Abstract
BIIE0246, a recently introduced non-peptide neuropeptide Y (NPY) Y2 receptor antagonist, was pharmacologically characterized in vivo, on vascular responses evoked in the anaesthetized pig.
The NPY Y2 receptor agonist N-acetyl[Leu28Leu31]NPY(24-36) evoked dose-dependent vasoconstriction in spleen. These vascular responses were potently and dose-dependently antagonized by BIIE0246. Significant inhibition was seen already at 1 nmol kg−1, whereas at 100 nmol kg−1 of BIIE0246 these responses were completely abolished. The ID50 value for this antagonism was 2.1 nmol kg−1.
Peptide YY (PYY) evoked dose-dependent vasoconstriction in both kidney and spleen, vascular responses mediated by the NPY Y1 receptor and both NPY Y1 and Y2 receptors, respectively. Only the splenic response was inhibited by BIIE0246, the effect of which reached significance at 1 nmol kg−1. Already 30 min after the last dose of BIIE0246 there was a significant recovery of the PYY-evoked splenic vasoconstriction, and a further 60 min later, this response was no longer significantly inhibited compared to control.
BIIE0246 (100 nmol kg−1) did not affect renal and splenic vasoconstrictor responses either to the NPY Y1 receptor agonist [Leu31Pro34]NPY, the α1-adrenoceptor agonist phenylephrine, the P2X1-purinoceptor agonist α,β-methylene ATP or angiotensin II, demonstrating both selectivity and specificity for the NPY Y2 receptor in vivo.
It is concluded that BIIE0246 is a highly potent and selective NPY Y2 receptor antagonist, albeit with rather short duration of action, in vivo. BIIE0246 thus represents the first interesting tool for studies on NPY Y2 receptor-mediated transmission in vivo.
Keywords: NPY Y2 receptor, antagonist, BIIE0246, vasoconstriction, in vivo
Introduction
Throughout the years, several studies have addressed the functions of neuropeptide Y (NPY) as a sympathetic cotransmitter (for review see Malmström, 1997). For example, NPY may evoke vasoconstriction (Lundberg & Tatemoto, 1982), enhance the effects of other vasoactive substances (Ekblad et al., 1984) and regulate sympathetic (Lundberg et al., 1982; Lundberg & Stjärne, 1984; Pernow et al., 1986; Stjärne et al., 1986; Pernow & Lundberg, 1989), as well as parasympathetic (Potter, 1987), transmitter release. Vascular effects of NPY, and its hormonal homologue peptide YY (PYY), are predominantly mediated via the NPY Y1 receptor subtype (Malmström, 1997). This was ultimately shown using subtype-selective antagonists, which is the most preferred approach for precise classification of receptor subtypes. Before the introduction of such non-peptide antagonists, the division of NPY receptors was based on the actions of a number of peptide analogues acting like agonists (Wahlestedt et al., 1986). NPY analogues that were modified in the C-terminal part of the peptide were shown to mimic most postjunctional effects, whereas C-terminal fragments of the peptide were found to mimic the prejunctional effects of NPY. This formed the basis for an initial division of NPY receptors in Y1 and Y2, respectively. Today, several receptors for NPY and its homologues have been cloned and classified as the Y1, Y2, Y4, Y5 and y6 subtypes on the basis of their molecular and pharmacological profiles (Michel et al., 1998). Except for the y6, all subtypes are functionally expressed in several species (Larhammar, 1996), including man, although the involvement of other NPY receptor subtypes than the Y1 and Y2 in sympathetic transmission still remains to be proposed. Shortly after the introduction of non-peptide antagonists selective for the NPY Y1 receptor, e.g. BIBP 3226 (Rudolf et al., 1994) and SR 120107A (Serradeil-Le Gal et al., 1994), evidence was presented that NPY, released from sympathetic nerves, mediates vasoconstrictor responses both in vitro (Malmström & Lundberg, 1995a, 1995b) and in vivo (Lundberg & Modin, 1995; Malmström & Lundberg, 1996; Malmström et al., 1996; 1997), acting preferably on the NPY Y1 receptor. However, as revealed by the use of the same antagonists, not all vascular effects exerted by NPY can be attributed NPY Y1 receptor mediated phenomena. For example, in pig spleen NPY Y1 receptor antagonists do not block the entire response to NPY and PYY (Lundberg & Modin, 1995; Malmström & Lundberg, 1996; Malmström et al., 1996; 1997). In addition, peptide analogues ‘selective' for NPY Y2 receptors may exert responses in this vascular bed (Modin et al., 1991; Malmström et al., 1998). Therefore, NPY Y2 receptors in sympathetic vascular control might not be purely prejunctional, involved in regulation of sympathetic transmitter release. Conversely, evidence for prejunctional NPY Y1 receptors has been presented e.g. in rabbit vas deferens by the use of BIBP 3226 (Doods et al., 1995), although such receptors are still in most instances purportedly of the Y2 subtype, as indicated by the use of peptide agonists (Doods & Krause, 1991; Potter et al., 1994). However, given that all such agonists are chemically related to NPY, none of these agonists developed this far is truly selective for one receptor subtype. Thus, conclusive evidence for the role of NPY Y2 receptors is still to be awaited due to the lack of selective antagonists. Most recently, Doods et al. (1999) reported on the development of BIIE0246, (S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide, as the first non-peptide antagonist selective for the NPY Y2 receptor subtype. BIIE0246 possesses high affinity for the NPY Y2 receptor and is devoid of affinity for NPY Y1, Y4 and Y5 receptors (or 60 other receptor types and enzymes) in various receptor binding assays (Doods et al., 1999; Dumont et al., 2000), and this compound also antagonizes NPY Y2 receptor-mediated responses in several in vitro bioassays, while not affecting those mediated by NPY Y1 or Y4 receptors (Doods et al., 1999; Dumont et al., 2000). In the present study, the pharmacological profile of BIIE0246 in vivo was investigated in detail. For this purpose the anaesthetized pig model was used, where vascular responses evoked by both NPY receptors proposed to be involved in sympathetic transmission (Y1 and Y2), as well as other receptor subtypes, were studied.
