Abstract
The effect of several nitric oxide releasing-non-steroidal anti-inflammatory drugs (NO-NSAID) and nitroprednisolone on blood vessel relaxation in vitro and in vivo was studied. Nitroflurbiprofen (NOF; EC50, 688.8±93.8 μM), nitroaspirin (NOA; EC50, 57.9±6.5 μM), nitroparacetamol (NOPARA; EC50, 71.5±14.6 μM) and nitroprednisolone (EC50, 15.1±1.4 μM) caused concentration-related relaxation of noradrenaline (NA)-contracted rat aortic rings. All NO releasing compounds tested were approximately three orders of magnitude less potent than sodium nitroprusside (SNP, EC50, 35.7±3.5 nM).
The vasorelaxant effect of NOF and NOPARA in the rat aorta was potentiated by zaprinast (5 μM) and reduced by ODQ (5 μM). Flurbiprofen and paracetamol (100 μM) caused minimal (<10%) relaxation of the rat aorta and did not affect the response to SNP. The effect of NOF was unchanged in the presence of L-NAME (100 μM; EC30, 181.8±35.1 μM cf. EC30, 125.1±17.0 μM, P>0.05) but increased by removal of the endothelium (EC30, 164.3±26.3 μM cf. EC50, 688.8±93.8 μM, P<0.05).
NOF (0.1–50 μM) produced a small but not concentration-related vasodilation of the NA-preconstricted (i.e. ‘high tone') perfused rat mesentery preparation (cf. SNP, EC30, 4.4±0.7 μM). In contrast, NOF (1–100 μM) produced concentration-related vasodilation of the ‘high tone' perfused rat kidney with an EC50 of 33.1±4.4 μM.
Neither NOF (74 mg kg−1, i.p.) nor NOA (91.9 mg kg−1, i.p.) nor equimolar doses of flurbiprofen (50 mg kg−1, i.p.) or aspirin (50 mg kg−1, i.p.) affected mean arterial blood pressure (MAP) or heart rate (HR) of pentobarbitone-anaesthetized rats over a 1 h period.
NO-NSAID relax blood vessels in vitro by an NO-dependent mechanism. The absolute vasorelaxant effect of NO releasing drug varies greatly with the choice of compound and between blood vessel preparations.
Keywords: NO-NSAID, nitroflurbiprofen, aorta, mesentery, kidney, nitroparacetamol, nitroaspirin, nitroprednisolone
Introduction
Aspirin and like nonsteroidal anti-inflammatory drugs (NSAID) have been employed, for many years, as a treatment for inflammation and hyperalgesia associated with such conditions as rheumatoid arthritis. However, the side effects of NSAID in the gastrointestinal tract (e.g. haemorrhage, ulceration), resulting from reduced formation of vasodilator and cytoprotective prostanoids in the gastric mucosa, limit the usefulness of these compounds in the clinic (Wallace, 1997).
In recent years, nitric oxide (NO)-releasing derivatives of NSAID (so-called ‘nitro-nonsteroidal anti-inflammatory drugs or NO-NSAID') have attracted considerable attention as possible therapeutic alternatives to the parent NSAID (reviewed by del Soldato et al., 1999). Thus, NO-NSAID cause significantly less gastrointestinal damage in experimental animals than do the parent NSAID (e.g. Muscara et al., 1998; Takeuchi et al., 1998; Ukawa et al., 1997; Wallace et al., 1994).
It has been suggested that nitro-NSAID most probably ‘spare' the gastrointestinal tract by the local release of NO resulting in increased stomach mucosal blood flow (Mariotto et al., 1995). Furthermore, NO released from NO-NSAID may also contribute to the anti-inflammatory effect of this group of drugs. For example, nitroaspirin (but not aspirin) inhibits caspase 1 activity and thereby reduces formation of pro-inflammatory, interleukin-1β (Fiorucci et al., 1999). Such an action may contribute to the augmented anti-inflammatory (Al-Swayeh et al., 2000) and anti-thrombotic (Wallace et al., 1995) effect of nitroaspirin (cf. aspirin). Thus, NO-NSAID may be considered as a novel class of ‘NO donor' drugs.
