Abstract
The pharmacological characteristics of muscarinic receptors in the male mice urinary bladder smooth muscle were studied.
(+)-Cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine induced concentration-dependent contractions of the urinary bladder smooth muscle (pEC50=6.6±0.1, 6.9±0.1, 6.7±0.1, 5.8±0.1 and 5.8±0.1, EMax=3.2±0.8 g, 2.7±0.4 g, 1.0±0.1 g, 2.7±0.3 and 0.9±0.2 g, respectively, n=4). These contractions were competitively antagonized by a range of muscarinic receptor antagonists (pKB values): atropine (9.22±0.09), pirenzepine (6.85±0.08), 4-DAMP (8.42±0.14), methoctramine (5.96±0.05), p-F-HHSiD (7.48±0.09), tolterodine (8.89±0.13), AQ-RA 741 (7.04±0.12), s-secoverine (8.21±0.09), zamifenacin (8.30±0.17) and darifenacin (8.70±0.09).
In this tissue, the pKB values correlated most favourably with pKi values for these compounds at human recombinant muscarinic M3 receptors. A significant correlation was also noted at human recombinant muscarinic m5 receptors given the poor discriminative ability of ligands between M3 and m5 receptors.
In recontraction studies, in which the muscarinic M3 receptor population was decreased, and conditions optimized to study M2 receptor activation, methoctramine exhibited an affinity estimate consistent with muscarinic M3 receptors (pKB=6.23±0.14; pA2=6.16±0.03).
Overall, these data study suggest that muscarinic M3 receptors are the predominant, if not the exclusive, subtype mediating contractile responses to muscarinic agonists in male mouse urinary bladder smooth muscle.
Keywords: Muscarinic receptors, M3-receptor, urinary bladder smooth muscle
Introduction
Muscarinic receptors are pharmacologically classified into four subtypes, M1, M2, M3 and M4 which equate with four of the known muscarinic receptor gene products (m1, m2, m3 and m4) (Hulme et al., 1990; Caufield, 1993; Eglen et al., 1996 for reviews). A fifth gene has been identified, m5, for which a role has yet to be unambiguously defined (see Eglen & Nahorski, 2000 for review).
Being widely distributed, muscarinic receptors play a key physiological role in peripheral organs, including the urinary bladder. In most smooth muscles, the muscarinic M2 receptor subtype accounts for 70–80% of the receptor population whereas the M3 receptor subtype forms only 20–30% (Eglen et al., 1996). In this tissue from rat, it was proposed that muscarinic M3 receptor activation primarily causes direct contraction of the smooth muscle and the muscarinic M2 receptor contracts the tissue indirectly, by reversing sympathetically mediated relaxation (Hegde et al., 1997). Pharmacological characterization of muscarinic receptors mediating contraction of detrusor muscle has been well established in rat (Longhurst et al., 1995; Hegde et al., 1997), rabbit (Tobin & Sjogren, 1995; Choppin et al., 1998), guinea-pig (Noronha-Blob et al., 1989) and human (Newgreen & Naylor, 1996). Several investigations of the muscarinic receptors mediating contractions of mouse bladder have been undertaken (Durant et al., 1991; Paravicini et al., 2000; Stengel et al., 2000; Welsh et al., 2000), and most suggest a major role of the muscarinic M3 receptor in the contractile response, with the role of the M2 receptor, if any, being unresolved. Recently, the situation has become clearer with the use of transgenic mice that lack either the muscarinic M2 (Stengel et al., 2000) or M3 receptor (Matsui et al., 2000). These data collectively indicate a minimal role for the former and that the latter mediates most of the contractile response. The objective of the present study was therefore to examine, using a range of defining antagonists, the pharmacological characteristics of muscarinic receptors present in male mouse urinary smooth muscle using isolated tissue studies.
A preliminary account of the findings has been presented previously to the 9th international symposium on subtypes of muscarinic receptors (Eglen & Choppin, 2001).
