin stimulates NO and inhibits DNA synthesis in hepatocytes, the latter effect is not mediated by NO or TGF-β, TNF-α, IL-6 and IL-1β.
Methods
Preparation of stellate cells
The experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health. Stellate cells were prepared from the livers of male Sprague–Dawley rats (450–500 g) as described previously (Gabriel et al., 1998). Briefly, following the collagenase/protease digestion of the liver and removal of the hepatocytes and cell debri by low speed centrifugation, stellate cells were purified from other nonparenchymal cells by density gradient centrifugation using 8.12% (w v−1) Nycodenz. Stellate cells were suspended in Dulbecco's modified Eagle's medium (DMEM) containing 250 u ml−1 penicillin, 250 μg ml−1 streptomycin and 10% foetal bovine serum/10% horse serum, and plated in 24-well culture plates at a density of 1×106 cells well−1. The viability of the cells was greater than 95% as determined by Trypan blue exclusion. The purity of the cells was determined by phase contrast microscopy, vitamin A autofluorescence, and immunohistochemically using specific markers for endothelial cells (antibody to factor VIII related antigen; DAKO, Carpintoria, CA, U.S.A.), stellate cells (antibody to desmin; DAKO), Kupffer cells (clone ED2; Serotec, Indianapolis, IN, U.S.A.) and epithelial cells (clone AE1/AE3; Boehringer Mannheim, Indianapolis, CA, U.S.A.) as described previously (Gandhi et al., 1999). Briefly, the cells were washed with phosphate buffered saline (PBS), fixed in 95% ethanol and antibodies were applied. Biotinylated IgGs were used as secondary antibodies, and avidin-biotin complex (Vectorstain, Burlingame, CA, U.S.A.) and peroxidase chromogen kit (Biomeda Corp. Foster City, CA, U.S.A.) were used for detection as described (Gandhi et al., 1999). The purity and the plating efficiency of the cells were greater than 95 and 75% respectively. Cells were used on day 10 of culture when more than 80% expressed α-smooth muscle actin as determined by immunohistochemistry (Gabriel et al., 1998).
Preparation of hepatocytes
Hepatocytes were prepared by collagenase (type CLSI, 0.05%) digestion of the liver as described previously (Kuddus et al., 2000). The cells were separated in ice-cold Hank's balanced salt solution (HBSS). The suspension was filtered and the filtrate centrifuged at 50×g for 1 min at 4°C. The cell pellet was washed three times with PBS containing 1 mM CaCl2 and suspended in this medium at 5×106 cells ml−1. The suspension was mixed with equal volume of 90% percoll in HBSS and centrifuged at 50×g for 10 min at 4°C. The pellet containing viable hepatocytes (>95%) was washed (2×), suspended in William's medium E supplemented with 2 mM L-glutamine, 250 u ml−1 penicillin, 250 μg ml−1 streptomycin, 10% foetal bovine serum, 10−6 M insulin (Eli Lilly Co., Indianapolis, IN, U.S.A.) and 10 mM HEPES, and plated in 24-well plates (0.125×106 cells well−1). The medium was renewed after a 3 h attachment period and the cells were used for experiments after an overnight incubation. The purity of the cells, determined immunohistochemically using specific markers for various nonparenchymal cells as described above in the preparation of stellate cells, was greater than 98%.
Determination of the effects of stellate cell-conditioned medium on hepatocytes
Stellate cells were washed and placed in serum-free DMEM containing 0.1% BSA (w v−1) and the test agents at concentrations indicated in the figure legends. After 24 h, the medium was filtered (0.2 μm; Gelman Laboratory, Ann Arbor, MI, U.S.A.) and used for NO determination. Medium from another set of stellate cells was transferred to the overnight culture of hepatocytes. Appropriate controls were used to determine the effects of the agents and unconditioned medium on hepatocytes. Hepatocytes were incubated for 24 h with the test agents or the medium conditioned by stellate cells after which various determinations were made.
Determination of nitric oxide
The concentration of NO end products nitrite and nitrate was determined by Griess method (Green et al., 1982) using a colorimetric assay kit (Canyan Chemicals, Ann Arbor, MI, U.S.A.). Briefly, Griess reagent was added to the culture supernatant after treatment with nitrate reductase. The optical density was determined spectrophotometrically at 550 nm. A standard curve was developed using concentrations of sodium nitrate (0 and 35 μM) treated similarly.
Determination of DNA synthesis
After aspirating the medium from stellate cells or hepatocytes, fresh medium containing 1 μCi ml−1 [3H]-thymidine was added to the culture wells. Following an incubation of 4 h at 37°C, the cells were washed with ice-cold HBSS containing 0.1% BSA, treated with ice-cold 10% trichloroacetic acid (TCA) for 10 min, and washed once with TCA followed by 95% ethanol. The cells were then digested with 5% (w v−1) SDS and the radioactivity was determined.
Western blot analysis for iNOS
Cells were scraped from the plate in ice-cold lysis buffer (10 mM Tris-HCl, pH 7.6, containing 0.1 M NaCl, 1 mM EDTA, 0.5 mM PMSF and 1 mM aprotinin). Following homogenization in an ice bath, the homogenate was kept in ice for 30 min and then centrifuged at 15,300×g for 10 min at 4°C. The supernatant was aspirated and its protein concentration was determined by Lowry's procedure (Lowry et al., 1951). The lysate containing 10 μg protein was subjected to SDS–PAGE on a 8% acrylamide gel and the separated proteins were transferred on to a Immobilon-P membrane (Millipore, Bedford, MA, U.S.A.). The membrane was treated with a rabbit monoclonal anti-rat iNOS antibody (Transduction Laboratories Co., Lexington, KY, U.S.A.). After washing, the membrane was incubated with peroxidase-linked anti-rabbit IgG (Amersham-Pharmacia), and detection was achieved using an ECL chemiluminescence kit (Amersham-Pharmacia).