With this in mind it is perhaps surprising that there is a paucity of published information concerning the cardiovascular effects of NO-NSAID. For example, in vivo, nitroflurbiprofen (NOF), nitroaspirin (NOA) and nitronaproxen are reportedly without effect on blood pressure of the anaesthetized rat (Fujihara et al., 1998; Wallace et al., 1994; 1995, Yamamoto et al., 2000). Similarly, a newly reported NO releasing derivative of salicylic acid (B-NOD) has also recently been reported to be without effect on rat blood pressure or heart rate following oral administration whilst potently inhibiting platelet aggregation in vitro (Bing et al., 2000). Furthermore, high concentrations of NOF have been shown to relax isolated rat aortic rings in vitro (Adami et al., 1996). However, to the best of our knowledge, there has been no systematic attempt to study the effect of NOF (and other NO-NSAID) on vascular responsiveness in a range of blood vessel preparations in vitro.
To this end, we now report the vasorelaxant effect of NOF and flurbiprofen in comparison with sodium nitroprusside (SNP) using a range of in vitro blood vessel preparations (i.e. rat aorta, perfused rat kidney and mesentery) and have additionally probed the mechanism of action of NOF-induced vasorelaxation and determined its potency (cf. other NO-NSAID) in the rat aorta preparation.
Methods
Effect of NO releasing compounds on rat aortic ring
Rat aortic rings were prepared essentially as described previously (Moore et al., 1990). Briefly, rats (male, Wistar, 220–300 g) were either killed by a blow to the head and exsanguination or by exposure to a gradually increasing concentration of CO2. The thoracic aorta was removed and cleared of extraneous connective tissue. Rings (approx. 2–4 mm diameter) were mounted using stainless steel clips under a tension of 1 g in 20 ml organ baths containing warmed (37°C), oxygenated (95% O2: 5% CO2) Krebs solution (composition, mM); NaCl 118, KCl 5.4, NaHCO3 25, MgSO4 1.2, CaCl2 2.5, glucose 11.1, pH 7.4. Changes in tension were recorded using Grass-FT03 force transducers connected to a MacLab 2E (AD Instruments Inc.) attached to a Macintosh Performa 475 computer. After equilibration (1 h), rings were contracted with 1 μM noradrenaline (NA), a concentration that produces approximately 70% of the maximum contraction and thereafter relaxed by cumulative addition (dose cycle time, 6 min) of increasing concentrations (10–1000 μM) of nitroflurbiprofen (NOF), nitroaspirin (NOA), nitroprednisolone (NOP), nitroparacetamol (NOPARA) or sodium nitroprusside (SNP: 0.01–20 μM). The vasorelaxant effect of flurbiprofen, aspirin, prednisolone and paracetamol (all 10–1000 μM) was also evaluated as was the effect of SNP in the presence of paracetamol or flurbiprofen (both 100 μM). In some experiments the vasorelaxant effect of NOF and NOPARA in the rat aorta was additionally determined after preincubation (15 min) with either [1,2,4] oxadiazolo [4,3a] quinoxalin-1-one (ODQ; 5 μM), zaprinast (5 μM) or L-NAME (100 μM) or in rings gently rubbed on the intimal surface to remove the endothelial cell layer (checked by failure to relax in response to 1 μM carbachol). All results are expressed as per cent relaxation of NA-induced tone.
Effect of NOF on perfused rat mesentery and kidney
Rat mesentery and kidney preparations were perfused as described previously (Moore et al., 1990). Briefly, rats (male, Wistar, 200–250 g, Charles River Ltd) were killed by a blow to the head and exsanguination. The superior mesenteric artery and/or renal artery were cannulated and the mesenteric vascular bed and/or kidney were immediately removed and perfused (5 ml min−1) with warmed (37°C), oxygenated (95% O2: 5% CO2) Krebs solution. Perfusion pressure was monitored continuously by means of a Druck pressure transducer connected to a Devices pen recorder. In order to assess the vasorelaxant effect of NOF (0.1–100 μM), flurbiprofen (1–100 μM) and SNP (0.1–100 μM) on perfusion pressure, NA at a concentration (31.3 μM) previously shown to cause approximately 70% maximal vasoconstrictor tone (Bhardwaj & Moore, 1988) was added to the perfusing Krebs solution. Under these circumstances no significant loss of vasoconstrictor tone in either mesentery or kidney was detected over a 3 h period which was the maximum length of each experiment. Experiments were carried out in a non-cumulative manner i.e. drugs were added to the perfusing Krebs solution for a period of 15 min to allow development of a full vasodilator response after which time preparations were returned to NA-containing Krebs before exposure to a higher concentration of test drug. Results indicate change in perfusion pressure (mm Hg).