Methods
In vivo study
This study was approved by the local ethics committee for animal research.
Surgical preparation
Domestic pigs (15–20 kg) of either sex were premedicated with ketamine (20 mg kg−1 intramuscular, i.m.) and atropine (0.02 mg kg−1 i.m.), and thereafter anaesthetized with sodium pentobarbitone (20 mg kg−1 intravenously, i.v.), tracheotomized and artificially ventilated by a respirator (Servo ventilator 900, Siemens-Elema, Sweden). Skeletal muscle relaxation was induced with pancuronium (0.5 mg kg−1 i.v.) after the anaesthesial depth was checked by pinching the interdigital skin. A catheter was inserted into the left femoral vein for infusion of drugs. For measurement of mean arterial pressure (MAP), a catheter, connected to a Statham P23 AC pressure transducer, was inserted into the left femoral artery. A tachograph unit triggered by the blood pressure recorded heart rate. The blood flows of the splenic and left renal arteries were measured by ultrasonic flow probes (2RB) connected to a Transonic flowmeter (T206, Transonic Instruments, Ithaca, NY, U.S.A.). All parameters were continuously recorded on Grass Polygraphs. Throughout the experiments, drugs were given to maintain anaesthesia (fentanyl, 10 μg kg−1 h−1, and midazolam, 0.2 mg kg−1 h−1), skeletal muscle relaxation (pancuronium, 0.5 mg kg−1 h−1), fluid balance (sodium chloride 154 mM and glucose 28 mM, 2 ml min−1), and for prevention of intravascular coagulation (heparin, 250 iu kg−1 h−1). The abdomen was closed and the pigs were allowed to stabilize for 1 h before experiments were undertaken.
Experimental procedures
Phenylephrine (15 nmol kg−1), α,β-methylene ATP (mATP, 6 nmol kg−1) and angiotensin II (240 pmol kg−1) were given as i.v. bolus injections. Dose-response curves were performed for the NPY Y2 receptor agonist N-acetyl[Leu28Leu31]NPY(24-36), the NPY Y1 receptor agonist [Leu31Pro34]NPY, and PYY (23, 70 and 230 pmol kg−1, each). The NPY Y2 receptor antagonist BIIE0246 was then given at increasing doses administered as i.v. injections, followed by i.v. infusions, at approximately 30 min intervals. The bolus doses of BIIE0246 were 0.3–100 nmol kg−1 (equal to 0.3–100 μg kg−1). Each i.v. bolus dose injection of BIIE0246 was followed by a 20 min i.v. infusion of the compound, during which the corresponding bolus dose was administered (e.g. a bolus of 30 nmol kg−1 was followed by an infusion when 30 nmol kg−1 was given during 20 min, i.e. at a rate of 1.5 nmol kg−1 min−1). The last dose of BIIE0246 (100 nmol kg−1) was followed by a prolonged infusion at the corresponding dose-rate (5 nmol kg−1 min−1 during 60 min) due to the length of the protocol (see below). Each dose of BIIE0246 will herein be referred to by their bolus injection amounts. A dose-response curve to either PYY or N-acetyl[Leu28Leu31]NPY(24-36) was repeated 10 min into each of the 20 min infusions that followed the first five doses (0.3–30 nmol kg−1) of BIIE0246. Thereafter, the entire protocol of agonist injections was repeated commencing 10 min into the infusion that followed the last dose (100 nmol kg−1) of BIIE0246. After cessation of the last infusion of BIIE0246, PYY (230 pmol kg−1) was injected every 30th min, during a recovery period of 2 h, to investigate the duration of action of the antagonist. The doses for the agonists were chosen on basis of previous studies in the pig in vivo (Malmström, 1997). All vascular responses studied are reproducible and not susceptible to any spontaneous decline as has been demonstrated in earlier studies (Malmström, 1997).
Calculations
The vascular responses are expressed as changes in vascular conductance, calculated as blood flow divided by MAP (Stark, 1968). ID50 values (the dose of antagonist needed to exert 50% inhibition of a given response) were determined by non-linear regression analysis. Data in the text are given as means±s.e.mean, and statistical significance was calculated with the multiple analysis of variance (ANOVA) followed by the post test of Tukey, or with the Student's t-test (paired samples) where applicable.
Drugs
Ketamine (Parke-Davis, CA, U.S.A.), sodium pentobarbitone (NordVacc, Sweden), atropine and sodium heparin (KabiVitrum, Sweden), pancuronium bromide (Organon, The Netherlands), fentanyl (Pharmalink, Sweden), midazolam (Roche, Sweden), PYY, [Leu31Pro34]NPY and N-acetyl[Leu28Leu31]NPY(24-36) (Auspep, Australia), angiotensin II, phenylephrine hydrochloride and α,β-methylene ATP (Sigma, MO, U.S.A.). BIIE0246, (S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin - 11 - yl] - 1- piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide, (Boehringer Ingelheim Pharma KG, Biberach, Germany). All drugs were dissolved in either saline or glucose (5%, w v−1) solution.