Determination of mRNA expression of iNOS and cytokines
Semiquantitative reverse transcriptase polymerase chain reaction (RT–PCR) assay was employed to assess the relative levels of the mRNA expression of iNOS, IL-1β, TNF-α, IL-6 and IFNγ in LPS-stimulated and unstimulated stellate cells as described previously (Gabriel et al., 1998; 1999). Levels of β-actin mRNA were determined to ascertain the efficiency of cDNA synthesis and reverse transcription. The PCR primers specific for iNOS cDNA: 5′-AGAATGTTCCAGAATCCCTCCCTGGACA-3′ (F) and 5′-GAGTGAGCTGGTAGGTTCCTGTTG-3′ (R) [product size, 356 bp (Kosuga et al., 1994)]; TNF-α cDNA; 5′-CACGCTCTTCTGTCTACTGA-3′ (F) and 5′-GGACTCCGTGATGTCTAAGT-3′ (R) [product size, 543 bp (Uemura et al., 2000); IL-1β cDNA; 5′-GAAGCTGTGGCAGCTACCTATGTCT (F) and CTCTGCTTGAGAGGTGCTGATGTACC-3′ (R) [product size, 522 bp (Uemura et al., 2000)]; IL-6 cDNA;5′-GAAAGTCAACTCCATCTGCC-3′ (F) and 5′-CATAGCACACTAGGTTTGCC-3′ (R) [product size, 681 bp (Uemura et al., 2000)]; IFN-γ cDNA: 5′-ATCTGGAGGAACTGGCAAAAGGACG-3′ (F) and 5′-CCTTAGGCTAGATTCTGGTGACAGC-3′ (R) [product size, 288 bp (Tian et al., 1997); and β-actin cDNA: 5′-TTCTACAATGAGCTGCGTGTG-3′ (F) and 5′-TTCATGGATGCCACAGGATTC-3′ (R) [product size, 561 bp (Nudel et al., 1983)] were used. PCR amplification reaction was performed essentially as described previously (Gabriel et al., 1998; 1999). PCR products were resolved in a 1.5% agarose gel and stained with 1×SYBR Green I (FMC Biproduct, Rockland, ME, U.S.A.). The gels were scanned under blue fluorescence light using a phosphorimager and the band intensity was quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Determination of cyclic GMP
Stellate cells or hepatocytes were treated with 1 μg ml−1 LPS for 24 h. Likewise, hepatocytes were incubated with stellate cell medium conditioned in the absence or presence of LPS for 24 h. During the last 30 min of incubation, 1 mM 3-isobutyl-1-methylxanthine was added to the medium to inhibit endogenous phosphodiesterase activity. At the end of the incubation period, the medium was aspirated and cells were scraped in 1 ml ice-cold ethanol and left at room temperature for 5 min. The suspension was centrifuged at 1500×g for 10 min at 4°C, and the supernatant was dried by vacuum centrifugation. The residue was reconstituted in phosphate buffer and cyclic GMP concentration was determined by ELISA using a commercial kit as per the instructions of the manufacturer (Cayman Chemicals, Ann Arbor, MI, U.S.A.). The assay is based on the competition between free cyclic GMP and a cyclic GMP tracer (cyclic GMP linked to acetylcholinesterase molecule) for a limited number of cyclic GMP-specific rabbit antiserum binding sites. Free acetylcholinesterase is then reacted with Ellman's reagent that contains the substrate for acetylcholinesterase, and the distinct yellor color of the reaction is read at 412 nm. The assay was performed in duplicate in 96 well plates using 50 μl of the reconstituted cell extract. For developing a standard curve, 0.23–30 pmol ml−1 cyclic GMP solution was used. Appropriate conditions for nonspecific binding and blank were also used. Cyclic GMP acetylcholinesterase tracer was added to all wells except the blanks. Cyclic GMP antiserum was added to the wells containing cell extracts and standards and the plate was incubated for 18 h at room temperature. The plate was emptied, and rinsed five times with the wash buffer provided by the manufacturer. Ellman's reagent was added to each well and the absorbance of the resultant colour was read at 412 nm.
Materials
The following were purchased from the indicated sources: Protease type XIV, Nycodenz, bovine serum albumin (fraction V) (BSA), N-[2-hydroxyethyl] piperazine-N′-[ethanesulfonic acid] (HEPES), endotoxin (Escherichia coli lipopolysaccharide, serotype 0111:B4) (Sigma Chemical Co., St. Louis, MO, U.S.A.), collagenase type IV and type CLS1 (Worthington Biochemical Corporation, Freehold, NJ, U.S.A.), Dulbecco's modified Eagle medium, William's medium E, penicillin G, streptomycin, foetal bovine serum and horse serum, transforming growth factor (TGF)-β (Life Technologies, Grand Island, N.Y., U.S.A.); monoclonal antibody for TGF-β (Genzyme Corporation, Cambridge, MA, U.S.A.); neutralizing anti-TGF-α polyclonal antibody (R&D Systems, Minneapolis, MN, U.S.A.); L-NG-monomethylarginine monoacetate (L-NMMA) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (Alexis Biochemicals, San Diego, CA, U.S.A.); [methyl-3H]thymidine (3.18TBq/mmol) (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, U.S.A.).
Data analysis
The values are presented as averages of triplicate determinations±s.e.mean. Each experiment was repeated at least three times using cells from different animals. Student's t-test was employed for statistical comparison between the groups. A P value of <0.05 was considered statistically significant.
Results
Effect of LPS on NO and DNA synthesis in stellate cells
LPS stimulated NO synthesis in stellate cells in a concentration-dependent manner (Figure 1A). The effect was significant at the lowest concentration of LPS tested (1 ng ml−1). LPS produced concentration-dependent inhibition of DNA synthesis in stellate cells, the effect being significant at the lowest concentration tested (1 ng ml−1) (Figure 1B).
