Abstract
Calcineurin is a ubiquitous calcium/calmodulin dependent protein phosphatase that has been shown to regulate the activity of ion channels, glutamate release, and synaptic plasticity. In the present study we show that CsA, a specific inhibitor of calcineurin, affects the survival of cultures developed from hippocampal dentate gyrus. Mixed neuronal-glial cultures exposed to 8–40 μM CsA undergo cell death characterized by apoptotic changes in cellular and nuclear morphology.
TUNEL-positive staining was observed only in neurons that developed pyknotic morphology after treatment with 8 μM CsA for 24–72 h.
Immunocytochemical staining with an anti-GFAP monoclonal antibody revealed that astrocytes from mixed neuronal/glial cultures were unaffected by exposure to CsA at doses toxic for neurons and all TUNEL-positive cells were neurons.
MK-801, a noncompetitive inhibitor of glutamate receptor, does not inhibit the appearance of TUNEL-positive neurons and apoptotic changes in nuclear morphology.
Preincubation of cells with 8 μM CsA increased basal intracellular calcium level in time dependent manner and decreased relative calcium response to glutamate. Application of 1 μM MK-801 had no effect on CsA-induced changes in Ca2+ level.
Our findings suggest that the neuronal death after CsA treatment is not a result of glutamate excitotoxicity and the increase in intracellular calcium concentration in neurons is not dependent on calcium influx via NMDA channel.
Keywords: Immunosuppressant, neurotoxicity, calcineurin, neuronal cell death, apoptosis, glutamate excitotoxicity, MK-801
Introduction
Calcineurin is a ubiquitous calcium-activated serine phosphatase that plays an important role in the control of intracellular Ca+2 signalling (Guerini, 1997; Yakel, 1997). The enzyme is a target of important immunosuppressive drugs–cyclosporin A (CsA) and FK506 (Liu et al., 1991; Clipstone & Crabtree, 1992; Furman et al., 1992). Although both CsA and FK506 inhibit calcineurin, they form of complexes with different immunophilines: FK506 binds to FK506-binding protein, whereas CsA binds to cyclophilin A. The effects of immunosuppressants have been mostly characterized in lymphocytes, and have been ascribed to the drug-mediated inhibition of the phosphatase calcineurin that, in turn, results in the blockade of the transcription factor Nuclear Factor of Activated T cells (NF-AT) (Rao et al., 1997).
This phosphatase is important in neuronal signal transduction as well, given its abundance in nervous tissues and the necessity for tightly controlled calcium-regulated phosphorylation. The brain contains 3–10 higher concentrations of calcineurin then most studied tissues (Su et al., 1995) However, physiological function of high level of calcineurin expression in the brain, remains largely unknown. Protein dephosphorylation by calcineurin might play an important role in neuronal signal transduction due to its ability to regulate the activity of ion channels, glutamate release, and synaptic plasticity (Yakel, 1997). Calcineurin has been shown to regulate the activity of N-methyl-D-aspartate (NMDA) receptor channels by both altering their ion gating properties and promoting desensitization in cultured hippocampal neurons (Nichols et al., 1994; Lieberman & Mody, 1994; Tong, 1995; Victor et al., 1995; Sihra et al., 1995). In cultured foetal rat cortices, the inhibition of calcineurin with CsA (5–20 μM) increased the rate of spontanous neuronal firing, which have been ascribed to the enhanced release of glutamate by the presynaptic cells (Victor et al., 1995).
A considerable amount of evidence suggests that excitotoxic overactivation of glutamate receptors, especially those of the N-methyl-D-aspartate-type, contributes to neuronal cell death. Since N-methyl-D-aspartate receptors are highly permeable to Ca2+, influx of extracellular Ca2+ is considered to be the primary event responsible for glutamate toxicity (Leist & Nicotera, 1998). Although the mechanism of Ca2+-induced neurotoxicity is not completely understood, calcineurin, a Ca2+-regulated protein, may be associated with the neurotoxicity (Asai et al., 1999).
Despite the direct and indirect evidence that high level of calcineurin activity predisposes or mediates apoptosis in some neuronal cells (Asai et al., 1999), the role of calcineurin in the survival of nervous system cells remains controversial. We have demonstrated that CsA induces apoptosis of glioma cells (Mosieniak et al., 1997, 1998). CsA-induced apoptosis was associated with persistent activation of stress activated protein kinases–c-Jun N-terminal kinase (JNK), and p38 that results in the stabilization and activation of some pro-apoptotic proteins (Pyrzynska et al., 2000). CsA and FK506 also affect the survival of reactive astrocytes from striatal trauma (Pyrzynska et al., 2001) and foetal astrocytes (unpublished). The use of CsA for transplant patients is asscociated with serious side-effects involving neurotoxicity (Hauben, 1996). Moreover exposure of mouse cortical cultures to 20 μM CsA resulted in apoptosis of neurons (Mcdonald et al., 1996). Recently, a novel target of calcineurin myocyte enhancer factor 2 (MEF2) has been shown to mediate activity-dependent neuronal survival in cultured cerebellar granule neurons. In these cells, calcineurin participates in transmitting calcium signals mediating cell survival.
