Abstract
N-acylhomoserine lactones (AHLs) are small, diffusible signalling molecules, employed by Gram-negative bacteria to coordinate gene expression with cell population density. Recent in vitro findings indicate that AHLs may function as virulence determinants per se, through modification of cytokine production by eukaryotic cells, and by stimulating the relaxation of blood vessels.
In the present study, we assessed the influence of AHLs on cardiovascular function in conscious rats, and draw attention to the ability of the N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL), a signal molecule produced by P. aeruginosa, to cause marked bradycardia. This bradycardic effect was blocked by atropine and atenolol, and did not occur in vitro. Furthermore, modification of the acyl side chain length resulted in the loss of activity, whereas removal of the homoserine lactone ring, did not. The bradycardic effect of 3-oxo-C12-HSL was also observed in endotoxaemic animals, albeit attenuated.
In normal rats, 3-oxo-C12-HSL caused initial mesenteric and hindquarters vasoconstriction, but only slight, and delayed signs of vasodilatation in the renal and mesenteric vascular beds. Furthermore, administration of 3-oxo-C12-HSL (pre-treatment or 2 h post-treatment) together with LPS, did not modify the established regional haemodynamic effects of the LPS, 6 h after the onset of its infusion.
Our observations do not provide any clear evidence for an ability of 3-oxo-C12-HSL to modify the haemodynamic responses to LPS infusion. However, they are not inconsistent with the hypothesis that some of the cardiovascular sequelae of bacterial infection may be modulated by an influence of bacterial quorum sensing signalling molecules on the host.
Keywords: Bacterial physiology; bacteremia; bradycardia, cardiovascular system; vasoconstriction; vasodilatation
Introduction
One crucial feature of almost all bacterial infections is the need for the invading pathogen to reach a critical cell population density, sufficient to overcome host defences and establish the infection. Controlling the expression of virulence determinants, such as exotoxins, in concert with cell population density may, therefore, confer a significant survival advantage on the pathogen, such that the host is overwhelmed before a defence response can be fully initiated. Many bacterial pathogens are now known to regulate diverse physiological processes, including virulence, in a cell density-dependent manner through cell-cell communication (Salmond et al., 1995; Williams et al., 2000; de kievit & Iglewski, 2000). This phenomenon, which relies upon the interaction of a diffusible signal molecule with a sensor, or transcriptional regulator, to couple gene expression with cell population density, has become known as ‘quorum sensing' (de kievit & Iglewski, 2000). In Gram-negative bacteria, the most intensively investigated quorum sensing signal molecules are the N-acylhomoserine lactones (AHLs). AHLs have been identified with N-acyl side chains of 4, 6, 8, 10, 12 and 14 carbons, with either an oxo-, or hydroxy-, or no, substituent at the C3 position of the N-linked acyl chain (Swift et al., 1999). They are produced by bacteria, such as Pseudomonas aeruginosa, an opportunistic human pathogen, commonly responsible for respiratory tract infections in cystic fibrosis patients, as well as infections of blood, skin, eye and genitourinary tract in patients immunocompromised by surgery, cytotoxic drugs, or burn wounds. P. aeruginosa produces a wide variety of exoproducts, many of which contribute to its virulence (Williams et al., 2000; de kievit & Iglewski, 2000). It is now apparent that regulation of the genes for many P. aeruginosa virulence determinants is achieved via hierarchical quorum sensing, involving the transcriptional regulators, LasR and RhlR, and their cognate activators, N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-L-homoserine lactone (C4-HSL) (Winson et al., 1995; Latifi et al., 1996; Pearson et al., 1997).
Although AHLs have, so far, largely been considered as effectors of prokaryotic gene expression, a notable development in this area of research has come with the finding that AHLs are capable of influencing eukaryotic cell behaviour (di mango et al., 1995; Telford et al., 1998; Saleh et al., 1999). For example, in murine and human leukocyte immunoassays in vitro, 3-oxo-C12-HSL inhibited lymphocyte proliferation and tumour necrosis factor alpha (TNF-α) production by lipopolysaccharide (LPS)-stimulated macrophages (Telford et al., 1998). These observations raise the possibility that AHLs may act both as bacterial signalling molecules, and as virulence determinants per se. More recently, Lawrence et al. (1999) showed that 3-oxo-C12-HSL inhibited vasoconstrictor tone in porcine coronary arteries and, to a lesser extent, pulmonary arteries, and suggested that, in vivo, 3-oxo-C12-HSL might exert regional haemodynamic effects of benefit to the invading bacteria (Lawrence et al., 1999).
In this context, the first objective of the present work was to assess the regional haemodynamic effects of 3-oxo-C12-HSL in conscious rats. Our second objective was to determine whether or not 3-oxo-C12-HSL influenced the cardiovascular changes associated with experimental endotoxaemia, achieved by infusion of LPS in conscious rats, since, in this model, we have evidence that cytokines, such as TNF-α, may contribute to the haemodynamic sequelae (Gardiner et al., 1999). An unexpected finding in our initial studies was a striking effect of 3-oxo-C12-HSL on heart rate. Therefore, follow-up, preliminary experiments were performed to assess the effects of a series of AHLs on heart rate and blood pressure in vivo, and the actions of 3-oxo-C12-HSL on the heart in vitro. Some of the results reported here have been presented to the British Pharmacological Society (Gardiner et al., 2000).