Methods
In vitro contractile studies
Male C57BL6 mice (25–30 g) were euthanized by CO2 asphyxiation. The urinary bladder was isolated, cleared of adhering adipose tissue and placed in oxygenated Krebs solution (composition in mM: NaCl 118.2, KCl 4.6, CaCl2 2.5, MgSO4.7H2O 1.2, KH2PO4 1.2, NaHCO3 24.8 and dextrose 10.0). The physiological solution contained indomethacin (10 μM) in order to reduce prostaglandin-induced spontaneous activity of the tissues. Four strips of urinary bladder smooth muscle were cut from the supratrigonal portion of the bladder (longitudinal section). The tissues were mounted in 10 ml organ baths containing Krebs solution, maintained at 37°C and constantly aerated with 95% O2/5% CO2 (pH=7.4). Grass FT03 transducers were used to measure changes in isometric tension of the tissues, which were displayed on a Grass 7E polygraph. The tissues were maintained at a resting tension of 1 g during an equilibration period of 60 min. Tension adjustments were made as necessary. The tissues were washed every 15 min.
The viability of each tissue was assessed by determining the contractile response to KCl (30 mM) at the start of the experimental protocol. After washing, tissues were re-equilibrated for 10 min and allowed to regain baseline tension. Cumulative concentration-effect curves to agonists ((+)-cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine; 1 nM–0.1 mM) were then constructed in each tissue. Thereafter, tissues were equilibrated in either the absence (time control) or presence of antagonist for a 90 min period during which tissues were washed every 10 min. Subsequently, a second concentration-effect curve to the same agonist was constructed.
Recontraction experiments
After an initial concentration-response curve to (+)-cis-dioxolane was established, the tissues were washed and equilibrated with 4-DAMP mustard (40 nM) for 60 min in the presence of methoctramine (0.3 μM). This procedure enabled selective alkylation of M3 but not M2 receptors (Hegde et al., 1997). 4-DAMP mustard was then removed from the tissues by overflow with Krebs solution containing methoctramine (0.3 μM) every 10 min for 60 min and subsequently with methoctramine-free Krebs solution every 10 min for 90 min. The tissues were then contracted with 90 mM of KCl and subsequently relaxed with isoproterenol (30 μM). Once the tissues had relaxed to baseline, a cumulative concentration-effect curve to (+)-cis-dioxolane (1 nM–0.1 mM) was constructed.
Effects of an M2 antagonist (methoctramine) on the recontractile responses to (+)-cis-dioxolane
After constructing two concentration-effect curves to (+)-cis-dioxolane under conditions described above, a third cumulative concentration effect curve to (+)-cis-dioxolane (1 nM–0.3 mM) was constructed after equilibration of tissue in absence (time control) or presence of methoctramine (0.1–1.0 μM) for 90 min.
Data analysis
Contractions were recorded as changes in tension from baseline and expressed as a percentage of the maximum response of the first agonist concentration-effect curve. Agonist concentration-response curves were fitted using a nonlinear iterative fitting program (Origin, Microcal Software, Inc., Northampton, MA, U.S.A.) using the relationship of Parker & Waud (1971). Agonist potencies and maximum response are expressed as pEC50 (− logarithm of the molar concentration of agonist producing 50% of the maximum response) and Emax, respectively. Concentration-ratios (CRs) were determined from EC50 values in the presence and absence of antagonist. Antagonist affinity estimates (pKB values) were determined with the equation described by Furchgott (1972) (pKB=−log ([antagonist]/CR-1)) or using the method of Arunlakshana & Schild (1959) using at least three concentrations of the antagonist (pA2 values). In cases where the slope of the linear regression was not significantly different from unity, the slope was constrained to unity and the data expressed as the pKB value. All data are expressed as mean±s.e.mean. Pearson correlation coefficients (r) and associated P-values were calculated using the method described by Dixon & Massey (1983). The sum of squares of differences in affinity estimates for each plot (σ (y–x)2, noted ssq) defines the proximity of the data points to the line of identity (y=x).