While the mechanisms underlying immunosuppressive effects of CsA are well known, mechanisms responsible for neurotoxicity remain to be established. We have studied the effects of CsA on survival of intact hippocampal dentate gyrus cultures, an involvement of glutamate toxicity and CsA ability to modulate intracellular calcium levels. Dentate gyrus cultures are very homogenous neuronal population of excitatory granule neurons.
Methods
Cell culture and treatment
Primary cultures of dentate gyrus were obtained from 5-day old rat pups using a modification of procedure described previously (Figiel & Kaczmarek, 1997a, 1997b). Cells were plated on poly-L-lysine coated glass coverslips at the concentration of 50 cells/mm2 and maintained for 24 h in Dulbecco's modified Eagle's medium (DMEM, Gibco) medium containing 10% foetal calf serum (with 25 mM K+, 1 mM sodium pyruvate, 2 mM glutamine and antibiotics). Then cells were transferred into chemically defined medium consisting of DMEM supplemented with 5 μg ml−1 bovine insulin, 50 μg ml−1 human transferrin, 20 nM progesterone, 100 μM putrescine (Sigma), 30 nM sodium selenite (Gibco).
Cyclosporin A (Sandimmun, Sandoz, stock solution 50 mg ml−1) was added at different concentrations 6 days after plating of cells. The effect of the compound was monitored at various times up to 72 h by phase-contrast microscopy. MK-801 (Tocris, U.K.), soluble in water, was added to the medium at concentration of 1 μM.
Nuclear Hoechst 33258 staining
For nuclear DNA staining cultures were set up at a density of 50 cells/mm2 on 11-mm glass coverslips coated with poly-L-lysine. Six days after plating, the cultures were treated with CsA for 24 or 72 h. After the indicated period of time culture medium was aspirated, cells were washed with PBS and fixed with 70% ethanol at 4°C overnight. Then cells were washed once with PBS and stained with 50 ng ml−1 Hoechst 33258 (in PBS, pH 7.4). Stained cells were washed again with PBS and observed under fluorescence microscope Nikon Optiphot-2 with excitation at 330–380 nm.
Immunocytochemistry
For immunocytochemical staining, cultures were fixed in 4% paraformaldehyde for 30 min at room temperature. After several rinses in phosphate-buffered saline (PBS), the cultures were incubated with Tris-A (0.1% Triton X-100 in Tris-saline buffer) and Tris-B (10 ml of 2% BSA in Tris-A supplemented with normal serum) for 15 min each. The cultures were then incubated overnight with primary antibody in Tris-B at 4°C, washed in PBS/0.05% Triton X-100, and incubated for 2 h at room temperature with biotinylated horse anti-mouse IgG followed by avidin-biotin complex ABC (ABC Elite immunoperoxidase kit, Vector). Primary antibodies were as follows: monoclonal anti-glial fibrillary acidic protein–GFAP (Boehringer Mannheim, Germany) diluted 1 : 50, monoclonal anti-MAP2 protein (Sigma) diluted 1 : 100. Stained cultures were examined and photographed with an inverted Nikon Diaphot microscope using phase-contrast and bright-field optics.
Tunel labelling
The labelling of DNA breaks in situ was performed according the procedure described by Gavrieli et al. (1992) with some modifications. The cultures were fixed in 4% paraformaldehyde for 30 min at room temperature. After rinsing in PBS, the cultures were washed for 2 min in increasing concentrations of ethanol: 25, 50, 70, 90 and 100%. Air-dried cells were incubated for 1 h at 37°C in terminal deoxynucleotidyl transferase (TdT) buffer (30 mM Tris-HCl, pH 7.2, 140 mM sodium cocodylate, 1 mM cobalt chloride) containing TdT (0.3 u ml−1) and digoxigenin-11dUTP (0.2 nM) (Boehringer Mannheim, Germany). In negative controls TdT enzyme was omitted. Detection of incorporated digoxigenin-labelled nucleotides was done using the Nucleic Acid Detection Kit (Boehringer).