Methods
Synthesis of AHLs
AHLs (Figure 1) were synthesized using the general method described previously (Chhabra et al., 1992). Each synthetic AHL was purified to homogeneity by preparative HPLC, and its structure confirmed by mass spectrometry and proton NMR spectroscopy.
Figure 1.
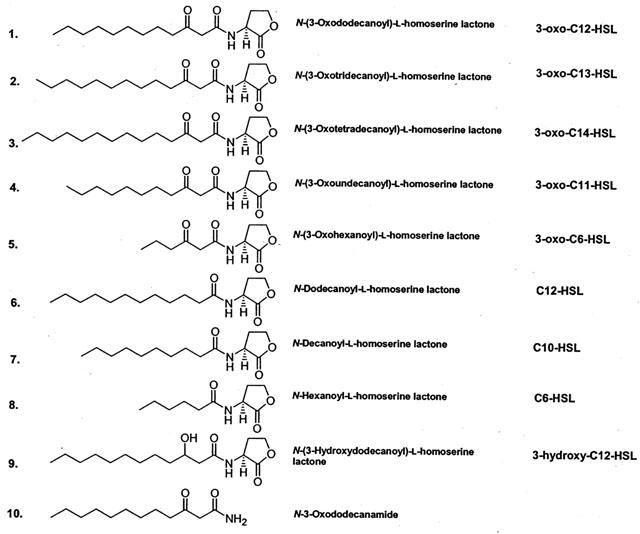
Structures of the compounds used in the present study.
In vivo studies
All experiments were carried out on conscious, male, Long Evans rats (350–450g), bred in the Biomedical Services Unit, University of Nottingham. Animals were housed under a 12 h light/dark cycle with lights on from 0600 to 1800 h. The project licence under which these experiments were performed was approved by Nottingham University Ethical Review Committee.
Under anaesthesia (sodium methohexitone, 40–60 mg kg−1 i.p., supplemented as required), miniaturized pulsed Doppler flow probes were implanted around the left renal and superior mesenteric arteries, and around the distal abdominal aorta (at a level to permit assessment of flow to the hindquarters). Animals recovered in their home cages for at least 14 days, with free access to food and water. Thereafter, under anaesthesia (as above), catheters were implanted in the distal abdominal aorta (via the caudal artery) and the right jugular vein. The former allowed continuous monitoring of intra-arterial blood pressures and heart rate, and the latter were used for administration of substances. Experiments were not begun until at least 24 h after catheter placement, with animals fully conscious and freely-moving, having free access to food and water. All surgical and technical procedures have been published in detail previously (Gardiner et al., 1995a).
For some experiments, animals were instrumented with catheters only (procedure as above).
Regional haemodynamic effects of 3-oxo-C12-HSL in normal animals
After a period of baseline recordings, rats (n=9 in each group), were given 3-oxo-C12-HSL (up to 10 mg kg−1) or the vehicle (50/50 acetonitrile/dextrose) i.v. in a volume of 0.1 ml, flushed in with 0.1 ml of isotonic saline. Continuous recordings of cardiovascular variables were made over the following 60 min, using a custom-designed microprocessor (University of Limburg, Maastricht, Netherlands) sampling every 2 ms, averaging per cardiac cycle, and storing to disc every 5 s.
Regional haemodynamic effects of 3-oxo-C12-HSL in endotoxaemic animals
Endotoxaemia was induced by a continuous infusion of lipopolysaccharide (LPS, E. coli serotype 0127:B8; Sigma, U.K.) as described previously (Gardiner et al., 1995a). Rats were given 3-oxo-C12-HSL (10 mg kg−1, as above), either 15 min before (n=3) or 2 h after (n=8) the onset of LPS infusion (see Discussion). As a control for these experiments, rats (n=8) were given LPS infusion alone. Measurements were made (as above) for 6 h after the start of the LPS infusion.
Effects on 3-oxo-C12-HSL analogues on blood pressure and heart rate
AHLs differing in N-acyl side chain length and C3 substituent (Figure 1) were administered at a dose of 10 mg kg−1 (n=2–4 in each group); the vehicle was as for 3-oxo-C12-HSL (0.1 ml of 50/50 acetonitrile/dextrose) except for N-3(hydroxydodecanoyl)-L-homoserine lactone (3-hydroxy-C12-HSL), which, due to poor solubility, was administered in 0.15 ml acetonitrile.
Effects of cardiac autonomic blockade on the cardiovascular effects of 3-oxo-C12-HSL
The experiments described above showed a striking bradycardic effect of 3-oxo-C12-HSL (see Results). Therefore, in three animals (instrumented only for the measurement of blood pressure and heart rate), the effects of 3-oxo-C12-HSL on blood pressure and heart rate were measured 30 min after combined administration of atropine and atenolol (1 mg kg−1 bolus; 1 mg kg−1 h−1 infusion for both), to block cardiac autonomic efferent control (Widdop et al., 1992).
In vitro studies
Male, Long Evans rats (n=3) were killed by stunning and cervical dislocation. Spontaneously beating right atria were dissected and placed in jacketed 20 ml organ baths. The baths contained Krebs' physiological salt solution of the following composition (mM); NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4.7H2O 1.2, NaHCO3 25, NaH2PO4.2H2O 0.9 and glucose 11.1, kept at 37°C and gassed with 95%O2 and 5%CO2. Atria were suspended under 1 g tension and contractions were recorded through a Grass force displacement transducer attached to a Grass polygraph, with a Grass tachograph to measure the frequency of contractions. A stock solution of 3-oxo-C12-HSL (10−2 M) was prepared in acetonitrile, and further dilutions were made with distilled water. A range of concentrations was tested, from 1×10−6 M up to 3×10−5 M (Lawrence et al., 1999).