Measurement of rat mean arterial blood pressure (MAP)
Rats (male, Wistar, 200–250 g, Tucks Ltd) were anaesthetized with sodium pentobarbitone (60 mg kg−1, i.p.). The right carotid artery was cannulated for measurement of blood pressure and heart rate via a Druck pressure transducer connected to a Powerlab (AD Instruments Ltd) attached to a Dell Inspiron 4000 computer running Chart for Windows. NOF (74 mg kg−1), NOA (91.9 mg kg−1), flurbiprofen (50 mg kg−1), aspirin (50 mg kg−1), sodium nitroprusside (50 μg kg−1) or vehicle (0.5% w v−1 carboxymethylcellulose, 2 ml kg−1) were administered i.p. and blood pressure and heart rate monitored continuously for 60 min thereafter.
Drugs and chemicals
Nitroflurbiprofen (2-fluoromethyl [1,1-biphenyl]-4-acetic acid 4-(nitrooxy) butyl ester), nitroparacetamol (4-(nitroxy)butanoic acid 4-acetylaminophenyl ester), nitroaspirin (2-(acetyloxy) benzoic acid 3-(nitrooxymethy) phenyl ester) and nitroprednisolone (prednisolone 21-[(4-nitrooxymethyl)benzoate]) were the generous gift of Dr P. del Soldato (NicOX Ltd, Sophia-Antipolis, France). Flurbiprofen, paracetamol, aspirin, noradrenaline bitartrate, prednisolone, ODQ, zaprinast, sodium nitroprusside and carboxymethylcellulose were purchased from Sigma Ltd. Noradrenaline and sodium nitroprusside were dissolved in distilled water and kept on ice throughout the experiment to minimize spontaneous breakdown. All other drugs were dissolved in DMSO.
Statistical analysis
Results indicate mean±s.e.mean with the number of observations shown in parenthesis. Statistical significance of differences between groups was determined one-way analysis of variance (ANOVA) to compare means in the same group followed by unpaired student's t-tests to compare means between groups where necessary. In all cases a probability (P) value of less than 0.05 was taken to indicate statistical significance.
Results
The effect of NOF and other NO releasing compounds on rat aorta
In this study, NA (1 μM) caused approximately 70% maximal contraction of the isolated rat aorta (0.95±0.03 g, n=130). Increasing concentrations of NO-NSAID and nitroprednisolone (1 μM–1 mM) caused graded relaxation of NA-precontracted aortic rings (Figure 1). The rank order of potency was NOP (EC50, 15.1±1.4 μM: Emax, 96.5±4.2%, n=5–12)>NOA (EC50, 55.4±6.2 μM: Emax, 83.8±8.7%, n=12)>NOPARA (EC50, 71.5±14.6 μM: Emax, 103.5±2.9%, n=6)>NOF (EC50, 688.8±93.7 μM, n=15). Due to the limitations of solubility of the highest concentration of NOF tested (1 mM) the greatest relaxation of tone observed with this compound was 54.0±4.1% (n=15). In all cases, relaxation of rat aortic rings to these NO releasing compounds was a gradual process, which at higher concentrations, required 3–4 min to complete. Control experiments were undertaken with SNP which also relaxed NA-precontracted aortic rings (EC50, 74.5±7.4 nM, Emax, 99.6±1.9%, n=4). For comparison, SNP was therefore approximately three orders of magnitude more potent than the other NO releasing compounds tested.
Figure 1.
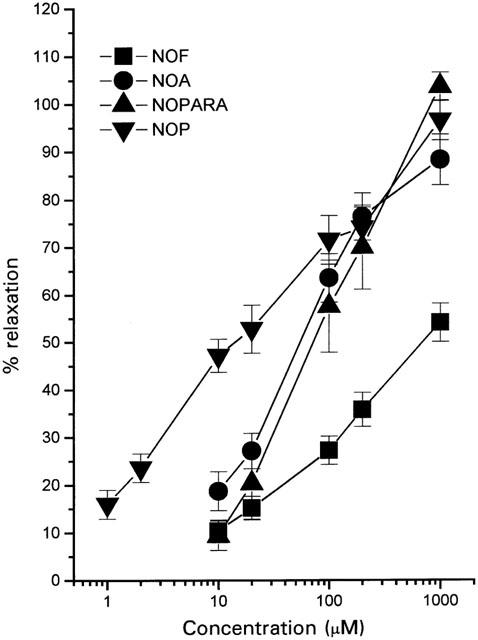
Vasorelaxant effect of a range of NO-NSAID on the rat aorta. Preparations were precontracted with NA (1 μM) and subsequently relaxed by cumulative addition of NO-NSAID. Results show per cent relaxation of NA-induced tone and are mean±s.e.mean, n=5–15.