Figure 1.
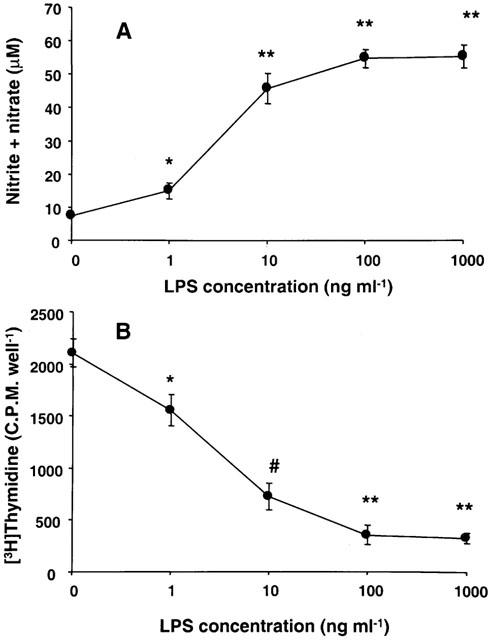
Effect of LPS concentration on NO and DNA synthesis in stellate cells. Stellate cells were incubated in serum-free medium for 24 h in the presence of indicated concentrations of LPS. The concentration of nitrite+nitrate in the medium (A) and DNA synthesis in the cells (B) were determined. *P<0.05 vs control; #P<0.01 vs control; **P<0.001 vs control.
Effect of medium conditioned by stellate cells with LPS on NO and DNA synthesis in hepatocytes
Cultured hepatocytes and hepatic endothelial cells spontaneously release NO, such release being 100 times greater in the former cell type (Zhang et al., 1997). We also observed considerable spontaneous release of NO by hepatocytes. This basal release of NO by hepatocytes was unaffected by LPS and medium conditioned by stellate cells in the absence of LPS (Figure 2A). However, exposure of hepatocytes to stellate cell medium conditioned with LPS caused an increase in the production of NO at LPS concentration of 10 ng ml−1 and was maximal at 100 ng ml−1 LPS.
Figure 2.
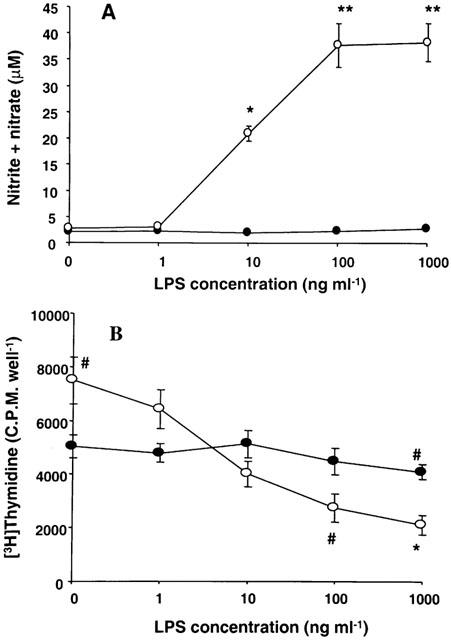
Effect of stellate cell medium conditioned with increasing concentrations of LPS on NO and DNA synthesis in hepatocytes. Stellate cells were incubated with indicated concentrations of LPS for 24 h in serum-free medium. The medium was then transferred to hepatocytes incubated in serum-free medium for 24 h. For control experiments, hepatocytes were incubated in serum-free medium with LPS. After 24 h, the concentration of nitrite+nitrate in the medium (A) and DNA synthesis in the cells (B) were determined. Solid symbols, hepatocytes; hollow symbols, hepatocytes incubated with stellate cell-conditioned medium. *P<0.01 vs control; **P<0.001 vs control; #P<0.05 vs control.
Incubation of hepatocytes with stellate cell-conditioned medium produced significant increase in the DNA synthesis (Figure 2B). In contrast, incubation of hepatocytes with medium conditioned by stellate cells in the presence of increasing concentrations of LPS produced progressive inhibition of DNA synthesis. LPS by itself caused some inhibition of DNA synthesis in hepatocytes at the highest (1000 ng ml−1) concentration tested.
Protein and mRNA expression of iNOS in stellate cells and hepatocytes
LPS did not affect the very low protein and mRNA expression of iNOS found in hepatocytes (Figure 3). Stellate cells did not contain detectable level of iNOS protein (Figure 3A) although the mRNA expression was feebly detectable (Figure 3B). Stimulation of stellate cells with LPS caused dramatic increase in the expressions of iNOS protein (Figure 3A) and mRNA (Figure 3B, C). Stellate cell-conditioned medium did not cause significant change in the expression of iNOS protein or mRNA in hepatocytes (Figure 3A–C). However, consistent with the stimulation of the production of NO, stellate cell medium conditioned with LPS produced robust increase in the expression of both iNOS protein and mRNA in hepatocytes (Figure 3).
Figure 3.
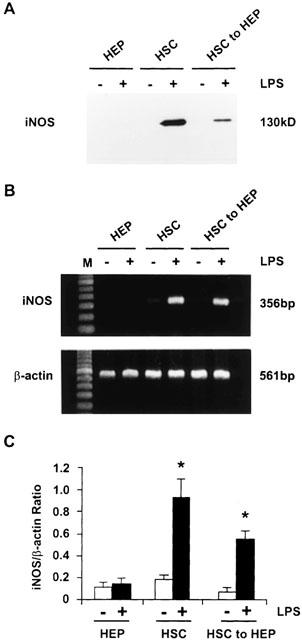
Protein and mRNA expression of iNOS in stellate cells and hepatocytes. Stellate cells were incubated in serum-free medium with or without 1 μg ml−1 LPS for 24 h. The medium was then transferred to hepatocytes and incubation was continued for 24 h. In parallel, hepatocytes were incubated in serum-free medium with or without LPS. The cells were then utilized for determination of iNOS protein expression by Western analysis (A) or for mRNA expression by semiquantitative RT–PCR (B). (C) The values show mRNA expression of iNOS standardized relative to the expression β-actin mRNA. HEP, hepatocytes; HSC, hepatic stellate cells; HSC to HEP, hepatocytes incubated with medium conditioned by stellate cells. *P<0.01 vs values in the absence of LPS.