Compounds used
Atropine sulphate, indomethacin and oxybutynin chloride were obtained from Sigma Chemical Co (MO, U.S.A.). (+)-Cis-dioxolane, acetylcholine, carbachol, oxotremorine-M, pilocarpine, pirenzepine dihydrochloride, methoctramine hydrochloride, 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) methiodide, 4-DAMP mustard and para fluoro hexahydrosiladifenidol (p-F-HHSiD) hydrochloride were obtained from Research Biochemicals Inc. (MA, U.S.A.). Darifenacin hydrobromide and zamifenacin fumerate were generously provided by Pfizer Central Research (Sandwich, Kent, U.K.). AQ-RA 741 (11-({4-[4-(diethylamino)butyl]-1-piperidinyl}acetyl)-5,11-dihydro-6H-pyrido(2,3-b)(1,4)benzodiazepine-6-one) was donated by Boehringer Ingelheim Pharmaceuticals, Inc. (Ridgefield, CT, U.S.A.). Isoproterenol, tolterodine and s-secoverine hydrochloride were synthesized at Roche Bioscience (Palo Alto, U.S.A.).
All compounds were diluted in distilled water except indomethacin which was diluted in polyethylene glycol.
Results
Characterization of muscarinic receptors mediating contractions of the mice isolated urinary bladder smooth muscle
(+)-Cis-dioxolane, oxotremorine-M, acetylcholine, carbachol and pilocarpine induced concentration-dependent contractions of the mice urinary bladder smooth muscle (pEC50=6.6±0.1, 6.9±0.1, 6.7±0.1, 5.8±0.1 and 5.8±0.1, Emax=3.2±0.8 g, 2.7±0.4 g, 1.0±0.1 g, 2.7±0.3 and 0.9±0.2 g, respectively, n=4). Time-control experiments showed that two consecutive concentration-effect curves to these agonists could be constructed in the same tissue with no significant temporal change in the agonist potency and maximum response (Figure 1).
Figure 1.
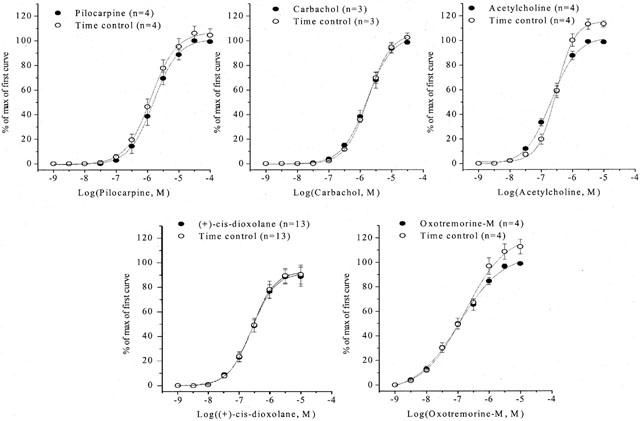
Effects of muscarinic agonists on mouse urinary bladder smooth muscle. Contractile effects were expressed as percentages of the maximum response of the control curve. The values shown are means±s.e.mean, n⩾4 animals.