Statistical analyses
Within-group analysis of the haemodynamic data was by Friedman's test (Theodorsson-Norheim, 1987), and between group-analysis was by the Mann-Whitney U-test, or Kruskal-Wallis test, as appropriate, applied to the integrated responses (i.e. areas under or over the curves); a P value <0.05 was taken as significant. Values are given as mean±s.e.mean.
Drugs
Atropine methyl nitrate and atenolol were obtained from Sigma (U.K.). They were dissolved in sterile saline and injected in a volume of 0.1 ml.
Results
In vivo studies
Regional haemodynamic effects of 3-oxo-C12-HSL in normal animals
There were no differences between the resting cardiovascular variables in the two groups of animals used in this part of the study (Figure 2). Administration of the vehicle caused a transient tachycardia, a modest rise in arterial blood pressure, and a short-lived increase in hindquarters vascular conductance (Figure 2), whereas administration of 3-oxo-C12-HSL (10 mg kg−1) caused pronounced bradycardia, a rise in mean arterial blood pressure, and falls in mesenteric and hindquarters vascular conductances, with a delayed fall in renal vascular conductance (Figure 2). Ten minutes following 3-oxo-C12-HSL administration, the bradycardia and hindquarters vasoconstriction were still present, but the changes in renal and mesenteric vascular conductances had waned (Figure 2). The integrated (0–10 min) effects of 3-oxo-C12-HSL on heart rate, and on mesenteric and hindquarters vascular conductances, were significantly different from the changes seen with administration of the vehicle (Figure 2). Sixty minutes following administration of 3-oxo-C12-HSL, heart rate and hindquarters vascular conductance had returned to baseline values. At that juncture, there was a modest hypotension, and renal and mesenteric vasodilatation (Figure 2).
Figure 2.
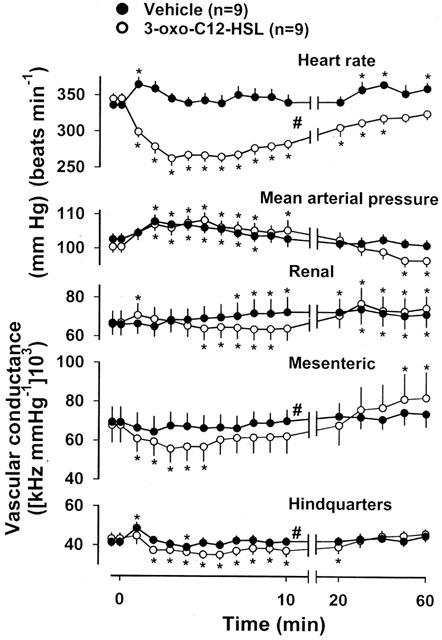
Resting cardiovascular variables, and the changes evoked by administration (at time=0 min) of vehicle or 3-oxo-C12-HSL in conscious, Long Evans rats. Values are mean and vertical bars show s.e.mean; *P<0.05 versus original baseline (Friedman's test), # P<0.05 for integrated response (area under or over curve 0–10 min) 3-oxo-C12-HSL versus vehicle (Mann-Whitney U test).
The effects of a lower dose (2.5 mg kg−1) of 3-oxo-C12-HSL were measured in three naive animals. The bradycardic effect was less marked than with the higher dose (maximum fall=−43±15 beats min−1 at 8 min), as was the fall in mesenteric vascular conductance (−8±6% at 6 min), although the fall in hindquarters vascular conductance was similar (−27±1% at 8 min).
Regional haemodynamic effects of 3-oxo-C12-HSL in endotoxaemic animals
The cardiovascular changes seen during infusion of LPS alone were as described previously (Gardiner et al., 1995a). Six hours after the start of LPS infusion, mean arterial blood pressure and heart rate were not different from the original baseline values (change from baseline=0±3 mmHg and +8±16 beat min−1, respectively), renal vascular conductance was significantly elevated (+58±6%), mesenteric vascular conductance was unchanged (+4±6%) and hindquarters vascular conductance was significantly reduced (−16±4%).
Administration of 3-oxo-C12-HSL, 15 min prior to the onset of LPS infusion, did not affect the subsequent changes in blood pressure, or renal, or mesenteric vascular conductances. The bradycardic and hindquarters vasoconstrictor effects of 3-oxo-C12-HSL were still apparent when the LPS infusion was started (−75±14 beats min−1 and −24±1%, respectively), but the ensuing LPS-induced profile of changes in these variables was not different from that of the group receiving LPS alone. Thus, at the end of the 6 h period of LPS infusion in animals pre-treated with 3-oxo-C12-HSL, there was a significant increase in renal vascular conductance (+54±12%), and a significant decrease in hindquarters vascular conductance (−15±6%), neither of which differed from the corresponding changes observed in animals receiving LPS alone (see above).
