Abstract
In addition to its role in hydrolyzing the neurotransmitter acetylcholine, the synaptically enriched enzyme acetylcholinesterase (AChE) has been reported to play an important role in the development and remodelling of neural processes and synapses.
We have shown previously that AChE causes an increase in binding of the specific AMPA receptor ligand (S)-[3H]-5-fluorowillardiine ([3H]-FW) to rat brain membranes.
In this study we have used quantitative autoradiography to investigate the regional distribution and age-dependence of AChE-evoked increases in the binding of [3H]-FW in rat brain.
Pretreatment of rat brain sections with AChE caused a marked enhancement of [3H]-FW binding to many, but not all, brain areas. The increased [3H]-FW binding was blocked by the specific AChE inhibitor BW 284c51.
The maximal potentiation of [3H]-FW binding occurred at different developmental age-points in different regions with a profile consistent with the peak periods for synaptogenesis in any given region.
In addition to its effects on brain sections, AChE also strongly potentiated [3H]-FW binding to detergent solubilized AMPA receptors suggesting a direct action on the receptors themselves rather than an indirect effect on the plasma membrane.
These findings suggest that modulation of AMPA receptors could provide one molecular mechanism for the previously reported effects of AChE on synapse formation, synaptic plasticity and neurodegeneration.
Keywords: AMPA receptors, acetylcholinesterase, CNS development, Fluorowillardiine, quantitative autoradiography, neuronal development, receptor solubilization, synaptogenesis
Introduction
α-Amino-3-hydroxy-5-methylisoxazolepropionate (AMPA) receptors mediate most excitatory synaptic transmission in the mammalian CNS. They play a central role in synapse formation, stabilization and plasticity and their prolonged activation is potently neurotoxic (for a review see Dingledine et al., 1999). Developmental and activity-dependent changes in the functional expression of AMPA receptors at synapses are strictly regulated. Several proteins that interact with the intracellular C-termini of AMPA receptor subunits and which appear to be involved in receptor targeting and surface expression have been identified (for reviews see Dev & Henley, 1998; Braithwaite et al., 2000). Recently, another protein called NARP has been shown to interact with the extracellular N-terminal region of AMPA receptors and cause receptor clustering (O'brien et al., 1999).
AChE is a member of the serine esterase domain family of proteins (Ichtchenko et al., 1996) and it exhibits a number of morphogenetic activities in addition to its role in hydrolysing acetylcholine (Anderson & Key, 1999; Grifman et al., 1998; Holmes et al., 1997; Koenigsberger et al., 1997). AChE is concentrated at synapses and has a neuroligin-homology domain (Ichtchenko et al., 1995) and a carbohydrate HNK epitope, a hallmark of adhesion molecules (Koenigsberger et al., 1997). It is now widely accepted that AChE has actions which are relevant to the development and remodelling of neural processes and synapses (for reviews see Layer & Willbold, 1995; Grisaru et al., 1999). Some of these actions, such as effects on cell differentiation and promotion of neurite extension appear to be related to the homology of AChE to certain cell adhesion proteins including the neuroligins (Grifman et al., 1998; Ichtchenko et al., 1995; Koenigsberger et al., 1997). Significantly, most of the morphogenetic actions attributed to this enzyme do not involve a catalytic activity (for a review see Grisaru et al., 1999) whereas its synaptogenic activity appears to require a catalytic action, but not membrane association (Sternfeld et al., 1998). Thus, the synaptogenic properties of AChE are separable from its neuritogenic effects which do not require an hydrolytic capacity.
We have reported previously that AChE pretreatment of rat brain membranes caused an upregulation of agonist binding to AMPA receptors (Olivera et al., 1999). In that study we showed that AChE caused a concentration-dependent increase in the number of binding sites (Bmax) for the AMPA receptor agonists (S)-[3H]-5-fluorowillardiine ([3H]-FW) and [3H]-AMPA but had no effect on [3H]-kainate binding to the related kainate-type glutamate receptors The actions of AChE were Ca2+- and temperature-dependent, suggesting an enzymatic action for AChE in this system.
Here we have used quantitative autoradiography to investigate the regional and age-dependant effects of AChE pretreatment on [3H]-FW binding to rat brain sections. In addition, we have investigated the action of AChE on [3H]-FW binding to detergent solubilized AMPA receptors.
Methods
Rat tissue preparations
Whole brains were removed from halothane/O2 anaesthetized Wistar rats, frozen in −40°C isopentane and stored at −80°C until use Cryostat sections (15 μm) were obtained in a OTF cryostat with a Bright 5030 microtome and mounted onto microscope slides with 0.1 mg/ml poly-L-lysine. Sections were dried and stored desiccated at −80°C for at least 24 h The age points studied were 8, 15, 21, 28 day-old (P8, P15, P21, P28), 6 and 10 weeks-old (PW6, PW10) rats.