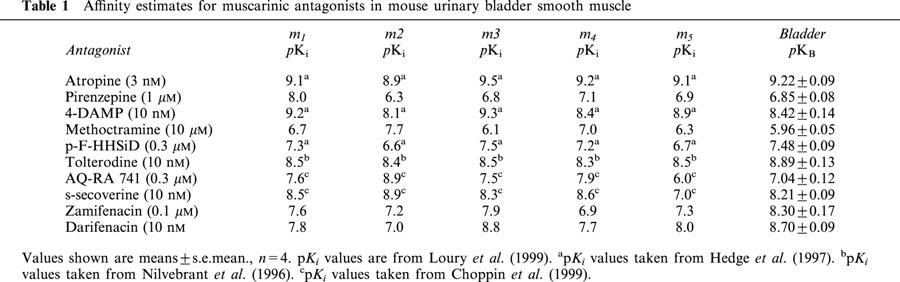
Pharmacological characterization of the muscarinic receptor mediating direct contractions was done by determination of antagonist affinities. Several antagonists (atropine, pirenzepine, 4-DAMP, methoctramine, p-F-HHSiD, tolterodine, AQ-RA 741, s-secoverine, zamifenacin and darifenacin) were tested for their ability to inhibit (+)-cis-dioxolane-induced responses and their functional affinity estimates (pKB) are summarized in Table 1. All these compounds, in a concentration-dependent fashion, with parallel rightward displacements, surmountably antagonized cumulative agonist concentration-response curves. The rank order of antagonist affinities (pKB) was: atropine (9.22±0.09), tolterodine (8.89± 0.13), darifenacin (8.70±0.09), 4-DAMP (8.42±0.14), zamifenacin (8.30±0.17), s-secoverine (8.21±0.09), p-F-HHSiD (7.48±0.09), AQ-RA 741 (7.04±0.12), pirenzepine (6.85± 0.08) and methoctramine (5.96±0.05).
Table 1.
Affinity estimates for muscarinic antagonists in mouse urinary bladder smooth muscle
Comparison of functional data for mice urinary bladder smooth muscle with binding data at human recombinant muscarinic receptors
Correlation analysis between the affinities of the antagonists at muscarinic receptors in the mice urinary bladder smooth muscle and the affinities at human recombinant muscarinic receptors showed a significant correlation (r=0.94, P<0.0001, ssq=1.42) at m3 but also at m5 receptors (r=0.84, P=0.002, ssq=3.70). In contrast, poor correlations were observed at m1, m2 and m4 (r=0.75, ssq=4.38; r=0.32, ssq=12.59; r=0.67, ssq=5.42) respectively (Figure 2).
Figure 2.
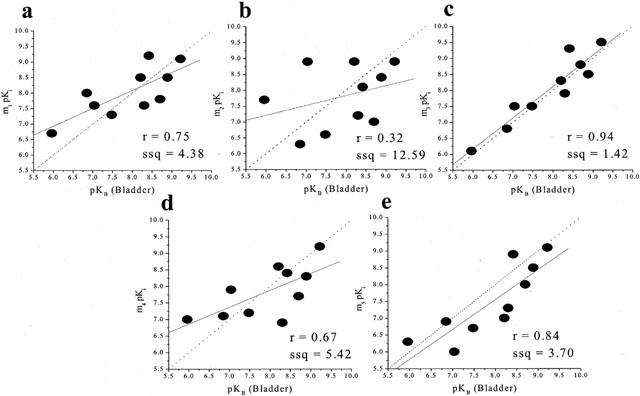
Correlation between the functional affinities (pKi values) of muscarinic antagonists at muscarinic receptor in mouse isolated urinary bladder smooth muscle and binding affinities (pKi values) at human recombinant muscarinic receptors (m1-m5; a–e respectively). The binding data were taken from Dörje et al., 1991; Eglen et al., 1997; Hegde et al., 1997; Nilvebrant et al., 1996. The broken line is the line of identity (x=y) while the solid line is the correlation plot (the inserts give the correlation factors (r) and the sum of squares values (ssq)).
Characterization of muscarinic receptors mediating the recontractions in mouse urinary bladder smooth muscle
Under control conditions, (+)-cis-dioxolane produced concentration-dependent contractions of the mouse urinary bladder smooth muscle (pEC50=6.57±0.05, n=4). After preferential alkylation of M3 receptor (exposure to 4-DAMP mustard in presence of methoctramine), (+)-cis-dioxolane produced recontractile (reversal of contraction) responses (pEC50=6.01±0.05, n=4) of KCl-precontracted tissues, which were relaxed with isoproterenol. The maximum recontractile response (expressed as per cent of the control curve) was 26±2% (n=4). No time-dependent changes in agonist sensitivity were observed during the construction of two consecutive concentration-recontractile effect curves. As shown in Figure 3, methoctramine produced surmountable antagonism of the recontractile response to (+)-cis-dioxolane. The affinity estimate (pA2) for methoctramine was 6.16±0.03 and the slope of the Schild plot was not significantly different than unity.