Mechanism of action of the vasorelaxant effect of NO-NSAID on rat aorta
A number of separate experiments were undertaken to investigate the mechanism of the vasorelaxant effect of NO-NSAID. Thus, relaxation of aortic rings in the presence of NO-NSAID is unlikely to be related to a vehicle effect since DMSO at a concentration (0.3% v v−1) which represents the highest concentration of vehicle achieved in the organ bath in these experiments did not relax NA-precontracted aortic rings. Similarly, a direct effect of prednisolone or NSAID (released by the cleavage of NO-NSAID in the organ bath) can be excluded since flurbiprofen, paracetamol, prednisolone and aspirin (concentrations up to 100 μM) caused minimal relaxation of NA-precontracted rat aortic rings (data not shown). It is also unlikely that, following NO-NSAID breakdown, the presence of the NSAID moiety significantly affected the vasorelaxant effect of released NO since exposure of tissues to flurbiprofen or paracetamol (both 100 μM) failed to affect the response to SNP (EC50, 30.3±2.4 nM cf. 40.4±4.6 and 51.5±7.4 nM in the presence of flurbiprofen and paracetamol respectively, n=4, P>0.05). Finally, pretreatment with L-NAME (100 μM) did not affect the vasorelaxant response to either NOF (EC30, 181.8±35.1 μM cf. 125.1±, 17.0 μM, n=4, P>0.05) or NOPARA (125.5±30.7 μM cf. 71.5±14.6 μM, n=4, P>0.05) suggesting that these NO-NSAID do not trigger the release of endogenous NO from aortic rings. In contrast, removal of endothelium significantly increased NOF-induced relaxation (EC50, 164.3±26.3 μM, n=7, P<0.05). Moreover, preincubation of aortic rings with zaprinast (selective inhibitor of cyclic GMP phosphodiesterase) significantly potentiated the vasorelaxant effect of NOF and NOPARA whilst ODQ (selective inhibitor of soluble guanylate cyclase) significantly reduced responses to both NO-NSAID (Figure 2).
Figure 2.
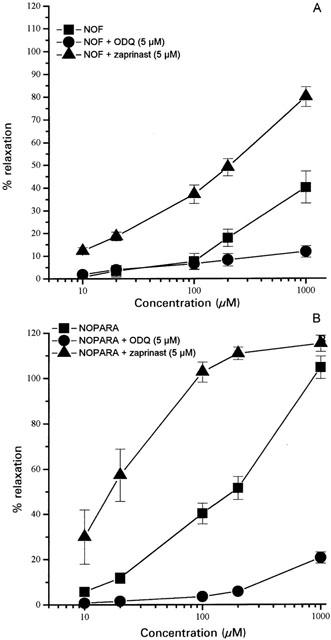
Vasorelaxant effect of NOF (A) and NOPARA (B) on the NA (1 μM) precontracted rat aorta following pretreatment (15 min) with either ODQ (5 μM) or zaprinast (5 μM). Results show per cent relaxation of NA-induced tone and are mean±s.e.mean, n=4–7.
Effect of NOF on isolated, perfused rat mesentery
Perfusion of rat mesentery preparations with NA (31.3 μM) resulted in so-called ‘high tone' preparations in which perfusion pressure was increased by approximately 100–180 mm Hg over basal values. Perhaps surprisingly, NOF (100 nM–50 μM) exhibited negligible vasodilator activity in this vascular bed. Indeed, the response to NOF was not dose related with a small but consistent vasodilatation being observed at all concentrations used (e.g. 50 μM: 15.7±5.7% of NA-induced tone, n=5). In control experiments, both DMSO (0.2% v v−1; highest concentration achieved) and flurbiprofen (100 μM) were without effect on vascular tone in these preparations (data not shown). SNP was considerably more active than NOF producing a maximal relaxation of NA-induced tone of 42.5±5.7 % (n=5) with an EC30 of 4.4±0.7 μM (n=5) (Figure 3). The failure of NOF to dilate the rat mesenteric vasculature in these experiments was not due to a non-specific inhibitory effect of the vehicle on the response to NO since DMSO (0.2% v v−1) did not affect vasodilation due to SNP in this preparation (data not shown).