AChE pre-treatment
Sections were slowly thawed (1–2 h) in air-tight containers and then immersed for 15 min in ice-cold 50 mM Tris-HCl buffer, pH 7.4 to remove debris due to mechanical damage during sectioning. A further 15 min wash in Tris-HCl buffer supplemented with 5 mM CaCl2 ensured the complete removal of endogenous glutamate (Dev et al., 1995). Slices were preincubated with AChE (0.5–10 u ml−1) in 50 mM Tris-HCl buffer plus 5 mM CaCl2, for 30 min Control sections were incubated with buffer alone The AChE was removed with a 15 min wash in ice-cold Tris-HCl buffer Inhibition experiments were performed using the same protocol but in the presence of 1×10−5–1×10−3 M 1,5-bis(4-allyl-dimethylammoniumphenyl)pentan-3-one dibromide (BW284c51) as previously described (Olivera et al., 1999).
Quantitative autoradiography
Each slide, containing 3–4 brain sections, was incubated in a total volume of 1 ml Tris-HCl buffer containing [3H]-FW (3–10 nM) or 100 nM [3H]-kainate for 60 min on ice. Slides were washed six times (total wash time 10 s) in ice-cold buffer, rinsed in water and immersed for 1 s in acetone. Sections were then dried rapidly in a steam of cool air. Non-specific binding was defined by inclusion of 1 mM L-glutamate for [3H]-FW or 100 μM kainate for [3H]-kainate. The sections were then exposed to Amersham Hyperfilm-3H alongside 3H-microscales standards (Amersham). After 28 days, films were developed for 2 min in Kodak D19, fixed and dried. Anatomical coordinates, structure names and abbreviations, were as in Paxinos & Watson (1986).
Image acquisition and quantitative analysis
Pictures were digitized from developed 3H-Hyperfilm autoradiograms using an EPSON Perfection 1200 U Scanner and Adobe Photoshop 4 Images were analysed with Scion Image Beta Release 3b, the NIH Image Software for Windows. Using the rectangular option of the selection tool we defined precise areas (6×6 pixels) from different brain regions (this area represents <1% of the entire image). The density values were calculated by the software from a grey scale calibrated in the range 0 (white)–256 (black) against 3H-microscales standards (Amersham). An exponential non-linear fitting curve best fit the density of the microscales and was used in all subsequent experimental analyses. Corresponding areas from control and AChE treated sections exposed to the same sheet of 3H-Hyperfilm were directly compared. Background from the film was determined by sampling three different areas surrounding the section, the mean value calculated and subtracted from each measurement. Data shown in Table 1 represent the ratio between the density of an area of brain in an AChE-treated slice and the corresponding brain area in an adjacent control slice.
Table 1.
Quantification of AChE effects on AMPA receptors binding autoradiography
In some experiments, to obtain quantitative analysis of the amount of radioligand binding, wet sections were wiped off the slide with Whatman GF/C glass microfibre filters. The filters were then transferred to scintillation vials containing 4 ml of Emulsifier-Safe scintillation fluid (Packard) and the [3H]-label counted in a scintillation counter (Beckman LS6500).
Preparation of rat cortical membranes and solubilization of AMPA receptors
Cortex from adult male Wistar rats (150–250 g) was homogenized in a glass-Teflon homogenizer in 10 volumes of ice-cold EDTA buffer (50 mM Tris pH 7.4 at 0°C with HCl, 1 mM EDTA, 1 mM EGTA) supplemented with 0.32 mM sucrose. Following centrifugation at 1000×g for 10 min the supernatant was centrifuged for 30 min at 40,000×g. The pellet (P2) was subjected to a freeze-thaw-wash protocol exactly as described previously (Dev et al., 1995). The final pellet was resuspended and used immediately Detergent extracts were prepared by incubating the membranes at a ratio ∼1 : 10 (w v−1) with 1% octylglucoside (OG) in EDTA buffer containing the following protease inhibitors (final concentrations; EDTA, 2 mM; EGTA, 2 mM; Soybean Trypsin Inhibitor, 1 mg/100 ml−1; Bacitracin, 20 mg 100 ml−1, Benzamidine, 15 mg 100 ml−1) overnight on a rotating wheel at 4°C The detergent-membrane mixture was then centrifuged for 1 h at 130,000×g and the supernatant containing soluble proteins collected and stored at −80°C. Where appropriate, 5 ml of the soluble extract was dialysed against EDTA buffer for 16 h with three changes of dialysis buffer [3H]-FW binding to membranes and detergent extracts was determined by filtration on Whatman GF/B filters soaked in 0.3% PEI (Henley & Barnard, 1989). Protein concentrations were assessed using the Pierce assay kit as described (Olivera et al., 1999).