Two hours after the start of LPS infusion, immediately before administration of 3-oxo-C12-HSL, there was a modest hypotension (−12±3 mmHg) and a marked increase in renal vascular conductance (+91±10%). The corresponding changes in the group that received LPS alone were −12±2 mmHg and +75±10%; in both groups, the changes were similar to those described previously (Gardiner et al., 1995a). The initial (0–10 min) effects of 3-oxo-C12-HSL in animals infused with LPS for 2 h are shown in Figure 3, together with the data from normal animals (from Figure 2). For comparison, data from the group receiving LPS alone are also shown (Figure 3). There was a clear bradycardic effect of 3-oxo-C12-HSL in the LPS-infused animals, although the integrated (0–10 min) change was significantly less than in the normal animals. A rise in mean arterial blood pressure, and falls in renal and hindquarters vascular conductances, occurred in both groups of animals given 3-oxo-C12-HSL, but not in the animals receiving LPS alone. Indeed, in the latter group, there was a rise in renal vascular conductance over the 10 min period. The reduction in mesenteric vascular conductance, seen following 3-oxo-C12-HSL administration in the absence of LPS, did not occur in the presence of LPS, whereas there was a rise in the mesenteric vascular conductance in the group receiving LPS alone.
Figure 3.
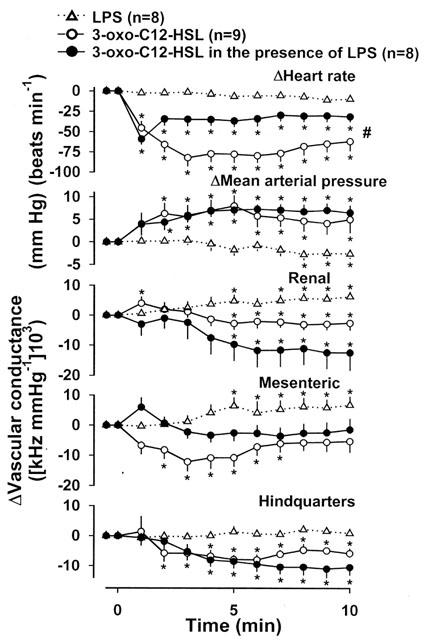
Changes in cardiovascular variables in conscious, Long Evans rats receiving LPS alone, or 3-oxo-C12-HSL alone, or 3-oxo-C12-HSL, 2 h after the onset of infusion of LPS. Values are mean and vertical bars show s.e.mean; * P<0.05 versus value shown at time=0 min (Friedman's test), # P<0.05 for integrated response (area under or over curve 0–10 min) to 3-oxo-C12-HSL in normal versus LPS-infused rats (Kruskal-Wallis test).
The initial effects of 3-oxo-C12-HSL, described above, did not persist, and hence, 6 h after the start of LPS infusion, in animals given 3-oxo-C12-HSL at the 2 h time-point, the increase in renal vascular conductance (+71±13%) and decrease in hindquarters vascular conductance (−21±3%) were similar to the changes observed in the other two groups of LPS-infused animals (see above).
Cardiovascular effects of different AHLs
Figure 4 shows the effects of representative examples from a series of AHLs differing in N-acyl side chain length, and in the presence or absence of a C3 substituent (oxo or hydroxy) on blood pressure and heart rate. There were falls in heart rate following administration of N-(3-oxoundecanoyl)-L-homoserine lactone (3-oxo-C11-HSL) and N-dodecanoyl-L-homoserine lactone (C12-HSL); in addition, C12-HSL caused a substantial fall in blood pressure. N-decanoyl-L-homoserine lactone (C10-HSL), and compounds with shorter N-acyl side chains, and AHLs with 13 and 14 carbon side chains, had no clear bradycardic effects (data not shown). Interestingly, N-oxododecanamide (Figure 1), which lacks the homoserine lactone ring, caused bradycardia and hypotension (like C12-HSL) (Figure 4). Although only two animals were given N-oxododecanamide, its bradycardic effect was similar (nadirs −95 and −101 beats min−1).
Figure 4.
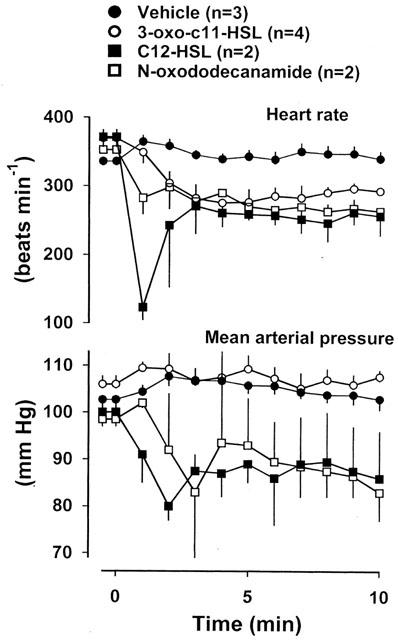
Resting values for heart rate and mean arterial blood pressure and changes in response to vehicle, 3-oxo-C11-HSL, C12-HSL, or N-oxododecanamide, administered at time=0 min, in conscious Long Evans rats. Values are mean and vertical bars show s.e.mean.
Effects of cardiac autonomic blockade on the cardiovascular effects of 3-oxo-C12-HSL
In the presence of atenolol and atropine, the bradycardic effect of 3-oxo-C12-HSL was totally abolished, but the rise in blood pressure (as seen with the vehicle) was unchanged (Figure 5).
Figure 5.