Figure 3.
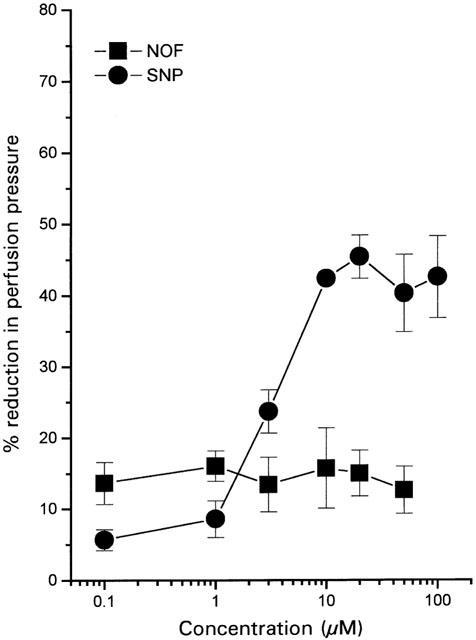
Vasodilator effect of NOF and SNP in the isolated, perfused rat mesentery. Preparations were preconstricted with NA (31.3 μM) and subsequently relaxed by non-cumulative addition of drug. Results show per cent reduction in NA-induced perfusion pressure and are mean±s.e.mean, n=5.
Effect of NOF on isolated, perfused rat kidney
Perfusion of rat kidney preparations with NA (31.3 μM) resulted in so-called ‘high tone' preparations in which perfusion pressure was increased by approximately 80–150 mm Hg over basal values. Both NOF (1–100 μM) and SNP (1–100 μM), but not flurbiprofen (10–100 μM), caused concentration-dependent relaxation of the NA-preconstricted renal vasculature (Figure 4). In contrast to data obtained using both rat aorta and rat mesentery, the maximum relaxation response following exposure to NOF (100 μM; 58.4±5.5%, n=5) was considerably greater than that observed with SNP (100 μM; 19.2±6.3%, n=5, P<0.05). In these experiments, the EC50 for NOF was 33.1±4.4 μM (n=5). Unfortunately, due to the lack of effect of SNP, no direct comparison of the potency of NOF and SNP is possible.
Figure 4.
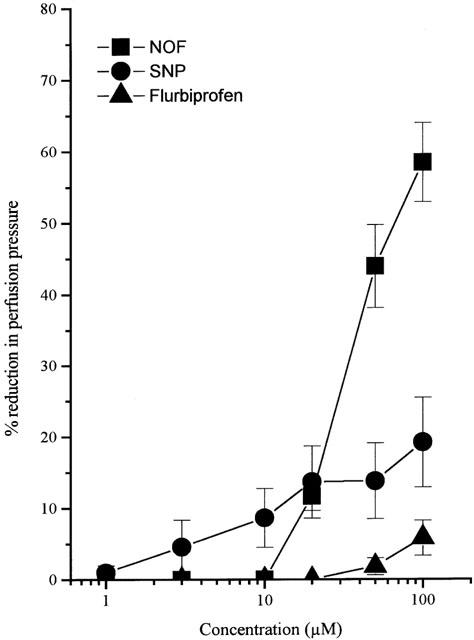
Vasodilator effect of NOF, SNP and flurbiprofen in the isolated, perfused rat kidney. Preparations were preconstricted with NA (31.3 μM) and subsequently relaxed by non-cumulative addition of drug. Results show per cent reduction in NA-induced perfusion pressure and are mean±s.e.mean, n=5.
Effect of NOF on MAP
Resting MAP (heart rate in parenthesis) of pentobarbitone-anaesthetized rats in these experiments was 110±3 mm Hg and 402±8 beats min−1 respectively (n=25). Injection (i.p.) of NOF (74 mg kg−1), NOA (91.9 mg kg−1), flurbiprofen (50 mg kg−1), aspirin (50 mg kg−1) or vehicle did not significantly affect MAP or heart rate over the following 60 min period (Table 1). In contrast, i.p. injection of a much lower dose of SNP (50 μg kg−1) significantly reduced MAP at 5 min post-injection.