Measurement of intracellular calcium
Intracellular Ca2+ level was measured as described in (Baranska et al., 1995) with the following modifications. Cells cultured on coverslips were washed once with PBS, and the solution containing (mM): NaCl 137, KCl 2.7, Na2HPO3 1, glucose 25, HEPES (pH 7.4) 20, MgCl2 1, 1% (w v−1) bovine serum albumin and CaCl2 2 (later referred as a standard buffer). Then cells were incubated for 30 min in the standard buffer with 1 μM Fura-2 AM at 37°C. Thereafter, the cells were washed three times with the standard buffer and coverslips were mounted in a chamber over a Nikon Diaphot inverted-stage microscope equipped with ×40 oil-immersion fluorescence objective lens. Digital fluorescence microscopy was used to determine the changes in [Ca2+]i. Experiments were carried out on a video imaging system (MagiCal, Applied Imaging Ltd.). The cells were alternatively illuminated with 340 and 380 nm wavelengths of light from a xenon lamp. The emitted light was passed through a 510 nm barrier filter into an image-intensified camera (Extended ISIS, Photonic Science). The 340 nm and 380 nm images (256 grey levels) were software averaged and captured every 2.85 s. The 340 nm and 380 nm signals were examined for real changes in [Ca2+]i. Ratio (R) values were converted to an estimate of [Ca2+]i using the following formula (Grynkiewicz et al., 1985):
R–fluorescence ratio recorded from the cell, Rmin–fluorescence ratio in the absence of Ca2+, Rmax–fluorescence ratio in saturating concentration of Ca2+, Kd–calcium dissociation constant of the fura-2, b–the ratio of the fluorescence of Fura-2 Ca2+-free form to the Ca2+-saturated form recorded at 380 nm. Intracellular calibration was carried out by addition of 4 μM ionomycin to glioma C6 cells placed in a solution containing 2 mM Ca2+ (Rmax=2.3) and no added calcium with 5 mM EGTA (Rmin=0.3). The b value was 4.5 and Kd of 224 nM was assumed. Data processing and ratio values conversion to an [Ca2+]i were carried out using Tardis V8.0 software.
All substances were added as solutions in the standard buffer at final concentrations indicated in figures.
Data analysis
Data are expressed as the means±s.d. for eight cells randomly chosen from different coverslips, tested in typical experiment. Experiments were reproduced on three independently derived dentate gyrus cultures. Statistical significance was assessed by the Mann-Whitney U-test.
Results
Cyclosporin A treatment results in apoptotic cell death of neurons in hippocampal cultures
The developing dentate gyrus consists of two cell types: astrocytes, which form a monolayer of flatten, large cells and neurons growing on the top of astrocyte monolayer in groups formed by cell bodies with extended processes (Figure 1A). In mixed neuron/glial cultures we observed the pattern of changes in neuronal morphology induced by a 24-h exposure to CsA. Neurons exposed to 4 or 8 μM CsA exhibited cell shrinkage and loss of perikarya; indeed most of the neurite structures have been lost. Remaining cell bodies appear shrunken and condensed (Figure 1B,C). Observations at earlier time points (1–6 h) indicated no significant disturbances in cell morphology, and we observed a delayed form of neuronal cell death occurring over 6–24 h exposure. In cultures exposed to higher concentrations of CsA (40–200 μM) both neurons and astrocytes were killed rapidly during first few hours, undergoing acute cellular swelling and lysis, indicative of necrosis (Figure 1D). To identify the kind of cells (astroglial vs neuronal) committed to cell death in cultures treated for 24 h with 8 μM CsA, staining of cultures with MAP-2 antibody was performed. Degenerative morphological changes of neurons, especially affecting cell processes, were apparent (Figure 2). Surprisingly astrocytes from mixed neuronal/glial cultures were unaffected by exposure to CsA at doses toxic for neurons, however most of astrocytes exhibited vacuolization (Figure 2).
Figure 1.

Dose-dependent effects of cyclosporin A on morphology and survival of hippocampal neuronal/glial cultures. Hippocampal dentate gyrus cultures were prepared as described in Materials and methods. Cells were exposed to various doses of cyclosporin A and monitored for 24–72 h by phase-contrast microscopy at 20×. The panels show untreated cells (A); cultures treated with CsA: 8, 40, 200 μM (B, C, and D respectively).
Figure 2.