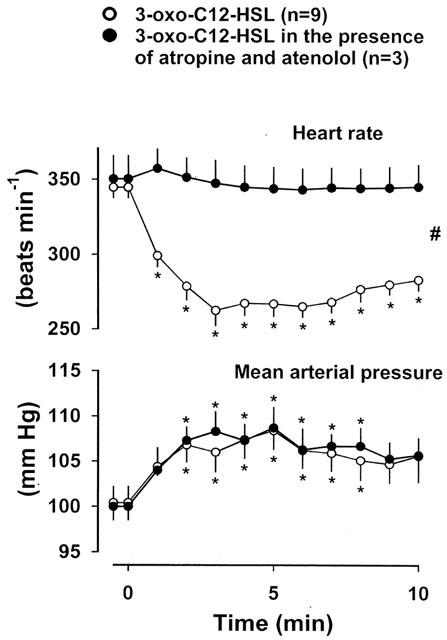
Changes in heart rate and mean arterial blood pressure in conscious Long Evans rats given 3-oxo-C12-HSL (at time=0 min) in the absence (data from Figure 2) or presence of atropine and atenolol. Values are mean and vertical bars show s.e.mean; *P<0.05 versus original baseline (Friedman's test), # P<0.05 for integrated response (area over curve 0–10 min) in the absence and presence of atropine and atenolol (Mann-Whitney U test).
In vitro studies
At concentrations from 1×10−6 to 3×10−5 M, the effects of 3-oxo-C12-HSL on right atrial frequency were small and inconsistent (n=3, maximum change=−28, −2 and −4 beats min−1).
Discussion
The major aims of the present study were to assess the haemodynamic effects of 3-oxo-C12-HSL in conscious rats, and to determine whether or not 3-oxo-C12-HSL influenced the cardiovascular sequelae of experimental endotoxaemia.
Cardiovascular effects of 3-oxo-C12-HSL in normal animals
The most striking, and unexpected, action of 3-oxo-C12-HSL in normal animals was its profound bradycardic effect. Although full dose-response studies were not performed, the findings indicate that the response was dose-dependent, with a fall of about 100 beats min−1 at the highest dose tested (10 mg kg−1; higher doses were not administered, due to problems with solubility). We have previously measured the cardiovascular effects of the novel bradycardic agent, S16257, under the same conditions as in the present experiments (Gardiner et al., 1995b). In those studies the fall in heart rate, which was of a similar degree to that observed with 3-oxo-C12-HSL, was accompanied by a modest reduction in mean arterial blood pressure, and by peripheral vasoconstriction, particularly in the mesenteric and hindquarters vascular beds (Gardiner et al., 1995b). There, we suggested the vasoconstrictor responses were due to reflex neurohumoral activation following a fall in cardiac index, and we would offer the same explanation for the effects associated with 3-oxo-C12-HSL although, notably, there was no overt fall in mean arterial blood pressure. Interestingly, we did not find any clear evidence for a vasorelaxant effect of 3-oxo-C12-HSL, in contrast to its actions in vitro (Lawrence et al., 1999). However, for the reasons outlined above, it is feasible that any direct vasorelaxation was offset by neurohumorally-mediated vasoconstriction. In this context it is notable that CGRP, which exerts marked mesenteric vasodilator effects in vitro (Marshall et al., 1986), causes significant mesenteric vasoconstriction in vivo (Gardiner et al., 1989), probably for the reasons given above. However, it is also apparent that some agonists, such as urotensin II, cause mesenteric vasodilatation both in vitro and in vivo (Gardiner et al., 2001), possibly because they cause less activation of vasoconstrictor mechanisms, such as the renin-angiotensin system (e.g., Gardiner et al., 1994).
Specific bradycardic agents, like S16257 (Thollon et al., 1994) and zatebradine (Kobinger & Lillie, 1984), are believed to act at the If channel in pacemaker tissue, and have proved problematic in clinical use due to their effects on vision (Frishman et al., 1995). Our findings that the bradycardic effect of 3-oxo-C12-HSL was blocked by combined treatment with atropine and atenolol in vivo, and was not straightforwardly reproduced in vitro, indicate its mechanism of action is not directly at the If channel, but is likely due to modulation of vagal and/or sympathetic function. Our present experiments do not permit us to dissect the mechanism(s) further. Acute administration of the β-adrenoceptor antagonist, propranolol, to conscious rats causes a fall in heart rate of a similar magnitude to that observed with 3-oxo-C12-HSL (Struyker-Boudier et al., 1979) with no fall in blood pressure, since there is peripheral vasoconstriction (Struyker-Boudier et al., 1979; Hatzinikolaou et al., 1983; Gardiner & Bennett, 1988). Thus, we cannot dismiss the possibility that 3-oxo-C12-HSL was acting at cardiac β-adrenoceptors, although, in our experience, β-adrenoceptor antagonists tend to reduce heart rate variability, and that was not observed in the present experiments (unpublished observations).
If 3-oxo-C12-HSL was affecting vagal activity to slow the heart, this could occur at a number of different levels. Clearly, it was not acting as a muscarinic agonist, since such an effect should have been seen in vitro, whereas, in the isolated, spontaneously beating right atrial preparations we studied, the compound had no consistent bradycardic effect. While it could be suggested that a failure to detect a bradycardic action under those conditions might have been due to a relatively low resting rate in vitro, the latter variable was not related to the change in heart rate seen on addition of 3-oxo-C12-HSL to the organ bath. Thus, the bradycardic responses to the compound in the three preparations studied were −28, −2, and −4 beats min−1, whereas the resting rates were 160, 232 and 220 beats min−1, respectively. It is feasible that the bradycardic effect of 3-oxo-C12-HSL in vivo was due to augmented vagal efferent tone, secondary to central and/or afferent activation. Marked vagal bradycardia is associated with cardiopulmonary afferent activation (i.e., the Bezold-Jarisch reflex (Veelken et al., 1990)), but the time-course of that effect is extremely rapid and transient, whereas the effects of 3-oxo-C12-HSL were relatively long-lasting. So, if the bradycardic effect of the compound was due to vagal afferent activation, then the process clearly differs from a classical Bezold-Jarisch reflex.