SDS–PAGE and Western blots
SDS–PAGE and Western blots using pan-AMPA subunit antibodies (Archibald et al., 1998) were performed as described previously (Henley, 1993). Immunoreactivity was visualized using horseradish peroxidase and/or alkaline phosphatase-coupled anti-rabbit IgG secondary antibodies (Sigma) and the Amersham ECL kit.
Materials
[3H]-FW was from Tocris-Cookson and [3H]-kainate was from New England Nuclear Hyperfilm-[3H] and [3H]-standards were from Amersham International. All other ligands and reagents were from Tocris-Cookson, Sigma or BDH.
Results
AChE effects on [3H]-FW binding in different brain areas
In a previous study we have performed a senes of [3H]-FW saturation binding experiments to rat brain membranes (Olivera et al., 1999). We detected two classes of binding sites and showed that the increase in [3H]-FW binding following treatment with AChE is due predominantly to an increase in the number of receptors available for radioligand binding (Bmax) rather than a conformational shift to a higher affinity state (KD). The values for [3H]-FW binding in control experiments were: high affinity site–KD1=62.2±6.5 nM, Bmax1 1.56±0.12 pmol mg−1 protein; low affinity site–KD2=1324±159 nM, Bmax2=7.92±0.64. Following AChE preincubation the values for [3H]-FW binding were: high affinity site–KD1=89.3±11.2 nM, Bmax1=2.48±0.33 pmol mg−1 protein; low affinity site–KD2=1039±130 nM, Bmax2=13.12±0.64. In the present study [3H]-FW binding was assessed by quantitative autoradiography of coronal brain sections from immature (P8, P15, P21, P28, P42) and adult rats following preincubation with 1 u ml−1 or 5 u ml−1 AChE. The levels of [3H]-FW binding in distinct anatomical regions at P8, P28 and adult (P42), expressed as a ratio of non-AChE-treated controls are given in Table 1.
Consistent with the results from membrane binding experiments AChE pre-treatment augmented [3H]-FW binding to most areas of the adult brain (Table 1, adult) but regional differences in the degree of AChE-evoked enhancement were observed. For example, amongst nuclear structures, there was a clear difference in the AChE enhancement of [3H]-FW binding to the caudate-putamen compared to that in the globus pallidus. In addition, marked AChE-evoked increases in [3H]-FW binding were observed in certain, but not all, thalamic and hypothalamic nuclei of the adult brain (Table 1).
Age-dependence of AChE-evoked increases in [3H]-FW binding
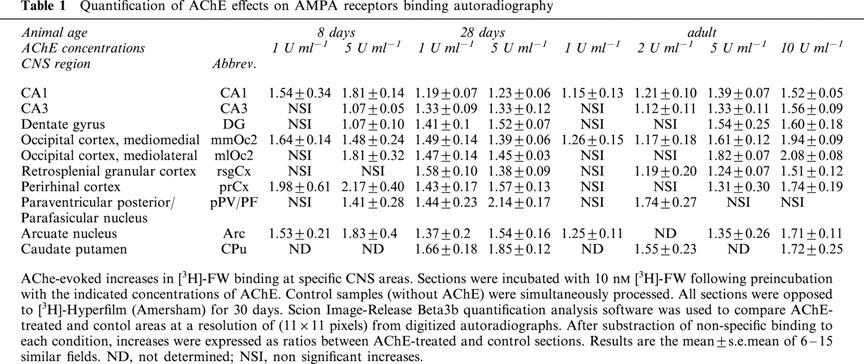
At each of the age points investigated there was substantial enhancement of [3H]-FW, but not [3H]-kainate, binding in discrete areas following AChE pre-treatment (Figures 1 and 2). The maximal enhancement of [3H]-FW binding by AChE occurred at different times in different areas but in general it seemed that the phylogenetically more primitive a given area (e.g., allocortex), the earlier in development it showed maximal AChE-enhanced [3H]-FW binding.
Figure 1.
Regional- and age-dependence of AChE-enhancement of [3H]-FW binding to AMPA receptors in the rat brain. Autoradiographs of 15 μm coronal cryostat brain sections from 8-, 28-day-old and adult rats. Experiments were performed as detailed in the Methods. Quantitative analysis is given in Table 1. The abbreviations used are: Amy, amygdala; Arc, arcuatus nucleus; CA1, CA2, CA3, CA4, CA1–4 fields of hippocampus proper (Cornu Ammonis); CPu, caudate putamen; DG, dentate gyrus; DHF, dorsal hippocampal formation; dIIIV, dorsal third ventricle; dTh, dorsal thalamus; grDG, granular layer of dentate gyrus; H, hypothalamus; hf, hippocampal fissure; hil, hilus; IIIV, third ventricle; molDG, molecular layer of dentate gyrus; mTh, medial thalamus; neoCx, neocortex; ocCx, occipital cortex; or, stratum onens of hippocampus; pCx, parietal cortex; pirCx, piriform cortex; prhCX, penrhinal cortex; pyr, pyramidal cell layer of hippocampus; rf, rhinal fissure; rsgCx, retrosplenial granular cortex; S, subiculum; temCx, temporal cortex; Th, thalamus.