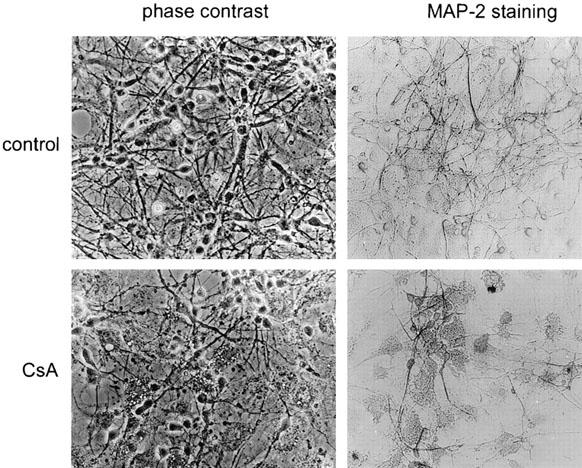
CsA affects survival of neuronal cell in dentate gyrus neuronal/astrocyte cultures. Staining with MAP-2 antibody revealed that treatment with 8 μM CsA affects preferentially neurons. Degenerative morphological changes of neurons, especially affecting cell processes, were apparent.
Staining with fluorescent dye Hoechst 33258 revealed the appearance of cells with apoptotic nuclei in mixed cultures exposed to 8 μM CsA for 24 h (Figure 3 upper panel). Visualization of in situ DNA fragmentation by TUNEL technique showed that in CsA-treated cultures, neurons exhibited significant DNA fragmentation indicated by the positively stained cells, as compared to control, untreated cultures. As shown in Figure 3 (lower panel) fragmented DNA is heavily labelled, and TUNEL-positive staining was observed only in neurons that developed pyknotic morphology. The number of TUNEL-positive cells increased with prolonged drug exposure.
Figure 3.

Nuclear alterations and DNA fragmentation in neurons of hippocampal neuronal/glial cultures treated with cyclosporin A. Representative micrographs show cultures exposed to 8 μM CsA for 0, 24 and 72 h; Upper panel shows CsA-treated cells with hypercondensed chromatin visualized by Hoechst 33258 staining (A, B, C), original magnification: ×200. Lower panel shows cells stained by the TUNEL staining method, typical TUNEL-positive cells indicated by arrows (D, E, F); original magnification: ×100. Cells were also stained with anti-GFAP antibody to visualize astrocytes in mixed cultures. All GFAP-positive cells are TUNEL-negative (G, H); original magnification: ×200.
Immunocytochemical staining with a monoclonal antibody that recognizes GFAP revealed that GFAP-positive cells (astrocytes) were not undergoing apoptosis as they were TUNEL-negative. All TUNEL-positive cells were neurons (Figure 3 lower panel, higher magnification). CsA at concentrations 8 μM did not affect astrocyte viability or alter the pattern of GFAP immunostaining (Figure 3).
Effect of MK-801 on hippocampal neuronal-glial cultures treated with CsA
In order to determine whether CsA-induced neuronal cell death is associated with activation of NMDA receptor, we investigated the effect of its selective antagonist–MK-801 (1 μM) in cultures treated with either CsA or glutamate for 24 h. As shown in Figure 4B, cells treated with MK-801 alone preserved their healthy morphology. In contrast cells exposed to either CsA (Figure 4C) or glutamate (Figure 4D) showed morphological changes typical of cell death such as somal shrinkage and rounding, dendrite fragmentation and/or regression. Pretreatment of the cultures with 1 μM MK-801 for 30 min prevents the neurotoxicity induced by glutamate (Figure 4F), whereas it has no effect on CsA-induced cell death (Figure 4E). Morphologically, apoptotic features such as nuclear condensation and fragmentation were prominent, as assessed by nuclear staining with Hoechst 33258. Detection of DNA fragmentation at the single cell level using the TUNEL method provided a clear demonstration of nuclear staining in cultures treated with CsA (Figure 5C) or CsA and MK-801 (Figure 5D). In control, untreated cultures (Figure 5A) and in cultures exposed to MK-801 alone (Figure 5B) positive staining could be seen only very rarely.
Figure 4.

Effect of MK-801 on morphological changes induced by CsA or glutamate in hippocampal mixed neuronal-glial cultures. The cultures were exposed for 24 h to MK-801–1 μM (B), CsA–8 μM (C) or glutamate–0.5 mM (D) and compared with control (untreated) cells (A). The lowest panel represents cultures treated with MK-801 and after 30 min exposed to CsA (E) or glutamate (F) for 24 h.
Figure 5.

Effect of MK-801 on DNA fragmentation induced by CsA treatment in mixed neuronal-glial cultures. Cyclosporin A was added 6 days after plating of cells. DNA fragmentation was detected using TUNEL method at 24 h after treatment in cells maintained under various conditions: in untreated cultures (A), cultures exposed to 1 μM MK-801 (B) or 8 μM CsA (C) alone, and MK-801 and CsA (D).