Effect of L-NMMA and ODQ on NO and DNA synthesis
A specific inhibitor of nitric oxide synthase, L-NMMA, was used to assess whether the effect of LPS on stellate cells and of stellate cell medium conditioned with LPS on hepatocyte DNA synthesis was mediated by NO. L-NMMA caused complete inhibition of LPS-induced production of NO by stellate cells (Figure 4A). L-NMMA did not alter the basal synthesis of NO in hepatocytes, but inhibited that stimulated by LPS-conditioned stellate cell medium (Figure 4A).
Figure 4.
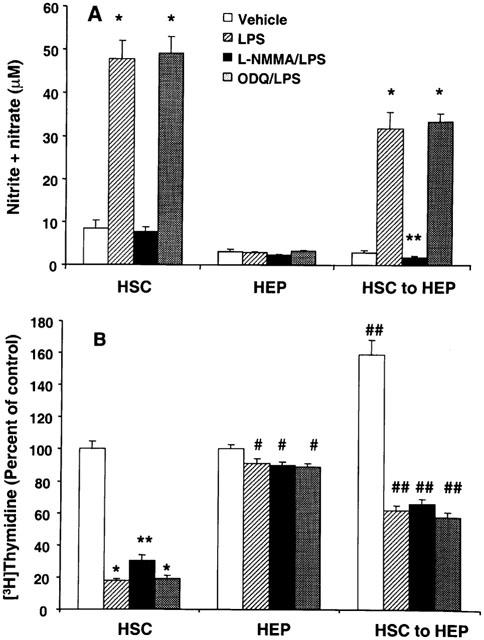
Effect of L-NMMA and ODQ on LPS-induced NO (A) and inhibition of DNA synthesis (B) in stellate cells and hepatocytes. Stellate cells were incubated with 1 μg ml−1 LPS±0.5 mM L-NMMA or 5 μM ODQ for 24 h in serum-free medium. The medium was used for measurement of nitrite+nitrate concentration and the cells for determination of DNA synthesis. Medium from another set of stellate cells was transferred to cultured hepatocytes and incubation as continued for 24 h. Nitrite+nitrate (medium) concentration and DNA synthesis (cells) were then determined. For control experiment, hepatocytes were incubated with LPS±L-NMMA or ODQ in unconditioned medium. HSC, hepatic stellate cells; HEP, hepatocytes; HSC to HEP, hepatocytes incubated with medium conditioned by stellate cells. (A) *P<0.001 vs vehicle; **P<0.05 vs vehicle. (B) *P<0.001 vs vehicle; **P<0.001 vs vehicle or P<0.01 vs LPS; #P<0.05 vs vehicle; ##P<0.01 vs hepatocytes with unconditioned medium.
L-NMMA partially reversed LPS-induced inhibition of DNA synthesis in stellate cells (Figure 4B). However, the inhibition of DNA synthesis in hepatocytes caused by LPS-conditioned stellate cell medium was unaltered by L-NMMA (Figure 4B).
Several effects of NO on the cellular processes are mediated by cyclic GMP (Schmidt et al., 1993). The concentration of cyclic GMP increased in stellate cells stimulated with LPS and in hepatocytes challenged with LPS-conditioned stellate cell medium (Table 1). Therefore, ODQ, an inhibitor of soluble guanyl cyclase (Garthwaite et al., 1995) was used to determine whether the effects of LPS on stellate cells and those of stellate cell-conditioned medium on hepatocytes were mediated by cyclic GMP. ODQ did not affect LPS-induced synthesis of NO in stellate cells (Figure 4A). Additionally, no difference was observed between the NO production by hepatocytes incubated with stellate cell medium conditioned with LPS in the presence and absence of ODQ. ODQ also did not affect LPS-induced inhibition of DNA synthesis in stellate cells and caused by LPS-conditioned stellate cell medium in hepatocytes (Figure 4B).
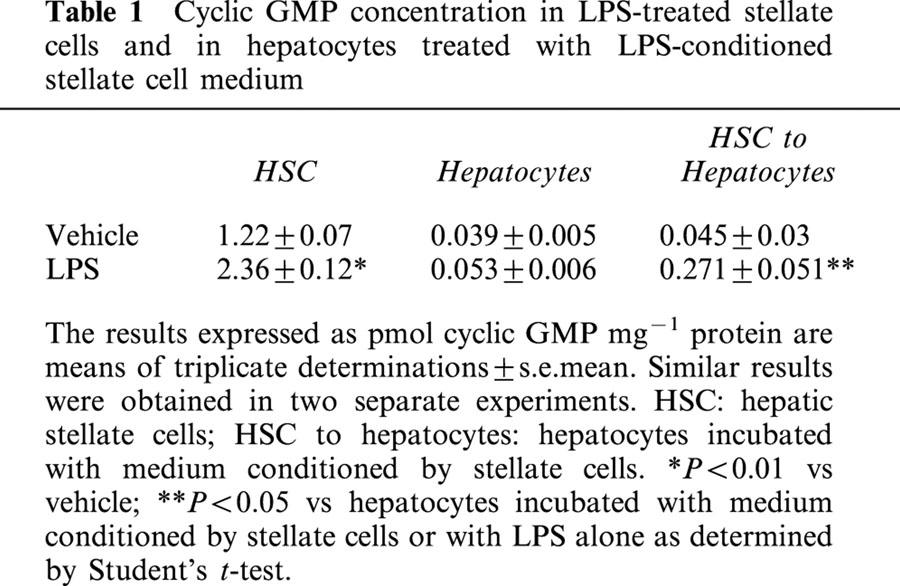
Table 1.