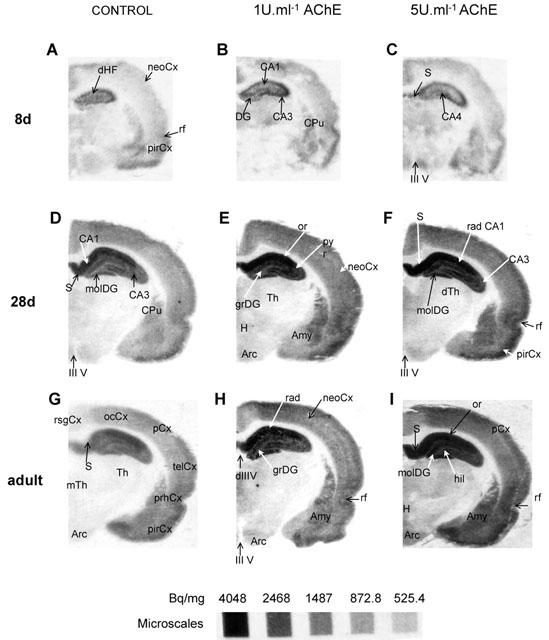
Figure 2.
AChE enhances [3H]-FW but not [3H]-kainate binding to rat brain sections. Autoradiographs show [3H]-kainate (A,B,D,E) and [3H]-FW (C,F) binding to brain sections from adult rats 1 u ml−1 AChE evoked increased [3H]-FW binding but did not significantly affect [3H]-kainate binding. Note that whereas [3H]-FW non-specific binding was negligible (less than 3% of total, autoradiographs not shown), [3H]-kainate non-specific binding (A,D) represented ∼50% of total binding. Independently from the agonist used, AChE alone (D) did not affect non-specific binding. Abbreviations, as in Figure 1; py(CA3), pyramidal cell layer of hippocampus (CA3 field); vlTh, ventrolateral thalamus; vmH, ventromedial hypothalamus; vmTh, ventromedial thalamus.
Developmental regulation of the AChE-evoked increase in [3H]-FW binding in the hippocampal formation
The maximal enhancement of [3H]-FW binding by AChE shifted during development, from a predominant subpyramidal (mainly at stratum onens) location at the P8 stage, to maximal enhancement in the adult, in stratum radiatum and lacunosum-moleculare of hippocampus and molecular layer of dentate gyrus. At P8, AChE potentiation of [3H]-FW binding was only detectable at CA1 as a thin, dense subpyramidal layer (Table 1) continuous with a wider layer of subpyramidal location at CA3 and hilus-CA4 fields (see Figures 1E and 3B; 1 u ml−1 AChE final concentration). At P15, this layer increased in intensity and widened to reach pyramidal cell layer, especially at CA3-CA4-hilus (Figure 3B). At P28, a striking AChE potentiation of [3H]-FW binding was seen at the hippocampal stratum radiatum and stratum lacunosum-moleculare, and also as a thin band beneath the granular layer of the dentate gyrus (roughly corresponding to stratum lucidum), and at the whole subiculum (2500–4000 Bq mg−1 with 5 u ml−1 AChE). At this stage of development AChE potentiation of [3H]-FW binding occurred within the two inner thirds (approximately 50% increase as compared to P8, Table 1) and hilus of dentate gyrus. At later stages of maturation differences in the AChE potentiation of [3H]-FW binding amongst regions/layers were less pronounced and, unlike at P8-P15, at P28, 5 u ml−1 of AChE was required for marked potentiation in the subiculum-CA1 regions.
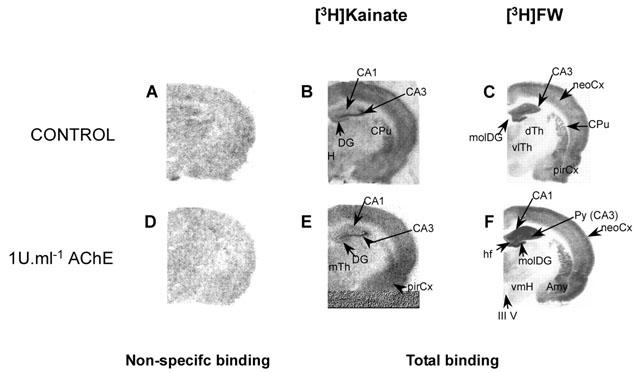
Figure 3.