CsA treatment results in the increase of intracellular calcium level that is not blocked by MK-801
To determine whether cyclosporin A effect on hippocampal dentate gyrus neurones survival is associated with calcium neurotoxicity evoked by NMDA receptor activation, we have measured intracellular calcium level in cells treated with CsA. We also tested the calcium response to glutamate (NMDA receptor agonist) under these conditions as a positive control. Preincubation of cells with 8 μM cyclosporin A increased basal intracellular calcium level in time dependent manner (Figure 6, also summarized in Figure 7A), and decreased relative calcium response to 100 μM glutamate (Figure 6). To investigate the involvement of NMDA receptor in calcium elevation elicited by cyclosporin A, we have treated cells with the noncompetitive NMDA receptor blocker–MK-801, together with CsA incubation. An application of 1 μM MK-801 was without any effect on changes in Ca2+ level caused by CsA (Figure 7). This suggests that an increase in intracellular calcium concentration in neurons exposed to CsA is not dependent on calcium influx via NMDA channel.
Figure 6.
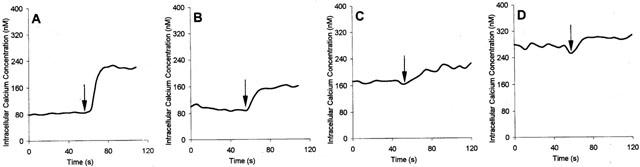
The effect of cyclosporin on intracellular calcium concentration and calcium response to glutamate in neurones. (A) Control cells, 100 μM glutamate added. Cells exposed to 8 μM CsA for: 1, 3 and 6 h (B–D, respectively). Each trace represents a mean value for eight cells tested in a typical experiment. Glutamate was added as indicated by arrows.
Figure 7.

MK-801–an antagonist of NMDA receptor–does not inhibit CsA-induced increase of intracellular calcium level. (A) The effect of 8 μM cyclosporin A on intracellular basal calcium concentration in hippocampal dentate gyrus neurons. The columns represent measurements of cytosolic calcium level in control cells, and after 1, 3 and 6 h of CsA treatment. The results are expressed as the means±s.d. of eight cells tested in typical experiment; similar results were obtained on three independently derived neuronal cultures (n=24). Asterisks indicate statistical significance: **P<0.005 (versus control) as estimated by the Mann-Whitney U-test. (B) The effect of NMDA receptor antagonist–MK-801 on changes in intracellular calcium elicited by 8 μM cyclosporin A after 6 h of incubation. The results are expressed as the means±s.d. of eight cells tested in typical experiment; similar results were obtained on two independently derived neuronal cultures. Differences were not statistically significant.
Discussion
In the present study we found that cyclosporin A, a highly specific inhibitor of calcineurin, affects the survival of neuronal/glial cultures from dentate gyrus. Hippocampal cultures exposed to 8–40 μg/ml CsA undergo cell death characterized by apoptotic changes in cellular and nuclear morphology. Immunocytochemical and TUNEL staining revealed that astrocytes from mixed neuronal/glial cultures were unaffected by exposure to CsA at dose toxic for neurons and all TUNEL-positive cells were neurons. CsA increased basal intracellular calcium level in time-dependent manner. However, changes in Ca2+ level caused by CsA were not blocked by a NMDA receptor antagonist–MK-801. Our findings demonstrate that changes in cell survival triggered by CsA might be dependent on an increase in intracellular calcium concentration. However the mechanism of this elevation is unlikely to be dependent on calcium influx via NMDA channel. CsA-induced morphological alterations of hippocampal neurons were similar to the one induced after exposure to 0.5 mM glutamate. Despite granule cells of dentate gyrus are believed to be particularly resistant to excitotoxic insult (Mattson & Kater, 1989), they undergo apoptosis after exposure to 0.5 mM glutamate (Figiel & Kaczmarek, 1997).
Our results showing the cytotoxic effects of CsA on hippocampal neurons are in agreement with those demonstrated by Mcdonald et al. (1996) on murine cortical cultures. In our hands, 8 μM CsA induced apoptosis of neurons in dentate gyrus neuronal/glial cultures. They have found that 5–20 μM CsA produced near-complete neuronal death in cortical cell cultures from foetal brains. The presence of DNA laddering and TUNEL staining of dying neurons in CsA-treated cultures (15 μM CsA) indicated the occurrence of apoptosis (McDonald et al., 1995).
Since the earlier visible effects of CsA treatment were those affecting neurite extentions, it is possible that the inhibition of calcineurin by CsA modifies cytoarchitectonics of neurons. Ferreira et al. (1993) demonstrated that calcineurin is associated with the cytoskeleton of cultured cerebellar neurons and has a role in the acquisition of polarity. Moreover, it has been found that calcineurin, enriched in growth cones, functions in neurite outgrowth and directs filopodial motility in cultured chick dorsal root ganglia neurons. Cyclosporin A and FK506 delayed neuritogenesis and inhibited neurite extension (Chang et al., 1995). Calcineurin A alpha−/− mice exhibited abnormalities in the cytoskeleton of their mossy fibres and a lower neurofilament/microtubule ratio than wild-type animals. These cytoskeletal changes are likely to have functional consequences on the affected neurons (Kayyali et al., 1997).