Cyclic GMP concentration in LPS-treated stellate cells and in hepatocytes treated with LPS-conditioned stellate cell medium
Effect of antiTGF-α antibody on DNA synthesis in hepatocytes stimulated by stellate cell-conditioned medium
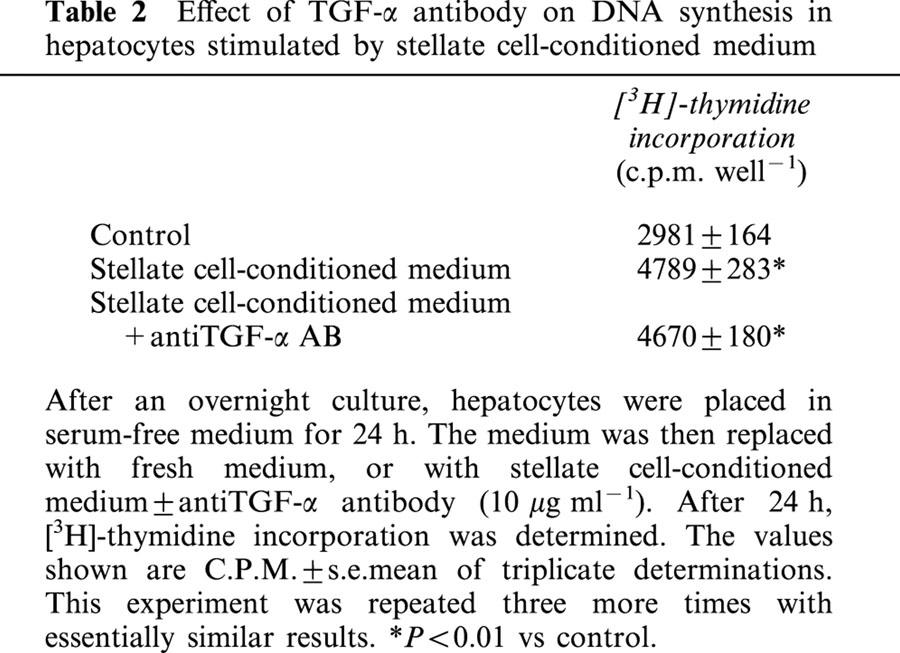
A neutralizing antibody for TGF-α did not affect the stimulation of DNA synthesis in hepatocytes caused by stellate cell-conditioned medium suggesting that this effect was not mediated by TGF-α (Table 2).
Table 2.
Effect of TGF-α antibody on DNA synthesis in hepatocytes stimulated by stellate cell-conditioned medium
Effect of neutralizing antibodies for TGF-β, TNF-α, IL-1β and IL-6 on inhibition of DNA synthesis in hepatocytes caused by LPS-conditioned stellate cell medium
LPS treatment caused increased mRNA expression of IL-1β, IL-6, but not of TNF-α (Figure 5). No expression of IFN-γ mRNA was observed in unstimulated or LPS-stimulated stellate cells (Figure 5). Therefore, we determined whether neutralizing antibodies against TGF-β, IL-1β, IL-6 and TNF-α reversed the inhibition of DNA synthesis in hepatocytes caused by LPS-conditioned stellate cell medium. As shown in Figure 6A, anti-IL-1β and TNF-α antibodies partially reversed NO production in hepatocytes stimulated by LPS-conditioned stellate cell medium. However, anti-TGF-β- and anti-IL-6 antibodies did not affect this process. None of these antibodies affected the inhibition of DNA synthesis in hepatocyte caused by LPS-conditioned stellate cell medium (Figure 6B).
Figure 5.
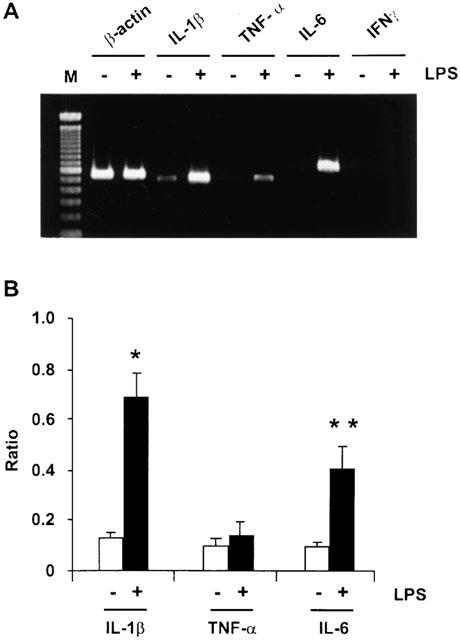
Effect of LPS on the mRNA expression of IL-1β, TNF-α, IL-6 and IFNγ in activated stellate cells. (A) Cells were incubated with 1 μg ml−1 LPS for 24 h. RNA was then extracted and cDNA was prepared. Semiquantitative RT–PCR analysis was performed to determine the concentrations of mRNA for the cytokines. (B) mRNA expression of the cytokines standardized relative to the expression of β-actin mRNA. *P<0.01 vs vehicle; **P<0.05 vs vehicle.
Figure 6.