BW284c51 blocks the AChE-evoked potentiation of [3H]-FW binding to AMPA receptors. The autoradiographs show [3H]-FW binding to serial coronal sections of the hippocampal formation from a 15-day-old rat. Sections were preincubated under the conditions shown as described in the Methods. Abbreviations, as in Figure 2; alv-or lim, limit between hippocampal alveus and stratum oriens; lac-mol, stratum lacunosum-moleculare; rad, stratum radiatum.
In parallel experiments using [3H]-kainate binding to adjacent sections from adult animals, no significant increases in binding were observed following AChE pretreatment (Figure 2). This was consistent with our previous observations using synaptic membrane preparations (Olivera et al., 1999) and demonstrates that the effects of AChE are selective for AMPA receptors over kainate receptors.
Effects of BW284c51 on AChE actions
Since dramatic changes were observed in the hippocampus we focused on this region in subsequent experiments to investigate the effects of the AChE inhibitor BW284c51 BW284c51 alone had no effect on [3H]-FW binding but, as shown in Figure 3, BW284c51 completely prevented the increase in [3H]-FW binding evoked by AChE.
AChE potently enhances [3H]-FW binding to soluble AMPA receptors
We have shown previously that pre-treatment of brain cortical membranes with AChE increases the Bmax for [3H]-FW binding (Olivera et al., 1999). A similar increase in the Bmax of [3H]-AMPA binding has been shown following detergent solubilization of brain membranes (Hall et al., 1992; Henley & Barnard, 1989). We therefore investigated the effects of AChE on [3H]-FW binding to octylglucoside-solubilized receptors. As shown in Figure 4A, AChE still caused a concentration-dependent increase in [3H]-FW binding demonstrating that the two treatments are not mutually exclusive. Preincubation of solubilized receptors with AChE in the presence of the specific inhibitor BW284c51 (final concentrations used 1 μM–1 mM) fully prevented the increase in [3H]-FW binding. BW284c51 alone had no effect on binding. Moreover, immunoblots (Figure 4B) using a pan-AMPA specific antibody (Archibald et al., 1998) showed that AChE did not result in receptor degradation.
Figure 4.
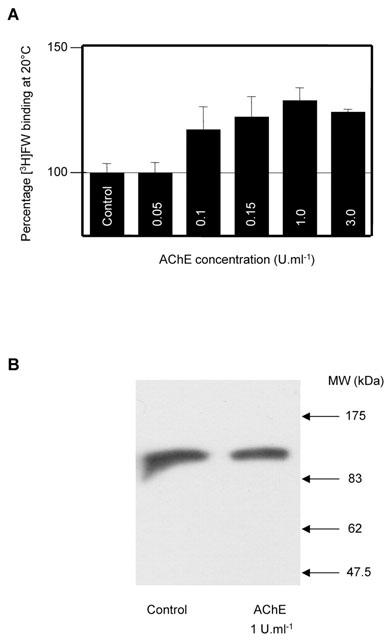
AChE enhances [3H]-FW binding to solubilized AMPA receptors. (A) Histogram showing the concentration curve for AChE enhancement of [3H]-FW binding to AMPA receptors solubilized with 1% octylglucopyranoside There was a significant increase (P<0.01, non paired Student's t-test) in binding to AChE-treated solubilized compared to solubilized control samples. (B) Western blot showing that AChE-treated solubilized AMPA receptors were not degraded.
Discussion
The autoradiographic data presented here demonstrate a substantial, anatomically-specific, and developmentally-modulated increase in [3H]-FW, but not [3H]-kainate, binding following pre-treatment with AChE. These data are consistent with our previous work showing that AChE causes an increase in the Bmax of [3H]-FW binding to rat brain membranes. The AChE potentiation of [3H]-FW binding followed well defined anatomical patterns throughout the rat CNS, suggesting that the AChE-responsive sites may be associated with specific subsystems. In the adult brain, the distribution of AChE-enhanced [3H]-FW binding sites does not parallel the localization of AChE (Matthews et al., 1974; Nadler et al., 1974).
There were developmental differences in sensitivity and in the chronology of the appearance of the AChE potentiation of [3H]-FW binding and, in general, phylogenetically older structures appear more strongly responsive to AChE in the adult and show the earliest appearance of the enhancement of [3H]-FW binding during development. For example, in the hippocampal formation the layer of enhanced binding occurs beneath CA1-CA3 pyramidal cell layer at P8 whereas at P15 it widens at CA3-CA4 to reach the pyramidal layer (Figure 1B,E). This pattern spatio-temporally parallels the localization of the septo-hippocampal afferent system that makes synapses with pyramidal cells at this time (Fiala et al., 1998; Matthews et al., 1974; Petralia et al., 1999).