A recent study showed an inhibitory effect of 3-oxo-C12-HSL on purine receptor expression and effects in tracheal gland cells (Saleh et al., 1999). There are a number of ways in which purines can affect the heart (see Ralevic & Burnstock (1998) for review), but inhibition of P2Y-mediated effects would be expected to increase, rather than decrease, heart rate, and, furthermore, such an effect would be independent of the autonomic nerves. Moreover, the studies of Saleh et al. (1999) reported effects of N-(3-oxohexanoyl)-L-homoserine lactone (3-oxo-C6-HSL) at similar concentrations to 3-oxo-C12-HSL whereas, in the conscious rat, 3-oxo-C6-HSL had no effect on heart rate. Therefore, we consider that interference with purine receptor-mediated events is an unlikely explanation for the bradycardic action of 3-oxo-C12-HSL.
The studies we performed with different AHLs demonstrated a clear influence of the carbon chain length on the effects observed. Thus, C12-HSL (12 carbon chain, like 3-oxo-C12-HSL) and 3-oxo-C11-HSL (11 carbon chain) were the only other AHLs with any reproducible bradycardic effects. However, it appears that the homoserine lactone ring is not essential, since N-3-oxododecanamide exerted clear bradycardic effects.
A full profiling of the bradycardic actions of the AHLs, and detailed analysis of the direct and/or indirect mechanisms involved will require experiments utilising specific antagonists in vivo, with their appropriate controls. As mentioned above, it appears that 3-oxo-C12-HSL might elicit bradycardia through mechanisms other than an action at the If channel, thus more soluble derivatives of this compound could be of therapeutic interest.
Cardiovascular effects of 3-oxo-C12-HSL in endotoxaemic animals
Since Telford et al. (1998) had shown an inhibitory effect of 3-oxo-C12-HSL on TNF-α production by LPS-stimulated macrophages, and since TNF-α may play a role in the haemodynamic effects of endotoxaemia (Gardiner et al., 1999), we hypothesised that 3-oxo-C12-HSL would influence the cardiovascular sequelae of LPS infusion. It is clear from our results that 3-oxo-C12-HSL, given either before the onset of the LPS infusion, or after 2 h, when plasma levels of TNF-α are high (Waller et al., 1995), had no effect on the established haemodynamic changes seen 6 h after the onset of LPS infusion. These findings can be interpreted in several ways, and further experimentation would be needed to address the various issues. One possibility is that 3-oxo-C12-HSL did not inhibit TNF-α production. Alternatively, it is feasible that TNF-α production was inhibited, but this did not influence the cardiovascular profile, since it is but one of many cytokines released under these conditions. From previous experiments, we know that administration of antibodies to TNF-α alone, or antibodies to TNF-α together with antibodies to IL-1β, do not greatly influence the haemodynamic effects of LPS in this model of endotoxaemia (Waller et al., 1995; Gardiner et al., 1998). However, we have also shown that inhibition of TNFα and IL-1β production, with FR 167653, does affect the early hypotensive effects of LPS (Gardiner et al., 1999). Thus, if 3-oxo-C12-HSL had inhibited cytokine production, we might have expected to see a similar inhibitory effect on the early fall in blood pressure, but that was not the case. Rather than inhibiting the effects of LPS, it is equally possible that 3-oxo-C12-HSL may have influenced host cell function in a way as to enhance the action of LPS. However, from our experiments, we could find no evidence for that. Clearly the use of different bacterial insults, and different AHLs, may reveal such interactions.
In the context of the LPS experiments, we noted that the bradycardic action of 3-oxo-C12-HSL, whilst still present, was less pronounced than in the normal animals. One possible explanation for this observation is that the extent of bradycardia depends on the existing autonomic tone, which would be expected to be altered during the LPS infusion (Gardiner et al., 1995a). However, it appears that the bradycardic action of 3-oxo-C12-HSL is not simply related to resting heart rate (see earlier).