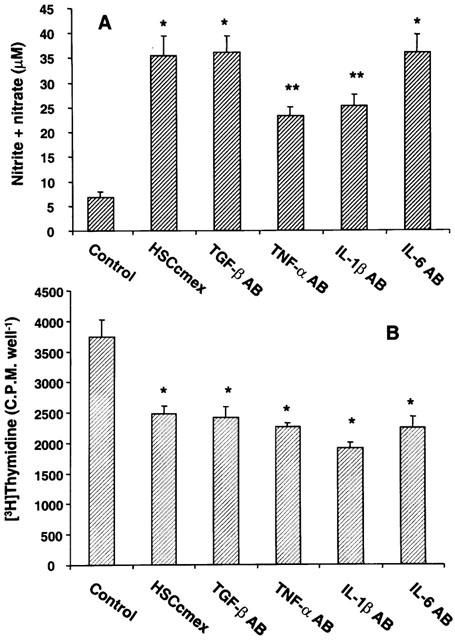
Effect of antibodies to TGF-β, TNF-α, IL-1β and IL-6 on the stimulation of NO and inhibition of DNA synthesis in hepatocytes induced by LPS-conditioned stellate cell medium. Hepatocytes were incubated with LPS-conditioned stellate cell medium in the presence of 15 μg ml−1 anti-TGF-β, 2.5 μg ml−1 anti-TNF-α, 2 μg ml−1 anti-IL-1β, 0.4 μg ml−1 and anti-IL-6 antibodies. After 24 h, concentration of nitrite+nitrate in the medium (A) and DNA synthesis in the cells (B) were determined. (A) *P<0.01 vs control:. **P<0.01 vs control or P<0.05 vs hepatocytes incubated with LPS-conditioned stellate cell medium (HSCcmex). (B) *P<0.05 vs control.
Discussion
This study demonstrates that activated stellate cells produce growth mediators that influence hepatocyte DNA synthesis. While medium conditioned by activated stellate cell stimulated DNA synthesis in hepatocytes, medium conditioned in the presence of endotoxin caused strong inhibition of this process. Physiologically, hepatocytes are quiescent with 1/20,000 undergoing mitosis at a given time (Fausto, 1990). During chronic injury, they regenerate in a unique fashion in nodules encapsulated by fibrous tissue produced mainly by activated stellate cells. Activated stellate cells have been shown to synthesize several potent growth mediators and cytokines (Blomhoff & Wake, 1991; Geerts et al., 1994; Ramadori, 1994). However, the precise mechanisms by which stellate cells modulate the growth of hepatocytes remain inconclusive. Since activated stellate cells do not produce HGF (Ramadori et al., 1992; Schirmacher et al., 1992), HGF cannot be responsible for the stimulation of DNA synthesis in hepatocytes caused by the medium conditioned by activated stellate cells. Our results showing inability of a neutralizing TGF-α antibody to inhibit DNA synthesis in hepatocytes stimulated by medium conditioned by activated stellate cells indicate that TGF-α may not be a major factor responsible for this effect.
Endotoxin produced by intestinal bacteria entering the circulation is taken up and destroyed by Kupffer cells in the liver (Nolan, 1981). During chronic liver injury, endotoxin concentration increases as a consequence of increased intestinal translocation (Triger et al., 1978; Lumsden et al., 1988), reduced hepatic clearance due to intra- and extrahepatic shunts and impaired activity of the reticuloendothelial system (Bomzon & Blendis, 1994). In such situations, it has been suggested that endotoxin may exert effects on other hepatic cell types including stellate cells (Nolan, 1981). Indeed, endotoxin stimulated iNOS expression and NO synthesis, and inhibited DNA synthesis in the activated stellate cells. The effect of endotoxin on DNA synthesis in stellate cells appears to be partially mediated by NO as evident by its incomplete inhibition by L-NMMA. Experiments of the present investigation were performed in serum-free medium. Since the presence of serum is required for providing LPS-binding protein (LBP) in the initiation of binding of LPS to its receptor CD14 (Martin et al., 1992), our findings suggest that either activated stellate cells may produce LBP or that LBP is not required for the action of LPS on stellate cells.
Interestingly, in contrast to the stimulatory effect of stellate cell medium conditioned in the absence of LPS, medium conditioned in its presence strongly inhibited DNA synthesis in hepatocytes. Since LPS did not alter DNA synthesis in hepatocytes, it is apparent that mediators produced by LPS-stimulated stellate cells are the responsible factors. NO has been shown to inhibit DNA synthesis in several cell types such as vascular smooth muscle cells (Paul et al., 1997), stellate cells (Helyar et al., 1994; Kawada et al., 1998), cardiac myocytes (Calderone et al., 1998), fibroblasts (Calderone et al., 1998) and renal mesangial cells (Rupprecht et al., 2000). A strong induction of iNOS activity in hepatocytes treated with LPS-conditioned stellate cell medium suggested that the effect on DNA synthesis may be mediated by NO. However, inhibition of NOS and guanylyl cyclase respectively by L-NMMA and ODQ did not alter inhibition of DNA synthesis in hepatocytes caused by LPS-conditioned stellate cell medium. These results indicate that factor(s) other than NO are most probably responsible for the effect of LPS-conditioned stellate cell medium on hepatocytes.
Cultured nonparenchymal cells (a mixture of endothelial and Kupffer cells) isolated from regenerating rat liver, but not the unmodified liver, were found to release factor(s) that inhibited DNA synthesis in hepatocytes (Boulton et al., 1997). Endotoxin causes increased production of several cytokines, such as IFNγ, IL-1β and TNF-α, in macrophages including Kupffer cells (Nathan, 1968; Adams & Hamilton, 1984; Decker, 1990; Klocker et al., 2000), that are known to modulate DNA and NO synthesis in hepatocytes (Feingold et al., 1988; Nakamura et al., 1988; Beyer et al., 1990; Kuma et al., 1990; Webber et al., 1998; Curran et al., 1990; Nussler et al., 1992). Activated stellate cells are also capable of producing such cytokines as well as TGF-β (Blomhoff & Wake, 1991; Geerts et al., 1994; Ramadori, 1994). In the present study, LPS increased the mRNA expression of IL-6 and IL-1β, but not of TNF-α, and no expression of IFN-γ was found in unstimulated or LPS-stimulated stellate cells. However, antibodies to TNF-α, IL-6, IL-1β as well as to TGF-β failed to exert any effect on the inhibition of DNA synthesis in hepatocytes induced by LPS-conditioned stellate cell medium. These results indicate that LPS stimulates the synthesis of an as yet unidentified growth modulator(s) by activated stellate cells that is capable of inhibiting DNA synthesis in hepatocytes.