Results
Basal splenic blood flow (155±20 ml min−1), renal blood flow (120±10 ml min−1), MAP (110±6 mmHg), and heart rate (140±10 beats min−1) were not significantly altered upon the injections of BIIE0246 (0.3–100 nmol kg−1). However, there was a trend of a slight, but non-significant, gradual increase in splenic blood flow, accompanied by a marginal fall in MAP and elevation in heart rate, upon increasing doses of BIIE0246. These effects were most pronounced upon the highest dose of BIIE0246 (100 nmol kg−1) when splenic blood flow was increased from 165±30 to 170±30 ml min−1, MAP decreased from 108±9 to 106±8 mmHg, and heart rate increased from 150±10 to 160±10 beats min−1.
PYY (23–230 pmol kg−1) evoked dose-dependent vasoconstrictor responses in spleen (Figure 1a), kidney (Figure 1b), and elevation of MAP. Splenic vascular conductance was reduced by 25±4, 51±5 and 78±2% upon 23, 70 and 230 pmol kg−1 of PYY, respectively. BIIE0246 dose-dependently inhibited the PYY-evoked splenic vascular responses (Figure 2a). Significant inhibition was seen already upon 1 nmol kg−1 of BIIE0246, after which splenic vascular conductance was reduced by 22±4% (P<0.01 vs the control response), 46±5% (P<0.05) and 68±4% (P<0.05) upon the corresponding doses of PYY, respectively. Greatest inhibition was observed after 100 nmol kg−1 of BIIE0246 when PYY (23–230 pmol kg−1) exerted reduction of splenic vascular conductance by 4±2, 15±2 and 37±3%, respectively (Figures 1a and 3a). The ID50 value for inhibition of the NPY Y2 receptor-mediated (see Discussion) part of splenic vasoconstriction exerted by PYY (230 pmol kg−1) was 3.4 nmol kg−1 (95% Confidence Interval 1.2–5.7 nmol kg−1). Thirty minutes after cessation of the last infusion of BIIE0246, the splenic vasoconstrictor response to PYY (230 pmol kg−1) was significantly less inhibited (Figure 4). A further 60 min after this, the PYY-evoked splenic vasoconstrictor response was no longer significantly inhibited compared to the control response (Figure 4).
Figure 1.
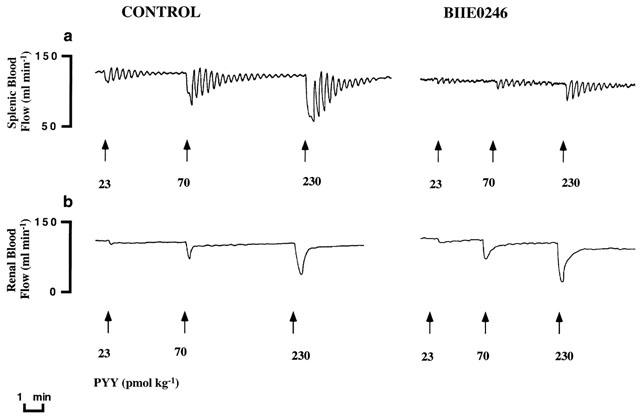
Original recording of (a) splenic and (b) renal blood flows in the anaesthetized pig. Vasoconstrictor responses to PYY (23–230 pmol kg−1, i.v.) are shown before (control) and after treatment with the NPY Y2 receptor antagonist BIIE0246 (100 nmol kg−1, i.v.). Note the inhibition seen upon the PYY-evoked vascular responses in spleen, whereas such an effect was not seen in kidney.
Figure 2.
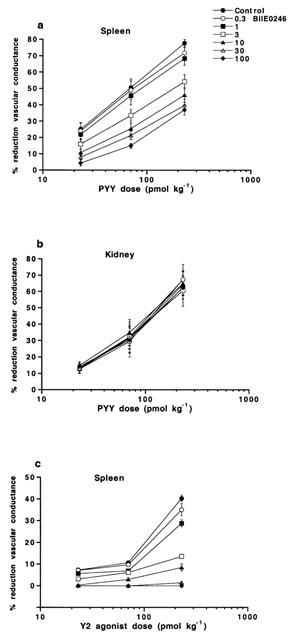
Dose response curves for vasoconstrictor responses (expressed as per cent reduction of vascular conductance compared to basal) evoked in (a) spleen and (b) kidney by PYY and (c) in spleen by the NPY Y2 receptor agonist N-acetyl[Leu28Leu31]NPY(24–36), in the absence and presence of increasing doses of the NPY Y2 receptor antagonist BIIE0246 (0.3–100 nmol kg−1), in the anaesthetized pig. Data are given as means±s.e.mean, n=4–9.
Figure 3.