Putative role of the cardiovascular actions of quorum sensing molecules in the pathogenicity of bacteria
As outlined in the Introduction, the starting point for this work was the finding that 3-oxo-C12-HSL can influence eukaryotic cells in vitro. Notably, the finding that this compound is able to inhibit vasoconstrictor tone in isolated blood vessels led Lawrence et al. (1999) to suggest that, in vivo, 3-oxo-C12-HSL might exert regional haemodynamic effects that would be beneficial to the invading bacteria producing this molecule. In this context, two important questions need to be answered, namely, are the doses of AHLs used here representative of the amounts produced by invading bacteria, and, more fundamentally, are AHLs produced in vivo during bacterial infection? There is evidence from in vitro systems that P. aeruginosa can produce 3-oxo-C12-HSL in amounts sufficient to raise its concentration to around 5 μM in the culture medium (Pearson et al., 1995). Furthermore, there is some evidence for AHL production in vivo (Williams et al., 2000). However, possibly for the reasons considered above, our results do not provide clear evidence to support the notion that production of 3-oxo-C12-HSL by invading bacteria could act to ensure availability of nutrients by increasing organ blood flow (Lawrence et al., 1999). Nonetheless, it is feasible that 3-oxo-C12-HSL (and other quorum sensing molecules) might exert important effects on the microcirculation, in the absence of overt effects on regional haemodynamics (because of activation of counter-regulatory systems; see above), Indeed, one could argue that an ability of AHLs to manipulate micro-haemodynamics stealthily, i.e., without significant actions on the systemic circulation (which might unnecessarily compromise the host) would be particularly beneficial to the pathogen. The possibility of microcirculatory changes in the absence of systemic effects could be assessed by determination of sub-regional blood flow distribution using coloured microspheres (Kemp et al., 1999).
In summary, we report here a remarkable bradycardic effect of 3-oxo-C12-HSL in conscious rats, which may be of relevance to many clinical conditions in which bacterial infection has been implicated in cardiovascular dysfunction.
Acknowledgments
Thanks to Mavis Daykin for technical assistance (HPLC purification of synthetic AHLs), to Phil Kemp and Julie March (haemodynamic studies), and to Dr Vince Wilson for his helpful comments on a draft of the manuscript. Work in the authors' laboratories is supported by grants from the Medical Research Council U.K. and the BHF, which are gratefully acknowledged.
Abbreviations
- AHLs
N-acyhomoserine lactones
- C4-HSL
N-butanoyl-L-homoserine lactone
- C12-HSL
N-dodecanoyl-L-homoserine lactone
- LPS
Lipopolysaccharide
- 3-oxo-C6-HSL
N-(3-oxohexanoyl)-L-homoserine lactone
- 3-oxo-C11-HSL
N-(3-oxoundecanoyl)-L-homoserine lactone
- 3-oxo-C12-HSL
N-(3-oxododecanoyl)-L-homoserine lactone
- TNF-α
Tumour-necrosis factor-α
References
- CHHABRA S.R., STEAD P., BAINTON N.J., SALMOND G.P.C., STEWART G.S.A.B., WILLIAMS P., BYCROFT B.W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone. J. Antibiot. 1992;46:441–447. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- DE KIEVIT T.R., IGLEWSKI B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MANGO E., ZUR H.J., BRYAN R., PRINCE A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRISHMAN W.H., PEPRINE C.J., WEISS R.J., BAIKER W.M. Addition of zatebradine, a direct sinus node inhibitor, provides no greater exercise tolerance benefit in patients with angina taking extended-release nifedipine: results of a multicenter, randomized, double-blind, placebo-controlled, parallel group study. J. Am. Coll. Cardiol. 1995;26:305–312. doi: 10.1016/0735-1097(95)80000-7. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., BENNETT T. Regional hemodynamic responses to adrenoceptor antagonism in conscious rats. Am. J. Physiol. 1988;255:H813–H824. doi: 10.1152/ajpheart.1988.255.4.H813. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T. Regional haemodynamic effects of calcitonin gene-related peptide. Am. J. Physiol. 1989;256:R332–R338. doi: 10.1152/ajpregu.1989.256.2.R332. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., HARTY C., WILLIAMS P., PRITCHARD D., BYCROFT B.W., BENNETT T. N-(3-oxododecanoyl)-L-homoserine lactone causes bradycardia in conscious rats. Br. J. Pharmacol. 2000;129:47P. doi: 10.1038/sj.bjp.0704174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Cardiac and regional haemodynamics, inducible nitric oxide synthase (NOS) activity, and the effects of NOS inhibitors in conscious, endotoxaemic rats. Br. J. Pharmacol. 1995a;116:2005–2016. doi: 10.1111/j.1476-5381.1995.tb16405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Acute and chronic cardiac and regional haemodynamic effects of the novel bradycardic agent, S16257, in conscious rats. Br. J. Pharmacol. 1995b;115:579–586. doi: 10.1111/j.1476-5381.1995.tb14971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Influence of FR 167653, an inhibitor of TNF-α and IL-1, on the cardiovascular responses to chronic infusion of lipopolysaccharide in conscious rats. J. Cardiovasc. Pharmacol. 1999;34:64–69. doi: 10.1097/00005344-199907000-00011. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., WOOLLEY J., BENNETT T. The influence of antibodies to TNF-α and IL-Iβ on haemodynamic responses to the cytokines, and to lipopolysaccharide, in conscious rats. Br. J. Pharmacol. 1998;125:1543–1550. doi: 10.1038/sj.bjp.0702250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., DAVENPORT A.P., BENNETT T. Depressor and regionally-selective vasodilator effects of human and rat urotensin II in conscious rats. Br. J. Pharmacol. 2001;132:1625–1629. doi: 10.1038/sj.bjp.0704051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., RAKHIT T., KEMP P.A., MARCH J.E., BENNETT T. Regional haemodynamic responses to pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide in conscious rats. Br. J. Pharmacol. 1994;111:589–597. doi: 10.1111/j.1476-5381.1994.tb14778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATZINIKOLAOU P., CHAROCOPOS F., GAVRAS I., GAVRAS H. Systemic and regional hemodynamic effects of propranolol in intact and anephric rats. Clin. Exp. Hypertens. 1983;A5:729–739. doi: 10.3109/10641968309081804. [DOI] [PubMed] [Google Scholar]