Figure 3.
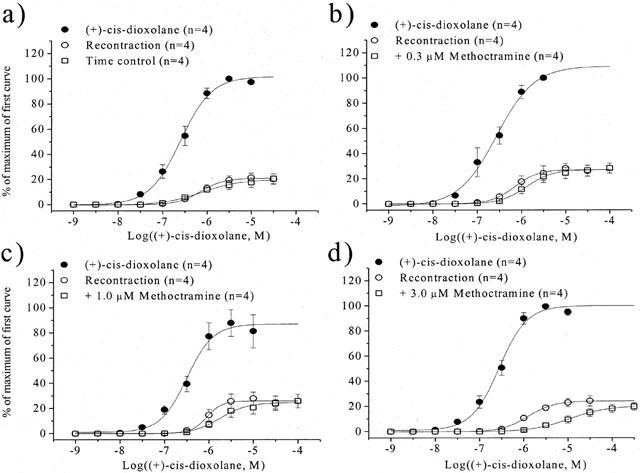
Recontraction experiments in mouse urinary bladder smooth muscle: effect of methoctramine on the recontractile concentration-effect to (+)-cis-dioxolane obtained after elevation of adenylyl cyclase activity following preferential alkylation of muscarinic M3 receptors (n=4). (a) Time control; (b) +0.3 μM methoctramine; (c) +1.0 μM methoctramine; (d) +3.0 μM methoctramine.
Discussion
Previous studies using mouse urinary bladder (Durant et al., 1991; Lundbeck & Sjögren, 1992) have demonstrated a muscarinic-induced contractile response but did not characterize the receptor subtype(s) involved. The present study has examined in detail the pharmacological characteristics of muscarinic receptors in this tissue.
Mouse urinary bladder smooth muscle
Inspection of the agonist potencies and maximal responses suggest that the muscarinic receptor mediating contraction was associated with a low efficacy. Thus, the partial agonist, pilocarpine, yielded a potency similar to the affinity and gave a lower maximal response than seen with the full agonists. Similar observations have been seen in urinary bladder tissue from rat (Hegde et al., 1997). (+)-Cis-dioxolane produced concentration-dependent contractions, which were inhibited in a concentration-dependent and competitive fashion by muscarinic antagonists. The apparent affinity estimates of these antagonists correlated most strikingly with the binding affinities of the antagonists at m3 recombinant muscarinic receptors (pKi are: atropine 9.5; 4-DAMP 9.3; AQ-RA 741, 7.5; darifenacin 8.8; methoctramine 6.1; tolterodine 8.5; pirenzepine 6.8; s-secoverine 8.3; p-F-HHSiD 7.5 and zamifenacin 7.9; r=0.94, ssq=1.42; Dörje et al., 1991; Eglen et al., 1997; Hegde et al., 1997; Nilvebrant et al., 1996) and are consistent with the exclusive involvement of M3 muscarinic receptors in the direct contractile response to muscarinic agonists. This accords with findings in the rabbit (Tobin, 1995; Tobin & Sjögren, 1995), rat (Longhurst et al., 1995; Hegde et al., 1997), human (Newgreen & Naylor, 1996) and preliminary results in female mice (Paravicini et al., 2000) bladder. It should be noted, however, that a significantly good correlation (r=0.84, ssq=3.70) was also obtained with the binding affinities of the antagonists at m5 recombinant muscarinic receptors (pKi values: atropine 9.1; 4-DAMP 8.9; AQ-RA 741, 6.0; darifenacin 8.0; methoctramine 6.3; tolterodine 8.5; pirenzepine 6.9; s-secoverine 7.0; p-F-HHSiD 6.7 and zamifenacin 7.3; Dörje et al., 1991; Eglen et al., 1997; Hegde et al., 1997; Nilvebrant et al., 1996). This is unsurprising since many ligands discriminate poorly between M3 and m5 receptors, and highlights the difficulty of excluding a role for the latter in M3 mediated responses.