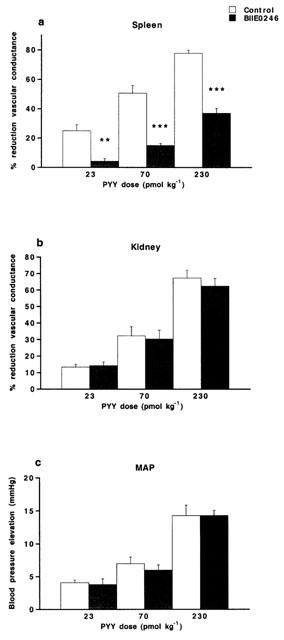
Vasoconstrictor responses (expressed as per cent reduction of vascular conductance compared to basal) evoked in (a) spleen and (b) kidney and (c) elevation of MAP (in mmHg) upon i.v. injected PYY (23–230 pmol kg−1) in the anaesthetized pig. The vascular responses are shown before (control) and after treatment with the NPY Y2 receptor antagonist BIIE0246 (100 nmol kg−1, i.v.). Data are given as means±s.e.mean, n=6–9. Significant differences compared to control are indicated **P<0.01, ***P<0.001.
Figure 4.
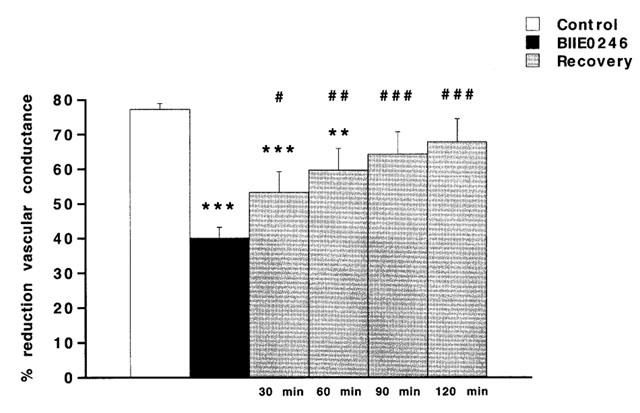
The splenic vasoconstrictor response (expressed as per cent reduction of vascular conductance compared to basal) upon i.v. injected PYY (230 pmol kg−1) in the anaesthetized pig. The vascular response is shown before (control), after treatment with the NPY Y2 receptor antagonist BIIE0246 (100 nmol kg−1, i.v.) and at every 30th min, during a recovery period of 120 min, after cessation of the corresponding infusion of BIIE0246. Data are given as means±s.e.mean, n=8. Significant differences compared to control are indicated **P<0.01, ***P<0.001. Significant differences between BIIE0246 and recovery are indicated #P<0.05, ##P<0.01, ###P<0.001.
Renal vascular conductance was reduced by 13±2, 32±6 and 67±5% upon 23, 70 and 230 pmol kg−1 of PYY, respectively. These vascular responses were not inhibited by BIIE0246 (Figures 1b, 2b and 3b). MAP was elevated by 4±0.4, 7±1 and 14±2 mmHg upon 23, 70 and 230 pmol kg−1 of PYY, respectively, and these effects were not affected by BIIE0246 (Figure 3c).
N-acetyl[Leu28Leu31]NPY(24–36) evoked dose-dependent splenic vasoconstriction but no, or only marginal, effects in kidney. Splenic vascular conductance was reduced by 7±1, 13±1 and 39±3% upon 23, 70 and 230 pmol kg−1 of N-acetyl[Leu28Leu31]NPY(24-36), respectively. These splenic vascular responses were dose-dependently antagonized by BIIE0246 (Figure 2c). Significant antagonism was seen already upon 1 nmol kg−1 of BIIE0246 when splenic vascular conductance was reduced by 5±1% (P<0.05 vs the control response), 7±1% (P<0.001) and 29±2% (P<0.001) upon the corresponding doses of N-acetyl[Leu28Leu31]NPY(24–36), respectively. The ID50 value for inhibition of the splenic vasoconstriction exerted by N-acetyl[Leu28Leu31]NPY(24–36) (230 pmol kg−1) was 2.1 nmol kg−1 (95% Confidence Interval 1.7–2.6 nmol kg−1). The splenic vasoconstrictor responses evoked by 23, 70 and 230 pmol kg−1 of N-acetyl[Leu28Leu31]NPY(24-36) were completely abolished by BIIE0246 at 10, 30 and 100 nmol kg−1, respectively (Figure 2c). A modest elevation of MAP (by 3±1 mmHg) was seen upon the highest dose of N-acetyl[Leu28Leu31]NPY(24–36) and this effect was also abolished by BIIE0246 (data not shown).
[Leu31Pro34]NPY evoked dose-dependent vasoconstriction in spleen and kidney, as well as elevation of MAP. Splenic vascular conductance was reduced by 6±1, 12±1 and 30±2% upon 23, 70 and 230 pmol kg−1 of [Leu31Pro34]NPY, respectively. Renal vascular conductance was reduced by 8±1, 17±2 and 47±7% upon the corresponding doses of [Leu31Pro34]NPY, respectively. Neither of these vasoconstrictor responses were affected by BIIE0246 (100 nmol kg−1) (Figure 5). MAP was elevated by 2±0.5, 4±0.5 and 8±0.5 mmHg upon these doses of [Leu31Pro34]NPY, respectively. BIIE0246 did not affect the elevation of MAP evoked by [Leu31Pro34]NPY (2±0.5, 5±0.5 and 9±1 mmHg, respectively, after BIIE0246).
Figure 5.