Discussion
NO-NSAID have been proposed as alternatives to the parent NSAID for the treatment of a number of clinical conditions associated with inflammation, pain and/or thrombosis (del Soldato et al., 1999). In this context, it is of interest that nitroprednisolone has also been reported to exhibit more potent anti-inflammatory activity than the parent compound, prednisolone (Paul-Clark et al., 2000). The major perceived therapeutic advantage of NO-NSAID (cf. NSAID) is that these compounds cause much reduced gastrointestinal haemorrhage and ulceration (Elliot et al., 1995; Muscara et al., 1998; Ukawa et al., 1997; Wallace et al., 1994). The mechanism of such gastroprotection has been proposed to involve NO-mediated dilatation of the gastric vasculature (del Soldato et al., 1999; Wallace et al., 1995). However, this possibility is not consistent with previous reports that NO-NSAID have no effect on MAP of the anaesthetized rat (Fujihara et al., 1998; Wallace et al., 1994; 1995, Yamamoto et al., 2000) and raise the intriguing possibility of a differential effect of NO-NSAID on vascular beds throughout the body.
In the present series of experiments we have extended our preliminary observation that NOF exhibits no vasodepressor activity in the urethane-anaesthetized rat (Mcloughlin et al., 1999). We now report that NOA (in addition to NOF) is devoid of vasodepressor activity in this species and have also employed pentobarbitone as the anaesthetic in order to avoid any potential inhibition of cardiovascular reflexes associated with urethane. In contrast, a 1000 fold lower dose of the NO donor, SNP, did as expected cause a marked but relatively transient fall in MAP in these animals. It should be pointed out that, at doses shown to be without effect on MAP in the present study, both NOF and NOA exhibit pronounced anti-inflammatory activity in the carrageenan-induced rat hindpaw oedema test (Al-Swayeh et al., 1999, 2000) and NOF attenuates endotoxin-induced gastric and intestinal lesions in the rat (Wallace et al., 1995). Thus, it may be concluded that pharmacologically active doses of NOF and NOA are devoid of hypotensive activity in vivo at least in the anaesthetized rat. Similarly, chronic oral dosing of conscious rats with nitroflurbiprofen also failed to reduce MAP (Fujihara et al., 1998). Interestingly, chronic treatment with nitronaproxen has been reported to reduce MAP in the hypertensive rat (Muscara et al., 2000). Thus, the consensus of published information (see Introduction for additional references) strongly suggests a lack of vasodepressor activity of the NO-NSAID class of compounds in normotensive rats. Although beyond the scope of the present investigation, it would clearly be of interest to probe further the mechanism(s) underlying the fall in MAP elicited by nitronaproxen in the hypertensive rat.
All of the NO-releasing compounds tested (i.e. NOF, NOA, NOP and NOPARA) relaxed the NA-precontracted rat aorta. Relaxation of aortic rings due to both NOF and NOPARA was increased by zaprinast and reduced by ODQ thereby suggesting that NO release from NOF and NOPARA was a pre-requisite for blood vessel relaxation to take place. This conclusion was supported by the weak vasorelaxant effect of flurbiprofen, paracetamol and prednisolone coupled with the inability of flurbiprofen and paracetamol to influence the vasorelaxant effect of SNP-derived NO in this preparation. The finding that the vasorelaxant effect of NOF and NOPARA was not reduced by L-NAME, taken together with the above findings, strongly suggest that NO-NSAID act as endothelium-independent, NO donors in this preparation. In this context, the augmented vasorelaxant effect of NOF in endothelium-denuded aortic rings is probably explained by improved access to the underlying smooth muscle cells in the absence of an endothelial barrier.
An interesting feature of the present study is that pronounced differences in the vasorelaxant potency of the various NO-releasing compounds used in this study are apparent. Thus, the rank order of potency to relax the NA-precontracted rat aorta was NOP > NOA > NOPARA > NOF. Based upon a comparison of EC50 measurements it is clear that NOP is approximately 50 times more potent than NOF in this preparation. In this context, it is also of interest that the vasorelaxant effect of one of the NO-NSAID tested, NOF, varied considerably between blood vessel preparations. Thus, NOF was markedly less potent than SNP in the rat aorta and also in the perfused rat mesentery but was considerably more potent than SNP in the perfused rat kidney.