In contrast to the rat urinary bladder and several gastrointestinal smooth muscle tissues, functional studies (recontraction experiments) in the mouse bladder revealed no indirect contractile role of M2 receptors, and only M3 receptor activation induced bladder contraction. The low affinity of methoctramine (pA2=6.16) argues against the involvement of an M2 receptor and the remaining 26% of the maximal response observed with (+)-cis-dioxolane after recontraction are likely due to an incomplete alkylation of muscarinic M3 receptors. Data from studies performed in M2 knockout mice (Stengel et al., 2000) suggest that muscarinic M2 receptors play a minor role in carbachol induced contraction of isolated bladder smooth muscle, since the potency of muscarinic agonists is only modestly reduced, and the maximal response unaffected. Concordantly, data from transgenic mice lacking the muscarinic M3 receptor also suggest predominant involvement of muscarinic M3 receptor, as the contraction in vitro was virtually abolished in these mice (Matsui et al., 2000). In vivo, urinary retention was marked in these animals, suggesting that a dominant, if not exclusive, role of this subtype prevails when voiding reflexes are intact (Matsui et al., 2000). The data obtained in the present study are consistent with these findings. Parasympathetic nerves innervating the urinary bladder are endowed with prejunctional inhibitory muscarinic receptors, which have been classified as muscarinic M2 receptors in the rabbit (Tobin & Sjogren, 1995) and rat (Somogyi & De Groat, 1992) urinary bladder but M4 in the guinea-pig urinary bladder. M2 receptors may also act prejunctionally in the mouse bladder but given the difficulty to distinguish between these two subtypes, this function has not been investigated in the present study.
Conclusions
The present study has shown that the pharmacological antagonist profile of the muscarinic receptors present in the mouse bladder equates most closely with the M3 muscarinic receptor. Moreover, these data suggest that only M3 receptors play a role in both direct and indirect contraction in accord with emerging data from knockout animals. It thus appears that the mouse urinary bladder differs from the mouse ileum and urinary bladder tissue from other species, including rat and possibly human. It therefore remains to be established if mouse tissue represents an optimal species to provide a useful model for disorders of human urinary bladder function.
Abbreviations
- AQ-RA 741
(11-({4-[4-(diethylamino)butyl]-1-piperidinyl}acetyl)-5,11-dihydro-6H-pyrido(2,3-b)(1,4)benzodiazepine-6-one)
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine
- p-F-HHSiD
para fluoro hexahydrosiladifenidol
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUFIELD M.P. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M., HEGDE S.S. Pharmacological characterisation of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br. J. Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., LOURY D.N., WATSON N., HEGDE S.S., EGLEN R.M. S-secoverine: a defining ligand in muscarinic M5 receptors characterization. Br. J. Pharmacol. 1999;128:33P. doi: 10.1038/sj.bjp.0702696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON W.J., MASSEY F.J.Introduction to statistical analysis 1983New York: McGraw-Hill Publishing Company; 4th edition [Google Scholar]
- DÖRJE F., WESS J., LAMBRECHT G., TACKE R., MUTSCHLER E., BRANN M.R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J. Pharmacol. Exp. Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- DURANT P.A., SHANKLEY N.P., WELSH N.J., BLACK J.W. Pharmacological analysis of agonist-antagonist interactions at acetylcholine muscarinic receptors in a new urinary bladder assay (1991) Br. J. Pharmacol. 1991;104:145–150. doi: 10.1111/j.1476-5381.1991.tb12399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., BONHAUS D.W., CALIXTO J.J., CHOPPIN A., LEUNG E., LOEB M., LOURY D., MOY T., WILDA M., HEGDE S.S. Characterization of the interaction of tolterodine at muscarinic receptor subtypes in vitro and in vivo. Br. J. Pharmacol. 