The finding that there are developmental differences in the sensitivity to AChE agrees with our previous demonstration that the sensitivity of [3H]-FW binding to AChE in cortical membranes prepared from 15-day-old rats is four orders of magnitude greater than that in membranes prepared from adult rats (EC50 value=4×10−5 unit ml−1 corresponding to ∼4×10−14 M) compared to adults (EC50 value=0.1 unit ml−1; Olivera et al., 1999). These observations are intriguing since AMPA receptors play an important role in synapse formation and the control of synaptic efficiency. Thus the fact that AMPA receptors are highly sensitive to AChE modulation and that the sensitivity is under developmental regulation is consistent with the hypothesis that AChE may be involved in neuronal development and synaptic stabilization.
Our results also demonstrate that AChE elicits a concentration-dependent increase in [3H]-FW binding to detergent solubilized AMPA receptors. The effects of AChE were blocked by the specific inhibitor BW284C51 and by heat treatment of the protein. These data suggest that the action of AChE on [3H]-FW binding is not due to any effect on the phospholipid membrane microenvironment surrounding the receptors as was previously suggested for AChE-evoked changes in Aplysia cholinergic receptors (Fossier et al., 1983). Furthermore, detergent solubilization of AMPA receptors has been shown to increase in apparent number of binding sites (Bmax) (Hall et al., 1992; Henley & Barnard, 1989). The observation that prior solubilization does not occlude the AChE-mediated increase in binding indicates separate modes of action and implies that there is a direct modulation of AMPA receptors by AChE. However, the possibility remains that AChE could act on proteins tightly associated with AMPA receptors such as the proposed inhibitory modulatory component (Dev & Henley, 1998) which, in turn, regulates [3H]-FW binding to the receptor complex. If this were the case AChE could cause the dissociation of the inhibitory modulatory component from the receptor complex thereby causing an apparent increase in the number of [3H]-FW binding sites. There are a number of candidate proteins that are known to interact with AMPA receptors, mainly via the C-terminal domain of the GluR2 subunit (Braithwaite et al., 2000). There is also at least one protein, Narp (O'brien et al., 1999), that interacts with the extracellular N-terminal domain that is accessible to AChE. Narp possesses several properties that make it likely to play a key role in the genesis of excitatory synapses It is selectively enriched at excitatory synapses and its overexpression increases the number of excitatory but not inhibitory synapses in cultured spinal neurons. Narp-expressing HEK 293T cells can induce the aggregation of neuronal AMPA receptors suggesting that Narp can function as an extracellular clustering factor for AMPA receptors (O'brien et al., 1999). Future experiments will be required to determine the precise mechanisms by which AChE pretreatment alters the number of AMPA receptor agonist binding sites.
AChE contains an HNK-like carbohydrate domain and this motif is considered a hallmark of adhesion proteins (Koenigsberger et al., 1997). Its neurite-growth promoting activity (Holmes et al., 1997) has been attributed both to the E6-encoded C terminal domain of the protein and to AChE sequence homology to adhesion molecules such as laminin and neuroligins (Scheiffele et al., 2000). It is the extracellular AChE-homology domain of neuroligin that binds to the extracellular domain of β-neurexin and is proposed to act as a recognition mechanism for triggering synapse formation (Rao et al., 2000; Scheiffele et al., 2000). The correlation between the localization of AChE-enhanced [3H]-FW binding sites and layers undergoing intensive synapse formation and maturation suggests that this protein may be involved in one or more step of synaptogenesis AChE has been implicated in the control of neuronal growth and synaptogenesis, as well as in synaptic plasticity. It is now widely accepted that this protein causes extension of neurites (neuritogenic action) and can promote an increase in both the surface area and the structural organization of the postynaptic membrane (Grifman et al., 1998; Sternfeld et al., 1998). These observations go some way towards explaining the fact that AChE is commonly expressed in developing neurones irrespective of the neurotransmitters they finally utilize (Layer & Willbold, 1995)
Our data are consistent with AChE potentiation of [3H]-FW binding occurring particularly in regions undergoing active synaptogenesis. The neuroligin-like domain of AChE may participate in recognition between pre- and post- synaptic membranes during synapse formation (Scheiffele et al., 2000; Rao et al., 2000), possibly competing with neurexins (Grifman et al., 1998). Whereas the catalytic domain of AChE can modify agonist binding to AMPA receptors AMPA receptor-mediated neuronal activity plays a crucial role in the establishment and stabilization of active synaptic connections (Wu et al., 1996).