The disparity in potency both between different NO-NSAID (and nitroprednisolone) in the same tissue and for the same NO-NSAID in different tissues is somewhat unexpected. To the best of our knowledge, release of NO from NO-NSAID and like drugs is stochiometric and as such NO-mediated blood vessel relaxation would be expected to be similar between different NO-NSAID and within different tissues.
One possible explanation for these observations is that the absolute amount and/or the time course of NO release varies from one NO-releasing compound to the next and also between vascular beds. The mechanism(s) by which NO release from these compounds take place are not fully understood. It is clear that the molecule comprises NO linked via a ‘spacer' molecule and an ester bridge to the parent NSAID (or prednisolone) and it is also clear that this ester linkage is broken by cell/tissue esterase enzymes in order to liberate free NO (see del Soldato et al., 1999). However, the identity, and tissue distribution of these endogenous esterases remains unknown. One interpretation of the present data is therefore that the rate of esterase-mediated cleavage of NO differs between compounds. Thus, we hypothesize that NOP is cleaved more efficiently by esterase enzyme(s) present in rat aortic rings than is NOF and the other NO-NSAID studied and consequently causes relaxation at lower concentrations. Similar differences in esterase activity (i.e. high in kidney, low in mesentery and aorta) may account for the variability in vasorelaxant activity of NOF between different blood vessel preparations.
In conclusion, NO-NSAID (and nitroprednisolone) relax isolated blood vessel preparations in vitro with a potency which varies with the individual compound and with choice of blood vessel preparation. The possibility that the activity and selectivity of the endogenous, tissue-based esterase enzyme(s) which release NO from NOA, NOF, NOPARA and NOP play a critical role in the cardiovascular response to these compounds is suggested. Further experiments to investigate the mechanism of NO release from NO-NSAID and related compounds both in vitro and in vivo are required.
Acknowledgments
J. Keeble was sponsored by the British Pharmacological Society. We would like to thank Dr P. del Soldato (NicOx Ltd, Sophia-Antipolis, France) for the generous gift of NO-NSAID and nitroprednisolone.
Abbreviations
- DMSO
dimethylsulphoxide
- HR
heart rate
- MAP
mean arterial blood pressure
- NA
noradrenaline
- NOA
nitroaspirin
- NOF
nitroflurbiprofen
- NO-NSAID
nitro-non-steroidal anti-inflammatory drug(s)
- NOP
nitroprednisolone
- NOPARA
nitroparacetamol
- NSAID
non-steroidal anti-inflammatory drug(s)
- ODQ
oxadiazolo [4,3a] quinoxalin-1-one
- SNP
sodium nitroprusside
References
- ADAMI A., CUZZOLIN L., MINUZ P., CRIVELLENTE F., LECHI A., BENONI G. Vasodilating properties of a new non-steroidal anti-inflammatory drug, nitroflurbiprofen, on rat aortic rings. Pharmacol. Res. 1996;33:239–244. doi: 10.1006/phrs.1996.0033. [DOI] [PubMed] [Google Scholar]
- Al-SWAYEH O.A., CLIFFORD R.H., DEL SOLDATO P., MOORE P.K. A comparison of the anti-inflammatory and anti-nociceptive activity of nitroaspirin and aspirin. Br. J. Pharmacol. 2000;129:343–350. doi: 10.1038/sj.bjp.0703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-SWAYEH O.A., CLIFFORD R.H., MOORE P.K. Anti-oedema and antinociceptive effect of nitroprednisone and nitroflurbiprofen. Br. J. Pharmacol. 1999;127:84. [Google Scholar]
- BHARDWAJ R., MOORE P.K. Endothelium derived relaxing factor and the effects of acetylcholine and histamine on resistance blood vessels. Br. J. Pharmacol. 1988;95:835–843. doi: 10.1111/j.1476-5381.1988.tb11712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BING R.J., YAMAMOTO T., KIM H., GRUBBS R.H. The pharmacology of a new nitric oxide donor: B-NOD. Biochem. Biophys. Res. Commun. 2000;275:350–353. doi: 10.1006/bbrc.2000.3304. [DOI] [PubMed] [Google Scholar]