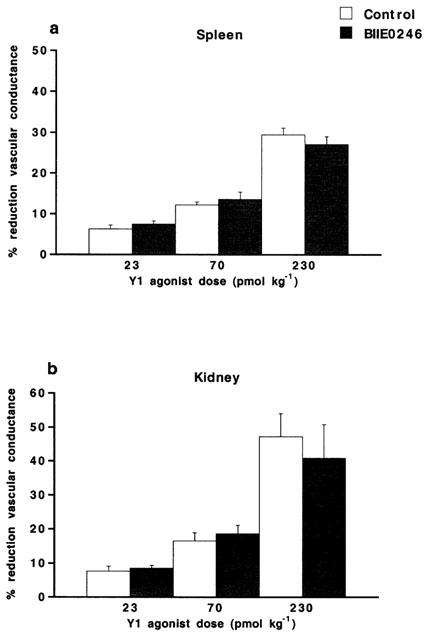
Vasoconstrictor responses (expressed as per cent reduction of vascular conductance compared to basal) evoked in (a) spleen and (b) kidney by the NPY Y1 receptor agonist [Leu31Pro34]NPY (23–230 pmol kg−1, i.v.), in the anaesthetized pig. The vascular responses are shown before (control) and after treatment with the NPY Y2 receptor antagonist BIIE0246 (100 nmol kg−1, i.v.). Data are given as means±s.e.mean, n=7. There were no significant differences compared to control.
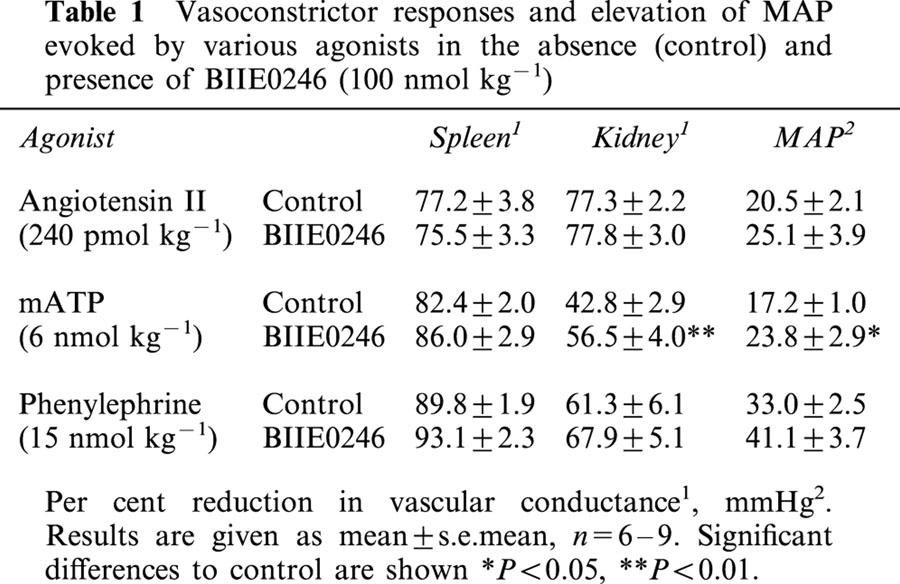
Phenylephrine (15 nmol kg−1), mATP (6 nmol kg−1) and angiotensin II (240 pmol kg−1) all evoked vasoconstriction in kidney and spleen as well as elevation of MAP (Table 1). These vascular responses were not affected by BIIE0246 (Table 1). In contrast, the vascular responses to mATP were slightly augmented upon repeated administration (Table 1).
Table 1.
Vasoconstrictor responses and elevation of MAP evoked by various agonists in the absence (control) and presence of BIIE0246 (100 nmol kg−1)
Discussion
In the present study, it was demonstrated that BIIE0246 acts like a highly potent and selective NPY Y2 receptor antagonist in vivo. In order to investigate the pharmacological profile of BIIE0246 in vivo two vascular beds of the anaesthetized pig were studied in detail: kidney and spleen. In these vascular beds both NPY and PYY evokes potent and dose-dependent vasoconstrictor effects (Modin et al., 1991; Malmström & Lundberg, 1996; Malmström, 1997; Malmström et al., 1998). In kidney, moderate doses of NPY Y1 receptor agonists potently exert vasoconstrictor responses (Modin et al., 1991; Malmström, 1997; Malmström et al., 1998), whereas NPY Y2 receptor agonists do not (Modin et al., 1991; Malmström, 1997; Malmström et al., 1998). In addition, NPY Y1 receptor antagonists can completely abolish the renal vascular responses to NPY and PYY (Lundberg & Modin, 1995; Malmström & Lundberg, 1996; Malmström et al., 1996; 1997; 2000). Therefore, pig kidney represents a vascular bed where the NPY Y1 receptor seems to be the sole NPY receptor involved in vasoconstriction (at least to moderate doses of exogenous agonists). In contrast, both NPY Y2 and Y1 receptor agonists potently evoke vasoconstriction in pig spleen (Modin et al., 1991; Malmström et al., 1998; 2000), only the latter of which can be antagonized by NPY Y1 receptor antagonists (Lundberg & Modin, 1995; Malmström et al., 1998; 2000). Furthermore, NPY Y1 receptor antagonists can only inhibit part of the splenic response to NPY and PYY (Lundberg & Modin, 1995; Malmström & Lundberg, 1996; Malmström et al., 1996; 1997; 2000). In fact, NPY Y1 receptor antagonists do not, or only marginally, affect the splenic responses to low doses of NPY and PYY (Malmström & Lundberg, 1996; Malmström et al., 1996), whereas such inhibitory effects are more clear-cut at higher doses of the agonists (Malmström & Lundberg, 1996). Thus, it was proposed that NPY, and PYY, preferentially activate NPY Y2 receptors in pig spleen to cause a vascular response, whereas at higher doses the involvement of NPY Y1 receptors increase and thus participate significantly in this response (Malmström, 1997). This notion was supported by results from the present study. Thus, in apparent contrast to what was observed using NPY Y1 receptor antagonists, the inhibitory effects of BIIE0246 were stronger on splenic vasoconstrictor responses to lower doses of PYY. Hence, the heterogeneous splenic population of NPY Y1 and Y2 receptors seems to be activated preferably upon high and low circulating level of NPY, respectively.