Influx of extracellular Ca2+ is considered to be the primary event responsible for glutamate toxicity. Since calcineurin is known to modulate activities of NMDA receptors that are highly permeable to Ca2+, we speculated that CsA induces progressive glutamate release and excitotoxic neuronal death due to disturbance of calcium homeostasis. We demonstrated that 8 μM CsA increased basal intracellular calcium level in time dependent manner. Relative calcium response to 0.1 mM glutamate (Figure 6) was decreased in CsA-treated cultures. However, the noncompetitive NMDA receptor blocker, MK-801 failed to prevent CsA-induced alterations in Ca2+ levels. This suggests that although neuronal death caused by cyclosporin may be dependent on the increase in cytoplasmic calcium level, the mechanism of this elevation is unlikely dependent on calcium influx via NMDA channels.
Immunosuppressants FK506 and cyclosporin A (both calcineurin inhibitors) have been shown to inhibit NMDA-evoked neurotoxicity in primary cortical cultures (Dawson et al., 1993; Ankarcrona et al., 1996). CsA inhibited the collapse of mitochondrial membrane potential observed during the exposure to glutamate, and this action was attributed rather to influence on CsA-binding protein (immunophilin D) which appears to be a component of mitochondrial megachannel (Ankarcrona et al., 1996). Dawson et al. (1993) showed that FK506 reduced the formation nitric oxide (NO) by inhibition the calcineurin mediated dephosphorylation of nitric oxide synthase (NOS). FK506 has neuroprotective effects on cerebral ischaemia and stroke (Sharkey & Butcher, 1994; Butcher et al., 1997). Although in vitro studies have implicated an antiexcitotoxic action involving nitric oxide and calcineurin, it has been shown that FK506 effect is rather mediated by FK506 binding proteins, as CsA did not have such neuroprotective effect (Butcher et al., 1997). All aforementioned studies were performed on neuronal cells exposed to excitotoxic insults, and did not address an issue of immunosuppressant effects on intact neurons.
CsA use as immunosuppressant is linked to central nervous system pathology as evidenced by a syndrome of confusion, agitation, psychosis, seizures and paralysis (Atkinson et al., 1984; de groen et al., 1987; Hauben, 1996). It is noteworthy that neurological alterations after CsA therapy are associated with the brain structures showing high calcineurin expression (Su et al., 1995). Our results strongly support the notion that side effects of the drug are due to promotion of the apoptotic cell death of brain neurons, glia and possibly other cell types. Although most data suggest that calcineurin is a target and mediates action of CsA on neuronal cells, we cannot exclude a possibility that inhibition of CsA binding proteins (immunophilines) may be partially responsible for this effect.
Some data suggest a novel role for calcineurin in the regulation of neuronal cell survival. Myocyte enhancer factor 2 (MEF2) has been shown recently to mediate activity-dependent neuronal survival in cultured cerebellar granule neurons. The specific inhibitors of calcineurin cyclosporin A and FK506 inhibited MEF2-dependent reporter gene expression which suggests that regulation of MEF2A by calcium signals requires the action of protein phosphatase calcineurin (Mao & Wiedmann, 1999). Furthermore another calcineurin target protein–NF-ATc4/NF-AT3 is expressed in hippocampal neurons and rapidly translocates from cytoplasm to nucleus activating NF-AT-dependent transcription in response to electrical activity or potassium depolarization (Graef et al., 1999). Recently it has been demonstrated that CsA and FK506 modulate the mechanisms involved in calcium homeostasis in cerebellar granule neurons. Immunosuppressants inhibited transcription of the IP3R1 (inositol 1,4,5-trisphosphate receptor type 1) gene regulated by calcium entry through L-type channels (Genazzani et al., 1999). Likewise, in hipocampal neurons the IP3R1 transcription is controlled by the calcium/calcineurin/NF-ATc pathway (Graef et al., 1999). Since IP3R1 is a major determinant of intracellular Ca2+ transients (Marks, 1997) and calcineurin modulates its expression and activity, inhibitors of calcineurin could alter the amplitude or distribution of the Ca2+ signals in neurons. The identification of novel targets of calcineurin may provide in part a biochemical explanation for the neurotoxic effects of CsA.
Acknowledgments
This work was supported by State Committee of Scientific Research (Poland) grant 4 P05A03709 (G. Mosieniak) and Phare SciTechII PL9611/003.10/4 (Center of Excellence for Studies on Neurodegeneration). B. Pyrzynska is a recipient of Foundation for Polish Science Fellowship.