AChE is released as a consequence of neuronal activation (Jones et al., 1994; Rodriguez-Ithurralde et al., 1997). Therefore, the synaptogenic action of AChE may, at least in part, be due to its effects on the molecular organization of postsynaptic membranes (Sternfeld et al., 1998), including an increase in available AMPA receptors. Thus activity-released AChE could provide a mechanism for transducing neuronal activity into structurally and functionally viable synapses. Further investigation into the molecular mechanisms underlying the AChE modulation of AMPA receptors should provide useful information about the roles of this enzyme in modulating synaptic plasticity and neuronal development and neurodegeneration.
Acknowledgments
We are grateful to the MRC, the Wellcome Trust and PEDECIBA (Biología) of Uruguay for financial support. S Olivera is supported by a PEDECIBA (Biología) of Uruguay travel fellowship.
Abbreviations
- [3H]-FW
[3H]-5-fluorowillardiine
- AChE
acetylcholinesterase
- alv-or lim
limit between hippocampal alveus and stratum oriens
- AMPA
α-Amino-3-hydroxy-5-methylisoxazolepropionate
- Amy
amygdala
- Arc
arcuatus nucleus
- BW284c51
1,5-bis(4-allyl-dimethylammoniumphenyl)pentan-3-one dibromide
- BW284c51
1,5-bis(4-allyl-dimethylammoniumphenyl)pentan-3-one dibromide
- CA1
CA2, CA3, CA4, CA1-4 fields of hippocampus proper (Cornu Ammonis)
- Cpu
caudate putamen
- DG
dentate gyrus
- DHF
dorsal hippocampal formation
- DIIIV
dorsal third ventricle
- DTh
dorsal thalamus
- GrDG
granular layer of dentate gyrus
- H
hypothalamus
- hf
hippocampal fissure
- hil
hilus
- IIV
third ventricle
- lac-mol
stratum lacunosum-moleculare
- molDG
molecular layer of dentate gyrus
- mTh
medial thalamus
- neoCx
neocortex
- ocCx
occipital cortex
- OG
octylgucoside
- or
stratum oriens of hippocampus
- pCx
parietal cortex
- pirCx
piriform cortex
- prhCx
perirhinal cortex
- py (CA3)
pyramidal cell layer of hippocampus (CA3 field)
- pyr
pyramidal cell layer of hippocampus
- rad
stratum radiatum
- rf
rhinal fissure
- rsgCx
retrosplenial granular cortex
- S
subiculum
- SDS–PAGE
SDS polyacrylamide gel electrophoresis
- temCx
temporal cortex
- Th
thalamus
- vlTh
ventrolateral thalamus
- vmH
ventromedial hypothalamus
- vmTh
ventromedial thalamus
References
- ANDERSON R.B., KEY B. Role of acetylcholinesterase in the development of axon tracts within the embryonic vertebrate brain. Int. J. Dev. Neurosci. 1999;17:787–793. doi: 10.1016/s0736-5748(99)00064-7. [DOI] [PubMed] [Google Scholar]
- ARCHIBALD K., PERRY M.J., MOLNAR E., HENLEY J.M. Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacology. 1998;37:1345–1353. doi: 10.1016/s0028-3908(98)00135-x. [DOI] [PubMed] [Google Scholar]
- BRAITHWAITE S.P., MEYER G., HENLEY J.M. Interactions between AMPA receptors and intracellular proteins. Neuropharmacology. 2000;39:919–930. doi: 10.1016/s0028-3908(99)00171-9. [DOI] [PubMed] [Google Scholar]
- DEV K.K., HENLEY J.M. Modulation of agonist binding to AMPA receptors. Mol. Neurobiol. 1998;17:33–58. doi: 10.1007/BF02802023. [DOI] [PubMed] [Google Scholar]
- DEV K.K., HONORÉ T., HENLEY J.M. Phospholipase A2 down-regulates the affinity of [3H]AMPA binding to rat cortical membranes. J. Neurochem. 1995;65:184–191. doi: 10.1046/j.1471-4159.1995.65010184.x. [DOI] [PubMed] [Google Scholar]
- DINGLEDINE R., BORGES K., BOWIE D., TRAYNELIS S.F. The glutamate receptor channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- FIALA J.C., FEINBERG M., POPOV V., HARRIS K.M. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSSIER P., BAUX G., TAUC L. Possible role of acetylcholinesterase in regulation of postsynaptic receptor efficacy at a central inhibitory synapse of Aplysia. Nature. 1983;301:710–712. doi: 10.1038/301710a0. [DOI] [PubMed] [Google Scholar]
- GRIFMAN M., GALYAM N., SEIDMAN S., SOREQ H. Functional redundancy of acetylcholinesterase and neuroligin in mammalian neuritogenesis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13935–13940. doi: 10.1073/pnas.95.23.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISARU D., STERNFELD M., ELDOR A., GLICK D., SOREQ H. Structural roles of acetylcholinesterase variants in biology and pathology. Eur. J. Biochem. 1999;264:672–686. doi: 10.1046/j.1432-1327.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- HALL R.A., KESSLER M., LYNCH G. Evidence that high-affinity and low-affinity DL-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) binding sites reflect membrane-dependent states of a single receptor. J. Neurochem. 1992;59:1997–2004. doi: 10.1111/j.1471-4159.1992.tb10086.x. [DOI] [PubMed] [Google Scholar]