The knowledge of the factors responsible for the abnormal liver growth as well as of those that prevent this condition is incomplete. The results of this study indicate that activated stellate cells are capable of producing both types of mediators. It is apparent that an imbalance in the production of such mediators, and modulation of their synthesis by endotoxin play an important role in hepatic pathology. Identification of these factors is, therefore, critical in formulating effective therapies to prevent abnormal liver growth.
Acknowledgments
This work was supported by a grant from NIH (DK 54411) and a VA Merit award. We thank Ms Kristin Anselmi for excellent technical assistance.
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbant assay
- GBSS
Gay's balanced salt solution
- HBSS
Hank's balanced salt solution
- LPS
lipopolysaccharide
- NO
nitric oxide, RT–PCR, reverse transcriptase polymerase chain reaction
- SDS–PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
References
- ADAMS D.O., HAMILTON T.A. The cell biology of macrophage activation. Annu. Rev. Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- BEYER H.S., STANLEY M., THEOLOGIDES A. Tumor necrosis factor-alpha increases hepatic DNA and RNA and hepatocyte mitosis. Biochem. Int. 1990;22:405–410. [PubMed] [Google Scholar]
- BLOMHOFF R., WAKE K. Perisinusoidal stellate cells of the liver: Important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- BOMZON A., BLENDIS L.M. The nitric oxide hypothesis and the hyperdynamic circulation in cirrhosis. Hepatology. 1994;20:1343–1350. [PubMed] [Google Scholar]
- BOULTON R., WOODMAN A., CALNAN D., SELDEN C., TAM F., HODGSON H. Nonparenchymal cells from regenerating rat liver generate interleukin-1α and -1β: A mechanism of negative regulation of hepatocyte proliferation. Hepatology. 1997;26:49–58. doi: 10.1053/jhep.1997.v26.pm0009214451. [DOI] [PubMed] [Google Scholar]
- CALDERONE A., THAIK C.M., TAKAHASHI N., CHANG D.L.F., COLUCCI W.S. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J. Clin. Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN R.D., BILLIAR T.R., STUEHR D.J., OCHOA J.B., HARBRECHT B.G., FLINT S.G., SIMMONS R.L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann. Surg. 1990;212:462–469. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECKER K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur. J. Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- FAUSTO N. Hepatology: A Textbook of Liver Disease. ed. Zakim, D. & Boyer, T.D. pp. Philadelphia: W.B. Saunders Co; 1990. Hepatic regeneration; pp. 49–65. [Google Scholar]
- FEINGOLD K.R., SOUED M., GRUNFELD C. Tumor necrosis factor stimulates DNA synthesis in the liver of intact rats. Biochem. Biophys. Res. Commun. 1988;153:576–582. doi: 10.1016/s0006-291x(88)81134-3. [DOI] [PubMed] [Google Scholar]
- GABRIEL A., KUDDUS R.H., RAO A.S., GANDHI C.R. Down-regulation of endothelin receptors by transforming growth factor β1 in hepatic stellate cells. J. Hepatol. 1999;30:440–450. doi: 10.1016/s0168-8278(99)80103-2. [DOI] [PubMed] [Google Scholar]
- GABRIEL A., KUDDUS R.H., RAO A.S., WATKINS W.D., GANDHI C.R. Superoxide-induced changes in endothelin (ET) receptors in hepatic stellate cells. J. Hepatol. 1998;29:614–627. doi: 10.1016/s0168-8278(98)80157-8. [DOI] [PubMed] [Google Scholar]
- GANDHI C.R., KUDDUS R., SUBBOTIN V.M., PRELICH J., MURASE N., RAO A.S., NALESNIK M.A., WATKINS S.C., DELEO A., TRUCCO M., STARZL T.E. A fresh look at augmenter of liver regeneration in rats. Hepatology. 1999;29:1435–1445. doi: 10.1002/hep.510290522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GEERTS A., DE BLESER P., HAUTEKEETE M.L., NIKI T., WISSE E.Fat-storing (Ito) cell biology The Liver: Biology and Pathobiology. 1994New York: Raven Press, Ltd; 819–838.ed. Arias, I.M., Boyer, J.L., Fasuto, N., Jakoby, W.B., Schachter, D.L. & Shafritz, D.A. pp [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- HELYAR L., BUNDSCHUH D.S., LASKIN J.D., LASKIN D.L. Induction of hepatic Ito cell nitric oxide production after acute endotoxemia. Hepatology. 1994;20:1509–1515. doi: 10.1002/hep.1840200621. [DOI] [PubMed] [Google Scholar]
- KAWADA N., SEKI S., KUROKI T., INOUE M. Regulation of stellate cell proliferation by lipopolysaccharide: Role of endogenous nitric oxide. J. Gastroenterol. Hepatol. 1998;13 Suppl.:S6–S13. doi: 10.1111/jgh.1998.13.s1.6. [DOI] [PubMed] [Google Scholar]
- KLOCKER U., SCHULTZ U., SCHALLER H., PROTZER U. Endotoxin stimulates liver macrophages to release mediators that inhibit an early step in hepadnavirus replication. J. Virol. 2000;74:5525–5533. doi: 10.1128/jvi.74.12.5525-5533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSUGA K., YUI Y., HATTORI R., SASE K., EIZAWA H., AOYAMA T., INOUE R., SASAYAMA S. Cloning of an inducible nitric oxide synthase from rat polymorphonuclear neutrophils. Endothelium. 1994;2:217–221. [Google Scholar]