In the current study, as anticipated, the NPY Y2 receptor agonist N-acetyl[Leu28Leu31]NPY(24–36) evoked vasoconstrictor responses in spleen only. These vascular responses were dose-dependently antagonized by BIIE0246. The calculated ID50 value for this inhibition indicated potent antagonism. Furthermore, BIIE0246 antagonized the NPY Y2 receptor-mediated part of the PYY-evoked response in spleen about equally potent. The NPY Y2 receptor-mediated part of the response to PYY was considered the part that was sensitive to the effects of BIIE0246. The remaining response to PYY, in the presence of the highest dose of BIIE0246, can be abolished by a NPY Y1 receptor antagonist and is thus mediated by the NPY Y1 receptor (unpublished observations). By judging from the results in the present study, it appears that BIIE0246 exerts highly potent NPY Y2 receptor antagonism in vivo. This extends the recently presented in vitro data showing that BIIE0246 antagonizes NPY Y2 receptor-mediated effects in dog saphenous vein and rat vas deferens with high potency (pA2 values at 8.6 and 8.1, respectively) (Doods et al., 1999; Dumont et al., 2000). As indicated by results from the present study, BIIE0246 possesses rather short duration of action in vivo. Thus, already at 30 min after the last dose of the antagonist, the PYY-evoked splenic vasoconstrictor response was significantly less inhibited. Furthermore, at a time-point 60 min later, there remained no significant inhibition of this response compared to control. Hence, it is suggested that in vivo studies, especially concerning longer protocols, with BIIE0246 be preferably performed during infusions of the compound.
Selectivity for NPY Y2 receptor-mediated events in vivo was demonstrated. Thus, whereas BIIE0246 potently antagonized splenic vascular responses to the NPY Y2 receptor agonist N-acetyl[Leu28Leu31]NPY(24–36), no inhibitory effects were observed on those exerted by the NPY Y1 receptor agonist [Leu31Pro34]NPY. The pig spleen represents a tissue expressing a heterogeneous population of NPY receptors (Malmström et al., 1998). The present results clearly demonstrate the ability of BIIE0246 to discriminate between NPY receptor subtypes within a tissue. In addition, as discussed above, BIIE0246 only blocked part of the splenic, NPY Y1 and Y2 receptor-mediated, vascular response evoked by PYY. Thus, BIIE0246 represents an interesting tool for establishing the genuine presence of NPY Y2 receptors in tissues with a divided population of NPY receptors. Fully in line with this, BIIE0246 was able to discriminate between NPY Y1 and Y4 receptors in rat colon in vitro (Dumont et al., 2000). Selectivity was strengthened in the current study as BIIE0246 did not affect renal NPY Y1 receptor-mediated vascular responses to either PYY or [Leu31Pro34]NPY. In accord with this, BIIE0246 do not inhibit contractions evoked by NPY in prototypical NPY Y1 receptor bioassays (rabbit saphenous vein and human cerebral arteries) in vitro (Dumont et al., 2000). As shown here, the MAP elevations exerted by PYY and [Leu31Pro34]NPY were not influenced by BIIE0246. These pressor responses have, by using selective NPY Y1 receptor antagonists, been pointed out to be predominantly, if not only, NPY Y1 receptor-mediated effects (Malmström, 1997). The lack of effect of BIIE0246 supports this, and also indicates that splenic NPY Y2 receptor-mediated vasoconstriction does not contribute significantly to the pressor response to PYY. BIIE0246 does not cross-react with a large number of receptors (other than NPY) in vitro (Doods et al., 1999). The specificity of action of BIIE0246 on NPY receptor-mediated events in vivo was shown in the present study by its lack of effect on vascular responses to other known vasoactive substances, phenylephrine, angiotensin II and mATP. The renal vascular response to mATP was slightly enhanced upon repeated administration, but this was not likely related to the effects of BIIE0246. Thus, similar results (although non-significant) have been observed e.g. in studies with NPY Y1 receptor antagonists as well (Malmström et al., 1996).
BIIE0246 did not significantly alter basal cardiovascular parameters per se. However, in nearly half of the studied subjects there was a gradual splenic vasodilatation upon increasing doses of the antagonist. Considering that spleen is yet the only known vascular bed in the pig where NPY Y2 receptors contribute significantly to vasoconstrictor events exerted by circulating NPY, splenic vasodilatation could be anticipated when giving BIIE0246, if the circulating (or locally released) NPY levels were high enough to exert vascular effects. It cannot be excluded that the role of NPY Y2 receptors in basal splenic blood flow regulation in the pig may vary with variations in environmental stress, although the pigs studied were all handled equally, and with efforts to be exposed to a minimum of stress. Alternatively, one may speculate on that the splenic vasodilatation was a non-specific effect. However, since most studied subjects in the present series did not respond with splenic vasodilatation to BIIE0246 per se, this may seem less likely. If possible, studies with an enantiomer to BIIE0246, inactive at NPY Y2 receptors, would eventually solve this issue.