1997;120:63P. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., CHOPPIN A. Pharmacological characterisation of muscarinic receptors in mouse urinary bladder smooth muscle. Life Sci. 2001;68:2634. doi: 10.1038/sj.bjp.0704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EGLEN R.M., NAHORSKI S.R. The muscarinic M5 receptor: a silent or emerging subtype. Br. J. Pharmacol. 2000;130:13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines, Handbook of Experimental Pharmacology 1972Berlin, Heidelberg, New York: Springer; 283–335.Vol. 33. Ed. Blaschko, H. & Muscholl, E. pp [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULME E.C., BIRDSALL N.J.M., BUCKLEY N.J. Muscarinic receptor subtypes. Ann. Rev. Pharmacol. Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE J.A.K. Characterization of functional muscarinic receptors in the rat urinary bladder. Br. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOURY D.N., HEGDE S.S., BONHAUS D.W., EGLEN R.M. Ionic strength of assay buffers influences antagonist binding affinity estimates at muscarinic M1-M5 cholinoceptors. Life Sci. 1999;64:6P. [Google Scholar]
- LUNDBECK F., SJÖGREN C. A pharmacological in vitro study of the mouse urinary bladder at the time of acute change in bladder reservoir function after irradiation. J. Urol. 1992;148:179–182. doi: 10.1016/s0022-5347(17)36548-5. [DOI] [PubMed] [Google Scholar]
- MATSUI M., MOTOMURA D., KARASAWA H., FUJIKAWA T., JIANG J., KOMIYA Y., TAKAHASHI S., TAKETO M.M. Multiple functional deficits in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl. Acad. Sci. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWGREEN D.T., NAYLOR A.M. Characterization of functional muscarinic receptors in human bladder. Br. J. Pharmacol. 1996;119:45P. [Google Scholar]
- NILVEBRANT L., SUNDQUIST S., GILLBERG P.-G.Tolterodine is not subtype (m1-m5) selective but exhibits functional bladder selectivity in vivo Neurourol. Urodyn. 19961534(abstract) [Google Scholar]
- NORONHA-BLOB L., LOWE V.C., PATTON A., CANNING B., COSTELLO D., KINNIER W.J. Muscarinic receptors: relationships among phosphoinositide breakdown, adenylate cyclase inhibition, in vitro detrusor muscle contractions and in vivo cystometrogram studies in guinea-pig bladder. J. Pharmacol. Exp. Ther. 1989;249:843–851. [PubMed] [Google Scholar]
- PARAVICINI T., PENNEFATHER J.N., LAU W.A.K., MA S., PATAK E. Muscarinic receptors mediating contraction of the urinary bladder from the female mouse. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 2000;7:1–12P. [Google Scholar]
- PARKER R.B., WAUD D.R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I. Agonists. J. Pharmacol. Exp. Ther. 1971;177:1–12. [PubMed] [Google Scholar]
- SOMOGYI G.T., DE GROAT W.C. Evidence for inhibitory nicotinic and facilitatory muscarinic receptors on cholinergic nerve terminals of the rat urinary bladder. J. Auton. Nerv. Syst. 1992;37:89. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- STENGEL P.W., GOMEZA J., WESS J., COHEN M.L. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J. Pharmacol. Exp. Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- TOBIN G. Muscarinic receptor subtypes in the submandibular gland and the urinary bladder of the rabbit: in vivo and in vitro functional comparisons of receptor antagonists. J. Auton. Pharmacol. 1995;15:451–463. doi: 10.1111/j.1474-8673.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- TOBIN G., SJÖGREN C. In vivo and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]-acetylcholine in the rabbit urinary bladder. Eur. J. Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- WELSH N.J., EGLEN R.M., SHANKLEY Pharmacological comparison of the muscarinic receptors mediating contraction of the guinea-pig left atrium, gastric smooth muscle and mouse urinary bladder. Br. J. Pharmacol. 2000;131:57P. [Google Scholar]