- KEMP P.A., GARDINER S.M., MARCH J.E., RUBIN P.C., BENNETT T. Assessment of the effects of endothelin-1 and magnesium sulphate on regional blood flows in conscious rats, by the coloured microsphere reference technique. Br. J. Pharmacol. 1999;126:621–626. doi: 10.1038/sj.bjp.0702342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBINGER W., LILLIE C. Cardiovascular characterization of UL-FS 49, 1,3,4,5-tetrahydro-7,8-dimethoxy-3-[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylimino]propyl]-2H-3-benzapin-2- on hydrochloride, a new “specific bradycardic agent”. Eur. J. Pharmacol. 1984;104:9–18. doi: 10.1016/0014-2999(84)90363-7. [DOI] [PubMed] [Google Scholar]
- LATIFI A., FOGLINO M., TANAKA K., WILLIAMS P., LAZDUNSKI A. A hierarchical quorum sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary phase sigma factor RpoS. Mol. Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- LAWRENCE R.N., DUNN W.R., BYCROFT B.W., CAMARA M., CHHABRA S.R., WILLIAMS P., WILSON V.G. The Pseudomonas aeruginosa quorum sensing signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone inhibits porcine arterial smooth muscle contraction. Br. J. Pharmacol. 1999;128:845–848. doi: 10.1038/sj.bjp.0702870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL I., AL-KAZWINI S.J., HOMAN J.J., CRAIG R.K. Human and rat α-CGRP but not calcitonin cause mesenteric vasodilatation in rats. Eur. J. Pharmacol. 1986;123:217–222. doi: 10.1016/0014-2999(86)90662-x. [DOI] [PubMed] [Google Scholar]
- PEARSON J.P., GRAY K.M., PASSADOR L., TUCKER K.D., EBERHARD A., IGLWESKI B.H., GRENNBERG E.P. A second N-acyl homoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 1995;192:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON J.P., PESCI E.C., IGLEWSKI B.H. Roles of Pseudomonas aeruginosa las and rhl quorum sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:415–492. [PubMed] [Google Scholar]
- SALEH A., FIGARELLA C., KAMMOUNI W., MARCHAND-PINATEL S., LAZDUNSKI A., TUBUL A., BRUN P., MERTEN M.D. Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone inhibits expression of P2Y receptors in cystic fibrosis tracheal gland cells. Infect. Immun. 1999;67:5076–5082. doi: 10.1128/iai.67.10.5076-5082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALMOND G.P.C., BYCROFT B.W., STEWART G.S.A.B., WILLIAMS P. The bacterial enigma: cracking the code of cell-cell communication. Mol. Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- STRUYKER-BOUDIER H.A.J., SMITS J.F., VAN ESSEN J. The role of the baro-receptor reflex in the cardiovascular effects of propranolol in the conscious spontaneously hypertensive rat. Clin. Sci. 1979;56:163–167. doi: 10.1042/cs0560163. [DOI] [PubMed] [Google Scholar]
- SWIFT S., WILLIAMS P., STEWART G.S.A.B.N-acylhomoserine lactones and quorum sensing in the proteobacteria Cell-cell Signaling in Bacteria 1999Washington D.C., ASM Press; 291–313.ed. Dunny G.M. and Winans S.C [Google Scholar]
- TELFORD G., WHEELER D., WILLIAMS P., TOMKINS P.T., APPLEBY P., SEWELL H., STEWART G.S.A.B., BYCROFT B.W., PRITCHARD D.I. The Pseudomonas aeruginosa quorum sensing signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect. Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODORSSON-NORHEIM E. Friedman and Quade tests: BASIC computer program to perform non-parametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput. Biol. Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- THOLLON C., CAMBARRAT C., VIAN J., PROST J.-F., RELGION J.L., VILAINE J.P. Electrophysiological effects of S16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br. J. Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEELKEN R., SAWIN L.L., DIBONA G.F. Epicardial 5-HT3 receptors in circulatory control in conscious Sprague Dawley rats. Am. J. Physiol. 1990;258:H466–H472. doi: 10.1152/ajpheart.1990.258.2.H466. [DOI] [PubMed] [Google Scholar]
- WALLER J., GARDINER S.M., JOSE J., BENNETT T. Lack of effect of TNF antibodies on the cardiovascular sequelae of lipopolysaccharide infusion in conscious rats. Br. J. Pharmacol. 1995;116:2487–2495. doi: 10.1111/j.1476-5381.1995.tb15100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDOP R.E., GARDINER S.M., KEMP P.A., BENNETT T. The influence of atropine and atenolol on the cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br. J. Pharmacol. 1992;105:653–656. doi: 10.1111/j.1476-5381.1992.tb09034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS P., CAMARA M., HARDMAN A., SWIFT S., MILTON D., HOPE V.J., WINZER K., MIDDLETON B., PRITCHARD D.I., BYCROFT B.W. Quorum sensing and the population-dependent control of virulence. Phil. Trans. Roy. Soc. Lond. Series B. Biol. Sci. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINSON M.K., CAMARA M., LATIFI A., FOGLINO M., CHHABRA S.R., DAYKIN M., BALLY M., CHAPON V., SALMOND P.C., BYCROFT B.W., LAZDUNSKI A., STEWART G.S.A.B., WILLIAMS P. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]