It remains to clearly establish the possible role(s) of NPY Y2 receptors in the organism. Only in the cardiovascular system, several functions have been attributed to NPY Y2 receptors. As shown here, NPY Y2 receptors are clearly involved in vasoconstrictor responses evoked in pig spleen. In addition, NPY exerts contractions of dog saphenous vein via NPY Y2 receptors, at least in vitro (Dumont et al., 2000). The NPY Y2 receptor has also been suggested to be involved in prejunctional regulation of transmitter release. Thus, the release of NPY (Pernow & Lundberg, 1989), ATP (Lundberg & Stjärne, 1984; Stjärne et al., 1986) and noradrenaline (Lundberg et al., 1982; Pernow et al., 1986; Pernow & Lundberg, 1989) from sympathetic nerves may all be inhibited by NPY presumably acting on the NPY Y2 receptor. In accord with this, evidence for prejunctional NPY Y2 receptors inhibiting electrically-induced contractions in rat vas deferens in vitro was recently presented (Doods et al., 1999; Dumont et al., 2000). Furthermore, BIIE0246 attenuated NPY Y2 receptor-mediated inhibition of cholinergic transmission in rat heart and guinea-pig trachea in vitro (Smith-White et al., 2001). Thus, BIIE0246 should prove most helpful to clearly establish whether the NPY Y2 receptor is truly involved in other cardiovascular responses that cannot be attributed the NPY Y1 (or other) receptor subtype, and finally to clarify the possible importance of such purported NPY Y2 receptor-mediated effects.
In summary, it was demonstrated that BIIE0246 is a highly potent and selective NPY Y2 receptor antagonist in vivo. The duration of action is rather short, and infusions of BIIE0246 may be advantageous when the compound is to be studied in vivo. BIIE0246 dose-dependently antagonized NPY Y2 receptor-mediated splenic vasoconstriction, without affecting vascular responses mediated by NPY Y1 (or other) receptors in spleen and kidney. Thus, BIIE0246 represents the first interesting tool to establish the potential role of NPY Y2 receptor-mediated transmission in vivo.
Acknowledgments
The author wishes to thank Dr Henri Doods at Boehringer Ingelheim Pharma, Biberach, Germany, for a generous supply of BIIE0246, and Margareta Stensdotter for expert technical assistance. This work was supported by grants from Karolinska Institutet, AstraZeneca R&D Mölndal, Magn. Bergvalls and Lars Hiertas Foundations.
Abbreviations
- BIIE0246
(S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5 (4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide;
- MAP
mean arterial pressure
- mATP
α,β-methylene ATP
- NPY
neuropeptide Y
- PYY
peptide YY
References
- DOODS H., GAIDA W., WIELAND H.A., DOLLINGER H., SCHNORRENBERG G., ESSER F., ENGEL W., EBERLEIN W., RUDOLF K. BIIE0246: a selective and high affinity neuropeptide Y Y2 receptor antagonist. Eur. J. Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- DOODS H.N., KRAUSE J. Different neuropeptide Y receptor subtypes in rat and rabbit vas deferens. Eur. J. Pharmacol. 1991;204:101–103. doi: 10.1016/0014-2999(91)90841-d. [DOI] [PubMed] [Google Scholar]
- DOODS H.N., WIENEN W., ENTZEROTH M., RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A. Pharmacological characterization of the selective nonpeptide neuropeptide Y Y1 receptor antagonist BIBP 3226. J. Pharmacol. Exp. Ther. 1995;275:136–142. [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., PHENG L.H., ABOUNADER R., HAMEL E., JACQUES D., REGOLI D., QUIRION R. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y2 receptor antagonist. Br. J. Pharmacol. 2000;129:1075–1088. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKBLAD E., EDVINSSON L., WAHLESTEDT C., UDDMAN R., HÅKANSON R., SUNDLER F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul. Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- LARHAMMAR D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., MODIN A. Inhibition of sympathetic vasoconstriction in pigs in vivo by the neuropeptide Y-Y1 receptor antagonist BIBP 3226. Br. J. Pharmacol. 1995;116:2971–2982. doi: 10.1111/j.1476-5381.1995.tb15952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG J.M., STJÄRNE L. Neuropeptide Y (NPY) depresses the secretion of 3H-noradrenaline and the contractile response evoked by field stimulation, in rat vas deferens. Acta Physiol. Scand. 1984;120:477–479. doi: 10.1111/j.1748-1716.1984.tb07410.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., TATEMOTO K. Pancreatic polypeptide family (APP, BPP, NPY, PYY) in relation to sympathetic vasoconstriction resistant to α-adrenoceptor blockade. Acta Physiol. Scand. 1982;116:393–402. doi: 10.1111/j.1748-1716.1982.tb07157.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., TERENIUS L., HÖKFELT T., MARTLING C.-R., TATEMOTO K., MUTT V., POLAK J., BLOOM S., GOLDSTEIN M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol. Scand. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E. Neuropeptide Y Y1 receptor mechanisms in sympathetic vascular control. Acta Physiol. Scand. 1997;160 Suppl 636:1–55. [PubMed] [Google Scholar]