- HENLEY J.M. Localization of AMPA receptor subunits in rat CNS using anti-peptide antibodies. Neuroreport. 1993;4:334–336. doi: 10.1097/00001756-199303000-00028. [DOI] [PubMed] [Google Scholar]
- HENLEY J.M., BARNARD E.A. Solubilisation and characterisation of a putative quisqualate-type glutamate receptor from chick brain. J. Neurochem. 1989;53:140–148. doi: 10.1111/j.1471-4159.1989.tb07305.x. [DOI] [PubMed] [Google Scholar]
- HOLMES C., JONES S.A., BUDD T.C., GREENFIELD S.A. Non-cholinergic, trophic action of recombinant acetylcholinesterase on mid-brain dopaminergic neurons. J. Neurosci. Res. 1997;49:207–218. [PubMed] [Google Scholar]
- ICHTCHENKO K., HATA Y., NGUYEN T., ULLRICH B., MISSLER M., MOOMAW C., SUDHOF T.C. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- ICHTCHENKO K., NGUYEN T., SÜDHOF T.C. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- JONES S.A., DICKIE B.G.M., KLEGERIS A., GREENFIELD S.A. The subthalamonigral pathway regulates movement and concomitant acetylcholinesterase release from the substantia-nigra. J. Neural Trans. 1994;98:23–37. doi: 10.1007/BF01277592. [DOI] [PubMed] [Google Scholar]
- KOENIGSBERGER C., CHIAPPA S., BRIMIJOIN S. Neurite differentiation is modulated in neuroblastoma cells engineered for acetylcholinesterase expression. J. Neurochem. 1997;69:1389–1397. doi: 10.1046/j.1471-4159.1997.69041389.x. [DOI] [PubMed] [Google Scholar]
- LAYER P.G., WILLBOLD E. Novel functions of cholinesterases in development, physiology and disease. Progr Histochem Cytochem. 1995;29:1–93. doi: 10.1016/s0079-6336(11)80046-x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS D.A., NADLER J.V., LYNCH G.S., COTMAN C.W. Development of cholinergic innervation in the hippocampal formation of the rat. Histochemical demonstration of acetylcholinesterase activity. Develop. Biol. 1974;36:130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- NADLER J.V., MATTHEWS D.A., COTMAN C.W., LYNCH G.S. Development of cholinergic innervation in the hippocampal formation of the rat II. Quantitative changes in choline acetyltransferase and acetylcholinesterase activities. Dev. Biol. 1974;36:142–154. doi: 10.1016/0012-1606(74)90197-3. [DOI] [PubMed] [Google Scholar]
- O'BRIEN R., XU D., PETRALIA R., STEWARD O., HUGANIR R., WORLEY P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- OLIVERA S., RODRIGUEZ-ITHURRALDE D., HENLEY J.M. Acetylcholinesterase potentiates [3H]fluorowillardiine and [3H]AMPA binding to rat cortical membranes. Neuropharmacology. 1999;38:505–512. doi: 10.1016/s0028-3908(98)00214-7. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotaxic coordinates 1986Academic Press: London; (Second Edition) [Google Scholar]
- PETRALIA R.S., ESTEBAN J.A., WANG Y.X., PARTRIDGE J.G., ZHAO H.M., WENTHOLD R.J., MALINOW R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- RAO A., HARMS K.J., CRAIG A.M. Neuroligation building synapses around the neurexin-neuroligin link. Nat. Neurosci. 2000;3:747–749. doi: 10.1038/77636. [DOI] [PubMed] [Google Scholar]
- RODRÍGUEZ-ITHURRALDE D., OLIVERA S., VINCENT O., MARURI-PIGNI A. In vivo and in vitro studies of glycine- and glutamate-evoked acetylcholinesterase release from spinal motor neurones: Implications for ALS/MND pathogenesis. J. Neurol. Sci. 1997;152:S54–S61. doi: 10.1016/s0022-510x(97)00245-1. [DOI] [PubMed] [Google Scholar]
- SCHEIFFELE P., FAN J., CHOIH J., FETTER R., SERAFINI T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- STERNFELD M., MING G., SONG H., SELA K., TIMBERG R., POO M., SOREQ H. Acetylcholinesterase enhances neurite growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J. Neurosci. 1998;18:1240–1249. doi: 10.1523/JNEUROSCI.18-04-01240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU G.Y., MALINOW R., CLINE H.T. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]