- KUDDUS R.H., NALESNIK M.A., SUBBOTIN V.M., RAO A.S., GANDHI C.R. Enhanced synthesis and reduced metabolism of endothelin-1 (ET-1) by hepatocytes-an important mechanism of increased endogenous levels of ET-1 in liver cirrhosis. J. Hepatol. 2000;33:725–732. doi: 10.1016/s0168-8278(00)80302-5. [DOI] [PubMed] [Google Scholar]
- KUMA S.-I., INABA M., OGATA H., INABA K., OKUMURA T., SAITO K., YAMAMOTO H., IKEHARA C. Effect of human recombinant interleukin-6 on the proliferation of mouse hepatocytes in the primary culture. Immunobiology. 1990;180:235–242. doi: 10.1016/s0171-2985(11)80331-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LUMSDEN A.B., HENDERSON J.M., KUTNER M.H.. Endotoxin levels measured by a chromogenic assay in portal, hepatic, and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- MARTIN T.R., MATHISON J.C., TOBIAS P.S., LETURCQ D.J., MORIARTY A.M., MAUNDER R.J., ULEVITCH R.J. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. J. Clin. Invest. 1992;90:2209–2219. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA T., ARAKAKI R., ICHIHARA A. Interleukin-1β is a potent growth inhibitor of adult rat hepatocytes in primary culture. Exp. Cell. Res. 1988;179:488–497. doi: 10.1016/0014-4827(88)90286-8. [DOI] [PubMed] [Google Scholar]
- NATHAN C.F.Interferon-gamma and macrophage activation in cell-mediated immunity Mechanisms of Host Resistance to Infectious Agents, Tumors, and Allografts. 1968New York: The Rockefeller University Press; 165–184.ed. Steinman, R.M. & North, R.J. pp [Google Scholar]
- NOLAN J.P. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- NUDEL U., ZAKUT R., SHANI M., NEUMAN S., LEVY Z., YAFFER D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983;11:1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUSSLER A.K., DI SILVIO M., BILLIAR T.R., HOFFMAN R.A., GELLER D.A., SELBY R., MADARIAGA J., SIMMONS R.L. Stimulation of nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J. Exp. Med. 1992;176:261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL A., BRYANT C., LAWSON M.F., CHILVERS E.R., PLEVIN R. Dissociation of lipopolysaccharide-mediated nitric oxide synthase and inhibition of DNA synthesis in RAW264.7 macrophages and rat aortic smooth muscle cells. Br. J. Pharmacol. 1997;120:1439–1444. doi: 10.1038/sj.bjp.0701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMADORI G. The stellate cell (Ito-cell, fat-storing cell, lipocyte, perisinusoidal cell) of the liver. New insights into pathophysiology of an intriguing cell. Virchows Arch. B Cell. Pathol. 1994;61:147–158. doi: 10.1007/BF02890417. [DOI] [PubMed] [Google Scholar]
- RAMADORI G., NEUBAUER K., ODENTHAL M., NAKAMURA T., KNITTEL T., SCHWOGLER S., MEYER ZUM BUSCHENFELDE K.-H. The gene of hepatocyte growth factor is expressed in fat-storing cells of rat liver and is downregulated during cell growth and by transforming growth factor-β. Biochem. Biophys. Res. Commun. 1992;183:739–742. doi: 10.1016/0006-291x(92)90545-v. [DOI] [PubMed] [Google Scholar]
- RUPPRECHT H.D., AKAGI Y., KEIL A., HOFER G. Nitric oxide inhibits growth of glomerular mesangial cells: role of the transcription factor EGR-1. Kidney Int. 2000;57:70–82. doi: 10.1046/j.1523-1755.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- SCHIRMACHER P., GEERTS A., PIETRANGELO A., DIENES H.P., ROGLER C.E. Hepatocyte growth factor/hepatopoietin A is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology. 1992;15:5–11. doi: 10.1002/hep.1840150103. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., LOHMANN S.M., WALTER U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim. Biophys. Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- TIAN L., STEPKOWSKI S.M., QU X., WANG M., KAHAN B.D. Cytokine mRNA expression in tolerant heart allografts after immunosuppression with cyclosporine, sirolimus or brequinar. Transpl. Immunol. 1997;5:189–198. doi: 10.1016/s0966-3274(97)80037-8. [DOI] [PubMed] [Google Scholar]
- TRIGER D.R., BOYER T.D., LEVIN J. Portal and systemic bactetriemia and endotoxemia in liver disease. Gut. 1978;19:935–939. doi: 10.1136/gut.19.10.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEMURA T., MIYAZAKI Y., HIRAI Y., MATSUMOTO H., OTA T., OHASHI R., SHIMIZU N., TSUJI T., INOUE Y., NAMBA M. Different expression of positive and negative regulators of hepatocyte growth in growing and shrinking hepatic lobes after portal vein ligation in rats. Int. J. Mol. Med. 2000;5:173–179. doi: 10.3892/ijmm.5.2.173. [DOI] [PubMed] [Google Scholar]
- WEBBER E.M., BRUIX J., PIERCE R.H., FAUSTO N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]
- ZHANG B., BORDERIE D., SOGNI P., SOUBRANE O., HOUSSIN D., CALMUS Y. NO-mediated vasodilation in the rat liver. Role of hepatocytes and liver endothelial cells. J. Hepatol. 1997;26:1348–1355. doi: 10.1016/s0168-8278(97)80471-0. [DOI] [PubMed] [Google Scholar]