- MALMSTRÖM R.E., ALEXANDERSSON A., BALMÉR K.C., WEILITZ J. In vivo characterization of the novel neuropeptide Y Y1 receptor antagonist H 409/22. J. Cardiovasc. Pharmacol. 2000;36:516–525. doi: 10.1097/00005344-200010000-00016. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., BALMÉR K.C., LUNDBERG J.M. The neuropeptide Y (NPY) Y1 receptor antagonist BIBP 3226: equal effects on vascular responses to exogenous and endogenous NPY in the pig in vivo. Br. J. Pharmacol. 1997;121:595–603. doi: 10.1038/sj.bjp.0701154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., HÖKFELT T., BJÖRKMAN J.-A., NIHLÉN C., BYSTRÖM M., EKSTRAND A.J., LUNDBERG J.M. Characterization and molecular cloning of vascular neuropeptide Y receptor subtypes in pig and dog. Regul. Pept. 1998;75–76:55–70. doi: 10.1016/s0167-0115(98)00053-6. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., LUNDBERG J.M. Endogenous NPY acting on the Y1 receptor accounts for the long-lasting part of the sympathetic contraction in guinea-pig vena cava: evidence using SR 120107A. Acta Physiol. Scand. 1995a;155:329–330. doi: 10.1111/j.1748-1716.1995.tb09981.x. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., LUNDBERG J.M. Neuropeptide Y accounts for sympathetic vasoconstriction in guinea-pig vena cava: evidence using BIBP 3226 and 3435. Eur. J. Pharmacol. 1995b;294:661–668. doi: 10.1016/0014-2999(95)00606-0. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., LUNDBERG J.M. Effects of the neuropeptide Y Y1 receptor antagonist SR 120107A on sympathetic vascular control in pigs in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:633–642. doi: 10.1007/BF00170839. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., MODIN A., LUNDBERG J.M. SR 120107A antagonizes neuropeptide Y Y1 receptor mediated sympathetic vasoconstriction in pigs in vivo. Eur. J. Pharmacol. 1996;305:145–154. doi: 10.1016/0014-2999(96)00164-1. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., WESTFALL T. XVI International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- MODIN A., PERNOW J., LUNDBERG J.M. Evidence for two neuropeptide Y receptors mediating vasoconstriction. Eur. J. Pharmacol. 1991;203:165–171. doi: 10.1016/0014-2999(91)90711-x. [DOI] [PubMed] [Google Scholar]
- PERNOW J., LUNDBERG J.M. Modulation of noradrenaline and neuropeptide Y (NPY) release in the pig kidney in vivo: involvement of alpha2, NPY and angiotensin II receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;340:379–385. doi: 10.1007/BF00167038. [DOI] [PubMed] [Google Scholar]
- PERNOW J., SARIA A., LUNDBERG J.M. Mechanisms underlying pre- and postjunctional effects of neuropeptide Y in sympathetic vascular control. Acta Physiol. Scand. 1986;126:239–249. doi: 10.1111/j.1748-1716.1986.tb07811.x. [DOI] [PubMed] [Google Scholar]
- POTTER E.K. Presynaptic inhibition of cardiac vagal postganglionic nerves by neuropeptide Y. Neurosci. Lett. 1987;83:101–106. doi: 10.1016/0304-3940(87)90223-0. [DOI] [PubMed] [Google Scholar]
- POTTER E.K., BARDEN J.A., MCCLOSKEY M.J.D., SELBIE L.A., TSENG A., HERZOG H., SHINE J. A novel neuropeptide Y analog, N-acetyl[Leu28Leu31]Neuropeptide Y(24-36), with functional specificity for the presynaptic (Y2) receptor. Eur. J. Pharmacol. 1994;267:253–262. doi: 10.1016/0922-4106(94)90148-1. [DOI] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A., WILLIM K.D., ENTZEROTH M., WIENEN W., BECK-SICKINGER A.G., DOODS H.N. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur. J. Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- SERRADEIL-LE GAL C., VALETTE G., ROUBY P.E., PELLET A., VILLANOVA G., FOULON L., LESPY L., NELIAT G., CHAMBON J.P., MAFFRAND J.P., LE FUR G. SR 120107A and SR 120819A: two potent and selective orally-effective antagonists for NPY Y1 receptors. Soc. Neurosci. Abstr. 1994;20:907. [Google Scholar]
- SMITH-WHITE M.A., HARDY T.A., BROCK J.A., POTTER E.K. Effects of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246, on Y2 receptors at peripheral neuroeffector junctions. Br. J. Pharmacol. 2001;132:861–868. doi: 10.1038/sj.bjp.0703879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK R.D. Conductance or resistance. Nature. 1968;217:779. doi: 10.1038/217779a0. [DOI] [PubMed] [Google Scholar]
- STJÄRNE L., LUNDBERG J.M., ÅSTRAND P. Neuropeptide Y- a co-transmitter with noradrenaline and adenosine-5′-triphosphate in the sympathetic nerves of the mouse vas deferens? A biochemical, physical and electropharmacological study. Neuroscience. 1986;18:151–166. doi: 10.1016/0306-4522(86)90184-3. [DOI] [PubMed] [Google Scholar]
- WAHLESTEDT C., YANAIHARA N., HÅKANSON R. Evidence for different pre- and post-junctional receptors for neuropeptide Y and related peptides. Regul. Pept. 1986;13:307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]