- DEL SOLDATO P., SORRENTINO R., PINTO A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends in Pharmacological Sciences. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- ELLIOT S.N., MCKNIGHT W., CIRINO G., WALLACE J.L. A nitric oxide releasing non-steroid anti-inflammatory drug accelerates gastric ulcer healing in rats. Gastroenterology. 1995;109:524–530. doi: 10.1016/0016-5085(95)90341-0. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., ANTONELLI E., SANTUCCI L., MORELLI O., MIGLIETTI M., FEDERICI B., MANNUCCI R., DEL SOLDATO P., MORELLI A. Gastrointestinal safety of nitric oxide derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- FUJIHARA C.K., MALHEIROS D.M.A.C., DONATO J.L., POLI A., DE NUCCI G., ZATZ R. Nitroflurbiprofen, a new nonsteroidal anti-inflammatory, ameliorates structural injury in the remnant kidney. Am. J. Physiol. 1998;274:F573–F579. doi: 10.1152/ajprenal.1998.274.3.F573. [DOI] [PubMed] [Google Scholar]
- MARIOTTO S., MENEGAZZI M., CARCERAN DE PRATI A., CUZZOLIN L., ADAMI A., SUZUKI H., BENONI G. Protective effect of NO on gastric lesions and inhibition of expression of gastric inducible NOS by flurbiprofen and its nitro-derivative, nitroflurbiprofen. Br. J. Pharmacol. 1995;116:1713–1714. doi: 10.1111/j.1476-5381.1995.tb16650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLOUGHLIN C., KEEBLE J., MOORE P.K. Effect of nitroflurbiprofen on lipopolysaccharide-induced sepsis. Mediators of Inflammation. 1999;8 suppl 1:P-16-1. [Google Scholar]
- MOORE P.K., AL-SWAYEH O.A., CHONG N.W.S., EVANS R.A., GIBSON A. L-NG-nitro arginine (L-NOARG) - a novel, L-arginine reversible inhibitor of endothelium-dependent vasodilatation. Br. J. Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCARA M.N., MCKNIGHT W., DEL SOLDATO P., WALLACE J.L. Effect of a nitric oxide-releasing naproxen derivative on hypertension and gastric damage induced by chronic nitric oxide inhibition in the rat. Life Sci. 1998;62:PL235–PL240. doi: 10.1016/s0024-3205(98)00072-1. [DOI] [PubMed] [Google Scholar]
- MUSCARA M.N., MCKNIGHT W., LOVREN F., TRIGGLE C.R., CIRINO G., WALLACE J.L. Antihypertensive properties of a nitric oxide releasing naproxen derivative in two-kidney, one-clip rat. Am. J. Physiol. 2000;279:H528–H535. doi: 10.1152/ajpheart.2000.279.2.H528. [DOI] [PubMed] [Google Scholar]
- PAUL-CLARK M., DEL SOLDATO P., FIORUCCI S., FLOWER R.J., PERRETTI M. 21-NO-prednisolone is a novel nitric oxide-releasing derivative of prednisolone with enhanced anti-inflammatory properties. Br. J. Pharmacol. 2000;137:1345–1354. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI K., UKAWA H., KONAKA A., KITAMURA M., SUGAWA Y. Effect of nitric oxide-releasing aspirin derivative on gastric functional and ulcerogenic responses in rats: comparison with plain aspirin. J. Pharm. Exp. Ther. 1998;286:115–121. [PubMed] [Google Scholar]
- UKAWA H., YAMAKUNI H., KATO S., TAKEUCHI K. Effects of cycloxygenase-2 selective and nitric oxide releasing nonsteroidal anti-inflammatory drugs on gastric ulcerogenic and healing responses in experimental animals. Dig. Dis. Sci. 1997;43:2003–2011. doi: 10.1023/a:1018846912032. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L. Nonsteroidal antiinflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., CIRINO G. Antithrombotic effects of a nitric oxide releasing gastric sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M., CIRINO G. Novel non-steroidal antiinflammatory derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., KAKAR N.R., VINA E.R., JOHNSON P.E., BING R.J. The effect of aspirin and two nitric oxide donors on the infarcted heart in situ. Life Sci. 2000;67:839–846. doi: 10.1016/s0024-3205(00)00678-0. [DOI] [PubMed] [Google Scholar]