Abbreviations
- CsA
cyclosporin A
- DMEM
Dulbecco's modified Eagle's medium
- EGTA
ethylene glycol-bis (b-amino ethyl ether) tetraacetic acid
- GFAP
glial fibrillary acidic protein
- JNK
c-Jun N-terminal kinase
- myocyte enhancer factor 2−MEF2; MAP-2
microtubule associated protein 2
- NF-AT
Nuclear Factor of Activated T cells
- NMDA
N-methyl-D-Aspartate
- NOS
nitric oxide synthase
- PBS
phosphate-buffered saline
- TUNEL
terminal transferase-mediated dUTP nick end labelling
References
- ANKARCRONA M., DYPBUKT J.M., ORRENIUS S., NICOTERA P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996;394:321–324. doi: 10.1016/0014-5793(96)00959-3. [DOI] [PubMed] [Google Scholar]
- ASAI A., QIU J.-H., NARITA Y., CHI S., SAITO N., SHINOURA N., HAMADA H., KUCHINO Y., KIRINO T. High level calcineurin activity predisposes neuronal cells to apoptosis. J. Biol. Chem. 1999;274:34450–34458. doi: 10.1074/jbc.274.48.34450. [DOI] [PubMed] [Google Scholar]
- ATKINSON K., BIGGS J., DARVENIZA P., BOLAND J., CONCANNON A., DODDS A. Cyclosporine-associated central nervous system toxicity after allogeneic bone marrow transplantation. Transplantation. 1984;38:34–37. doi: 10.1097/00007890-198407000-00009. [DOI] [PubMed] [Google Scholar]
- BARANSKA J., CHABAN V., CZARNY M., SABALA P. Changes in Ca2+ concentration in phorbol ester and thapsigargin treated glioma C6 cells . The role of protein kinase C in regulation of Ca2+ entry. Cell Calcium. 1995;17:207–215. doi: 10.1016/0143-4160(95)90035-7. [DOI] [PubMed] [Google Scholar]
- BUTCHER S.P., HENSHALL D.C., TERAMURA Y., IWASAKI K., SHARKEY J. Neuroprotective actions of FK506 in experimental stroke: in vivo evidence against an antiexcitotoxic mechanism. J. Neurosci. 1997;17:6939–6946. doi: 10.1523/JNEUROSCI.17-18-06939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG H.Y., TAKEI K., SYDOR A.M., BORN T., RUSNAK F., JAY D.G. Asymmetric retraction of growth cone filopodia following focal inactivation of calcineurin. Nature. 1995;376:686–690. doi: 10.1038/376686a0. [DOI] [PubMed] [Google Scholar]
- CLIPSTONE N.A., CRABTREE G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- DAWSON T.M., STEINER J.P., DAWSON V.L., DNIERMAN J.L., UHL G.R., SNYDER S.H. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9808–9812. [Google Scholar]
- DE GROEN P.C., AKSAMIT A.J., REKELA J. Central nervous system toxicity after liver transplantation. N. Engl. J. Med. 1987;317:861–886. doi: 10.1056/NEJM198710013171404. [DOI] [PubMed] [Google Scholar]
- FERREIRA A., KINCAID R., KOSIK K.S. Calcineurin is associated with the cytoskeleton of cultured neurons and has a role in the acquisition of polarity. Mol. Biol. Cell. 1993;4:1225–1238. doi: 10.1091/mbc.4.12.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIGIEL I., KACZMAREK L. Cellular and molecular correlates of glutamate-evoked neuronal programmed cell death in the in vitro cultures of rat hippocampal dentate gyrus. Neurochem. Int. 1997a;31:229–240. doi: 10.1016/s0197-0186(96)00152-0. [DOI] [PubMed] [Google Scholar]
- FIGIEL I., KACZMAREK L. Orthovanadate induces cell death in rat dentate gyrus primary culture. Neuroreport. 1997b;8:2465–2470. doi: 10.1097/00001756-199707280-00011. [DOI] [PubMed] [Google Scholar]
- FURMAN D.A., KLEE C.B., BIERER B.E., BURAKOFF S.J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK506 and cyclosporin A. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAVRIELI Y., SHERMAN Y., SASSON B.S.A. Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENAZZANI A.A., CARAFOLI E., GUERINI D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5797–5801. doi: 10.1073/pnas.96.10.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAEF I.A., MERMELSTEIN P.G., STANKUNAS K., NEILSON J.R., DEISSEROTH K., TSIEN R.W., CRABTREE G.R. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GUERINI D. Calcineurin: not just a simple protein phosphatase. Biochem. Biophys. Res. Commun. 1997;235:271–275. doi: 10.1006/bbrc.1997.6802. [DOI] [PubMed] [Google Scholar]
- HAUBEN M. Cyclosprine neurotoxicity. Neuropharmacology. 1996;16:576–583. [PubMed] [Google Scholar]
- KAYYALI U.S., ZHANG W., YEE A.G., SEIDMAN J.G., POTTER H. Cytoskeletal changes in the brains of mice lacking calcineurin A alpha. J. Neurochem. 1997;68:1668–1678. doi: 10.1046/j.1471-4159.1997.68041668.x. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN D.N., MODY I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- LEIST M., NICOTERA P. Apoptosis, excitotoxicity, and neuropathology. Exp. Cell Res. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- LIU J., FARMER J.D., JR, LANE W.S., FRIEDMAN J., WEISSMAN I., SCHREIBER S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MAO Z., WIEDMANN M. Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J. Biol. Chem. 1999;274:31102–31107. doi: 10.1074/jbc.274.43.31102. [DOI] [PubMed] [Google Scholar]
- MARKS A.R. Intracellular calcium release channels: regulators of life and death. Am. J. Physiol. 1997;272:597–605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- MATTSON M.P., KATER S.B. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989;490:110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- MCDONALD J.W., GOLDBERG M.P., GWAG B.J., CHI S.I., CHOI D.W. Cyclosporine induces neuronal apoptosis and selective oligodendrocyte death in cortical cultures. Ann. Neurol. 1996;40:750–758. doi: 10.1002/ana.410400511. [DOI] [PubMed] [Google Scholar]
- MOSIENIAK G., FIGIEL I., KAMINSKA B. Cyclosporin A, an immunosuppressive drug, induces programmed cell death in rat C6 glioma cells by a mechanism that involves the AP-1 transcription factor. J. Neurochem. 1997;68:1142–1149. doi: 10.1046/j.1471-4159.1997.68031142.x. [DOI] [PubMed] [Google Scholar]
- MOSIENIAK G., PYRZYNSKA B., KAMINSKA B. Nuclear Factor of Activated T cells (NFAT) as a new component of the signal-transduction pathway in glioma cells. J. Neurochem. 1998;71:134–141. doi: 10.1046/j.1471-4159.1998.71010134.x. [DOI] [PubMed] [Google Scholar]
- NICHOLS R.A., SUPLICK G.R., BROWN J.M. Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate. J. Biol. Chem. 1994;269:23817–23823. [PubMed] [Google Scholar]
- PYRZYNSKA B., LIS A., MOSIENIAK G., KAMINSKA B. Cyclosporin A-sensitive signalling pathway involving calcineurin regulates survival of reactive astrocytes. Neurochem. Int. 2001;38:409–415. doi: 10.1016/s0197-0186(00)00105-4. [DOI] [PubMed] [Google Scholar]
- PYRZYNSKA B., MOSIENIAK G., KAMINSKA B. Changes in the transactivating potential of the AP-1 transcription factor and activation of JNK pathway during cyclosporin A-induced apoptosis of glioma cells modify its transactivating potential. J. Neurochem. 2000;74:42–51. doi: 10.1046/j.1471-4159.2000.0740042.x. [DOI] [PubMed] [Google Scholar]
- RAO A., LUO C., HOGAN P.G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- SHARKEY J., BUTCHER S.P. Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature. 1994;371:336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- SIHRA T.S., NAIRN A.C., KLOPPENBURG P., LIN Z., POUZAT C. A role for calcineurin (protein phosphatase-2B) in the regulation of glutamate release. Biochem. Biophys. Res. Commun. 1995;212:609–616. doi: 10.1006/bbrc.1995.2013. [DOI] [PubMed] [Google Scholar]
- SU Q., ZHAO M., WEBER E., EUGSTER H.P., RYFFEL B. Distribution and activity of calcineurin in rat tissues. Evidence for post-translational regulation of testis-specific calcineurin. Eur. J. Biochem. 1995;230:469–474. doi: 10.1111/j.1432-1033.1995.0469h.x. [DOI] [PubMed] [Google Scholar]
- TONG G., SHEPHERD D., JAHR C.E. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- VICTOR R.G., THOMAS G.D., MARBAN E., O'ROURKE B. Presynaptic modulation of cortical synaptic activity by calcineurin. Proc. Natl. Acad. Sci. 1995;92:6269–6273. doi: 10.1073/pnas.92.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAKEL J.L. Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharmacol. Sci. 1997;18:124–134. doi: 10.1016/s0165-6147(97)01046-8. [DOI] [PubMed] [Google Scholar]


